Abstract
Anti-Thy1 nephritis is a well-established experimental mesangial proliferative nephritis model. Exploring the molecular mechanisms of pathophysiology in anti-Thy1 nephritis may elucidate the pathogeneses of mesangial proliferation. We examined the roles and acting mechanisms of differentially expressed proteins (DEPs) by bioinformatics analysis of glomeruli proteomic profiles during the course of anti-Thy1 nephritis. In total, 108 DEPs were found by two-dimensional fluorescence difference gel electrophoresis (2D-DIGE), and 40 DEPs were identified by matrix-assisted laser desorption ionization/time of flight and liquid chromatography-MS. DEPs were classified into five clusters (Clusters 1–5), according to their expression trends using Cluster 3.0 software, involved in regulating biological processes such as the stress response, cell proliferation, apoptosis, energy metabolism, transport, and the actin cytoskeleton. The expression patterns of ten DEPs, distributed across five clusters, including AKR1A1, AGAT, ATP6V1B2, HIBADH, MDH1, MPST, NIT2, PRDX6, PSMB7, and TPI1, were validated by Western blotting. Based on Western blotting and immunohistochemistry, we also found that the DEP FHL2, which was primarily expressed in the mesangial region, was down-regulated on days 3 and 5, and up-regulated on day 10. In vitro, we found that FHL2 overexpression induced mesangial cell proliferation by increasing the number of S-phase cells and decreasing G2/M-phase cells, whereas inhibiting FHL2 had the opposite effect. This study explored novel DEPs and their expression patterns during anti-Thy1 nephritis, and elucidated FHL2's effect on mesangial cell proliferation. These results will contribute to our understanding of the pathogenesis of mesangial proliferation.
Mesangial proliferative glomerulonephritis (MesPGN)1 is the most common chronic kidney disease and is characterized by pathological changes in mesangial cell proliferation and extracellular matrix accumulation. As an important cause of end-stage renal disease, MesPGN has been a major focus of renal disease research. Examining the molecular mechanisms of MesPGN may elucidate its pathogenesis and thereby facilitate the development of clinical treatments. Animal models, such as rat anti-Thy1 nephritis, provide a useful tool to explore these mechanisms.
Anti-Thy1 nephritis is a well-established model of mesangial proliferative glomerulonephritis with two major physiological phases (1): the mesangial proliferative phase (e.g. 5 and 7 days after anti-Thy1 antibody injection) and the recovery phase (e.g. 10 and 14 days after anti-Thy1 antibody injection). Biological functions such as stress (2), cytokine action (3, 4), cell proliferation (5, 6), and apoptosis (7, 8) mediate these changes in pathology during the process of anti-Thy1 nephritis. Many proteins have been reported to be involved in regulating these biological functions. For example, Porst (9) reported that fibrillin-1 may regulate mesangial cell proliferation and migration in anti-Thy1 nephritis. Sasaki et al. (6) showed that Galectin-3 modulates rat mesangial cell proliferation and matrix synthesis during experimental glomerulonephritis. However, these studies were limited to a relatively small number of proteins, and it is therefore necessary to examine additional proteins involved in the regulation of the biological functions in anti-Thy1 nephritis. New and powerful tools, such as proteomics, should be used to explore these proteins on a larger scale, as such research could improve current understanding of the molecular processes of anti-Thy1 nephritis.
Nazeer et al. (10) used two-dimensional electrophoresis (2DE) techniques to study differences in the proteomic profiles between control and anti-Thy1 nephritis at day 7 and found 16 differentially expressed proteins, providing new insight into the mechanisms of anti-Thy1 nephritis. However, the study examined only day-7 proteins (within the proliferation phase), whereas other time points such as days 10 and 14, which are within the resolution phase, were not analyzed. Here, we examined the different expressed proteins (DEPs) of anti-Thy1 nephritis, including both the proliferative and recovery phases, using 2D fluorescence difference gel electrophoresis (2D-DIGE), which has higher sensitivity and is more reproducible than traditional 2D methods.
Among the DEPs, four and a half LIM domain protein 2 (FHL2), whose expression was localized to the mesangial region, was down-regulated on day 3 and 5, and up-regulated on day 10 in anti-Thy1 nephritis, which suggests that the changes in FHL2 expression may be associated with mesangial cell proliferation. It was previously reported that FHL2 has multiple functions associated with cell proliferation. Overexpression of FHL2 inhibits the growth of specific cells, such as colon cancer (11) and hepatoma cells (12), and FHL2 may also promote cell proliferation in mouse fibroblast (13) and glioblastoma cells (14). However, FHL2's effect on mesangial cell proliferation is not known. In the present study, we explored whether FHL2 induces mesangial cell proliferation by mediating the cell cycle, and hypothesized a role for FHL2 in activating mesangial proliferation during anti-Thy1 nephritis.
EXPERIMENTAL PROCEDURES
Anti-Thy1 Nephritis Model
Anti-Thy1 nephritis was induced in 30 male Wistar rats (200 g) by a single intravenous injection (2.5 mg/kg) of the monoclonal anti-Thy1 antibody. Six rats that served as controls were injected with the same volume of phosphate buffered saline. Anti-Thy1-treated animals were sacrificed on days 3, 5, 7, 10, and 14 following injection (six rats per time point), and the control group was sacrificed at day 0. Glomeruli from the renal cortex of each rat (>90% purity) were isolated by selective sieving as described previously (15). Isolated glomeruli were snap frozen and stored at −80 °C for further analyses as described below.
Glomerular Histology and Immunohistochemical Staining
Renal tissues for light microscopy were fixed in 10% buffered formalin and embedded in paraffin. Sections (4 μm) were stained with periodic acid-Schiff reagent and counterstained with hematoxylin. Examination of proliferating cell nuclear antigen (PCNA) and FHL2 expression was performed using the indirect immunoperoxidase technique on paraffin-embedded sections as described previously (16).
2D-DIGE and Imaging
Minimal Fluorescent-Dye Labeling
Three rats were randomly selected from each group for gel electrophoretic analysis. Glomeruli protein from each rat in each group (three rats) was separately analyzed on a 2-D gel. The proteins from the glomeruli were isolated by lysis buffer (30 mmol/L Tris, 7 mol/L Urea, 2 mol/L Thiourea, 4% (w/v) 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate (CHAPS, pH 8.5). The pH of the proteins was adjusted to 8.5 by 50 mmol/L NaOH, and the concentration was adjusted to 5 mg/ml with lysis buffer. Equal amounts of proteins from the 18 samples were pooled together as the internal standard. A 50 μg proteins was labeled with 400 pmol of Cy3, Cy5, or Cy2 (Cy2 was used to label the internal standard) on ice for 30 min and then quenched with 1 μl lysine (10 mmol/L).
2-DE
The 50 μg Cy3- and Cy5-labeled samples from different groups were combined before mixing with 50 μg of Cy2-labeled internal standard. An equal volume of 2× sample buffer [7 mol/L Urea, 2 mol/L Thiourea, 2% (w/v) CHAPS, 130 mmol/L dithiothreitol (DTT), 1% IPG buffer] was then added to the sample, which was topped up to a total volume of 450 μl with rehydration buffer (7 mol/L Urea, 2 mol/L Thiourea, 2% (w/v) CHAPS, 65 mmol/L DTT, 0.5% IPG buffer). Samples were applied to 24-cm pH3–11 (NL) IPG strips, and isoelectric focusing was performed using the IPGphor IEF system. The isoelectric focusing program was set as follows: 30 V for 12 h, Grd 200 V for 6 h, Grd 500 V for 3 h, Grd 10,000 V for 1 h, and step 10000 V 64000 V h. The IPG strips were equilibrated in equilibration buffer A (50 mmol/L Tris-HCl, pH 8.8, 6 mol/L urea, 30% glycerol, 2% SDS, 10 mg/ml DTT) for 15 min at room temperature followed by equilibration buffer B (50 mmol/L Tris-HCl, pH 8.8, 6 mol/L urea, 30% glycerol, 2% SDS, 25 mg/ml iodoacetamid) for another 15 min incubation at room temperature. IPG strips were placed on top of 12% homogeneous polyacrylamide gels that had been precast with low fluorescence glass plates using an Ettan DALT six-gel caster. Strips were overlaid with 0.9% agarose in 1× running buffer containing bromphenol blue and were run for 12–16 h (5 W per gel, overnight) at 15 °C in an Ettan DALT six electrophoresis system. After the run was completed, the 2D gels were scanned with a Typhoon 9400 imager.
