Abstract
Background
An increase in gastrointestinal (GI) symptoms, including bowel discomfort, abdominal pain/discomfort, bloating, and alterations in bowel patterns, has been reported during premenses and menses menstrual cycle phases and the perimenopause period in women with and without irritable bowel syndrome (IBS).
Objective
This article reviews the literature related to one possible physiological mechanism—declining or low ovarian hormone levels—that may underlie the occurrence or exacerbations of abdominal pain/discomfort at times of low ovarian hormones (menses, menopause) in women with or without IBS.
Methods
To identify English-only review and data-based articles, PubMed was searched between January 1980 and September 2008 using the following terms: irritable bowel syndrome, functional gastrointestinal disorders, gastrointestinal motility, immune, pain, hyperalgesia, menstrual cycle, menopause, pregnancy, estrogen, estradiol (E2), and progesterone. Studies in animals and in humans were included; drug trials were excluded.
Results
From our review of the literature, 18 papers were identified that were related either to the mechanisms accounting for menstrual cycle fluctuations (n = 12) or to the impact of menopausal status on symptoms of IBS (n = 6). One study reported that visceral pain sensitivity was significantly higher during menses than at other menstrual cycle phases in women with IBS (P < 0.05). Other menstrual cycle phase–linked symptoms, dysmenorrheal symptoms (cramping pain) in particular, were more intense in women with IBS. Animal studies have shed some light on the relationship of ovarian hormones to GI sensorimotor function.
Conclusion
The increase in GI symptoms around the time of menses and early menopause occurs at times of declining or low ovarian hormones, suggesting that estrogen and progesterone withdrawal may contribute either directly or indirectly. This review highlights the need for confirmatory preclinical and clinical studies to unravel the role of ovarian hormones in women with IBS.
Keywords: irritable bowel syndrome, menstrual cycle, menopause, estrogen, progesterone, gastrointestinal symptoms, pregnancy, immune, pain
INTRODUCTION
Irritable bowel syndrome (IBS) is a gastrointestinal (GI) sensory and motility disorder characterized by abdominal pain or discomfort associated with a change in bowel habits. The Rome III diagnostic criteria define the abdominal pain or discomfort of IBS as originating 6 months before diagnosis, being currently active for 3 months, and being associated with at least 2 of 3 features: (1) relieved with defecation; (2) onset associated with change in stool frequency; and (3) onset associated with change in stool form.1 IBS is subclassified based on stool consistency as IBS with constipation, IBS with diarrhea, or IBS with mixed or alternating constipation and diarrhea. The underlying pathophysiology of IBS has not been fully elucidated. Physiological abnormalities may include increased visceral sensitivity; impaired GI motility; imbalanced autonomic nervous system function; disrupted intestinal flora, including potential small intestinal bacterial overgrowth; and altered intestinal secretion.1,2
IBS is highly prevalent and affects 10% to 15% of the Western population.3,4 Population studies have reported that the occurrence of IBS seems to be related to sex and age, but not to race. In Western cultures, the proportion of women seeking health care services for IBS is approximately twice that of men.3–5 A recently published 10-year natural history survey assessed symptoms and factors that influence consultation behavior in IBS patients.6 Female gender, in addition to lower quality of life and presence of dyspepsia, was found to be a significant independent risk factor for new-onset IBS (odds ratio [OR] = 2.14; 95% CI, 1.56–2.94). The majority of women with IBS who seek health care are of reproductive age. However, population surveys have reported that the prevalence of IBS declined after the age of 40 years.7,8 In analyzing the United Kingdom General Practice Research Database, Garcia Rodriquez et al9 found that although there was a much higher incidence rate of IBS diagnosed in women than in men in young adulthood, there was a steady decline with age among women, converging with the incidence rate in men by the seventh decade of life. In contrast, the incidence in men remained relatively stable between the ages of 20 and 69 years. Whether this decrease in IBS incidence with age in women is linked to declines in ovarian hormone levels is not known. Population surveys have also noted that women more frequently reported abdominal pain than did men.9,10 Furthermore, studies have found that within the general as well as the IBS patient population, women were more likely to report nonpainful symptoms (eg, bloating, abdominal distention) than were men.10,11 There is increasing evidence that physiological differences (eg, ovarian vs testicular hormones) between men and women may contribute, at least in part, to the gender gap in abdominal pain and IBS symptom reporting.11
There are multiple points at which gonadal hormones may influence visceral pain/discomfort sensations.11 The etiology of IBS is multifaceted, with potential abnormalities in central and peripheral neural pathways and neurotransmitter systems. The interactions of female gonadal hormones with the many pathways involved in pain transmission are complex and occur at multiple levels, including primary afferents and neuromodulator systems.12 Because of the range and multiplicity of these effects, potential mechanisms underlying ovarian hormonal influence on pain perception or neural activity have been difficult to elucidate.
The severity of GI symptoms, including abdominal pain, altered bowel habits, and bloating, varies across phases of the menstrual cycle as well as during the menopause transition in some women with or without IBS or other functional bowel disorders (FBDs).13–15 This review addresses one possible physiological mechanism, declining or low ovarian hormone levels, that may underlie the occurrence or exacerbations of abdominal pain/discomfort across the menstrual cycle and the perimenopause–early menopause transition in women with or without IBS.
METHODS
PubMed was used to identify appropriate English-language review and data-based articles published between January 1980 and September 2008. The following search terms were used: irritable bowel syndrome, functional gastrointestinal disorders, gastrointestinal motility, immune, pain, hyperalgesia, menstrual cycle, menopause, pregnancy, estrogen, estradiol (E2), and progesterone. In addition to animal studies, both preclinical and clinical studies were included. Drug trials were excluded.
RESULTS
Symptoms and Menstrual Cycle
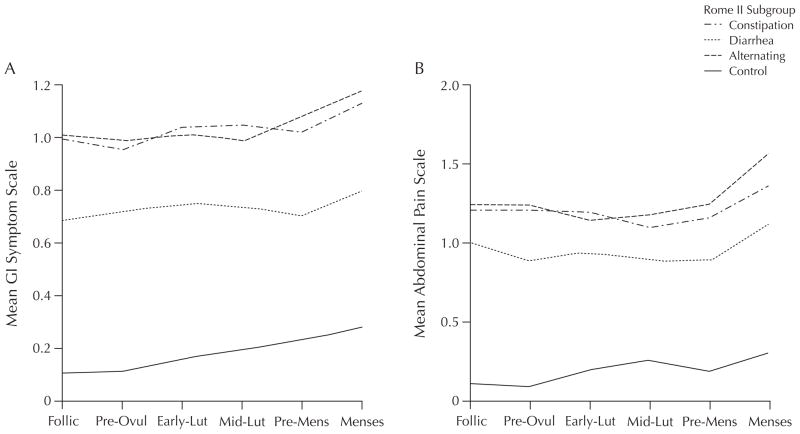
Studies (n = 34,14 n = 369,15 n = 5816) of the relationship between menstrual cycle phase and abdominal discomfort and pain symptoms indicate that premenses (days immediately preceding the onset of menses) and menses, compared with other cycle phases, are periods of increased complaints in women aged 19 to 37 years (Figure 1). Menses-related increases in GI symptoms are noted both in women with and without FBDs such as IBS. A systematic review of data from symptom-based questionnaires revealed that approximately one third of otherwise asymptomatic women experienced GI symptoms at the time of menstruation.19 In a descriptive correlational study of 30 healthy young women (mean age, 24.4 years), abdominal pain increased in the early to midluteal phase and progressively worsened during premenses and the menses phase.13 In this sample, back pain and headache negatively correlated with progesterone levels (r = −0.42, P < 0.05; r = −0.41, P < 0.05, respectively), whereas there were no significant relationships between levels of ovarian hormones and mood symptoms.
