Abstract
Background
Previously demonstrated safe and highly immunogenic in non-human primates, this study assessed DNA (pHIS-HIV-AE) prime, recombinant fowlpox (rFPV-HIV-AE) boost vaccines in humans.
Results
Eight participants (6 active vaccine, 2 placebo) received all vaccinations; local and systemic reactions were mild to moderate. The percentage CD4+ and CD8+ T cells responding to HIV-1 Gag antigens by ICS (mean ± SD) was 0.16 ± 0.12 and 0.10 ± 0.12 for active and 0.01 ± 0.01 and 0.00 ± 0.00 for placebo vaccine respectively. The percentage of T cells responding did not reach pre-defined thresholds to be considered positive responses. Consequently, the Data Safety Monitoring Board recommended cessation of further recruitment. Existing volunteers were followed to 52 weeks.
Methods
Vectors expressing homologous HIV-1 clade A/E gag, pol, env and regulatory genes or matched placebo were administered intramuscularly at weeks 0, 4, 8 (6 mg pHIS-HIV-AE) and week 12 (3.0 × 108 pfu rFPV-HIV-AE) in this randomized, double-blind, placebo-controlled phase I/IIa study in healthy Thai adults at low risk of HIV infection. Immunogenicity was determined by interferon-gamma and IL-2 expression using intracellular cytokine staining assay (ICS ), 13 weeks after randomization. Interim analysis was performed when eight volunteers reached 16 weeks follow-up.
Conclusions
Vaccine candidates were generally well tolerated, but showed limited immunogenicity. Better vaccines and delivery systems are required.
Key words: HIV, AIDS vaccine, DNA/fowlpox, clinical trial, Thailand
Introduction
Since 1992, extensive national education and prevention programs in Thailand have lead to a substantial decline in HIV prevalence, including in women attending antenatal clinics, military recruits and in certain groups of commercial sex workers.1,2 The number of infections in Thailand has fallen, from a peak of approximately 143,000 new infections per year in 1991 to around 14,000 new infections estimated for 2006.2,3 Current data suggest that these new infections are in injecting drug users and women infected by their husbands or sexual partners.2 In addition, 17.3% and 28.3% of men who had sex with men were HIVpositive in Thai Ministry of Public Health surveys conducted in Bangkok in 2003 and 2005, respectively and in 2006, only 19.2% reported always using a condom with sexual partners in the previous three months.4
Although effective treatments for HIV infection exist with reasonable availability in Thailand, prevention remains the most sustainable strategy to curb the HIV-1 pandemic. A safe and effective vaccine for HIV-1 infection is urgently needed. Intensive efforts are underway world wide to develop vaccines to prevent or reduce the pathogenicity of HIV infection. In Thailand, the major dominant HIV-1 subtype is the circulating recombinant form, CRF01_AE, although very few candidate vaccines have been targeted specifically to this subtype.5–12
The generation of CTL and T-helper lymphocytes was widely seen to be critical to the success of an HIV vaccine, due to the temporal correlation with the control of acute viremia in humans.13–18 Stimulation of these effector responses following administration of exogenous antigens is dramatically improved using vector-delivered vaccines, such as those based on recombinant vaccinia and avian pox viruses (canarypox and fowlpox viruses), genetically engineered to express HIV-1 proteins.
Kelleher et al. conducted a phase I/IIa clinical trial in Sydney, Australia to assess the safety and immunogenicity of a clade B DNA prime (pHIS-HIV-B) and recombinant fowlpox virus boost (rFPV-HIV-B).19 Similar vaccines had shown significant T-cell immunogenicity in non-human primate models.20 The DNA vaccine contained 65% of the HIV-1 genome including gag, pol, env, tat and rev, while the recombinant fowlpox virus contained gag and pol only. One milligram of pHIS-HIV-B was injected at weeks 0 and 4, followed by 5 × 107 pfu rFPV-HIV-B boost at week 8. Although the vaccine regimen was safe, no significant vaccineinduced CTL responses were observed. It was postulated that the differences between macaques and humans might be due to the relative dose response curve difference between the two species.21 The dose that had been used in the clade B trial was equivalent to the lowest dose examined in cynomolgus monkeys (0.8 mg/m2 for pHIS-HIV-B, boosted with rFPV-HIV-B 4 × 107 pfu/m2). Furthermore, the lowest dose in macaques that produced broad immunogenicity was 3.9 mg/m2 for pHIS-HIV-B, boosted by rFPV-HIV-B 1.9 × 108 pfu/m2. Therefore, increased doses of the vaccine may be desirable to produce CTL responses in humans.
