Abstract
Inflammation and oxidative stress, elicited by Trypanosoma cruzi infection, are important pathologic events during progressive Chagasic cardiomyopathy. In this study, we infected Sprague-Dawley rats with T. cruzi, and treated with phenyl-α-tert-butylnitrone (PBN-antioxidant) and/or benznidazole (BZ-anti-parasite). We employed two-dimensional gel electrophoresis/mass spectrometry to investigate (a) the plasma proteomic changes associated with infection and disease development, and (b) the beneficial effects of PBN and BZ in controlling the disease-associated plasma profile. Matrix-assisted laser desorption ionization/time of flight (MALDI-TOF) tandem MS (MS/MS) analysis of differentially expressed (total 146) and oxidized (total 48) protein spots yielded 92 unique proteins. Our data showed that treatment with PBN and BZ restored the differential expression of 65% and 30% of the disease-associated proteins to normal level, respectively, and PBN prevented development of oxidative adducts on plasma proteins. Western blotting to detect dinitrophenyl-derivatized carbonyl-proteins revealed plasma proteins were maximally oxidized during acute infection. Functional and disease/disorder analyses allocated a majority of the differentially expressed and oxidized proteins into inflammation/immunity and lipid metabolism categories and to molecular pathways associated with heart disease (e.g. cardiac infarction, contractile dysfunction, hypertrophy, and hypertension) in chagasic rats, and to curative pathways (e.g. ROS scavenging capacity, immune regulation) in infected rats treated with PBN and/or BZ. We validated the two-dimensional gel electrophoresis results by Western blotting, and demonstrated that the disease-associated increased expression of gelsolin and vimentin and release of cardiac MYL2 in the plasma of chagasic rats was returned to control level by PBN/BZ treatment. Increased plasma levels of gelsolin, MYL2 and vimentin were directly correlated with the severity of cardiac disease in human chagasic patients. Together, these results demonstrate the plasma oxidative and inflammatory response profile, and plasma detection of cardiac proteins parallels the pathologic events contributing to Chagas disease development, and is of potential utility in diagnosing disease severity and designing suitable therapy for management of human chagasic patients.
Chagas disease continues to pose a serious threat to health in Latin America and Mexico, and is an emerging parasitic disease in developed countries. According to World Health Organization reports, the overall prevalence of human Trypanosoma cruzi infection is at ∼16–18 million cases, and ∼120 million people, i.e. 25% of the inhabitants of Latin America, are at risk of infection (1, 2). It is estimated that >300,000 infected patients live in the United States (3). Of those infected, 30–40% progress to an irreversible cardiomyopathy several years following infection, which results in considerable morbidity and mortality (1). Moreover, no vaccines are available. Benznidazole, the available drug therapy, is effective in controlling parasitemia in acutely infected individuals (4, 5); however, its efficacy in arresting or reversing disease progression in chronically infected patients is not clearly established (6, 7). It is crucial that molecular markers are identified that could allow classification of disease state and detection of asymptomatic individuals who are at risk of developing chagasic cardiomyopathy, and new therapies are developed to arrest or prevent the progression of symptomatic clinical disease.
The red and white blood cells are dynamic components of the circulatory system and interact with all cells, tissues, and organs, specifically the heart. It is, therefore, logical to assume that the pathologic processes during the development of Chagas disease would cause characteristic changes in the circulating proteins (e.g. level, oxidation) and generate a detectable, disease-specific molecular phenotype. With long-term cardiac injury, as noted in a majority of chronic chagasic patients (8, 9), the progression of disease severity is presented by an increasing order of cell death, heart decompensation, and a drop in cardiac output, leading to heart failure (10, 11). Cell death during this process may result in the sustained release of cardiac proteins in the peripheral system. These cardiac proteins and their disease-dependent modified forms in plasma are the potential cardiac-specific biomarkers (12, 13).