Figure 1.
Patterns of mean (A) gastrointestinal (GI) symptoms (constipation, diarrhea, alternating) and (B) abdominal pain alone (constipation, diarrhea, alternating) in Rome II subgroups across 6 menstrual cycle phases in women with and without irritable bowel syndrome. The definitions of Rome II subgroups are based on 3 symptoms of constipation (hard stools, <3 stools/wk, straining) and diarrhea (loose or watery stools, >3 stools/d, urgency).17 Follic = follicular phase; Pre-Ovul = preovulatory phase; Early-Lut = early-luteal phase; Mid-Lut = midluteal phase; Pre-Mens = premenses phase. Adapted with permission.18
Findings from a retrospective comparative study noted that women with FBDs reported an increase in symptoms (abdominal pain, diarrhea, constipation) during premenses and menses (OR = 1.1; 95% CI, 0.9–1.2) (P < 0.01).20 Chang et al21 found that ~40% of women with IBS (n = 542) reported that their symptoms were influenced by the menstrual cycle. In another prospective study, bloating was found to worsen premenstrually in up to two thirds of women with IBS.22 Whitehead et al15 found that women with FBDs including IBS (n = 140) reported that their bowel symptoms were affected by menstruation to a greater degree than did women without FBDs (P < 0.05). Because no differences were observed in psychological test scores between women with or without FBDs, the increase in GI symptoms likely had a physiological rather than a psychological basis. A more recent prospective, descriptive study of Korean women (n = 193), however, found no significant menstrual cycle phase differences in abdominal pain reports.23 Such differences may be methodological (retrospective vs prospective measures) or cultural.
Much of the literature previously mentioned relied on symptom recall, in which women were asked about their usual experiences at various cycle phases. Symptom recall may be biased, with women frequently reporting their worst experience as well as their inherent beliefs about the influence of the menstrual cycle.24 To address this issue, other investigators have used a daily diary to study patterns of abdominal pain/discomfort symptoms, bowel function, mood, and somatic symptoms across the menstrual cycle in women with or without FBDs. Data from one study that used a daily diary found that the mean severity of GI symptoms was higher on both menses and nonmenses days (P < 0.05) in those with IBS (n = 149) than in those without IBS (n = 42).18 Even when predominant bowel pattern (ie, constipation, diarrhea, alternating predominant IBS) was considered, a similar pattern of symptom reports was found. However, responses on daily diaries may also be influenced by personal beliefs about menstruation.25
Gastrointestinal Symptoms and Oral Contraceptives
Given the hypothesis that the increase in abdominal pain/discomfort symptoms at premenses and menses is the result of the natural decline in progesterone and estrogen levels during the late luteal phase, a logical extension might be that women who have regulated hormone levels (ie, due to oral contraceptives [OCs]) would not experience an increase in symptoms at menses. Yet, in one prospective comparative study in women with IBS (n = 149) who were taking OCs (monophasic or triphasic preparations), the subjects continued to experience an increase in GI symptoms at menses.18 Overall, the women with IBS who were taking OCs containing both estrogen and progestin appeared to have reduced levels of abdominal pain/discomfort compared with the women with IBS who were not taking OCs (mean [SD] symptom severity, 1.30 [0.70] vs 1.20 [0.76]; P = 0.025 unadjusted). After adjusting for multiple comparisons, there were no significant differences between the groups. Similar to the women not taking OCs, those who took OCs experienced a decrease in ovarian hormone levels before menses.26 Studies with continuous OCs would help to determine if steady levels of ovarian hormones are associated with a greater reduction in abdominal pain and other IBS symptoms.
Visceral Sensitivity
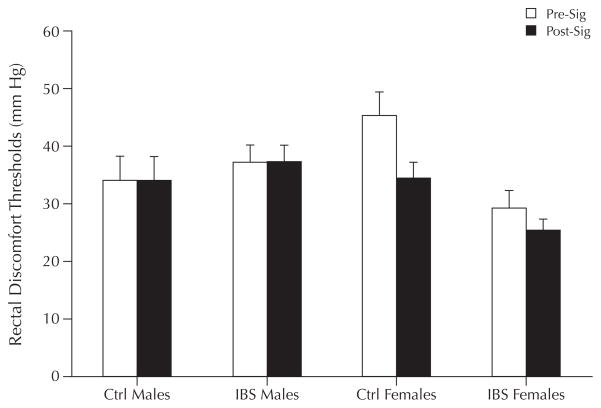
Enhanced visceral perception has been reported in IBS patients compared with healthy controls.27–29 Three studies assessed sex differences in lower GI perception in IBS patients,30–32 although menstrual cycle phase and hormonal therapy were not measured. In a study of 52 adult women and men with IBS (39 women, total sample median age 40 years), Ragnarsson et al30 reported that women had a more pronounced decrease in maximal tolerable rectal distention than did men (mean pressure threshold cm H2O before and after meal [range], 46 [20–70] to 41 [20–70] vs 46 [30–60] to 45 [30–60]; P < 0.05). Chang et al32 compared 58 patients with IBS (34 men, 24 women) and 26 controls (9 men, 17 women) (total sample mean [SD] age, 42 [1] years) on perception of rectosigmoid stimuli. They found that women with IBS had lower discomfort thresholds for phasic rectal distention compared with their male counterparts (P < 0.01) and healthy controls (P < 0.001). In addition, they reported that although healthy women had reduced baseline perceptual responses (decreased sensitivity), women as a group, regardless of a diagnosis of IBS, had evidence of sensitization after repeated noxious sigmoid stimulation, compared with men (Figure 2). In contrast, no significant gender differences were found between IBS and controls in a study by Kim et al,31 in which sensory thresholds were compared in a Korean sample of men and women with IBS (n = 28 and n = 31, respectively) and without IBS (n = 10 and n = 12) using a dual-drive barostat. However, pain thresholds were not measured and a slow ramp distention paradigm was used, which has not been found to elicit differences between IBS and controls.33
Figure 2.