Data Analysis
Analysis of 2D-DIGE was performed using DeCyder 6.0 software (GE Healthcare) according to the manufacturer's recommendation. Briefly, the DeCyder biological variation analysis module was used to detect spots (the estimated number of spots was 2500) and simultaneously match all 27 protein spot maps from nine gels. All matches were also confirmed manually. Standard deviation of a protein spot data in each group was calculated according to the differences among each of the three rats. A one-way analysis of variance (ANOVA) was used for statistical analysis of the protein spot data, and a p value of <0.05 indicated that a protein spot was significantly changed across the six groups.
Protein Identification
In-gel Digestion
Spot picking was carried out with preparative gels. Two-dimensional electrophoresis was performed as described under “2D-DIGE and Imaging” except that the IPG strips were loaded with 500–1000 μg of protein, and gels were stained with silver stain and Coomassie Brilliant Blue. Protein spots of interest were excised and destained with 100 mm sodium thiosulfate, 30 mmol/L ferricyanatum Kalium, and 25 mmol/L ammonium bicarbonate, 50% acetonitrile. The destained gels were incubated in 10 mmol/L DTT at 56 °C for 1 h and in 55 mmol/L IAM at room temperature for 45 min in the dark. Gels were then dried completely by centrifugal lyophilization and digested overnight at 37 °C with gentle agitation using 0.05 μg/μl of sequence grade-modified trypsin (Promega, Madison, WI). The reaction was stopped by 2% trifluoroacetic acid.
MALDI-TOF Analysis
A 1-μl aliquot of tryptic peptide was mixed with 1 μl of matrix (4-hydroxy-α-cyanocinnamic acid in 30% acetonitrile, 0.1% trifluoroacetic acid) before spotting on the target plate. MS analyses were performed on an AXIMA matrix-assisted laser desorption ionization/time of flight (MALDI-TOF) instrument (Shimadzu Corp., Kyoto, Japan). Peptide mass maps were acquired in the positive reflection mode, averaging 1500 laser shots per MALDI-TOF spectrum by KOMPACT V2.4.0 software. The mass spectra were analyzed using MASCOT 1.2.0 software and searched against the NCBI rattus protein database (released April 2009, 68457 sequences). Peptide tolerances were set at 200 ppi and maximum missed cleavage was set as 2 with fixed modifications: carbamidomethyl (C)/variable: oxidation (M). Known contaminant ions (keratin) were excluded. PMF scores (probability-based MOWSE score) >60 were considered statistically significant (p values ≦ 0.05). The molecular weight and pI values of most proteins were consistent with the gel regions from which the spots were excised.
Liquid Chromatography-MS (LC-MS) Analysis
The tryptic peptides were extracted with 5% formic acid/50% CH3CN, and the analysis was performed using an LTQ instrument (Thermo) supplied with C18 capillary columns. Mobile phase A was 0.1% formic acid and mobile phase B was 0.1% formic acid in acetonitrile. The program was set as follows: L: 100% (0 min)–100% A (5 min)–5% B (5.1 min)–65% B (60 min)–100% B (75 min)–100% B (85 min), Flow rate: 200–800 nl/min. The peak list was generated by Xcalibur version 2.0 SR2 software and the data were analyzed using SEQUEST V27 software and searched against the NCBI rattus database (released March 2009′14820 sequences). Scoring criteria were set as follows: Del CN value ≧ 0.1, xCorr ≧ 1.9 for charge state 1+; ≧ 2.5 for charge state 2+; and ≧ 3.75 for charge state 3+.
Hierarchical Cluster Analysis
Hierarchical cluster analyses were carried out using the Cluster program 3.0, and the results were compiled using Eisensoftware-TreeView. The proteins in a given cluster exhibited similar expression profiles in a synergistic manner.
Western Blotting
Renal glomeruli and cellular protein were isolated using RIPA (containing 50 mmol/L Tris-HCl pH 7.5,150 mmol/L NaCl, 0.5% deoxycholate, 1% Nonidet P-40, 0.1% SDS, 1 mm phenylmethylsulfonyl fluoride, and a variety of protease inhibitors). AKR1A1, AGAT, PRDX6, MDH1, PSMB7, and HIBADH antibodies were purchased from ABCAM (Cambridge, MA); ATP6V1B2, MPST, TPI1, cyclin D1 and FHL2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The NIT2 antibody was produced by our laboratory. β-actin (Sigma) was used as the control. Approximately 80 μg of protein were loaded for 12% SDS-PAGE electrophoresis. The membrane was incubated in primary antibodies at suitable concentrations. The blots were developed with ECL reagent (Santa Cruz Biotechnologies) according to the manufacturer's instructions and exposed to x-ray film. The protein bands were quantified using Quantity One software (Bio-Rad, Hercules, CA).
Primary Mesangial Cell (MC) Culture
Primary MCs were cultured as reported previously (17). Briefly, glomeruli were isolated from Wistar rats weighing 200 g using a stainless-steel sieve. Isolated glomeruli were cultured in RPMI 1640 with 15% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C. First passage MCs were grown from glomeruli 5–7 days later. MCs were identified by detecting MC markers such as desmin, vimentin, α-SMA et al. MCs from passages 10 to 15 were used in our study.
Cell Transfection
Cells at 70–80% confluency in six-well plates were prepared for plasmid transfection. The pcDNA-FHL2 plasmid was a gift from Professor Qinong Ye. Plasmid (2 μg/ml) and 4 μl/ml Jetprime (Polyplus-transfection) were applied for 8 h at 37 °C, then replaced with fresh medium (RPMI 1640, 15% fetal bovine serum) for 16 h. For siRNA transfection, 100 nm siRNA were transfected into 3 × 105 cells in six-well plates using lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's reverse transfection protocol. The siFHL2 target sequence was 5′-GCAAGGACTTGTCCTACAA-3′(18). The cell number was determined for detecting cell proliferation prior to harvesting. FHL2 expression in cells was detected by Western blotting, as described previously.
Determination of the Cell Cycle Phase by PI Staining
Cells were harvested and fixed in 75% ethanol for 24 h at 4 °C. The nuclei were stained with 50 μg/ml propidium iodide (PI) in phosphate-buffered saline containing 200 μg/ml DNase-free RNase, and the DNA content was analyzed by flow cytometry using FACS Calibur (BD, USA). The percentage of cells in each phase of the cell cycle was determined by the Modfit LT program (Verity Software House, Topsham, ME).
Statistical Analysis
A chi-square analysis was applied to compare cell phase distribution (%). t-tests and an ANOVA were conducted for two-way and multiclass comparisons, respectively.
RESULTS
Mesangial Proliferation and Recovery Were Two Major Phases During Anti-Thy1 Nephritis
Following intravenous injection of anti-Thy1 antibodies, a partial complement-dependent mesangiolysis appeared on day 3; obvious mesangial cell proliferation (more than three MCs in a single mesangial region) and extracellular matrix accumulation occurred on day 5 and peaked on day 7. Recovery from glomerular injury began on day 10 and the number of MCs showed an obvious decrease as extracellular matrix accumulation attenuated at day 14 (Fig. 1). Detection of PCNA expression reflects the state of the cell cycle. High levels of PCNA are consistent with onset of S-phase, whereas low levels are consistent with nonproliferative cells in G1-phase. We found that there were higher levels of PCNA at every time point during anti-Thy1 nephritis as compared with the control (0 d) (Fig. 2), which suggests that the cell cycle remained active from days 3 to 14. PCNA expression levels had a unique pattern during anti-Thy1 nephritis. PCNA increased on days 3 and 5, peaked on day 7, and decreased from days 10 to 14. This suggests that cell cycle activity increased from days 3 to 7, and subsequently gradually decreased from days 10 to 14. Thus, our results imply that mesangial proliferation and recovery are the two major phases during the process of anti-Thy1 nephritis.