Similar candidate vaccines to those used in the Sydney trial were constructed containing homologous HIV-1 A/E sequences. These produced reactive HIV-specific responses in both CD4+ and CD8+ T cells, with a peak one week after the rFPV-HIV-AE boost in vaccinated pigtail macaques.22–25 The pHIS-HIV-AE/rFPV-HIV-AE vaccine regimen was well tolerated and broad induction of T-cell responses to multiple HIV antigens was observed in all animals studied.20,25 In the current phase I/IIa clinical trial in healthy, Thai volunteers, doses of pHIS-HIV-AE/rFPV-HIV-AE vaccine were increased to 6 mg DNA and 3 × 108 pfu.
Results
Recruitment, disposition and baseline characteristics.
In the initial phase of recruitment, 14 individuals were screened of whom seven were ineligible; two were HIV antibody-positive, one had hemolytic anaemia, one was hepatitis B surface antigen-positive, one had elevated liver function tests and two had uncontrolled hypertension. One volunteer was re-screened once their hypertension was under therapeutic control. Eight eligible volunteers were randomly allocated to receive either active vaccine (n = 6) or placebo (n = 2). All volunteers attended all visits and received all vaccinations, as per protocol. One volunteer was lost to follow up after week 36 and the remaining volunteers were followed-up until 52 weeks. Selected baseline characteristics are summarized (Table 1). Participants were exclusively Thai and male, and were well matched for age.
Table 1.
Selected baseline characteristics
| Total | Vaccine | Placebo | |
| Volunteers, N | 8 | 6 | 2 |
| Gender, male (%) | 8 (100.0) | 6 (100.0) | 2 (100.0) |
| Age, mean ± SD years | 40.5 ± 7.9 | 42.0 ± 8.6 | 38.0 ± 6.4 |
Immunogenicity.
The CMV, EBV and Influenza combined peptide pool and SEB-control ICS responses were robust and stable throughout 16 weeks for each group (data not shown).
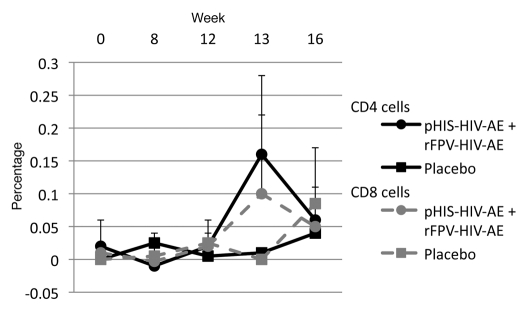
Borderline CD4+ T-cell responses to the HIV-1 A/E Gag pool were detected in four of six vaccinees (Fig. 1); this response was transient in two recipients and was sustained between week 13 and 16 in a further two recipients. When compared to our predetermined cutoffs, none of these responses satisfied the criteria for a positive response and at best were borderline positive (≤0.30%). The CD4+ T-cell response to the Gag antigen pool was the best response, although notably below or equal to that of CMV at baseline (Table 2). There were no responses to any other antigen pool in CD4+ T cells.
Figure 1.
Intracellular Cytokine Staining in Response to A/E Clade Gag Pool Peptides. ICS in CD4+ and CD8+ T cells in response to A/E Clade Gag Pool peptides + CD28/CD49d. Data are presented as mean ± SD for pHIS-HIV-AE/rFPV-HIV-AE recipients (n = 6) and mean only for placebo recipients (n = 2).
Table 2.