Sex differences in rectal thresholds at baseline (Pre-Sig) and after noxious sigmoid distention (Post-Sig) in patients with irritable bowel syndrome (IBS) and healthy controls (Ctrl). There was a significant interaction between sex and disease group (P < 0.01), and sex and condition (P < 0.05). Adapted with permission.32
A limited number of studies have examined visceral pain perception across the menstrual cycle. In a study comparing selected menstrual cycle stages (menses, days 1–4; follicular, days 8–10; luteal, days 18–20; and premenses, days 24–28) in healthy females (N = 20), no significant changes in rectal sensitivity, distention-induced rectal motility, or rectal compliance were observed, despite looser stools at menses (mean 2.32; 95% CI, 2.1–2.5).34 However, in a subsequent study of women with IBS (N = 29), Houghton et al35 found that rectal sensitivity (induced by distention of a rectal balloon controlled by a barostat) was increased at menses compared with all other cycle phases (P < 0.05). This is consistent with the hypothesis that reduced or decreasing ovarian hormones may contribute to pain sensitivity.
Ovarian Hormones and Pain Sensitivity
As noted previously, the relationships between gonadal hormones and somatic and visceral pain sensitivity in humans are complex. Stening et al36 used a cold pressor stimulus with 16 healthy volunteers (mean age, 27 years) to compare menstrual cycle phase points (early follicular, late follicular, early luteal, late luteal [second cycle]). Cycle phases were verified by estrogen and progesterone assays and subject diaries. The time to reach 30 on a visual analogue scale (0 = no pain to 100 = maximum pain) was decreased during the luteal phase compared with the other phases (P < 0.05). When the investigators examined the relationships among the levels of ovarian hormones collected on the day of testing with the cold pressor results, marked differences were noted. In particular, increased levels of progesterone during the luteal phase were related to increased reports of pain intensity. However, this relationship was reduced after controlling for increasing estradiol (E2) levels. Thus, E2 and progesterone may play both antinociceptive and pronociceptive roles, depending on their relative concentrations as well as the duration of exposure. It can also be conjectured that it is the dynamic changes of gonadal hormones as well as their absolute levels that may contribute to somatic pain sensitivity differences across the menstrual cycle.
Sex differences in pain in humans and animals are well described.37,38 Animal experiments have reported that gonadal steroids substantially influence visceral and somatic sensitivity to a variety of painful stimuli.37–44 In rodents, the response sensitivities of afferent fibers in the pelvic and hypogastric nerves are differentially affected by hormonal variations occurring across the estrous cycle.45 There is increased sensitivity to ureter stimuli during the metestrus/diestrus cycle phase, which corresponds to the perimenstrual phase in women.39 Other studies have examined the absence of ovarian hormones on visceral pain sensitivity. Approximately 4 weeks after surgery, Sanoja and Cervero40 found that female mice that underwent an ovariectomy subsequently developed robust mechanical hyperalgesia and allodynia in the abdomen, hind limbs, and proximal tail, but not in the forelimbs. Estrogen replacement prevented the development of hyperalgesia after ovariectomy.
At each of the sensory pathway components (ie, afferent nerve fibers, spinal cord, central nervous system [CNS]), ovarian hormones can modulate the response to painful or noxious stimuli. In rodents, the response sensitivities of afferent fibers in the pelvic and hypogastric nerves are differentially affected by hormonal variations occurring across the estrous cycle.40,45 There are, however, other animal studies that have reported increased sensitivity due to estrogen-dependent pathways. For example, in one study, ovariectomy abolished restraint stress-induced visceral hypersensitivity produced by colorectal distention in cycling female rats via neurokinin-1 receptor activation.41 Ovariectomy plus the administration of 17β-E2 or 17β-E2 plus progesterone, but not progesterone alone, was associated with stress-induced hypersensitivity. There may be a number of reasons for the seemingly conflicting reports of the role of estrogen and viscerosomatic hypersensitivity. The neuronal effects of estrogen are both rapid and, due to its genomic effects, prolonged. In women, the exposure to higher as well as lower levels of estrogen and progesterone relative to the estrous cycle of rodent models is greater. Thus, additional studies that consider these 2 potential routes for estrogen’s action are needed.
Gonadal hormones may exert their pain sensitivity effects via a variety of mediators including serotonin (5-hydroxytryptamine [5-HT]), which is ubiquitous throughout the GI tract. Clinical trials illustrate the role of 5-HT in abdominal pain perception as well as motility in the GI tract.46,47 In the rat CNS, the estrous cycle influences the expression of tryptophan hydroxylase, the synthetic enzyme for 5-HT.48 In a study of 39 women with IBS diarrhea (mean age, 33.4 years) and 20 healthy volunteers (mean age, 28.2 years) during the luteal phase, fasting levels of platelet-depleted plasma 5-HT concentrations were not significantly different between the 2 groups.49 However, ingestion of a standardized meal produced a greater increase in 5-HT concentrations in the women with IBS diarrhea compared with the healthy subjects (adjusted geometric mean, 211.3 h × nmol/L vs 151.1 h × nmol/L; P = 0.04). In a follow-up study, platelet-free 5-HT levels were measured in women with IBS diarrhea at menses (n = 16) and at the luteal phase (n = 39) and in men with IBS (n = 18) (age range, 18–52 years). Under fasting and fed conditions, men with IBS and women with IBS who were studied during the luteal phase, but not at menses, had significant elevations in platelet-poor 5-HT after standardized meal ingestion (P < 0.05), compared with healthy men (n = 24; age range, 18–46 years) and women (n = 40; age range, 19–50 years).50 Furthermore, men with IBS had higher 5-HT levels than did women with IBS at menses.
Estrogens are also known to enhance serotonergic postsynaptic responsiveness in the CNS. Al- though less is known about their influence on neurotransmission in the GI tract, ovarian hormones have been reported to influence the expression of 5-HT3 receptors in rat colon with restraint stress-induced bowel dysfunction. Li et al51 noted that the expression of 5-HT3 receptor mRNA was significantly decreased in rats after E2 plus progesterone treatment compared with sham-treated ovariectomized rats (mean [SD], 0.65 [0.05] vs 0.85 [0.06]; P < 0.01). There was a trend toward lower expression of 5-HT3 receptor mRNA in the colon with increasing serum levels of E2 and progesterone. These preclinical observations are consistent with observed increases in GI symptoms, including bowel discomfort or pain, during premenses and menses in women with or without IBS.
5-HT3 receptors are expressed on intrinsic primary afferent nerves and on both extrinsic spinal receptors and vagal primary afferent nerves. 5-HT3 located on the central terminals of spinal afferents are believed to be involved in the mediation of descending pain facilitatory pathways.52 Furthermore, antagonists of 5-HT3 receptors (eg, alosetron) have been found to be efficacious in the treatment of IBS, including the relief of abdominal pain and discomfort.53,54 Clinical trials of a 5-HT3 receptor antagonist (alosetron) have also reported a sex difference in therapeutic efficacy. It is not known if there is a sex-related difference in 5-HT3 receptor expression accounting for the more robust symptom relief with alosetron observed in women with IBS relative to men with IBS. However, other reasons to explain this sex difference include that alosetron clearance is 28% lower in women, resulting in ~30% to 50% higher concentrations in women compared with men for a given dose,55 and greater 5-HT synthesis in certain brain regions in men with IBS (n = 6) compared with women with IBS (n = 5), when both received treatment with alosetron (P < 0.05).56
Taken together, there is a conceivable link between abdominal pain, 5-HT3 receptors, and ovarian hormones that merits further study.