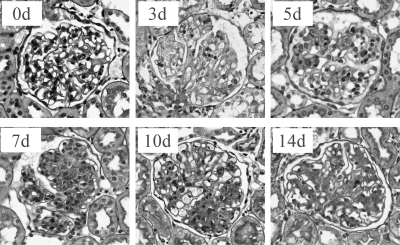
Fig. 1.
Histopathology of rat anti-Thy1 nephritis (PAS staining 400×). On day 0 (0d), the control group was injected with PBS. Controls showed normal glomerular capillaries with no cell proliferation or extracellular matrix accumulation. On day 3 (3d), following injection of anti-Thy1 antibody (3d), an initial complement-dependent mesangiolysis appeared. On day 5 (5d), the number of MCs began to increase and mild to moderate extracellular matrix accumulation was apparent. By day 7 (7d), MC proliferation peaked, and extracellular matrix accumulation became severe. On day 10 (10d), the mesangial proliferation began to resolve, and on day 14 (14d), the number of MCs was significantly reduced with attenuation of extracellular matrix accumulation.
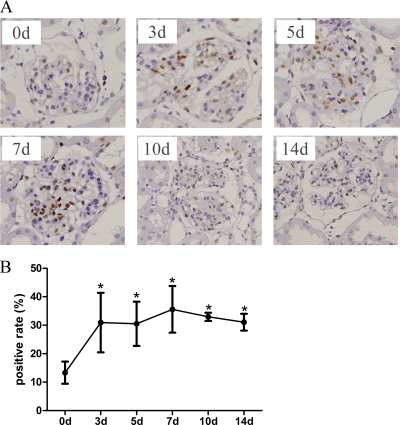
Fig. 2.
PCNA immunohistochemistry of anti-Thy1 nephritis. A, Immunohistochemistry graph of PCNA. B, PCNA-positive rates (mean ± std) in each group (three rats per group). The rate was obtained by calculating the percentage of PCNA-positive nuclei in 10 glomeruli per rat. We found that the rate increased at 3d and 5d, peaked at 7d, and subsequently decreased at 10d and 14d. However, the rate at any of these time points was higher than the control, 0d (* mean p < 0.05).
Forty DEPs, Identified by 2D-DIGE, Were Classified Into Five Clusters According to Their Expression Patterns During Anti-Thy1 Nephritis
We analyzed the proteomic profiles of each group by 2D-DIGE, and the Cy2, Cy3, and Cy5 channels of each gel were individually imaged (Figs. 3A, 3B, 3C). In total, 108 proteins were significantly changed (p < 0.05), and 40 proteins were identified by MALDI-TOF (Table I) and LC-MS (Table II). The corresponding sites of these DEPs, including identified proteins in DIGE profiles, are provided in Fig. 3D. The DEPs contained proteins such as enzymes, cytoskeletal proteins, and heat-shock proteins (Table III). The enzymes included oxido-reductases (PRDX3, PRDX6, DLD, HIBADH, MDH1, AKR1A, CAS1), transferases (ACAT1, AGAT, AGT2, GLYAT, MPST, COMT, AK3L1, Pgk1), ligases (SUCLA2, SUCLG2), hydrolases (FAHD2A, NIT2, ATP6V1A, Atp6v1b2, PSMA3, PSMB7), isomerases (TPI1), and lyases (CA2, ECHS1, ECH1). Cytoskeletal-related proteins included CAPZA, CAPZB, SEPT2, ANXA2, FHL2, and PDLIM1. The heat-shock proteins HSPD1 and HSPA8 were identified along with other types of proteins such as NDRG1, SPI6, TOLLIP, and ERP29.
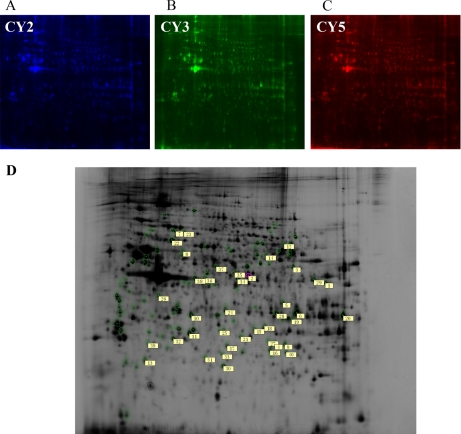
Fig. 3.
Representative 2D-DIGE gels showing the protein expression profiles obtained from anti-Thy1 nephritis. Proteins from the samples obtained for each group were differentially labeled with Cy3 and Cy5 (B, C). Equal amounts of each sample were combined and labeled with Cy2 and set as an internal standard (A). Labeled samples (50 μg of each of the Cy3- and Cy5-labeled samples and of the Cy2-labeled internal standard) were loaded onto 24-cm pH 3–11 NL-IPG strips and subjected to IEF. Proteins were further separated by SDS-PAGE (12.5%) in the second dimension. Numbers allocated by the DeCyder software indicate spots with significant changes in intensity (p < 0.05), which were identified by MALDI-TOF or LC-MS (D).
Table I. DEPs were identified by MALDI-TOF.
| Spot no. | NCBI no. | Name of identified protein | Symbol | Theoretical molecular mass (Da) | Theoretical isoelectric point (pI) | One-way -ANOVA p value | MALDI-TOF |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Mascot scores | Peptides matched | Peptides unmatched | Coverage (%) | |||||||
| 1 | NP_058771.1 | Acetyl-Coenzyme A Acetyltransferase 1 | ACAT1 | 45009 | 8.92 | 4.30E-03 | 192 | 24 | 15 | 58 |
| 2 | NP_112293.1 | l-Arginine:Glycine Amidinotransferase | AGAT | 48724 | 7.17 | 1.90E-03 | 151 | 22 | 40 | 52 |
| 3 | NP_114023.1 | Alanine-Glyoxylate Aminotransferase 2 | AGT2 | 57869 | 8.46 | 2.90E-03 | 139 | 11 | 2 | 30 |
| 4 | NP_058831.1 | Adenylate Kinase 3-Like 1 | AK3L1 | 25301 | 7.79 | 2.50E-03 | 65 | 10 | 45 | 48 |
| 5 | NP_112262.1 | Aldo-Keto Reductase Family 1, Member A1 | AKR1A1 | 36711 | 6.84 | 3.90E-03 | 86 | 7 | 3 | 21 |