T-cell immunogenicity by ICS to A/E clade HIV antigen stimulation at weeks 0 and 13 in vaccine and placebo recipients
| CD4+ T cells | Vaccine (n = 6) | Placebo (n = 2) | p* | ||
| Antigen pool | Week 0 | Week 13 change | Week 0 | Week 13 change | |
| SEB | 5.21 (1.65) | 1.26 (2.23) | 4.96 (2.63) | −0.98 (2.14) | 0.24 |
| CMV | 0.77 (0.96) | −0.05 (0.33) | 0.44 (0.43) | −0.06 (0.02) | 1.00 |
| AE clade Gag Pool + CD28/CD49d | 0.02 (0.04) | 0.16 (0.12) | 0 | 0.01 (0.01) | 0.13 |
| AE clade Pol Pool + CD28/CD49d | 0.03 (0.04) | 0.03 (0.04) | 0 | 0 (0) | 0.39 |
| AE clade Tat/Rev Pool + CD28/CD49d | 0.04 (0.06) | 0.03 (0.05) | 0 | 0.02 (0.03) | 0.74 |
| AE clade Env Pool + CD28/CD49d | 0.06 (0.08) | 0.08 (0.09) | 0.015 (0.02) | −0.01 (0.01) | 0.24 |
| CD8+ T cells | Vaccine (n = 6) | Placebo (n = 2) | p* | ||
| Antigen pool | Week 0 | Week 13 change | Week 0 | Week 13 change | |
| SEB | 3.95 (1.89) | 1.88 (2.41) | 3.64 (2.06) | −0.88 (1.75) | 0.24 |
| CMV | 0.31 (0.54) | 0.25 (0.51) | 0.09 (0.04) | −0.02 (0.01) | 0.18 |
| AE clade Gag Pool + CD28/CD49d | 0.01 (0.01) | 0.10 (0.12) | 0 | 0 | 0.59 |
| AE clade Pol Pool + CD28/CD49d | 0.01 (0.01) | 0.02 (0.08) | 0.04 (0.04) | −0.04 (0.04) | 0.23 |
| AE clade Tat/Rev Pool + CD28/CD49d | 0.02 (0.02) | 0.03 (0.05) | 0.05 (0.06) | −0.05 (0.07) | 0.18 |
| AE clade Env Pool + CD28/CD49d | 0.02 (0.02) | 0.08 (0.15) | 0.07 (0.05) | −0.02 (0.12) | 0.62 |
Responses are mean (SD).
p represents a formal comparison of change from baseline to week 13 for each antigen between vaccine and placebo arms using a Mann Whitney U test.
CD8+ T-cell responses to HIV-1 A/E Gag pool were observed in three of six vaccinees; the response in one recipient was sustained between weeks 13 and 16 and was transient in the two other recipients. None of these responses satisfied the criteria for a positive response. These were the same volunteers with CD4+ responses and one of these volunteers also had a transient borderline positive response to the HIV-1 A/E Pol pool. There was no response recorded to the other HIV genes encoded in the vaccine, either in CD4+ or CD8+ T cells. In light of the lack of immunogenicity, scheduled ELISpot and fowlpox reactogenicity assays were not performed. There were no responses above background response to any of the other HIV proteins included in the vaccines.
There were no positive responses to the HIV antigens in the placebo group. No individual exhibited HIV antibody bands developed in western blots at any tests up to week 24.
Safety.
There were no serious adverse events (Table 3) and there were no new HIV infections. The vaccines were generally well tolerated, although all vaccine recipients experienced chills or fevers. Two of six vaccine recipients reported severe chills or fevers in the days following rFPV-HIV-AE vaccination. There were no other local or systemic adverse events or laboratory abnormalities graded greater than II. Events were equally distributed between the two groups. Mild to moderate pain at the injection site was experienced by four of six vaccine recipients. Myalgia, asthenia, headaches nausea, influenza-like symptoms and upper respiratory tract infections and other events were equally distributed between vaccine groups.
Table 3.
Summary of vaccine safety
| Table 3A. Number of adverse events and number of volunteers experiencing adverse events during 52 weeks on study. | ||||
| Treatment | Vaccine (n = 6) | Placebo (n = 2) | ||
| Severity | No. events | No. vaccine recipients with these events (%) | No. events | No. placebo recipients with these events (%) |
| Mild | 56 | 6 (100) | 8 | 2 (100) |
| Moderate | 23 | 6 (100) | 2 | 2 (100) |
| Severe | 2 | 2 (33) | 0 | 0 (0) |
| Total | 81 | 6 (100) | 10 | 2 (100) |
| Table 3B. Number of protocol-defined laboratory adverse events during 52 weeks on study. | ||||
| Treatment | Vaccine (n = 6) | Placebo (n = 2) | ||
| Grade | No. events | No. vaccine recipients with these events (%) | No. events | No. placebo recipients with these events (%) |
| I | 14 | 4 (66) | 3 | 1 (50) |
| II | 0 | 0 (0) | 1 | 1 (50) |
| III | 0 | 0 (0) | 0 | 0 (0) |
| IV | 0 | 0 (0) | 0 | 0 (0) |
| Total | 14 | 4 (66) | 4 | 1 (50) |
In light of the coincident data from the STEP Study, the Protocol Steering Committee concluded that it was appropriate to review the vanguard cohort week 16 immunogenicity data at the same time as the pre-scheduled review of the safety data.27 As a result, the DSMB and Protocol Steering Committee recommended cessation of any further recruitment and closure of the trial.