Several studies by us and others have implicated the role of central and peripheral inflammatory mechanisms and oxidative stress in Chagas disease (reviewed in (7, 14, 15)). It is documented in experimental animal models and human patients that parasite persistence results in consistent activation of inflammatory responses and leads to the development and/or propagation of pathological lesions in the heart (16–18). In other studies, myocardial production of reactive oxygen species (ROS)1 because of mitochondrial dysfunction of the electron transport chain and release of electrons to molecular oxygen has been found to be the major source of oxidative stress in chagasic hearts (19–22). Recent studies demonstrated that an increase in myocardial oxidative damage correlated with an antioxidant inefficiency and cardiac dysfunction. Further, treatment of infected animals with an antioxidant was effective in arresting the oxidative cardiac pathology (18) and preventing the loss of cardiac LV function in chronic hearts (23), thus, establishing the pathological significance of oxidative overload in Chagas disease.
Blood serves as a useful tissue capable of detecting and responding to the changes induced in the body during the course of T. cruzi infection and disease development. The changes in immune response, oxidative stress, and antioxidant imbalance are detectable in peripheral blood of infected mice (20), and, notably, a strong positive correlation was detected for the disease state-specific changes in the heart-versus-blood level of oxidative stress markers and antioxidants (e.g. glutathione peroxidase, glutathione, and manganese superoxide dismutase) (20). Distinct plasma protein-nitrotyrosylation profiles have also been documented in acutely- and chronically-infected chagasic animals (24). These studies, along with documentation of oxidative overload in chagasic humans (25, 26), support the idea that characterization of plasma proteomes will be useful in identifying the molecular mechanisms that are disturbed during the progression of Chagas disease.
In this study, we investigated the host physiological changes at the protein level associated with oxidative stress induced by T. cruzi infection. Sprague-Dawley rats were infected with T. cruzi and treated with phenyl-α-tert-butyl nitrone (PBN), a nitrone-based antioxidant that scavenges a wide variety of free radical species and inhibits free radical generation (27). Some of the infected rats were treated with benznidzole (BZ) that is currently the treatment of choice for chagasic patients (6). We employed a two-dimensional gel electrophoresis (2D-GE) approach in identifying plasma proteomic changes in response to T. cruzi infection and disease development and determined whether the beneficial effects of treatment with PBN and BZ (individually or in combination) in controlling myocardial oxidative stress, parasite persistence, and the resultant left ventricular LV dysfunction were reflected in the plasma proteome profile. Our findings of a number of proteins differentially expressed and oxidized in a disease-specific manner that returned to control level by PBN and BZ treatment have provided clues to the mechanisms of parasite-host interactions that contribute to disease pathogenesis. We discuss the potential utility of plasma levels of gelsolin (GSN), myosin light chain 2 (MYL2), and vimentin (VIM) in diagnosing disease severity and evaluating efficacy of therapeutic treatments in managing disease progression in human chagasic patients.
EXPERIMENTAL PROCEDURES
Animals and Parasites
Sprague-Dawley rats (4–5-weeks-old, Harlan Labs) were infected with T. cruzi (SylvioX10/4, 50,000 trypomastigotes/rat, intraperitoneal). Rats were given 1.3 mm PBN (beginning day 0, throughout the course of infection) and/or 0.7 mm BZ (beginning day 40 pi, for 3 weeks) in drinking water. The plasma samples were obtained at acute (27–45 days post-infection (dpi)) and chronic (>150 dpi) stages of infection and disease development. Animal experiments were performed according to the National Institutes of Health Guide for Care and Use of Experimental Animals and approved by the UTMB Animal Care and Use Committee.
Rat Plasma Collection and Removal of High-Abundance Proteins
Whole blood was collected in K3EDTA vacutainers and kept on ice for 30 min. The samples were centrifuged for 15 min at 3000 × g, and the lipid-containing upper layer was removed. The abundant proteins (e.g. albumin, α1-acid glycoprotein, α1-antitrypsin, α2-macroglobulin, apolipoproteins (ApoA-I, ApoA-II), fibrinogen, haptoglobin, immunoglobulins (IgA, IgG, and IgM), and transferrin) in plasma were removed using ProteomeLab IgY-12 LC10 affinity spin columns (Beckman Coulter, Fullerton, CA). The plasma samples depleted of abundant proteins were then concentrated by using Amicon® ultra centrifugal filters (3 kDa cutoff, from Millipore) and desalted by PD-10 desalting columns (GE Healthcare). Protein content was determined by using the Bradford assay (Bio-Rad).