Pain Sensitivity and Pregnancy
In addition to ovariectomy, pregnancy is another model with which to examine the role of ovarian hormones in modulating pain. Pregnancy is a time of elevated ovarian hormone levels as well as opioid-mediated antinociception. Cogan and Spinnato43 found that somatic pain thresholds were elevated (ie, they experienced decreased sensitivity) in 6 pregnant women at the end of their third trimester compared with 6 women who were not pregnant (F1,10 = 14.46; P < 0.05). Gintzler and Liu,57 in a series of experiments using a physiological pregnancy (increasing concentrations of estrogen and progesterone) rat model, observed a 60% increase in hypogastric nerve impulse propagation as well as activation of spinal κ and δ opioid systems and α2-noradrenergic pathways. Hypogastric neurectomy abolishes pregnancy-related antinociception, likely through loss of augmented α2-noradrenergic tone.
Considerable preclinical and clinical evidence is consistent with the concept that female sex hormones (estrogen, in particular), in interaction with neuroendocrine systems co-opted from attachment/caregiving processes (such as opioids and oxytocin), may underlie biobehavioral sex differences in response to stress.44,58–61
Viscero–Visceral Pain Sensitivity
Women with IBS report more extraintestinal conditions (eg, migraine, fibromyalgia) compared with their male counterparts.62 Of particular interest is the overlap between IBS and primary dysmenorrhea (painful menstruation). Women with dysmenorrhea report significantly more GI symptoms occurring prior to or concurrent with uterine cramping pain at menses than do nondysmenorrheic women (P < 0.05).20 Dysmenorrhea is one of the most common problems identified in primary care clinics, with estimates as high as 90% in menstruating women.62 Compared with those who do not have IBS, women with IBS are more likely to receive a diagnosis of dysmenorrhea.14,63–66 In a prospective study, daily diaries were used to record symptoms of women with IBS and coexisting self-reported premenstrual syndrome symptoms (n = 59), dysmenorrhea (n = 15), and both premenstrual syndrome symptoms and dysmenorrhea (n = 37).64 There was a significant group effect in abdominal and stomach pain (P < 0.05) observed at the luteal phase, with the 3 groups reporting more severe pain relative to an IBS-only group (n = 114). A similar, although nonsignificant, effect in abdominal pain was noted at menses.
Primary dysmenorrhea is linked to an excess or imbalance of prostaglandins and arachidonic acid metabolites originating in secretory endometrium during menstruation. A recent prospective, non-comparative multicenter study of 406 women with dysmenorrhea found that an estrogen-free OC was associated with resolution or improvement of dysmenorrhea symptoms in 93% of patients.67
Similar findings of increased sensitivity to painful stimuli at menses have been observed in women with dysmenorrhea.68 Bajaj et al69 reported significantly reduced somatosensory pain thresholds to nociceptive heat, pressure, and pinch stimuli (P < 0.05), both within and outside areas of referred menstrual pain at menses, compared with other cycle phases (ovulatory, luteal, premenstrual) in women with dysmenorrhea (n = 15; mean age, 25 years) versus women without dysmenorrhea (n = 15; mean age, 28 years). Similar findings were noted by Brinkert et al,68 who compared 11 women with dysmenorrhea with 10 healthy age-matched controls. Significantly lower distention volume detection (57%) and pain thresholds (39%) were observed in dysmenorrheic women (P < 0.05), particularly in the sigmoid colon. However, no significant differences were found in electrical stimulation pain thresholds.
Although colonic perception in dysmenorrhea needs to be further assessed in high-quality studies with larger sample sizes, the fact that increased colonic perception is noted midcycle (no measurement was made premenses or at menses) suggests that an inherent viscero–visceral hyperalgesia may be present and requires further exploration. For example, does excessive uterine contractility sensitize peripheral afferents or spinal cord elements? Or is the overall sensitivity to visceral afferent input due to alterations in descending inhibitory regulation? In addition, is the subclinical intestinal hypersensitivity noted in women with dysmenorrhea greater during premenses or menses when ovarian hormone levels are decreasing or low? Potent cross-system, viscero–visceral interactions in which pathophysiology in one organ influences physiology including pain sensitivity in another organ has been reported between the vagina, uterus, and urinary system in animal models of endometriosis and uterine inflammation, and between the colon and bladder in animal models of colon and bladder irritation.70–72
There are limited data on the overlap of IBS and endometriosis. In one study, 50 women with IBS were compared with 52 gynecology patients (21 with chronic pelvic pain, 30 with endometriosis).73 Based on responses to the symptom questionnaire, women with IBS reported significantly more upper abdominal pain (P < 0.001; 95% CI, 1.2–68.6), colicky pain (P < 0.001; 95% CI, 2.2–114), and altered bowel habits (eg, pasty stools) (P < 0.001; 95% CI, 3.5–55.6) than did patients with laparoscopically confirmed endometriosis or pelvic inflammatory disease. However, there are additional reports that patients with endometriosis and intestinal involvement, and even those without intestinal involvement, have symptoms of IBS.74–76 In fact, 32% of women who had chronic pelvic pain and suspected endometriosis without bowel lesions (n = 362) met Rome II criteria for IBS.74,75 Similar to dysmenorrhea, the pathogenesis of endometriosis has been linked to E2. Endometriosis is associated with inflammatory changes in the peritoneal fluid. In women with endometriosis, high levels of E2 are believed to increase chemokine secretion, which has been identified as a potential contributor to the pathogenesis and the progression of endometriosis.77
An overall association of low-grade mucosal immune activation and IBS symptoms has been suggested. There is evidence of small increases in immune cells, such as T lymphocytes and mast cells, in the intestinal mucosa of patients with IBS and a history of gastroenteritis (ie, postinfectious IBS78) and in a subset of patients with IBS but without a previous GI infection.79–81 Female sex is an independent risk factor for developing postinfectious IBS.80 Enthusiasm for this hypothesis is furthered by the finding that mast cells in close proximity to mucosal nerve fibers and their mediators are associated with abdominal pain.81 The number of colonic mucosal mast cells was found to be higher in women with IBS (n = 31) than in men with IBS (n = 13) (46%; P < 0.05).82 It is not known whether sex differences in immune function or reactivity are related to the increased reporting of abdominal pain, increased prevalence of IBS, or greater vulnerability to developing post-infectious IBS in women.
The cumulative findings that females produce stronger cellular and humoral immune reactions, have a greater resistance to bacterial infections, and are more likely to develop autoimmune diseases compared with males, and that symptoms of these conditions are affected by reproductive status and menstrual cycle, support the evidence of sex differences in immune responses.81–83 A comprehensive review of the role of estrogen in inflammation and immune function was recently published. Straub84 concluded that depending on its level at a given point in time (menstrual cycle phase, pregnancy, menopause status), estrogen influences important proinflammatory and anti-inflammatory pathways. For example, pregnancy levels of E2 typically inhibit proinflammatory cytokines, but low levels have no, or even stimulatory, effects. Thus, postmenopausal women could be more vulnerable to a proinflammatory condition.