Discussion
An initial randomized, placebo-controlled clinical trial of heterologous HIV-1 B clade DNA prime expressing mutated gag, pol, env, tat and rev sequences, followed by a recombinant fowlpox vector expressing gag and pol boost found that the regimen was well tolerated, but failed to generate significant immunogenicity in humans.19 This was despite the broad and marked immunogenicity seen by IFNγ ELISpot in non-human primates at all doses.20 Notably, in the recent STEP Study with the MRK Ad5 gag/pol/env vector, there was good T-cell immunity generated as measured by ELISpot, but this did not translate into sterilizing immunity and did not reduce viral load in those becoming infected.27,28
In preclinical studies in pigtail macaques using the current A/E clade DNA/rFPV HIV vaccine candidates, 12 and seven out of 12 vaccinated animals produced significant CD4+ and CD8+ T-cell responses to HIV-1 A/E Gag antigen pools, respectively, as measured by ICS.20 In these macaque studies and human studies with the B clade vaccine, ICS and ELISpot produced immune response curves with similar characteristics.19,20 ICS was chosen at the primary end point in the current study due to its ability to discern between CD4+ and CD8+ responses and therefore yield further information. However, when these same vaccines were administered to humans, there were no positive anti-Gag responses in any vaccine, when compared to the pre-determined cutoff levels. In the preclinical macaque experiments, there were also antibodies to all HIV-1 antigens found within the vaccines detected, although there were none to the antigens assayed by Western blot in any volunteer in the current human trial.
The vaccines were given by the same route, using the same regimen in both macaque and human studies. The dose required to induce similar immune responses may be higher in humans. It has been suggested that the method of calculating the relative normalized dose may be underestimating the amount of vaccine required to produce similar immunogenicity as seen in macaques.21 Macaques are on average 2 to 4 kilograms in weight and de Rose and colleagues suggested that a dose normalized for body surface area (BSA) may actually be more informative when calculating the dose response curve of the current DNA and rFPV vaccines. When calculated in terms of BSA, the doses used in the current clinical trial were 4.0 mg/m2 pHIS-HIV-AE and 1.3 × 108 pfu/m2 rFPV-HIV-AE. These doses were close to the maximum that were ethically and physically practical; the pHIS-HIV-AE vaccine was produced at the maximum concentration to avoid precipitation and maintain biologically active conformation; the DNA and fowlpox vaccinations were given intramuscularly in 4.0 and 6.0 mL respectively. The doses were equivalent to those at the minimum that achieved readily detectable T-cell immunity in macaques and six times that utilized in the previous B clade trial.19,20 Data from other groups suggest that DNA vaccines only become immunogenic at doses of approximately 4.0 mg.29,30 The dose may well have been below a threshold to induce T-cell immunogenicity and production of higher concentration presents challenges for vaccine development and manufacturing of these particular DNA constructs.
The DNA vaccine used here has included in its design a number of novel factors that were included to boost the immunogenicity of the vaccine, including the intron to increase protein expression and the CpG motif, intended to act as an endogenous adjuvant. Despite these putative enhancers of expression and immunogenicity, the vaccines remain suboptimally immunogenic, even at higher doses. This may indicate that these enhancers are not effective and even may be problematic. Their inclusion increases the size of the plasmid, which then limits the extent to which the vaccine can be concentrated while maintaining criteria of GLP manufacture. As a result, the relatively low concentration limited the dose of vaccine able to be given at any one physical site. Therefore, multiple doses had to be administered at multiple sites in order to achieve the desired total dose. This may have limited the extent of cell transfection and antigen expression at any site. This generation of potentially suboptimal antigen expression at several different locations, rather than a large amount of antigen at one site may limit the efficacy of the priming of the response. Therefore, this may have limited the immunogenicity of the entire regimen, especially in an environment such as muscle where low levels of antigen presenting cells exist.
Following vaccine development and commencement of the clinical study, it was noted that the fowlpox strain used successfully to induce antigenic responses in other disease states was the FP9 strain, while our studies utilized the Webster's mild vaccine strain.31,32 This strain has subsequently been shown to be less able to induce potent immune responses when used as a viral vaccine vector and may explain the low immunogenicity of the vaccine candidate.33
Better vaccine delivery systems may assist in enhancing immunogenicity, particularly for the DNA vaccine. Promising studies in mice and non-human primate models have shown significant increases in HIV-specific T-cell responses when DNA vaccine was immunized by in vivo electroporation.34,35 However, the STEP trial indicates that T-cell immunogenicity as judged by IFN-g/IL-2 responses does not imply protection.26,27 Of note, current technology does not allow for the induction of potent neutralizing antibodies that may prove valuable in the search for an active vaccine. However, the recent results of an ALVAC prime, protein boost regimen that does not induce neutralizing antibodies, may offer some protection. Therefore challenge is twofold; the type of immune response required for prevention of infection is not understood and the most efficient way to induce these responses is also far from clear. If a DNA vaccine is going to play a role in an effective HIV vaccine, it will need to be either a small plasmid capable of being given at large doses in relatively small volumes or a simple mechanism for enhancing the immunogenicity of DNA vaccines in humans.