| 7 | NP_001101788 | Atpase, H+ Transporting, Lysosomal V1 Subunit A | ATP6V1A | 68564 | 5.42 | 1.20E-03 | 154 | 13 | 2 | 23 |
| 9 | NP_062164.1 | Carbonic Anhydrase II | CA2 | 29267 | 6.89 | 6.70E-05 | 68 | 6 | 8 | 26 |
| 10 | NP_031630 | Capping Protein (Actin Filament) Muscle Z-Line, Alpha 2 | CAPZA2 | 33118 | 5.57 | 4.60E-02 | 137 | 14 | 28 | 61 |
| 12 | NP_062164.1 | Catalase | CAS1 | 59931 | 7.15 | 3.70E-02 | 81 | 22 | 72 | 45 |
| 14 | NP_955417 | Dihydrolipoamide Dehydrogenase | DLD | 54574 | 7.96 | 7.40E-03 | 74 | 7 | 5 | 12 |
| 16 | NP_511178.1 | Enoyl Coenzyme A Hydratase, Short Chain, 1, Mitochondrial | ECHS1 | 28498 | 6.41 | 2.10E-02 | 109 | 11 | 9 | 35 |
| 17 | NP_446413.1 | Endoplasmic Retuclum Protein 29 | ERP29 | 28614 | 6.23 | 1.90E-03 | 58 | 7 | 12 | 31 |
| 18 | NP_001128306.1 | Fumarylacetoacetate Hydrolase Domain Containing 2A | FAHD2A | 34958 | 8.63 | 1.70E-02 | 78 | 6 | 2 | 24 |
| 19 | NP_113865.1 | Four And A Half LIM Domains 2 | FHL2 | 34060 | 7.31 | 1.50E-04 | 230 | 22 | 14 | 68 |
| 20 | NP_001009648.1 | Glycine-N-Acyltransferase | GLYAT | 34162 | 8.82 | 1.30E-02 | 61 | 11 | 33 | 56 |
| 21 | NP_071579.1 | 3-Hydroxyisobutyrate Dehydrogenase, Mitochondrial Precursor | HIBADH | 35679 | 8.73 | 2.60E-02 | 88 | 13 | 44 | 44 |
| 22 | NP_071565.1 | Heat Shock Protein (Hsp60) Precursor | HSPD1 | 61088 | 5.91 | 1.30E-03 | 98 | 14 | 11 | 32 |
| 25 | NP_620198.1 | 3-Mercaptopyruvate Sulfurtransferase | MPST | 33074 | 5.88 | 2.50E-03 | 66 | 11 | 56 | 46 |
| 27 | NP_001029298.1 | Nitrilase Family, Member 2 | NIT2 | 31024 | 6.9 | 7.70E-03 | 76 | 10 | 19 | 45 |
| 29 | NP_445743.2 | Phosphoglycerate Kinase 1 | PGK1 | 44909 | 8.02 | 1.20E-03 | 126 | 12 | 4 | 34 |
| 31 | NP_446028 | Peroxiredoxin 6 | PRDX6 | 24860 | 5.64 | 8.20E-03 | 65 | 5 | 4 | 22 |
| 34 | NP_476489.1 | Septin 2 | SEPT2 | 41737 | 6.15 | 6.60E-05 | 71 | 11 | 18 | 39 |
| 38 | NP_001094220.1 | GTP-Specific Succinyl-Coa Synthetase Beta Subunit | SUCLG2 | 47042 | 7.57 | 8.70E-03 | 222 | 29 | 31 | 53 |
| 40 | NP_075211.2 | Triosephosphate Isomerase 1 | TPI1 | 31676 | 5.56 | 4.00E-05 | 111 | 8 | 3 | 45 |
Table II. DEPs were identified by LC-MS.
| Spot no. | NCBI no. | Name of identified protein | Symbol | Theoretical molecular mass (Da) | Theoretical isoelectric point (pI) | One-way -ANOVA p value | LC-MS |
||
|---|---|---|---|---|---|---|---|---|---|
| Score | Peptide matched | Coverage (%) | |||||||
| 6 | NP_063970.1 | Annexin A2 | ANXA2 | 38678 | 7.55 | 1.70E-03 | 254.34 | 67 | 63 |
| 8 | NP_476561 | Vacuolar H+Atpase B2 | Atp6v1b2 | 56550 | 5.57 | 5.90E-04 | 100.25 | 23 | 15 |
| 11 | NP_001005903 | Capping Protein (Actin Filament) Muscle Z-Line, Beta = 20 | CAPZB | 30628 | 5.69 | 5.00E-03 | 90 | 9 | 23 |
| 13 | NP_036663.1 | Catechol-O-Methyltransferase | COMT | 29597.3 | 5.3 | 2.50E-02 | 80.22 | 16 | 34 |
| 15 | NP_072116.1 | Enoyl Coenzyme A Hydratase 1, Peroxisomal | ECH1 | 36201 | 8.14 | 7.80E-03 | 126.30 | 35 | 25 |
| 23 | NP_077327 | Heat Shock Protein 8 | HSPA8 | 70870 | 5.32 | 2.90E-02 | 130.24 | 26 | 22 |
| 24 | NP_150238.1 | Malate Dehydrogenase 1, NAD (Soluble) | MDH1 | 36482 | 6.16 | 1.80E-02 | 50.27 | 16 | 22 |
| 26 | NP_001011991 | N-Myc Downstream Regulated Gene 1 | NDRG1 | 42954.5 | 5.3 | 1.50E-04 | 50.23 | 9 | 12 |
| 28 | NP_059061.1 | PDZ And LIM Domain 1 | PDLIM1 | 35525 | 6.56 | 1.90E-02 | 100.24 | 18 | 28 |
| 30 | NP_071985.1 | Peroxiredoxin-3 | PRDX3 | 28321 | 7.14 | 2.10E-03 | 20 | 2 | 8 |
| 32 | NP_001004094 | Proteasome Subunit Alpha Type 3-Like | PSMA3 | 28419.1 | 5.29 | 1.90E-02 | 40.17 | 6 | 13 |
| 33 | NP_445984.1 | Proteasome (Prosome, Macropain) Subunit, Beta Type 7 | PSMB7 | 29927 | 8.14 | 6.10E-03 | 80.21 | 26 | 22 |
| 35 | NP_446263.1 | Solute Carrier Family 9 Isoform 3 Regulator 2 | SLC9A3R2 | 37670 | 7.21 | 3.20E-02 | 170.26 | 72 | 46 |
| 36 | NP_001007733.1 | Serine Protease Inhibitor 6 | SPI6 | 42301.4 | 5.61 | 5.90E-03 | 50 | 5 | 15 |
| 37 | NP_001101857.2 | Succinate-Coenzyme A Ligase, ADP-Forming, Beta Subunit | SUCLA2 | 50305.9 | 7.57 | 5.20E-03 | 120.24 | 12 | 24 |
| 39 | NP_001103138.1 | Toll Interacting Protein | TOLLIP | 30314.6 | 4.89 | 3.20E-02 | 40.21 | 13 | 19 |
Table III. DEPs were composed with enzymes, cytoskeleton proteins etc.