Together, these findings suggest a possible association of ovarian hormones in IBS and other pelvic visceral pain disorders, such as dysmenorrhea and endometriosis, that frequently coexist with IBS. Women with IBS are likely to experience an increase in abdominal pain and bowel symptoms at a time of declining or low ovarian hormone levels. Whether this is a direct effect of hormone withdrawal on various sensorimotor (ie, perception, motility) or immune-related mechanisms remains to be elucidated.
Further studies are needed to determine if gender differences in immune function play a pathogenic role in IBS, and if these differences could, in part, along with estrogen and progesterone interactions with the hypothalamic-pituitary-adrenal axis, autonomic nervous system, and sensory afferent pathways, explain women’s increased vulnerability to developing IBS.85
Furthermore, the question remains as to whether menses-linked increases in GI and other somatic and visceral pain symptoms are due to sensitivity to dynamic changes in ovarian hormones in women. The answer to this question will require better approaches than are currently employed. In their review of research on somatic pain and the menstrual cycle, Sherman and LeResche86 recommended that future studies adopt a standardized approach to defining cycle phases, such as the use of luteinizing hormone assessments to detect ovulation, direct measurement of hormones on a day-to-day basis, exclusion of women who are taking exogenous hormone preparations, and utilization of a standardized pain stimulus. These same criteria would allow for better clarification of the roles of ovarian hormones in GI pain/discomfort reports.
Gastrointestinal Symptoms and Menopause
The natural process of menopause provides another model for addressing the question of whether declining or low levels of estrogen and progesterone play a role in abdominal pain/discomfort symptoms. However, the transition to menopause is highly variable from woman to woman. Fluctuations occur in hypothalamus (gonadotropin- releasing hormone), pituitary (follicle-stimulating hormone), and ovarian hormone levels in addition to a conversion from ovarian E2 production to adrenal estrogen (estriol) production. Despite these well-established physiological changes, little is known about the impact of the menopause transition on FBDs including IBS.
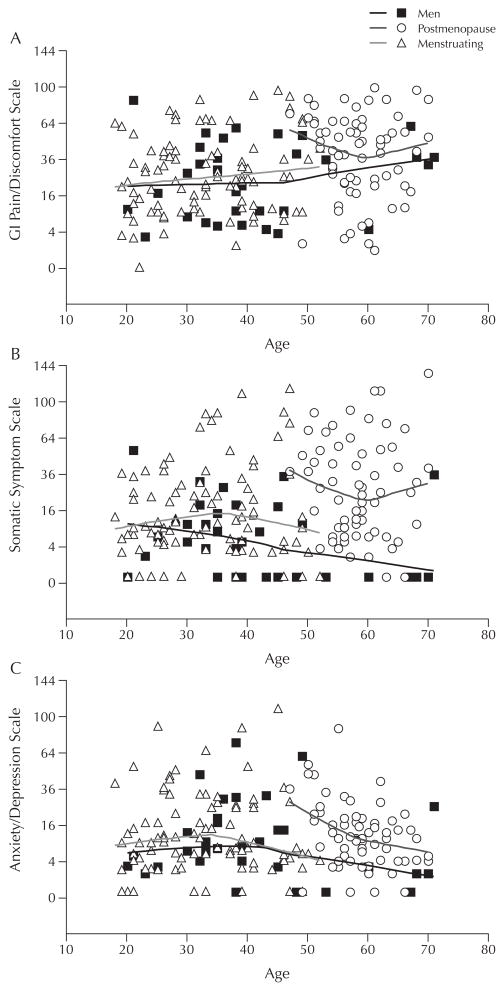
Several reports provide somewhat conflicting evidence. Triadafilopoulos et al87 surveyed premenopausal (n = 58) and postmenopausal (n = 170) women in a primary care practice and compared the percentages of women in clustered age groups (20–29, 30–39, 40–49, 50–59, 60–69 years). They found that perimenopausal and postmenopausal women had a high prevalence (38% vs 14% for premenopausal women) of self-reported altered bowel function (P < 0.001). The prevalence (36%) of IBS-type complaints peaked in the aged 40 to 49 group. Cain et al88 used a daily diary with men (n = 32; mean age, 41.2 years), menstruating women (n = 66; mean age, 32.9 years), and post-menopausal women (n = 66; mean age, 58.4 years), all of whom had IBS, to compare reports of GI, somatic, and psychological distress. Postmenopausal women reported higher levels (percentage of days with moderate to severe symptoms) of GI pain-related symptoms (abdominal distention, bloating, intestinal gas) than did men (P < 0.05). However, when controlled for age, the differences in GI discomfort symptoms became nonsignificant. Postmenopausal women also reported higher levels of somatic discomfort symptoms (joint and muscle pain) than did men and menstruating women (P < 0.05), and these differences persisted after controlling for age. In contrast, there were no significant differences in psychological distress indicators (anxiety, depression) among the 3 comparison groups (Figure 3).
Figure 3.
Scatterplots of percent of days (n = 28) when (A) gastrointestinal (GI) pain/discomfort, (B) somatic, and (C) anxiety/depression symptoms were rated as moderate to severe in men and women with irritable bowel syndrome. Reprinted with permission.88
In an analysis of the General Practice Research Database of women aged 50 to 69 years in the United Kingdom, Ruigomez et al89 found that the incidence rate of IBS per 1000 person-years was 3.8 among hormone replacement therapy (HRT) users compared with 1.7 in the cohort of never-users. It is conceivable that female sex hormones have different antinociceptive and pronociceptive effects on symptoms, based on absolute concentration of or dynamic changes in hormone levels in the premenopausal versus postmenopausal state. Another possible explanation is that women with IBS may be more likely to report various perimenopausal and postmenopausal symptoms such as hot flashes and insomnia, and thus may be prescribed HRTs to a greater degree.
In clinical studies of IBS, information on menopause status or HRT use is often not described. Women who have experienced hysterectomy or ovariectomy may be excluded from studies of patients with IBS. One study utilizing a large American health maintenance organization database (IBS, n = 3205; no IBS, n = 39,644) found an ~2-fold higher rate of hysterectomy in women with IBS (IBS, n = 1063 [32%]; no IBS, n = 6751 [17%]).90 Similarly, a study in the United Kingdom noted a higher rate of hysterectomy in women with IBS (OR = 1.6; 95% CI, 1.1–2.2) (P < 0.01).91 However, another more recent survey conducted in the community failed to find higher rates of hysterectomy in white and African American women with IBS.92
Because of the variability in age of menopause transition, age may not be an appropriate surrogate marker for menopause transition stage. In 2001, the Stages of Reproductive Aging Workshop developed bleeding and endocrine criteria to standardize the early-to-late menopausal transition stages.93 The use of standardized criteria to denote late perimenopausal, early menopausal, and late-menopausal periods may help to clarify the relationship between ovarian hormones and symptom experiences in women with IBS.