Volunteers and Methods/Materials
Vaccine design and construction.
The DNA vaccine, based on pHIS-64 (Coley Pharmaceutical Group, Inc., Boston, MA) was previously described and designated pHIS-HIV-AE.24 This construct contains approximately 65% of the HIV-1 genome including modified gag, modified pol, rev, tat, truncated env and truncated nef derived from the subtype A/E isolate p93TH253.3 (NIH AIDS Research and Reference Reagent Program, NIH, Bethesda, MD, USA). To enhance safety, the gag zinc finger RNA encapsidation signal was deleted, various regions of reverse transcriptase, RNase H and integrase were deleted or modified and the CD4 binding domain was deleted, while maintaining the Rev responsive element and rev coding region.
Insert expression, under the control of the CMV intermediate early promoter, was optimized by a synthetic intron placed immediately prior to HIV-1 gene coding sequences. Humanized CpG motifs were included with the intention of increasing immunogenicity of this vector.20 The construct, synthesized to GMP, was resuspended in 0.9% saline at 1.5 mg/mL (Qiagen, Germany).
The rFPV-HIV-AE construct was based on attenuated strain FPV-M3 and contained subtype A/E HIV-1 genes; modified gag/pol, truncated env and modified tat/rev expressed under the control of the fowlpox early/late promoter.22–24 Both vaccines are essentially homologous for inserted HIV-1 sequences. The construct, manufactured to GMP (Virax, Melbourne, Australia), was diluted to 5 × 107 pfu/mL in 0.9% saline, 10% glycerol. Identical placebos for both constructs were manufactured to GMP standards and contained diluent only.
Study design.
This was a single-centre, randomized, placebo-controlled, double blind phase I/IIa clinical study that aimed to enrol 24 eligible volunteers to either vaccine or matched placebo in a 3:1 ratio, stratified by gender. Recruitment was planned in two phases; eight participants were to be recruited prior to an independent DSMB review of all safety and immunogenicity data at week 16. Recruitment of the remaining 16 participants was contingent on satisfactory DSMB and Protocol Steering Committee review of the interim analysis.
Eligible volunteers were healthy HIV-negative Thais at no identifiable risk for HIV infection, 18 to 55 years old, in good health by clinical status and laboratory parameters, neither hepatitis B surface antigen or hepatitis C virus positive and had not received active candidate HIV vaccines previously, nor any other vaccine or investigative agent within the past 60 days.
The primary objectives were safety and immunogenicity; the primary immunogenicity endpoint was the mean difference in change in percent of CD4+ and/or CD8+ T cells expressing IFNγ or IL-2 by intracellular cytokine staining (ICS) assay in response to a Gag peptide pool from baseline to week 13.
The study protocol was approved by the institutional review boards of Chulalongkorn University, the Ministry of Public Health, Sub-committee on HIV/AIDS Vaccine Development, Bangkok, Thailand and the University of New South Wales, Sydney, Australia. Informed consent was obtained from each volunteer in accordance with guidelines of both the local institution and regulatory bodies.
Vaccine administration.
Vaccine recipients received 6.0 mg pHIS-HIV-AE in 4.0 mL sterile 0.9% saline at weeks 0, 4 and 8 and 3.0 × 108 pfu rFPV-HIV-AE in 6.0 mL sterile PBS 10% glycerol at week 12 into the deltoid, thigh and/or gluteal muscles. Placebo recipients received diluent only. Multiple injections were given at each visit to allow for the large volumes of administration.
Immunogenicity assays.
ICS assays were validated and performed at Chula-MRC, Faculty of Medicine, Chulalongkorn University. The ICS assay required 500 µL aliquots of fresh heparinized whole blood incubated for 6 hours at 37°C in the presence of monoclonal antibodies (mAbs) CD28 and CD49d, various antigens (peptide pools representing each of Gag, Pol, Env, Tat/Rev) with 10 µg/mL brefeldin A (Sigma-Aldrich Co., USA) added after 2 hours. 2 mM EDTA was then added, red cells lysed (FACS Lysing Solution, Becton Dickinson, NJ, USA), cells fixed, permeabilized (FACS Permeabilizing 2 Solution, Becton Dickinson), stained with mAbs: CD3-PerCP, CD4-APC, CD8-PE, anti-IFNγ-FITC and anti-IL-2-FITC (Becton Dickinson), resuspended in 0.5% paraformaldehyde and analyzed on FACS Calibur Flow Cytometer (Becton Dickinson). Results were expressed as % CD3+CD4+ or % CD3+CD8+ cells expressing IL-2 and/or IFNγ. Antigens were pooled 15mer peptides (overlapping by 11 amino acids) representing the A/E Gag pool, Env pool, combined Tat/Rev pool and Pol pool. These peptides were homologous to the sequences within the vaccine and were synthesized to >90% purity (Auspep, Melbourne, Victoria). A pool of peptides of previously described CD8+ T-cell epitopes from CMV, EBV and Influenza (Auspep, Melbourne Victoria) (Hansasuta P, personal communication) combined with a CMV lysate (Advanced Biotechnologies, MD, USA) was used as a positive control for antigen driven responses. SEB (Sigma, final concentration 1 µg/ml) superantigen was used as mitogen positive control. All peptides were used at a final concentration of (2 µg/ml).