| Cluster | Enzyme (27) |
Cytoskeleton protein | Others | |||||
|---|---|---|---|---|---|---|---|---|
| Transferase | Oxido-reductases | Hydrolase | Lyases | Ligase | Isomerases | |||
| (8) | (7) | (6) | (3) | (2) | (1) | (4) | (9) | |
| 1 | ACAT1, AGAT, AGT2 | PRDX3, HIBADH, AKR1A, CAS1 | FAHD2A, NIT2, ATP6V1A, Atp6v1b2 | ECHS1 | SUCLA2 | HSPD1, SPI6 | ||
| 2 | AK3L1, COMT, PGK1 | DLD | PSMA3 | CA2 | SUCLG2 | TPI1 | NDRG1 | |
| 3 | MPST | PRDX6, MDH1 | HSPA8 | |||||
| 4 | ECH1 | CAPZB, CAPZA2, SEPT2, FHL2, PDLIM1 | ANXA2 | |||||
| 5 | GLYAT | PSMB7 | ERP29, SLC9A3R2, TOLLIP | |||||
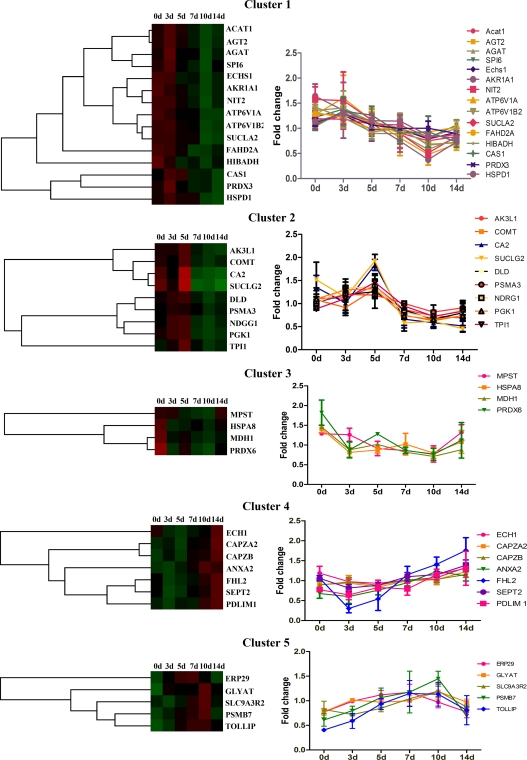
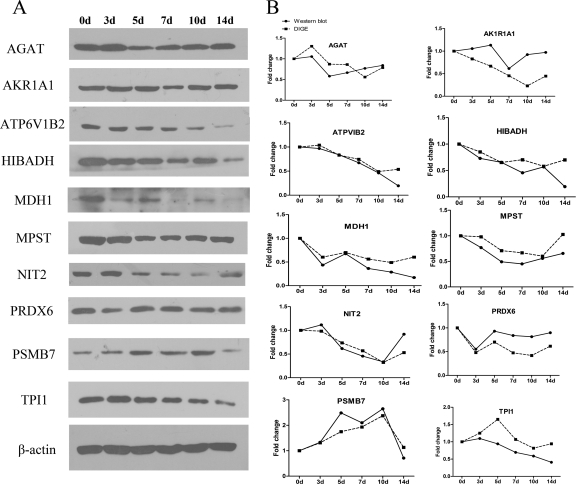
Cluster analysis was performed to investigate the expression trends of DEPs during anti-Thy1 nephritis. In total, 40 DEPs were classified into five clusters based on their expression signals (Fig. 4 and Table III). Proteins in Cluster 1 showed decreased expression starting on day 5 and/or 7 and continued to decline on days 10 and 14. Proteins in Cluster 2 were up-regulated on day 5 and down-regulated at days 7, 10, and 14. Cluster 3 proteins were down-regulated on days 3, 5, 7, and 10 and showed recovered expression on day 14. Cluster 4 proteins were down-regulated on days 3 and/or 5 and up-regulated on days 10 and/or 14. Cluster 5 proteins were up-regulated on days 5, 7, and/or 10 and recovered by day 14. All DEPs identified in our study have known roles in process such as the stress response, cell proliferation, apoptosis, energy metabolism, transport, and actin cytoskeleton regulation, suggesting that these processes may also be operative in different phases of anti-Thy1 nephritis (Tables III and IV). We harvested the glomeruli from three rats in each group, pooled the protein, and evaluated the expression of DEPS distributed across five clusters including AKR1A1, AGAT, ATP6V1B2, HIBADH, MDH1, MPST, NIT2, PRDX6, PSMB7, and TPI1 (Fig. 5). Based on Western blotting, we found that the expression patterns of all 10 proteins were altered at one or several time points as compared with the control. Western blotting and DIGE revealed similar expression patterns for eight DEPs (less than two time points showed significant differences), except for AK1R1A1 and PRDX6 (three time points showed significant differences).
Fig. 4.
DEPs were classified into five clusters according to their expression patterns. The colored graph displays the five DEP clusters (red: up-regulated, green: down-regulated), and the curve graph shows the expression of DEPs in each cluster (mean ± std) during the process of anti-Thy1. Cluster 1 showed decreased expression starting on days 5 and/or 7, and expression continued to decline on days 10 and 14. Cluster 2 showed up-regulation on day 5 and down-regulation on days 7, 10, and 14. Cluster 3 showed down-regulation on days 3, 5, 7, and 10 and recovery on day 14. Cluster 4 showed down-regulation on days 3 and/or 5 and up-regulation on days 10 and/or 14. Cluster 5 showed up-regulation on days 5, 7, and/or 10 and recovery by day 14.
Table IV. DEPs regulated biological functions such as stress, cell proliferation, apoptosis, etc.
| Cluster | Stress | Cell proliferation | Apoptosis | Actin cytoskeleton regulation | Energy metabolsim | Transport |
|---|---|---|---|---|---|---|
| 1 | AGAT, PRDX3, NIT2, HSPD1 | ACAT1, PRDX3, HSPD1, NIT2 | PRDX3, HSPD1, SPI6, ECHS1, ATP6V1B2, ATP6V1A | SUCLA2 | ACAT1, ATP6V1B2, ATP6V1A | |
| 2 | CA2, COMT, DLD, PGK1, AK3L1, TPI1, MPST | DLD, COMT, PGK1, NDRG1 | DLD, COMT, NDRG1 | DLD, PGK1, TPI1, SUCLG2 | MPST, CA2, COMT, DLD, TPI1 | |
| 3 | MPST, HSPA8 PRDX6 | PRDX6 | HSPA8, PRDX6 | MDH1 | HSPA8, MDH1 | |
| 4 | ANXA2, PDLIM1, FHL2 | SEPT2, FHL2, ANXA2 | FHL2, ANXA2 | CAPZB, CAPZA2, SEPT2, FHL2, PDLIM1, ANXA2 | CAPZB, CAPZA2, SEPT2, ANXA2 | |
| 5 | ERP29 | ERP29 | ERP29 | ERP29 |
Fig. 5.
Validating DEP expression by Western blotting. The expression of 10 DEPs, distributed in five clusters, including AKR1A1, AGAT, ATP6V1B2, HIBADH, MDH1, MPST, NIT2, PRDX6, PSMB7, and TPI1, were validated by Western blotting. A, DEP expression changes during the process of anti-Thy1 were detected by Western blotting. B, DEPs' expression trends in DIGE were validated by Western blotting. The broken-line curve represents the expression change of DEPs analyzed by DIGE (expression of the control group was arbitrarily set as 1.0), and the full-line curve represents the densitometry analysis of DEPs' expression detected by Western blotting (the intensities of these protein bands are presented as their ratios to the β-actin levels and data from the control group were arbitrarily set as 1.0).
Down-regulation of FHL2 Expression in MCs on Days 3 and 5, and Up-regulation on Day 10 During Anti-Thy1 Nephritis
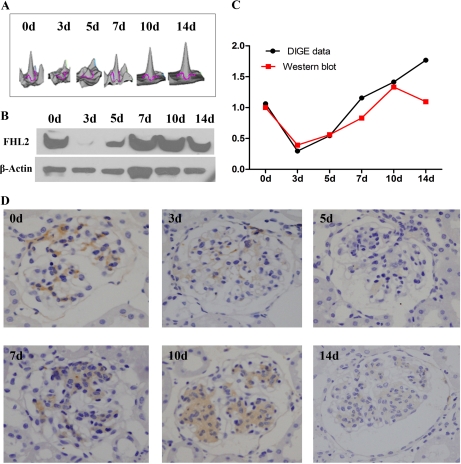
FHL2 (a member of the LIM protein family) was closely associated with cell proliferation. It is known that FHL2 expression is altered when detected using DIGE. We used Western blotting and immunohistochemistry to monitor changes in FHL2 expression during anti-Thy1 nephritis using independent glomeruli samples in each group. Both methods showed that FHL2 expression in the glomeruli mesangial region was significantly down-regulated on days 3 and 5, and up-regulated on day 10 (Fig. 6). This suggests that changes in FHL2 expression may be related to cellular processes such as proliferation in MCs.
Fig. 6.
FHL2 expression changes during the process of anti-Thy1 were validated by Western blotting and immunohistochemistry. A, Changes in FHL2 expression during the process of anti-Thy1 are shown by three-dimensional (3D) views of the fluorescence intensity of the spots in DIGE profiles. B, The expression changes of FHL2 during the process of anti-Thy1 were detected by Western blotting. C, The Western blotting validated that FHL2 was down-regulated on days 3 and 5, up-regulated on day 10. The black curve represents the expression change of FHL2 analyzed by DIGE (the expression of the control group was arbitrarily set as 1.0), and the red curve represents the densitometry analysis of FHL2 expression detected by Western blotting (the intensities of these protein bands are presented as their ratios to the β-actin levels and data from the control group were arbitrarily set as 1.0). D, FHL2 expression during anti-Thy1 nephritis was detected by immunohistochemistry. FHL2 expression in the glomeruli mesangial region was significantly down-regulated on days 3 and 5 and up-regulated on day 10.