CONCLUSIONS
Animal and human studies support a potential role for ovarian hormones in visceral pain sensitivity and pelvic visceral pain conditions. It is possible that estrogen as well as progesterone may have multiple effects that could vary at different stages in the reproductive cycle. For example, estrogen may increase the vulnerability of women to develop IBS, compared with men. However, the actual fluctuations in ovarian hormones may be more related to changes in pain and nonpainful symptoms and to pain sensitivity within the menstrual cycle.
Observations in women with IBS suggest that there may be links among GI symptoms, including abdominal pain, motility/transit, visceral sensitivity, and the ovarian hormone decline around menses. Both preclinical and clinical studies are needed to characterize the multiple locations at which dynamic changes in ovarian hormones modulate contractility, transit, visceral sensitivity, and immune function. The strong covariance of menstrual and bowel symptoms suggests there may be a common physiological basis involving both peripheral and central mechanisms. It can be hypothesized that in young adult women with IBS, exacerbation of bowel symptoms and other somatic complaints at premenses and menses may represent expressions of an overall increased somatic and visceral sensitivity linked temporally to dynamic decreases in, or in low, ovarian hormones. Descriptive and mechanistic studies that consider the stages of the menopause transition are required to further clarify the roles of ovarian hormones in the development of GI symptoms.
This review addressed one avenue of inquiry in response to the question of one possible physiological mechanism, declining or low ovarian hormone levels, that may underlie the occurrence or exacerbations of abdominal pain/discomfort across the menstrual cycle and the perimenopause–early menopause transition in women with or without IBS. A recent consensus report that summarized what is currently known about sex and gender differences in pain and analgesia stated that there are a number of clinical factors, other than gonadal hormones, that are relevant to sex and gender influences on pain perception.94 These factors include age; sociocultural and racial differences; previous pain and abuse history; comorbidities; physical variables such as blood pressure, height and weight; and perceptions and beliefs about pain, coping, and mood. With regard to IBS, additional physiological effects of ovarian hormones on GI functions (including motility and secretion) also need to be considered as potential areas of further investigation.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NR01094, P30 NR04001, and P50 DK64539). The authors would like to thank Ms. Cathy Liu, Ms. Sang-Eun Jun, and Ms. Wimon Deechakawan for their assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders [published correction appears in Gastroenterology. 2006;131:688] Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA. What does the future hold for irritable bowel syndrome and the functional gastrointestinal disorders? J Clin Gastroenterol. 2005;39 (Suppl 3):S251–S256. doi: 10.1097/01.mcg.0000156107.13247.69. [DOI] [PubMed] [Google Scholar]
- 3.Hungin AP, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: Prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21:1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 4.Müller-Lissner SA, Bollani S, Brummer RJ, et al. Epidemiological aspects of irritable bowel syndrome in Europe and North America. Digestion. 2001;64:200–204. doi: 10.1159/000048862. [DOI] [PubMed] [Google Scholar]
- 5.Taub E, Cuevas JL, Cook EW, III, et al. Irritable bowel syndrome defined by factor analysis. Gender and race comparisons. Dig Dis Sci. 1995;40:2647–2655. doi: 10.1007/BF02220455. [DOI] [PubMed] [Google Scholar]
- 6.Ford AC, Forman D, Bailey AG, et al. Irritable bowel syndrome: A 10-yr natural history of symptoms and factors that influence consultation behavior. Am J Gastroenterol. 2008;103:1229–1239. doi: 10.1111/j.1572-0241.2007.01740.x. quiz 1240. [DOI] [PubMed] [Google Scholar]
- 7.Kumano H, Kaiya H, Yoshiuchi K, et al. Comorbidity of irritable bowel syndrome, panic disorder, and agoraphobia in a Japanese representative sample. Am J Gastroenterol. 2004;99:370–376. doi: 10.1111/j.1572-0241.2004.04048.x. [DOI] [PubMed] [Google Scholar]
- 8.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: An international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 9.García Rodríguez LA, Ruigómez A, Wallander MA, et al. Detection of colorectal tumor and inflammatory bowel disease during follow-up of patients with initial diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2000;35:306–311. doi: 10.1080/003655200750024191. [DOI] [PubMed] [Google Scholar]
- 10.Talley NJ. Spectrum of chronic dyspepsia in the presence of the irritable bowel syndrome. Scand J Gastroenterol Suppl. 1991;182:7–10. doi: 10.3109/00365529109109530. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang A, Wrzos HF. Contribution of gender to pathophysiology and clinical presentation of IBS: Should management be different in women? Am J Gastroenterol. 2006;101(Suppl 12):S602–S609. doi: 10.1111/j.1572-0241.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 12.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 13.Laessle RG, Tuschl RJ, Schweiger U, Pirke KM. Mood changes and physical complaints during the normal menstrual cycle in healthy young women. Psychoneuroendocrinology. 1990;15:131–138. doi: 10.1016/0306-4530(90)90021-z. [DOI] [PubMed] [Google Scholar]
- 14.Heitkemper MM, Shaver JF, Mitchell ES. Gastrointestinal symptoms and bowel patterns across the menstrual cycle in dysmenorrhea. Nurs Res. 1988;37:108–113. [PubMed] [Google Scholar]
- 15.Whitehead WE, Cheskin LJ, Heller BR, et al. Evidence for exacerbation of irritable bowel syndrome during menses. Gastroenterology. 1990;98:1485–1489. doi: 10.1016/0016-5085(90)91079-l. [DOI] [PubMed] [Google Scholar]
- 16.Heitkemper MM, Jarrett M. Pattern of gastrointestinal and somatic symptoms across the menstrual cycle. Gastroenterology. 1992;102:505–513. doi: 10.1016/0016-5085(92)90097-i. [DOI] [PubMed] [Google Scholar]
- 17.Drossman D, Corazziari E, Thompson W, et al., editors. Rome II: The Functional Gastrointestinal Disorders. McLean, Va: Degnon Associates; 2000. [Google Scholar]
- 18.Heitkemper MM, Cain KC, Jarrett ME, et al. Symptoms across the menstrual cycle in women with irritable bowel syndrome. Am J Gastroenterol. 2003;98:420–430. doi: 10.1111/j.1572-0241.2003.07233.x. [DOI] [PubMed] [Google Scholar]
- 19.Moore J, Barlow D, Jewell D, Kennedy S. Do gastrointestinal symptoms vary with the menstrual cycle? Br J Obstet Gynaecol. 1998;105:1322–1325. doi: 10.1111/j.1471-0528.1998.tb10014.x. [DOI] [PubMed] [Google Scholar]
- 20.Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: A prevalence study. Am J Gastroenterol. 1998;93:1867–1872. doi: 10.1111/j.1572-0241.1998.540_i.x. [DOI] [PubMed] [Google Scholar]