Safety monitoring.
Safety was assessed by clinical review of laboratory parameters. HIV serology by routine ELISA to HIV 1–2 Env10, Env13, p24 and Env AL antigens (Vitros Immunodiagnostic Anti-HIV 1 + 2; Ortho-Clinical Diagnostics Inc., USA; Johnson and Johnson Company, USA) and gelatin particle agglutination (Trinity Biotech Capillus™ HIV1/HIV2, Carlsbad, USA) was performed at screening, randomization and all scheduled or unscheduled follow-up visits. Study investigators were blinded to HIV serology results. Pre and post HIV test counselling, including risk behavior assessment and safe sex and injecting counselling were performed at each visit.
Statistical analysis.
The week 16 interim analysis included data from all randomized volunteers who received any blinded study vaccines. Safety data, including adverse events and clinical laboratory data were listed by treatment group to week 24. Adverse event severity was graded using the DAIDS toxicity scale.
All ICS data were summarized according to active or placebo vaccine received, study week and number of volunteers with predefined positive responses. Extensive data were generated to optimize and validate the ICS assay at the Bangkok laboratory using both HIV-negative and positive controls. Positive cutoffs were determined using the quality-assured and SOP-adherent dataset by an in-house statistician prior to volunteer recruitment. A positive response (minus background) was defined as IL-2/IFNγ expression in response to the HIV-1 A/E Gag pool in either CD4+ or CD8+ T cells at levels: (1) greater than two times background and (2) greater than the predetermined level for a true positive response in this population of 0.3% and 0.65% for CD4+ and CD8+ T cells, respectively. To be consistent with the STEP Study MRKAd5 HIV-1 Gag/Pol/Nef trivalent vaccine ELISpot data, the proportion of vaccinees exhibiting a positive response had to be greater than two thirds, i.e., four out of six volunteers in order to be deemed a positive result.26 As there were only two volunteers in the control arm, mean responses were presented.
Acknowledgements
NCHECR is funded by the Australian Government Department of Health & Ageing and is affiliated with the Faculty of Medicine, The University of New South Wales. The vaccines were designed with the assistance of NIH-NIAID-DAIDS HVDDT Award N01-AI-05395.
The authors would like to thank Wadchara Pumpradit for his assistance in the preparation and initial drafting of trial protocols and regulatory documents. We also wish to thank members of the Australia Thai HIV Vaccine Consortium including the CSIRO, Australian National University, University of Melbourne, Australian Federation of AIDS Organisations, National Centre in HIV Social Research, University of New South Wales for the design and manufacture of vaccines and assistance with study protocol drafting and initiation.
We would like to acknowledge Ormrudee Rit-im, Wendy Lee, Noorul Absar, Charles Tran, Pitch Boonrak and Ekkasit Thodsanit for database and statistical assistance, Nounpen Sangthong, Narumon Subsri, Kanitta Pussadee and Peeraporn Keaw-on for clinical care, Parinya Sutheerasak for pharmacy expertise and Meeling Munier, Kate Merlin, Supranee Buranapraditkun, Pornsupa Chatkulkawin for their laboratory input.
Abbreviations
- BSA
body surface area
- CMV
cytomegalovirus
- CTL
cytotoxic T lymphocytes
- DSMB
data safety monitoring board
- ICS
intracellular cytokine staining assay
- mAbs
monoclonal antibodies
- pHIS-HIV-AE
AE clade DNA vaccine
- pHIS-HIV-B
B clade HIV vaccine
- rFPV-HIV-AE
AE clade recombinant fowlpox HIV vaccine
- rFPV-HIV-AE
B clade recombinant fowlpox HIV vaccine
- SEB
staphylococcal enterotoxin B
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/12635
Financial Support
Salaries for those working in the Vaccine and Cellular Immunology Laboratory were kindly supported in part by grants from the National Center for Genetic Engineering and Biotechnology, Thailand.