Two-dimensional Gel Electrophoresis
The 11-cm immobilized pH gradient (IPG) strips (pH: 3–10 or 5–8; Bio-Rad, Hercules, CA) were rehydrated at 50 V for 12 h with 250-μl rehydration buffer (1 m thiourea, 8 m urea, 2% 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate, 1% dithiothreitol, and 0.2% ampholytes) containing 200-μg protein samples and 0.002% of bromphenol blue. Isoelectric focusing was performed at 500 V for 1 h, 1000 V for 1 h, 8000 V for 2 h, and then at 8000 V for a total of 50,000 Vh (24). The IPG strips were suspended in equilibration buffer (50 mm Tris-HCl, pH 6.8, 6 m urea, 20% glycerol) and sequentially incubated for 15 min each in presence of 2% dithiothreitol/2% SDS (reducing conditions) and 2.5% iodoacetamide/2% SDS (alkylating conditions). Equilibrated IPG strips were subjected to second-dimension electrophoresis by using 8–10% linear gradient precast Tris-HCl gels (BioRad) on a PROTEAN plus Dodeca Cell System at 75 V for 1 h and then at 120 V until the dye front reached the bottom of the gel. Gels were fixed in 10% methanol/7% acetic acid, stained with SYPRO Ruby (BioRad), destained in 10% ethanol/7% acetic acid, and imaged by using a high-resolution ProXPRESS Proteomic Imaging System (Perkin Elmer).
Image Analysis
In total, 28 SYPRO Ruby-stained 2D gels (n = 4/group, two replicas/animal) were digitalized on a ProXPRESS Proteomic Imaging System (Perkin Elmer), and the images were analyzed on Progenesis SameSpotst™ software 2.0 (NonLinear Dynamics). Normalized spot volumes, i.e. the volume of each spot over the volume of all spots in the gel, were used for comparison of the different groups, and candidates were identified as protein spots that changed at least twofold versus their specific control. Statistical significance was assessed by a two-tailed Student's t test and analysis of variance test (ANOVA) (28), and p values of <0.05 were considered significant for comparison between control and experimental data.
Detection of Carbonyl Proteins
IPG strips were loaded with protein samples and subjected to first-dimension isoelectric focusing, as above. IPG strips were then incubated with 1 ml of 3% SDS and 5 mm 2,4-dinitrophenylhydrazine (DNPH)/10% trifluoroacetic acid for 25 min. After neutralization with 1.5 ml of 2 m Tris, 30% glycerol, DNP-derivatized protein samples in IPG strips were subjected to second-dimension electrophoresis by using 8–10% gradient precast gels (BioRad), and transferred to polyvinylidene difluoride membranes. Membranes were hybridized with rabbit anti-DNP antibody (1:300 dilution, Invitrogen) and HRP-conjugated goat anti-rabbit secondary antibody (1:4000 dilution, Sigma), and signal detected by using an Amersham BiosciencesTM ECL Plus system, according to the manufacturer's protocol (29, 30). Images were visualized, digitized, and signal was quantified by densitometry by using a FluorChem 8800 image analyzer (Alpha Innotech, San Leandro, CA).
Mass Spectrometry and Protein Identification
Selected spots (1-mm) were excised from gels and submitted to trypsin proteolysis as described (24). In brief, gel spots were incubated at 37 °C for 30 min in 50 mm NH4HCO3, dehydrated twice for 5 min each in 100-μl acetonitrile, dried, and in-gel proteins were digested at 37 °C for 6 h with 10 μl of trypsin solution (1% trypsin in 25 mm ammonium bicarbonate). After digestion, 1 μl of peptide mixture was directly spotted onto a MALDI TOF MS/MS target plate with 1 μl of α-cyano-4-hydroxycinnamic acid matrix solution (5 mg/ml in 50% acetonitrile). Peptides were analyzed by using a MALDI TOF/TOF ABI 4800 Proteomics Analyzer (Applied Biosystems, Foster City, CA). The Applied Biosystems software package included the 4000 Series Explorer (version 3.6 RC1) with Oracle Database Schema Version (version 3.19.0) and Data Version (3.80.0) to acquire and analyze MS and MS/MS spectral data. The instrument was operated in a positive ion reflectron mode with the focus mass set at 1700 Da (mass range: 850–3000 Da). For MS data, 1000–2000 laser shots were acquired and averaged from each protein spot. Automatic external calibration was performed by using a peptide mixture with the reference masses 904.468, 1296.685, 1570.677, and 2465.199. MALDI MS/MS was performed on several (five to ten) abundant ions from each protein spot. A 1 kV positive ion MS/MS method was used to acquire data under postsource decay conditions. The instrument precursor selection window was ± 3 Da. Automatic external calibration was performed by using reference fragment masses 175.120, 480.257, 684.347, 1056.475, and 1441.635 (from precursor mass 1570.700).