Overexpression of FHL2 Induces MC Proliferation by Increasing the Percentage of S Phase Cells, Whereas Inhibition of FHL2 Has the Opposite Effect
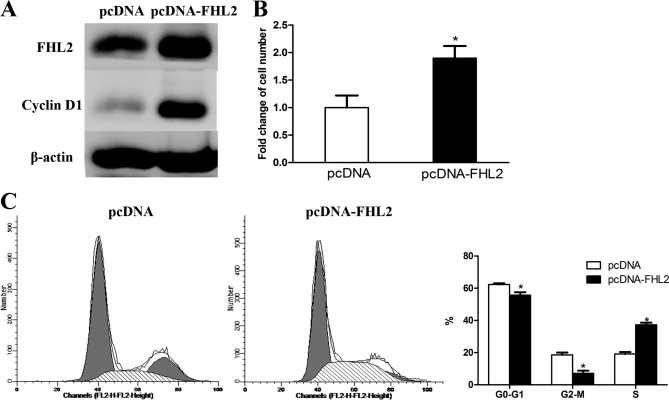
We wanted to evaluate the effects of FHL2 overexpression on cells. To accomplish this, we used Western blotting to examine FHL2 protein expression in cells transfected with the pcDNA-FHL2 plasmid (FHL2 group). Cells transfected with the pcDNA plasmid were used as a control. We found that FHL2 and cyclin D1 significantly increased in the FHL2 group as compared with the control at 24 h post-transfection (Fig. 7A). Next, we compared the number of cells in each group. More cells were present in the FHL2 group than in the control group at 24 h post-infection (1.9-fold increase, Fig. 7B). We monitored the cell cycle distribution in two groups using a flow cytometric DNA array. Overexpression of FHL2 increased the percentage of S phase cells and decreased the percentage of G0/G1 and G2-M phase cells significantly (Fig. 7C).
Fig. 7.
Overexpression of FHL2 induced MC proliferation by increasing the number of cells in S phase. A, FHL2 and cyclinD1 were overexpressed in the pcDNA-FHL2-transfected group when analyzed by Western blotting. B, Overexpression of FHL2 increased the number of MCs. There were more cells in the pcDNA-FHL2-transfected group than the pcDNA-transfected group, based on cell counts at 24 h post-transfection. *p < 0.05 versus pcDNA transfected group. C, Overexpression of FHL2 increased the number of MCs in S phase. There was a higher percentage of S phase cells and a lower percentage of G0/G1 and G2-M phase cells in the pcDNA-FHL2 transfected group at 24 h post-transfection. *p < 0.05 versus pcDNA transfected team.
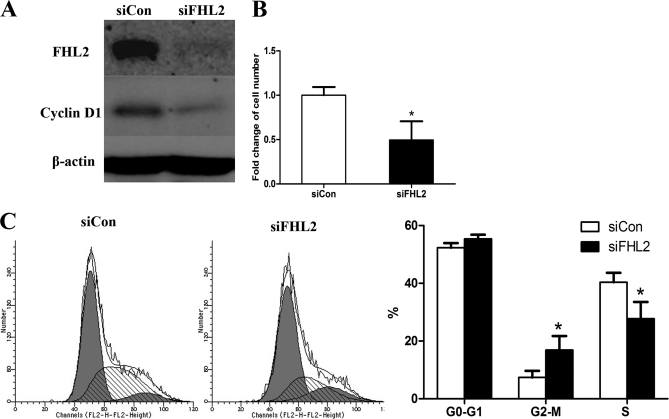
Next, we used siRNA transfection to inhibit FHL2 expression in MCs. We found that siFHL2 transfection completely inhibited FHL2 expression and suppressed cyclin D1 expression 48 h post-transfection, as detected by Western blotting (Fig. 8A). Fewer cells were present in the siFHL2-transfected group than in the control group (siCon) at 72 h post-transfection (0.5-fold decrease, Fig. 8B). The flow cytometric DNA array revealed that siFHL2 significantly decreased the percentage of S phase cells and increased the percentage of G2-M phase cells at 48 h post-transfection (Fig. 8C). These results confirm that overexpression of FHL2 induces MC proliferation by increasing the percentage of S phase cells, whereas siFHL2 has the opposite effect.
Fig. 8.
Inhibition of FHL2 suppressed MC proliferation by decreasing the number of cells in S phase. A, siFHL2 transfection completely inhibited expression of FHL2 and suppressed cyclin D1 expression at 48 h post-transfection, when detected by Western blotting. B, There were fewer cells in the siFHL2-transfected group than the siCon group at 72 h post-transfection, based on cell counts (0.5 fold). C, Flow cytometric DNA arrays revealed that siFHL2 significantly decreased the percentage of S phase cells and increased the percentage of G2-M phase cells at 48 h post-transfection. *p < 0.05 versus siCon-transfected team.
DISCUSSION
In this study, we bioinformatically analyzed anti-Thy1 nephritis and explored the effect of FHL2 on MC proliferation. In our classical mesangial proliferative model, anti-Thy1 nephritis induced obvious MC proliferation and recovery of proliferation. In our study, we determined that anti-Thy1 nephritis has two distinct mesangial phases: proliferation (days 5 to 7) and recovery (days 10 to 14) (Fig. 1). We also applied the PCNA stain to determine the state of the cell cycle during anti-Thy1 nephritis (Fig. 2) and found that cell cycle activation in MCs increased on days 5 to 7, and declined gradually from days 10 to 14, consistent with the proliferation process of MCs. However, the higher PCNA-positive level on day 3, suggesting activation of the cell cycle, did not correlate with pathological change in days (only partial mesangiolysis appeared with no mesangial proliferation). At this time, we also found that positive regulation cyclin proteins (such as cyclin D1 and E) were increased (data not shown), suggesting that cell cycle activation may begin on day 3. Based on these observations, we hypothesize that cell cycle activation on day 3 may trigger subsequent cell proliferation (cell cycle activation occurs prior to an increase in cell number). The relationship between cell cycle activation and mesangiolysis, however, requires further investigation.
We found that DEPs consisted mainly of enzymes and cytoskeletal components, which is supported by previous findings (10, 19, 20). According to the recent literature, DEP enzymes mediate biological functions such as energy and lipid metabolism and transport, by way of their catalytic activity. For example, ACAT1 (21, 22) and ECHS1 (23, 24) may be involved in lipid metabolism, whereas ATP6V1A and ATP6V1B2 mediate H+ transport (25, 26). PRDX3 (27, 28) and PRDX6 (29, 30) have antioxidant effects, regulating apoptosis and cell damage by way of DLD (31). SUCLG2, SUCLA2, and TPI1 (32) play a role in ATP and/or GTP metabolism. Another major DEP component are the cytoskeletal proteins, which may be involved in actin cytoskeleton regulation, according to recent reports. For example, CAPZA2 and CPAZB function in actin filament regulation (33), SEPT2 (34) plays a role in stabilizing or maintaining actin bundles, while FHL2 (35) and PDLIM1 (36) may bind actin-based cytoskeletons. Other than functioning as enzymes or cytoskeleton modulators, DEPs such as heat shock proteins (HSPA8, HSPD1) and toll-related proteins (TOLLIP) may function during stress or inflammatory responses. To corroborate the expression patterns of specific DEPs, we selected 10 proteins from five distinct clusters, representing different general expression trends (Fig. 4), and monitored any expression changes during the process of anti-Thy1 nephritis by Western blotting. We found that Western blotting and DIGE detected similar DEP expression patterns from 8 of the 10 proteins evaluated (Fig. 5). However, it should be mentioned that the actual function of these DEP expression changes during anti-Thy1 nephritis is still uncertain, despite the fact that their biological functions are well characterized. Thus, we chose a specific DEP (FHL2) and evaluated its effect on MC proliferation and hypothesized a role for FHL2 in anti-Thy1 nephritis. FHL2 is an important LIM domain protein and is involved in regulating cell processes such as proliferation, apoptosis, and signal transduction. We found that expression of FHL2 in the mesangial region was significantly altered during the process of anti-Thy1 nephritis when detected by immunohistochemistry and Western blotting (Fig. 6), suggesting that FHL2 may be involved in mediating cell processes such as proliferation in MCs during anti-Thy1 nephritis. Therefore, we investigated FHL2's effect on MC proliferation in vitro. In this study, we found that overexpression of FHL2 induced MC proliferation by increasing the number of S phase cells and decreasing the number of G2/M cells, whereas inhibition of FHL2 had the opposite effect. Moreover, we also found that cyclin D1 was up-regulated in FHL2 overexpressed cells, and down-regulated in FHL2 inhibited cells, suggesting that cyclin D1 is involved in the proliferative effect of FHL2. The specific effects of FHL2 on proliferation depend on the cell type by various mechanisms. For example, FHL2 has pro-proliferative effects on breast cells, mouse embryonic fibroblasts, and U2OS osteosarcoma cells by interacting with cyclins (p21Cip1/Waf1, cyclin D), signaling pathways (beta-catenin, MAPK signal pathway), and transcription factors (E4F1) (, 13, 37–39). FHL2 inhibits cell growth in liver cancer cells, human neuroblastoma cells, prostate cancer cells, or cardiac myocytes by interacting with transcription factors (Id2), signaling pathways (TGF beta), and enzymes (SK1) (12, 18, 40). In MC, FHL2 promote proliferation probably by activating cyclin D1. However, other mechanisms, such as signal transduction involved in FHL2 regulation on MC proliferation are still unknown.