References
- 1.Ruxrungtham K, Brown T, Phanuphak P. HIV/AIDS in Asia. Lancet. 2004;364:69–82. doi: 10.1016/S0140-6736(04)16593-8. [DOI] [PubMed] [Google Scholar]
- 2.UNGASS Country Progress Report: Thailand, National AIDS Prevention and Alleviation Committee. National Plan for the Prevention and Alleviation of AIDS in Thailand; 2008. [Google Scholar]
- 3.Brown T, Peerapatanapokin W For the Thai Working Group on HIV/AIDS Projection, author. Projections for HIV/AIDS in Thailand: 2000–2020. Bangkok: Division of AIDS, Department of Disease Control, Ministry of Public Health; 2001. [Google Scholar]
- 4.van Griensven F, Thanprasertsuk S, Jommaroeng R, Mansergh G, Naorat S, Jenkins RA, et al. Evidence of a previously undocumented epidemic of HIV infection among men who have sex with men in Bangkok, Thailand. AIDS. 2005;19:521–526. doi: 10.1097/01.aids.0000162341.50933.e8. [DOI] [PubMed] [Google Scholar]
- 5.Mason CJ, Kitsiripornchai S, Markowitz LE, Chanbancherd P, Supapongse T, Jugsudee A, et al. Nationwide surveillance of HIV-1 prevalence and subtype in young Thai men. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:165–173. doi: 10.1097/00042560-199810010-00010. [DOI] [PubMed] [Google Scholar]
- 6.Ruxrungtham K, Phanuphak P. Update on HIV/AIDS in Thailand. J Med Assoc Thai. 2001;84:1–17. [PubMed] [Google Scholar]
- 7.Weniger BG, Takebe Y, Ou CY, Yamazaki S. The molecular epidemiology of HIV in Asia. AIDS. 1994;82:13–28. [PubMed] [Google Scholar]
- 8.Karnasuta C, Paris RM, Cox JH, Nitayaphan S, Pitisuttithum P, Thongcharoen P, et al. Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine. 2005;23:2522–2529. doi: 10.1016/j.vaccine.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Nitayaphan S, Pitisuttithum P, Karnasuta C, Eamsila C, de Souza M, Morgan P, et al. Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative Thai adults. J Infect Dis. 2004;190:702–706. doi: 10.1086/422258. [DOI] [PubMed] [Google Scholar]
- 10.Pitisuttithum P, Nitayaphan S, Thongcharoen P, Khamboonruang C, Kim J, de Souza M, et al. Safety and immunogenicity of combinations of recombinant subtype E and B human immunodeficiency virus type 1 envelope glycoprotein 120 vaccines in healthy Thai adults. J Infect Dis. 2003;188:219–227. doi: 10.1086/376506. [DOI] [PubMed] [Google Scholar]
- 11.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, van Grievsen MH, Hu D, et al. Randomized, doubleblind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 12.Thongcharoen P, Suriyanon V, Paris RM, Khamboonruang C, de Souza MS, Ratto-Kim S, et al. A phase 1/2 comparative vaccine trial of the safety and immunogenicity of a CRF01_AE (subtype E) candidate vaccine: ALVAC-HIV (vCP1521) prime with oligomeric gp160 (92TH023/LAI-DID) or bivalent gp120 (CM235/SF2) boost. J Acquir Immune Defic Syndr. 2007;46:48–55. doi: 10.1097/QAI.0b013e3181354bd7. [DOI] [PubMed] [Google Scholar]
- 13.Clerici M, Berzofsky JA, Shearer GM, Tacket CO. Exposure to human immunodeficiency virus (HIV) type I indicated by HIV-specific T helper cell responses before detection of infection by polymerase chain reaction and serum antibodies. J Infect Dis. 1991;164:178–182. doi: 10.1093/infdis/164.1.178. [DOI] [PubMed] [Google Scholar]
- 14.Clerici M, Giorgi JV, Chou CC, Gudeman VK, Zack JA, Gupta P, et al. Cell-mediated immune response to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure to HIV-1. J Infect Dis. 1992;165:1012–1019. doi: 10.1093/infdis/165.6.1012. [DOI] [PubMed] [Google Scholar]
- 15.Kaul R, Rowland-Jones SL, Kimani J, Dong T, Yang HB, Kiama P, et al. Late seroconversion in HIV-resistant Nairobi prostitutes despite pre-existing HIV-specific CD8+ responses. J Clin Invest. 2001;107:341–349. doi: 10.1172/JCI10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMichael AJ, Ogg G, Wilson J, Callan M, Hambleton S, Appay V, et al. Memory CD8+ T cells in HIV infection. Philos Trans R Soc Lond B Biol Sci. 2000;355:363–367. doi: 10.1098/rstb.2000.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 18.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 19.Kelleher AD, Puls RL, Bebbington M, Boyle D, Ffrench R, Kent SJ, et al. A randomized, placebo-controlled phase I trial of DNA prime, recombinant fowlpox virus boost prophylactic vaccine for HIV-1. AIDS. 2006;20:294–297. doi: 10.1097/01.aids.0000199819.40079.e9. [DOI] [PubMed] [Google Scholar]