Applied Biosystems GPS ExplorerTM (version 3.6) software was used in conjunction with MASCOT (version 2.2.07) to search the NCBI rat protein database (release date: Jan 25, 2010; 68,555 entries) by using both MS and MS/MS spectral data for protein identification. Protein match probabilities were determined by using expectation values and/or MASCOT protein scores. The MS peak filtering included the following parameters: a mass range of 800 Da to 3000 Da; minimum S/N filter = 10; mass exclusion list tolerance = 0.5 Da; and mass exclusion list for trypsin and keratin-containing compounds included masses 842.51, 870.45, 1045.56, 1179.60, 1277.71, 1475.79, and 2211.1. The MS/MS peak filtering included the following parameters: minimum S/N filter = 10, maximum missed cleavages = 1, fixed modification of carbamidomethyl (C), variable modifications because of oxidation (M), precursor tolerance = 0.2 Da, MS/MS fragment tolerance = 0.3 Da, mass = monoisotopic, and peptide charges = +1. The significance of a protein match, based on the peptide mass fingerprint in the MS and the MS/MS data from several precursor ions, is presented as expectation values (p < 0.001).
Functional Analysis
All data sets were assessed by using Ingenuity Pathways Analysis (Ingenuity Systems®) and GOA-UniProt knowledge base (released on Feb 20, 2011, (http://www.uniprot.org/) to recognize the localization and function of the identified proteins. These two software programs retrieve a set of biological information such as gene name, subcellular location, tissue specificity, function, and association with disease; and then integrate the identified proteins into networks and signaling pathways with biological meaning and significance. A list of all gene ontology (GO) terms was obtained, and the frequency of annotation and information content of each term were calculated as described (31). Briefly, an e-value was calculated by estimating the probability of a random set of proteins having a frequency of annotation for that term greater than the frequency obtained in the real set, and a threshold of 10−3 was set to retrieve significant molecular functions and biological processes. With these parameters, we were able to highlight the most informative and significantly over-represented GO terms in the data set.
Human Plasma Samples
We previously described the criteria for characterization of seropositive chagasic subjects exhibiting variable degrees of cardiac disease (CD0-CD3), seronegative cardiomyopathy patients of other etiologies, and seronegative, healthy individuals exhibiting no history or clinical symptoms of cardiac disease (25). Human sera samples were collected according to protocols approved by the Institutional Review Board at the University of Texas Medical Branch (UTMB). Samples (n = 10/group) were analyzed by Western blotting as below.
Western Blotting
A 20-μg aliquot of each protein sample was resolved on 10% acrylamide gels and transferred to polyvinylidene difluoride membranes by using a wet, vertical Criterion Blotter (Bio-Rad). Membranes were blocked for 1 h with 5% nonfat dry milk (Bio-Rad) in 50 mm Tris-HCl (pH 7.4) containing 150 mm NaCl and 0.05% Tween-20 (TBST). All antibody dilutions were made in 5% nonfat dry milk-TBST. Membranes were incubated overnight at 4 °C with antibodies against GSN, MYL2 and VIM (1:2000 dilution, Santa Cruz). Membranes were washed thrice, incubated with HRP-conjugated secondary antibody (1:4000, Sigma) for 1 h, and signal was developed by using the Amersham BiosciencesTM ECL Plus system. Membranes were stained with Coomassie blue G250 (Bio-Rad) to confirm an equal loading of samples. Images were visualized and digitized, and densitometric analysis was performed by using a FluorChem 8800 system.