- 21.Chang L, Lee OY, Naliboff B, et al. Sensation of bloating and visible abdominal distention in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96:3341–3347. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan SN. A prospective study of unexplained visible abdominal bloating. N Z Med J. 1994;107:428–430. [PubMed] [Google Scholar]
- 23.Lee SY, Kim JH, Sung IK, et al. Irritable bowel syndrome is more common in women regardless of the menstrual phase: A Rome II–based survey. J Korean Med Sci. 2007;22:851–854. doi: 10.3346/jkms.2007.22.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marván ML, Cortés-Iniestra S. Women’s beliefs about the prevalence of premenstrual syndrome and biases in recall of premenstrual changes. Health Psychol. 2001;20:276–280. doi: 10.1037//0278-6133.20.4.276. [DOI] [PubMed] [Google Scholar]
- 25.Slade P. Premenstrual emotional changes in normal women: Fact or fiction? J Psychosom Res. 1984;28:1–7. doi: 10.1016/0022-3999(84)90034-5. [DOI] [PubMed] [Google Scholar]
- 26.Ross C, Coleman G, Stojanovska C. Prospectively reported symptom change across the menstrual cycle in users and non-users of oral contraceptives. J Psychosom Obstet Gynaecol. 2003;24:15– 29. doi: 10.3109/01674820309042797. [DOI] [PubMed] [Google Scholar]
- 27.Truong TT, Naliboff BD, Chang L. Novel techniques to study visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2008;10:369–378. doi: 10.1007/s11894-008-0071-2. [DOI] [PubMed] [Google Scholar]
- 28.Posserud I, Syrous A, Lindström L, et al. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113–1123. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragnarsson G, Hallböök O, Bodemar G. Abdominal symptoms and anorectal function in health and irritable bowel syndrome. Scand J Gastroenterol. 2001;36:833–842. [PubMed] [Google Scholar]
- 31.Kim HS, Rhee PL, Park J, et al. Gender-related differences in visceral perception in health and irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21:468–473. doi: 10.1111/j.1440-1746.2005.04060.x. [DOI] [PubMed] [Google Scholar]
- 32.Chang L, Mayer EA, Labus JS, et al. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;291:R277–R284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- 33.Lembo T, Munakata J, Mertz H, et al. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome [published correction appears in Gastroenterology. 1997;113:1054] Gastroenterology. 1994;107:1686–1696. doi: 10.1016/0016-5085(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 34.Jackson NA, Houghton LA, Whorwell PJ, Currer B. Does the menstrual cycle affect anorectal physiology? Dig Dis Sci. 1994;39:2607–2611. doi: 10.1007/BF02087697. [DOI] [PubMed] [Google Scholar]
- 35.Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–474. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stening K, Eriksson O, Wahren L, et al. Pain sensations to the cold pressor test in normally menstruating women: Comparison with men and relation to menstrual phase and serum sex steroid levels. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1711–R1716. doi: 10.1152/ajpregu.00127.2007. [DOI] [PubMed] [Google Scholar]
- 37.Fillingim RB. Sex, gender, and pain: Women and men really are different. Curr Rev Pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- 38.Giamberardino MA, Affaitati G, Valente R, et al. Changes in visceral pain reactivity as a function of estrous cycle in female rats with artificial ureteral calculosis. Brain Res. 1997;774:234–238. doi: 10.1016/s0006-8993(97)81711-8. [DOI] [PubMed] [Google Scholar]
- 39.Bradshaw HB, Temple JL, Wood E, Berkley KJ. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187–197. doi: 10.1016/S0304-3959(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 40.Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: A model of functional abdominal pain. Pain. 2005;118:243–253. doi: 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 41.Bradesi S, Eutamene H, Garcia-Villar R, et al. Stress-induced visceral hypersensitivity in female rats is estrogen-dependent and involves tachykinin NK1 receptors. Pain. 2003;102:227–234. doi: 10.1016/S0304-3959(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 42.Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cogan R, Spinnato JA. Pain and discomfort thresholds in late pregnancy. Pain. 1986;27:63–68. doi: 10.1016/0304-3959(86)90223-X. [DOI] [PubMed] [Google Scholar]
- 44.Strine TW, Chapman DP, Ahluwalia IB. Menstrual-related problems and psychological distress among women in the United States. J Womens Health (Larchmt) 2005;14:316–323. doi: 10.1089/jwh.2005.14.316. [DOI] [PubMed] [Google Scholar]
- 45.Robbins A, Berkley KJ, Sato Y. Estrous cycle variation of afferent fibers supplying reproductive organs in the female rat. Brain Res. 1992;596:353–356. doi: 10.1016/0006-8993(92)91572-v. [DOI] [PubMed] [Google Scholar]
- 46.Talley NJ. Serotoninergic neuroenteric modulators. Lancet. 2001;358:2061–2068. doi: 10.1016/S0140-6736(01)07103-3. [DOI] [PubMed] [Google Scholar]
- 47.Beglinger C. Tegaserod: A novel, selective 5-HT4 receptor partial agonist for irritable bowel syndrome. Int J Clin Pract. 2002;56:47–51. [PubMed] [Google Scholar]
- 48.Sumner BE, Grant KE, Rosie R, et al. Raloxifene blocks estradiol induction of the serotonin transporter and 5-hydroxytryptamine2A receptor in female rat brain. Neurosci Lett. 2007;417:95–99. doi: 10.1016/j.neulet.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 49.Houghton LA, Atkinson W, Whitaker RP, et al. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–670. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houghton LA, Perry H, Morris J, et al. Plasma 5-hydroxytryptamine concentration varies with gender and menstrual status in irritable bowel syndrome patients with diarrhea (IBS-D) but not healthy volunteers. Gastroenterology. 2008;134 (Suppl 1):A681. [Google Scholar]
- 51.Li TJ, Yu BP, Dong WG, et al. Ovarian hormone modulates 5-hydroxytryptamine 3 receptors mRNA expression in rat colon with restraint stress-induced bowel dysfunction. World J Gastroenterol. 2004;10:2723–2726. doi: 10.3748/wjg.v10.i18.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer EA, Tillisch K, Bradesi S. Review article: Modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment Pharmacol Ther. 2006;24:919–933. doi: 10.1111/j.1365-2036.2006.03078.x. [DOI] [PubMed] [Google Scholar]
- 53.Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: A meta-analysis of randomized controlled trials. Neurogastroenterol Motil. 2003;15:79–86. doi: 10.1046/j.1365-2982.2003.00389.x. [DOI] [PubMed] [Google Scholar]
- 54.Bueno L, de Ponti F, Fried M, et al. Serotonergic and non-serotonergic targets in the pharmacotherapy of visceral hypersensitivity. Neurogastroenterol Motil. 2007;19(Suppl 1):89–119. doi: 10.1111/j.1365-2982.2006.00876.x. [DOI] [PubMed] [Google Scholar]