FHL2 modulation of MC proliferation was involved in mesangial proliferation during anti-Thy1 nephritis. Interestingly, we found that on days 3 and 5, when cell cycle activation in MCs began (PCNA results shown), down-regulation of FHL2 antagonized cell cycle activity. Other cell cycle inhibitory factors may exist during anti-Thy1 nephritis. For example, up-regulation of p-p38 MAP kinase on day 3 (41) and up-regulation of p53, p21, and bax (data not shown) on days 5 and 7 may also inhibit the cell cycle. We hypothesize that these antagonistic effects, derived from genes such as FHL2, may be associated with negative feedback regulation of cell proliferation. However, further studies are required to explore the role of FHL2 in a potential negative feedback mechanism during anti-Thy1 nephritis.
CONCLUSIONS
Understanding the mechanisms underlying the pathogenesis of MesPGN could help to elucidate the pathophysiology of this disease and thus facilitate the development of therapy strategies. Here, we explored 40 DEPs and their expression patterns during the process of anti-Thy1 nephritis, and FHL2's effect on MC proliferation. FHL2 in anti-Thy1 nephritis could be a molecular therapeutic target for treating MesPGN.
Acknowledgments
We thank Professor J. Y. Dai and Dr. Z. M. Shi in the Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, Chinese Academy of Sciences.
Footnotes
* This work was supported by the National Natural Science Foundation of China (No. 30630033, No. 81000772) and the National Basic Research Program of China (No. 2011CB944004, No. 2007CB507400).
1 The abbreviations used are:
- MesPGN
- mesangial proliferative glomerulonephritis
- DEP
- differentially expressed protein
- 2D-DIGE
- two-dimensional fluorescence difference gel electrophoresis
- PCNA
- proliferative cell nuclear antigen
- DTT
- dithiotreitol.
REFERENCES
- 1. Jefferson J. A., Johnson R. J. (1999) Experimental mesangial proliferative glomerulonephritis (the anti-Thy-1.1 model). J. Nephrol. 12, 297–307 [PubMed] [Google Scholar]
- 2. Inagi R., Kumagai T., Nishi H., Kawakami T., Miyata T., Fujita T., Nangaku M. (2008) Preconditioning with endoplasmic reticulum stress ameliorates mesangioproliferative glomerulonephritis. J Am. Soc. Nephrol. 19, 915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar D., Bhaskaran M., Alagappan L., Tori D., Yadav I., Konkimalla S., Magoon S., Singhal P. C. (2010) Heme oxygenase-1 modulates mesangial cell proliferation by p21 Waf1 upregulation. Ren. Fail. 32, 254–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hartner A., Hilgers K. F., Bitzer M., Veelken R., Schöcklmann H. O. (2003) Dynamic expression patterns of transforming growth factor-beta(2) and transforming growth factor-beta receptors in experimental glomerulonephritis. J. Mol. Med. 81, 32–42 [DOI] [PubMed] [Google Scholar]
- 5. Jyo-Oshiro Y., Sasaki T., Kawakami Y., Nohno T., Itoh N., Osawa G., Kashihara N. (1999) Expression of the fibroblast growth factor receptor 1–4 genes in glomeruli in anti-Thy1.1 mesangial proliferative glomerulonephritis. Virchows Arch. 435, 501–508 [DOI] [PubMed] [Google Scholar]
- 6. Sasaki S., Bao Q., Hughes R. C. (1999) Galectin-3 modulates rat mesangial cell proliferation and matrix synthesis during experimental glomerulonephritis induced by anti-Thy1.1 antibodies. J Pathol 187, 481–489 [DOI] [PubMed] [Google Scholar]
- 7. Bokemeyer D., Ostendorf T., Kunter U., Lindemann M., Kramer H. J., Floege J. (2000) Differential activation of mitogen-activated protein kinases in experimental mesangioproliferative glomerulonephritis. J. Am. Soc. Nephrol. 11, 232–240 [DOI] [PubMed] [Google Scholar]
- 8. Shimizu A., Kitamura H., Masuda Y., Ishizaki M., Sugisaki Y., Yamanaka N. (1995) Apoptosis in the repair process of experimental proliferative glomerulonephritis. Kidney Int. 47, 114–121 [DOI] [PubMed] [Google Scholar]
- 9. Porst M., Plank C., Bieritz B., Konik E., Fees H., Dötsch J., Hilgers K. F., Reinhardt D. P., Hartner A. (2006) Fibrillin-1 regulates mesangial cell attachment, spreading, migration and proliferation. Kidney Int. 69, 450–456 [DOI] [PubMed] [Google Scholar]
- 10. Nazeer K., Janech M. G., Lin J. J., Ryan K. J., Arthur J. M., Budisavljevic M. N. (2009) Changes in protein profiles during course of experimental glomerulonephritis. Am. J. Physiol. Renal Physiol 296, F186–193 [DOI] [PubMed] [Google Scholar]
- 11. Amann T., Egle Y., Bosserhoff A. K., Hellerbrand C. (2010) FHL2 suppresses growth and differentiation of the colon cancer cell line HT-29. Oncol. Rep. 23, 1669–1674 [DOI] [PubMed] [Google Scholar]
- 12. Ding L., Wang Z., Yan J., Yang X., Liu A., Qiu W., Zhu J., Han J., Zhang H., Lin J., Cheng L., Qin X., Niu C., Yuan B., Wang X., Zhu C., Zhou Y., Li J., Song H., Huang C., Ye Q. (2009) Human four-and-a-half LIM family members suppress tumor cell growth through a TGF-beta-like signaling pathway. J. Clin. Invest. 119, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Labalette C., Nouët Y., Sobczak-Thepot J., Armengol C., Levillayer F., Gendron M. C., Renard C. A., Regnault B., Chen J., Buendia M. A., Wei Y. (2008) The LIM-only protein FHL2 regulates cyclin D1 expression and cell proliferation. J. Biol. Chem. 283, 15201–15208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li M., Wang J., Ng S. S., Chan C. Y., Chen A. C., Xia H. P., Yew D. T., Wong B. C., Chen Z., Kung H. F., Lin M. C. (2008) The four-and-a-half-LIM protein 2 (FHL2) is overexpressed in gliomas and associated with oncogenic activities. Glia 56, 1328–1338 [DOI] [PubMed] [Google Scholar]
- 15. Paul R. V., Wackym P. S., Budisavljevic M. N. (1998) Destabilization of natriuretic peptide C-receptor mRNA by phorbol myristate acetate. J. Am. Soc. Nephrol. 9, 26–32 [DOI] [PubMed] [Google Scholar]
- 16. Johnson R. J., Garcia R. L., Pritzl P., Alpers C. E. (1990) Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am. J. Pathol. 136, 369–374 [PMC free article] [PubMed] [Google Scholar]
- 17. Li Z., Chen X., Xie Y., Shi S., Feng Z., Fu B., Zhang X., Cai G., Wu C., Wu D., Gu Y. (2004) Expression and significance of integrin-linked kinase in cultured cells, normal tissue, and diseased tissue of aging rat kidneys. J. Gerontol. A Biol. Sci. Med. Sci. 59, 984–996 [DOI] [PubMed] [Google Scholar]