RESULTS
Serum Depletion of High-abundance Proteins Enhanced the Detection of Low-abundance Proteins
Plasma is the most clinically abundant specimen; however, because of a dynamic range of protein concentration, the high-abundance proteins interfere with the detection of low-abundance potential biomarker proteins. To enhance our ability to detect low abundance proteins, we employed IgY-12 LC10 immuno-affinity columns that provided a >90% depletion of 12 major proteins from the plasma samples (supplemental Fig. S1A). To verify whether depletion of abundant proteins enhanced detection of low-abundance proteins, we performed 2D-GE, and analyzed the gel images acquired after staining with SYPRO Ruby (supplemental Fig. S1B-D). When using pH 3–10 IPG strips, whole plasma and depleted plasma proteins resolved on the acidic side or in the middle portion of the second-dimension 8–16% gradient gel (supplemental Fig. S1B to S1D). The narrow-range IPG strips (pH 5–8) provided an enhanced resolution of depleted plasma proteins and the detection of fivefold more spots than were detected in whole plasma (1524 versus 265, supplemental Fig. S1B and 1E). We, therefore, used pH 5–8 IPG strips and depleted plasma for all additional studies.
2D-GE Analysis of Changes in Plasma Proteome by T. cruzi Infection
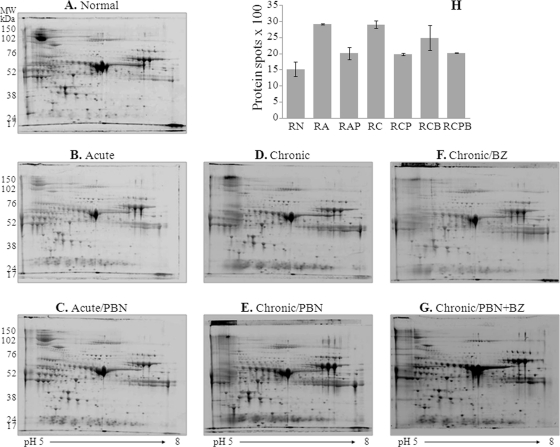
We analyzed the depleted plasma proteome of normal rats (RN), acutely infected rats (RA) treated with PBN (RAP), and chronically infected rats (RC) treated with PBN (RCP), BZ (RCB), or PBN and BZ (RCPB). Plasma samples were resolved to obtain eight gel replicas per group. Representative gel images are shown in Fig. 1A–1G. Gel images were aligned by using Progenesis SameSpots TM and protein spots identified. In total, 1524, 2915, 2015, 2900, 1979, 2486, and 2026 protein spots were observed in the RN, RA, RAP, RC, RCP, RCB, and RCPB groups, respectively (Fig. 1H). The maximal increase in the number of protein spots was associated with acute infection (RA) or chronic disease (RC) status. PBN treatment resulted in normal expression level of 65% and 63.6–66.9% of the proteins that were differentially expressed in acutely (RAP) and chronically (RCP or RCPB) infected rats, respectively. BZ treatment controlled the expression level of 30.1% of the proteins differentially expressed in chronically infected rats (Fig. 1H).
Fig. 1.
Sprague-Dawley rats were infected with T. cruzi and treated with phenyl-α-tert-butyl nitrone (PBN, antioxidant). Some rats were treated with benznidazole (BZ, anti-parasite drug) at the end of the acute infection phase. Plasma samples were collected during acute infection (25 dpi) and chronic disease (150 dpi) phases, and depleted of high-abundance proteins to enrich the detection of low-abundance proteins. (A–G) Shown are representative, two-dimensional gel electrophoresis (2D-GE) patterns of depleted plasma from normal rats (A), acutely infected rats (B) treated with PBN (C), and chronically infected rats (D) treated with PBN (E), BZ (F), or PBN and BZ (G). H, The bar graph shows the average number of protein spots identified in depleted plasma from each experimental group. Proteins altered in expression by >twofold were identified by mass spectrometry (listed in Table I).