- 20.De Rose R, Chea S, Dale CJ, Reece J, Fernandez CS, Wilson KM, et al. Subtype AE HIV-1 DNA and recombinant fowlpoxvirus vaccines encoding five shared HIV-1 genes: safety and T cell immunogenicity in macaques. Vaccine. 2005;23:1949–1956. doi: 10.1016/j.vaccine.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 21.De Rose R, Batten CJ, Smith MZ, Fernandez CS, Peut V, Thomson S, et al. Comparative efficacy of subtype AE simian-human immunodeficiency virus priming and boosting vaccines in pigtail macaques. J Virol. 2007;81:292–300. doi: 10.1128/JVI.01727-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle DB, Anderson MA, Amos R, Voysey R, Coupar BE. Construction of recombinant fowlpox viruses carrying multiple vaccine antigens and immunomodulatory molecules. Biotechniques. 2004;37:104–106. doi: 10.2144/04371RR02. [DOI] [PubMed] [Google Scholar]
- 23.Coupar BE, Purcell DF, Thomson SA, Croom HA, Thomson S, Coupar BE, et al. Fowlpox virus vaccines for HIV and SHIV clinical and pre-clinical trials. Vaccine. 2006;24:1378–1388. doi: 10.1016/j.vaccine.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 24.Dale CJ, De Rose R, Wilson KM, Croom HA, Thomson S, Coupar BE, et al. Evaluation in macaques of HIV-1 DNA vaccines containing primate CpG motifs and fowlpoxvirus vaccines co-expressing IFNgamma or IL-12. Vaccine. 2004;23:188–197. doi: 10.1016/j.vaccine.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 25.De Rose R, Sullivan MT, Dale CJ, Kelleher AD, Emery S, Cooper DA, et al. Dose-response relationship of DNA and recombinant fowlpox virus prime-boost HIV vaccines: implications for future trials. Hum Vaccin. 2006;2:134–136. doi: 10.4161/hv.2940. [DOI] [PubMed] [Google Scholar]
- 26.Dubey S, Clair J, Fu TM, Guan L, Long R, Mogg R, et al. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J Acquir Immune Defic Syndr. 2007;45:20–27. doi: 10.1097/QAI.0b013e3180377b5b. [DOI] [PubMed] [Google Scholar]
- 27.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cellmediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham BS, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, et al. for the Vaccine Research Center 004 Study Team, author. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J Infect Dis. 2006;194:1650–1660. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McConkey SJ, Reece WH, Moorthy VS, Webster D, Dunachie S, Butcher G, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9:729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 31.Walther M, Thompson FM, Dunachie S, Keating S, Todryk S, Berthoud T, et al. Safety, immunogenicity and efficacy of prime-boost immunization with recombinant poxvirus FP9 and modified vaccinia virus Ankara encoding the full-length Plasmodium falciparum circumsporozoite protein. Infect Immun. 2006;74:2706–2716. doi: 10.1128/IAI.74.5.2706-2716.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webster DP, Dunachie S, McConkey S, Poulton I, Moore AC, Walther M, et al. Safety of recombinant fowlpox strain FP9 and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in nonimmune volunteers. Vaccine. 2006;24:3026–3034. doi: 10.1016/j.vaccine.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 33.Cottingham MG, van Maurik A, Zago M, Newton AT, Anderson RJ, Howard MK, et al. Different levels of immunogenicity of two strains of fowlpox virus as recombinant vaccine vectors eliciting T-cell responses in heterologous prime-boost vaccination strategies. Clin Vaccine Immunol. 2006;13:747–757. doi: 10.1128/CVI.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007;81:5257–5269. doi: 10.1128/JVI.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim CY, Kang ES, Kim SB, Kim HE, Choi JH, Lee DS, et al. Increased in vivo immunological potency of HB-110, a novel therapeutic HBV DNA vaccine, by electroporation. Exp Mol Med. 2008;40:669–676. doi: 10.3858/emm.2008.40.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]