- 18. Sun J., Yan G., Ren A., You B., Liao J. K. (2006) FHL2/SLIM3 decreases cardiomyocyte survival by inhibitory interaction with sphingosine kinase-1. Circ. Res. 99, 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perluigi M., Sultana R., Cenini G., Di Domenico F., Memo M., Pierce W. M., Coccia R., Butterfield D. A. (2009) Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer's disease: Role of lipid peroxidation in Alzheimer's disease pathogenesis. Proteomics Clin. Appl. 3, 682–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nilo R., Saffie C., Lilley K., Baeza-Yates R., Cambiazo V., Campos-Vargas R., González M., Meisel L. A., Retamales J., Silva H., Orellana A. (2010) Proteomic analysis of peach fruit mesocarp softening and chilling injury using difference gel electrophoresis (DIGE). BMC Genomics 11, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Antalis C. J., Arnold T., Rasool T., Lee B., Buhman K. K., Siddiqui R. A. (2010) High ACAT1 expression in estrogen receptor negative basal-like breast cancer cells is associated with LDL-induced proliferation. Breast Cancer Res. Treat. 122, 661–670 [DOI] [PubMed] [Google Scholar]
- 22. Su Y. R., Dove D. E., Major A. S., Hasty A. H., Boone B., Linton M. F., Fazio S. (2005) Reduced ABCA1-mediated cholesterol efflux and accelerated atherosclerosis in apolipoprotein E-deficient mice lacking macrophage-derived ACAT1. Circulation 111, 2373–2381 [DOI] [PubMed] [Google Scholar]
- 23. Liu X., Feng R., Du L. (2010) The role of enoyl-CoA hydratase short chain 1 and peroxiredoxin 3 in PP2-induced apoptosis in human breast cancer MCF-7 cells. FEBS Lett. 584, 3185–3192 [DOI] [PubMed] [Google Scholar]
- 24. Zhang X., Yang J., Guo Y., Ye H., Yu C., Xu C., Xu L., Wu S., Sun W., Wei H., Gao X., Zhu Y., Qian X., Jiang Y., Li Y., He F. (2010) Functional proteomic analysis of nonalcoholic fatty liver disease in rat models: enoyl-coenzyme a hydratase down-regulation exacerbates hepatic steatosis. Hepatology 51, 1190–1199 [DOI] [PubMed] [Google Scholar]
- 25. Supino R., Scovassi A. I., Croce A. C., Dal Bo L., Favini E., Corbelli A., Farina C., Misiano P., Zunino F. (2009) Biological effects of a new vacuolar-H,-ATPase inhibitor in colon carcinoma cell lines. Ann. N.Y. Acad. Sci. 1171, 606–616 [DOI] [PubMed] [Google Scholar]
- 26. Sasazawa Y., Futamura Y., Tashiro E., Imoto M. (2009) Vacuolar H+-ATPase inhibitors overcome Bcl-xL-mediated chemoresistance through restoration of a caspase-independent apoptotic pathway. Cancer Sci. 100, 1460–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cox A. G., Brown K. K., Arner E. S., Hampton M. B. (2008) The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem. Pharmacol. 76, 1097–1109 [DOI] [PubMed] [Google Scholar]
- 28. Nonn L., Berggren M., Powis G. (2003) Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol. Cancer Res. 1, 682–689 [PubMed] [Google Scholar]
- 29. Walsh B., Pearl A., Suchy S., Tartaglio J., Visco K., Phelan S. A. (2009) Overexpression of Prdx6 and resistance to peroxide-induced death in Hepa1–6 cells: Prdx suppression increases apoptosis. Redox. Rep. 14, 275–284 [DOI] [PubMed] [Google Scholar]
- 30. Chang X. Z., Li D. Q., Hou Y. F., Wu J., Lu J. S., Di G. H., Jin W., Ou Z. L., Shen Z. Z., Shao Z. M. (2007) Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 9, R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jackman S. A., Hough D. W., Danson M. J., Stevenson K. J., Opperdoes F. R. (1990) Subcellular localisation of dihydrolipoamide dehydrogenase and detection of lipoic acid in bloodstream forms of Trypanosoma brucei. Eur. J. Biochem. 193, 91–95 [DOI] [PubMed] [Google Scholar]
- 32. Ahlborn G. J., Delker D. A., Roop B. C., Geter D. R., Allen J. W., Deangelo A. B., Winnik W. M. (2009) Early Alterations in Protein and Gene Expression in Rat Kidney Following Bromate Exposure. Food Chem. Toxicol. 47, 1154–1160 [DOI] [PubMed] [Google Scholar]
- 33. Barron-Casella E. A., Torres M. A., Scherer S. W., Heng H. H., Tsui L. C., Casella J. F. (1995) Sequence analysis and chromosomal localization of human Cap Z. Conserved residues within the actin-binding domain may link Cap Z to gelsolin/severin and profilin protein families. J. Biol. Chem. 270, 21472–21479 [DOI] [PubMed] [Google Scholar]
- 34. Schmidt K., Nichols B. J. (2004) Functional interdependence between septin and actin cytoskeleton. BMC Cell Biol. 5, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Canault M., Tellier E., Bonardo B., Mas E., Aumailley M., Juhan-Vague I., Nalbone G., Peiretti F. (2006) FHL2 interacts with both ADAM-17 and the cytoskeleton and regulates ADAM-17 localization and activity. J. Cell. Physiol. 208, 363–372 [DOI] [PubMed] [Google Scholar]
- 36. Ohno K., Kato H., Funahashi S., Hasegawa T., Sato K. (2009) Characterization of CLP36/Elfin/PDLIM1 in the nervous system. J. Neurochem. 111, 790–800 [DOI] [PubMed] [Google Scholar]
- 37. Martin B. T., Kleiber K., Wixler V., Raab M., Zimmer B., Kaufmann M., Strebhardt K. (2007) FHL2 regulates cell cycle-dependent and doxorubicin-induced p21Cip1/Waf1 expression in breast cancer cells. Cell Cycle 6, 1779–1788 [DOI] [PubMed] [Google Scholar]
- 38. Paul C., Lacroix M., Iankova I., Julien E., Schäfer B. W., Labalette C., Wei Y., Le Cam A., Le Cam L., Sardet C. (2006) The LIM-only protein FHL2 is a negative regulator of E4F1. Oncogene 25, 5475–5484 [DOI] [PubMed] [Google Scholar]
- 39. Ng C. F., Ng P. K., Lui V. W., Li J., Chan J. Y., Fung K. P., Ng Y. K., Lai P. B., Tsui S. K. (2011) FHL2 exhibits anti-proliferative and anti-apoptotic activities in liver cancer cells. Cancer Lett. 304, 97–106 [DOI] [PubMed] [Google Scholar]
- 40. Han W., Wu Z., Zhao Y., Meng Y., Si Y., Yang J., Fu X., Yu L. (2009) FHL2 interacts with and acts as a functional repressor of Id2 in human neuroblastoma cells. Nucleic Acids Res. 37, 3996–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bokemeyer D., Guglielmi K. E., McGinty A., Sorokin A., Lianos E. A., Dunn M. J. (1998) Different activation of mitogen-activated protein kinases in experimental proliferative glomerulonephritis. Kidney Int. Suppl. 67, S189–191 [DOI] [PubMed] [Google Scholar]