Densitometric analysis of the protein spot signal by Progenesis SameSpots TM identified ∼1000 spots that were altered in expression by ≥twofold (pANOVA<0.05). Of these, 325 protein spots were reproducibly identified to be differentially expressed in response to T. cruzi infection and/or treatment in at least three different experiments. Expanded view of the 2D-gel images of the selected protein spots altered by ≥twofold in expression in plasma of infected (±PBN/BZ-treated) rats is shown in Fig. 2 and supplemental Fig. S2. Pair-wise comparison of differentially expressed protein-spots is shown in supplemental Fig. S3A. We identified 86 and 91 protein spots that were differentially expressed in acutely and chronically infected rats as compared with normal controls. When comparing differentially expressed proteins in response to a particular treatment versus total differentially expressed proteins, we found that 37 protein spots were strictly stimulated in response to T. cruzi, 28 protein spots were up-regulated by PBN treatment in chronic plasma, and 61 protein spots were altered by PBN/BZ treatment in chronically infected rats (supplemental Fig. S3B). Together these results indicated that during Chagas disease, oxidative stress and parasite persistence contribute to alterations in the plasma proteome profile.
Fig. 2.
A–F, Shown is an expanded view of the 2D-gel images for selected plasma proteins that were altered in expression (>twofold) in response to T. cruzi infection, disease state, and/or treatment with PBN and BZ. The number on the left side represents the spot number identified on 2D-gels. The gene name of identified proteins, determined by mass spectrometry/BLAST analysis, is shown at right side. The detailed gene/protein names, along with spot numbers, are listed in Table I. Arrows mark the disease- or treatment-specific change in plasma proteins in chagasic rats. Abbreviations (color at top of each micrograph): RN, normal rats (pink); RA, acutely infected rats (blue); RAP, RA/PBN-treated (purple); RC, chronically infected rats (brown); RCP, RC/PBN-treated (mauve); RCB, RC/BZ-treated (red); and RCPB, RC/PBN+BZ-treated (green). The intensity of color is indicative of the extent of change in the protein level in chagasic plasma.
Mass Spectrometric Identification of Differentially Expressed Proteins in Chagasic Plasma
Toward discovery of maximally altered proteins that may be of potential significance in characterizing the disease state and severity, we submitted 146 protein spots to MALDI-TOF MS/MS for protein identification. The protein spots for sequencing were chosen based upon their maximal differential expression in infected and/or treated groups. The MS and MS/MS spectral data were submitted to MASCOT and homology searches were conducted against NCBI rat database to validate protein identity. These analyses identified with high probability 110 of the 146 proteins submitted for sequencing. Of the 110 differentially expressed identified proteins, 31 proteins were identified to be similar proteins resulting in 79 unique proteins (Table I). Those differentially expressed proteins identified by mass spectrometry as uniquely associated with or shared among different groups are shown in a Venn diagram in supplemental Fig. S4. Among these, 67 (65 + 2) appeared to be up-regulated in infected rodents, and PBN controlled the expression of 16 of the proteins that were otherwise dysregulated in acute plasma (supplemental Fig. S4A). A comparison of plasma profiles between normal and infected rats revealed that 33 of the proteins were consistently up-regulated during the acute infection and chronic disease phases, and hence may potentially be useful for diagnosis of Chagas disease (supplemental Fig. S4B). Two of the protein spots were strictly associated with the acute phase and 21 protein spots with the chronic phase and these are potentially useful for diagnosing disease states (supplemental Fig. S4B). When we analyzed the effect of PBN and BZ treatment on the proteome profile, we found that 28 and two proteins, respectively, were stimulated in chronic chagasic plasma in a treatment-specific manner, and that the increased plasma levels of 14 proteins in chronic rats was not altered by PBN and/or BZ treatment (supplemental Fig. S4Ca–b). Instead PBN+BZ treatment resulted in the altered expression of 61 (39 + 22) proteins, of which 22 were also altered in plasma of BZ-treated rats. Together, these results confirm that the plasma profile is altered by parasite persistence and oxidative stress, and treatment with PBN and BZ, known to control oxidative damage and inhibit parasite persistence, respectively (23), achieves its beneficial effects by controlling the expression of disease-associated proteins and up-regulation of other proteins.