- 55.Koch KM, Palmer JL, Noordin N, et al. Sex and age differences in the pharmacokinetics of alosetron. Br J Clin Pharmacol. 2002;53:238–242. doi: 10.1046/j.0306-5251.2001.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakai A, Diksic M, Kumakura Y, et al. The effects of the 5-HT3 antagonist, alosetron, on brain serotonin synthesis in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:212– 221. doi: 10.1111/j.1365-2982.2004.00615.x. [DOI] [PubMed] [Google Scholar]
- 57.Gintzler AR, Liu NJ. The maternal spinal cord: Biochemical and physiological correlates of steroid- activated antinociceptive processes. Prog Brain Res. 2001;133:83–97. doi: 10.1016/s0079-6123(01)33007-8. [DOI] [PubMed] [Google Scholar]
- 58.Lu CL, Hsieh JC, Tsaur ML, et al. Estrogen rapidly modulates mustard oil-induced visceral hypersensitivity in conscious female rats: A role of CREB phosphorylation in spinal dorsal horn neurons. Am J Physiol Gastrointest Liver Physiol. 2007;292:G438–G446. doi: 10.1152/ajpgi.00210.2006. [DOI] [PubMed] [Google Scholar]
- 59.Smith YR, Stohler CS, Nichols TE, et al. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26:5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 62.Chang L, Toner BB, Fukudo S, et al. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 63.Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol. 1996;87:55–58. doi: 10.1016/0029-7844(95)00360-6. [DOI] [PubMed] [Google Scholar]
- 64.Altman G, Cain KC, Motzer S, et al. Increased symptoms in female IBS patients with dysmenorrhea and PMS. Gastroenterol Nurs. 2006;29:4–11. doi: 10.1097/00001610-200601000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 66.Crowell MD, Dubin NH, Robinson JC, et al. Functional bowel disorders in women with dysmenorrhea. Am J Gastroenterol. 1994;89:1973– 1977. [PubMed] [Google Scholar]
- 67.Ahrendt HJ, Karckt U, Pichl T, et al. The effects of an oestrogen-free, desogestrel-containing oral contraceptive in women with cyclical symptoms: Results from two studies on oestrogen-related symptoms and dysmenorrhoea. Eur J Contracept Reprod Health Care. 2007;12:354–361. doi: 10.1080/13625180701536771. [DOI] [PubMed] [Google Scholar]
- 68.Brinkert W, Dimcevski G, Arendt-Nielsen L, et al. Dysmenorrhoea is associated with hypersensitivity in the sigmoid colon and rectum. Pain. 2007;132(Suppl 1):S46–S51. doi: 10.1016/j.pain.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. A comparison of modality-specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clin J Pain. 2002;18:180–190. doi: 10.1097/00002508-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 70.Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: Modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1592–R1601. doi: 10.1152/ajpregu.00455.2006. [DOI] [PubMed] [Google Scholar]
- 71.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: Implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: An afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol. 2006;290:F1478–F1487. doi: 10.1152/ajprenal.00395.2005. [DOI] [PubMed] [Google Scholar]
- 73.Lea R, Bancroft K, Whorwell PJ. Irritable bowel syndrome, chronic pelvic inflammatory disease and endometriosis: A comparison of symptomatology. Eur J Gastroenterol Hepatol. 2004;16:1269–1272. doi: 10.1097/00042737-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Remorgida V, Ragni N, Ferrero S, et al. The involvement of the interstitial Cajal cells and the enteric nervous system in bowel endometriosis. Hum Reprod. 2005;20:264–271. doi: 10.1093/humrep/deh568. [DOI] [PubMed] [Google Scholar]
- 75.Ferrero S, Abbamonte LH, Remorgida V, Ragni N. Irritable bowel syndrome and endometriosis. Eur J Gastroenterol Hepatol. 2005;17:687. doi: 10.1097/00042737-200506000-00017. [DOI] [PubMed] [Google Scholar]
- 76.Griffiths AN, Koutsouridou RN, Penketh RJ. Predicting the presence of rectovaginal endometriosis from the clinical history: A retrospective observational study. J Obstet Gynaecol. 2007;27:493–495. doi: 10.1080/01443610701405721. [DOI] [PubMed] [Google Scholar]
- 77.Yu J, Wang Y, Zhou WH, et al. Combination of estrogen and dioxin is involved in the pathogenesis of endometriosis by promoting chemokine secretion and invasion of endometrial stromal cells. Hum Reprod. 2008;23:1614–1626. doi: 10.1093/humrep/den125. [DOI] [PubMed] [Google Scholar]
- 78.Marshall JK, Thabane M, Garg AX, et al. for the Walkerton Health Study Investigators. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445–450. doi: 10.1053/j.gastro.2006.05.053. quiz 660. [DOI] [PubMed] [Google Scholar]
- 79.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 80.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 81.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 82.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 83.Guilarte M, Santos J, de Torres I, et al. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 85.Alonso C, Guilarte M, Vicario M, et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation [published correction appears in Gastroenterology. 2008;135:1017] Gastroenterology. 2008;135:163–172. doi: 10.1053/j.gastro.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 86.Sherman JJ, LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. Am J Physiol Regul Integr Comp Physiol. 2006;291:R245–R256. doi: 10.1152/ajpregu.00920.2005. [DOI] [PubMed] [Google Scholar]
- 87.Triadafilopoulos G, Finlayson M, Grellet C. Bowel dysfunction in postmenopausal women. Women Health. 1998;27:55–66. doi: 10.1300/J013v27n04_04. [DOI] [PubMed] [Google Scholar]
- 88.Cain KC, Jarrett ME, Burr RL, et al. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig Dis Sci. doi: 10.1007/s10620-008-0516-3. [published online ahead of print November 1, 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruigómez A, García Rodríguez LA, Johansson S, Wallander MA. Is hormone replacement therapy associated with an increased risk of irritable bowel syndrome? Maturitas. 2003;44:133–140. doi: 10.1016/s0378-5122(02)00321-3. [DOI] [PubMed] [Google Scholar]
- 90.Longstreth GF, Yao JF. Irritable bowel syndrome and surgery: A multivariable analysis. Gastroenterology. 2004;126:1665–1673. doi: 10.1053/j.gastro.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 91.Kennedy TM, Jones RH. The epidemiology of hysterectomy and irritable bowel syndrome in a UK population. Int J Clin Pract. 2000;54:647–650. [PubMed] [Google Scholar]
- 92.Feld AD, Von Korff M, Levy RL, et al. Excess surgery inirritable bowel syndrome(IBS) Gastroenterology. 2003;124:A388. [Google Scholar]
- 93.Harlow SD, Crawford S, Dennerstein L, et al. for the ReSTAGE Collaboration. Recommendations from a multi-study evaluation of proposed criteria for staging reproductive aging. Climacteric. 2007;10:112–119. doi: 10.1080/13697130701258838. [DOI] [PubMed] [Google Scholar]
- 94.Greenspan JD, Craft RM, LeResche L, et al. for the Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP. Studying sex and gender differences in pain and analgesia: A consensus report. Pain. 2007;132(Suppl 1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]