Table I. Depleted plasma profile of Chagasic rats treated with PBN antioxidant (±benznidazole). Sprague Dawley rats were infected with T. cruzi and treated with phenyl-α-tert-butyl nitrone (PBN, antioxidant) with or without benznidazole (BZ, anti-parasite drug). Plasma samples were collected at day 25 (acute stage) and 150 (chronic stage) post-infection, and depleted of high-abundance proteins to enrich detection of low-abundance proteins by 2D-gel electrophoresis. Gel images of plasma samples from normal rats (RN), rats that were acutely infected (RA) and treated with PBN (RAP), and chronically infected rats (RC) that were treated with PBN (RCP), BZ (RCB), or PBN and BZ (RCPB) were analyzed on Progenesis SameSpotst™ software and normalized spot volumes were used for comparison of different groups. Proteins spots with >2-fold change in chagasic plasma were subjected to MALDI-TOF MS/MS analysis. The significance of a protein match, based on the peptide mass fingerprint in the MS and the MS/MS data, is presented as expectation values (p < 0.001). The putative biological function and cellular location were identified using Ingenuity Pathway Analysis and UniProt software. N/A: No match available in public information databases, pI: Isoelectric pH, MW: molecular weight.
| Spot # | Gene name | Protein name | Accession no. | MW (Da) | pI | e-value (p < 0.001) | Average normalized density (x100000) |
Putative biological function | Putative cellular localization | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RN | RA | RAP | RC | RCP | RCB | RCPB | |||||||||
| 12, 180 | KRT1 | Keratin, type II | gi 120474989 | 65059.2 | 8.04 | 9.102E-09 | 4.70 | 4.03 | 21.47 | 9.92 | 7.22 | 9.47 | 11.17 | Oxidative stress response | Membrane |
| 34 | TTC37 | KIAA0372 gene product | gi 149058911 | 68849.3 | 6.22 | 0.001 | 2.29 | 0.64 | 0.37 | 0.31 | 0.45 | 0.37 | 0.25 | Protein binding | N/A |
| 37, 593 | SRPRB | Ba1–667 | gi 33086638 | 109546 | 8.35 | 5.319E-08 | 2.14 | 5.33 | 14.38 | 6.46 | 6.48 | 3.21 | 8.61 | Iron homeostasis | Extracellular |
| 49, 673 | IGH-1α | Igh-1a protein | gi 299352 | 52500 | 7.23 | 0.132 | 7.89 | 9.90 | 19.12 | 50.91 | 13.39 | 3.98 | 4.48 | Antigen binding | Extracellular |
| 52, 54, 302 | TF | Transferrin | gi 1854476 | 78538.3 | 6.94 | 5.55E-08 | 9.83 | 5.80 | 9.80 | 2.85 | 14.45 | 0.60 | 8.06 | Transport | Extracellular |
| 53, 225 | SroTP | Serotransferrin | gi 61556986 | 78512.5 | 7.14 | 1.671E-07 | 6.78 | 6.20 | 8.76 | 2.05 | 15.12 | 1.72 | 5.60 | Proteolysis | Membrane |
| 72 | IDH3A | Isocitrate dehydrogenase 3 a | gi 149041700 | 30334.3 | 5.83 | 6.694E-07 | 39.31 | 10.26 | 18.10 | 76.03 | 16.43 | 83.09 | 13.00 | Metabolism | Mitochondria |
| 73, 705, 787 | ALI3 | Alpha-1-inhibitor 3 | gi 83816939 | 165038 | 5.7 | 6.561E-09 | 86.41 | 19.56 | 46.94 | 136.2 | 38.67 | 216.9 | 28.67 | Inflammatory response | Extracellular |
| 77 | MUG1 | Murinoglobulin-1 | gi 12831225 | 166590 | 5.68 | 7.856E-05 | 28.44 | 16.28 | 16.3 | 47.41 | 8.675 | 107.7 | 20.1 | Acute-phase response | Extracellular |
| 88 | IGHG-γ | Ig-2a immunoglobulin heavy chain | gi 1220486 | 52242.6 | 8.15 | 1.24E-08 | 2.54 | 1.45 | 74.61 | 38.51 | 40.49 | 4.75 | 17.20 | Antigen binding | N/A |
| 94 | FGB | Fibrinogen beta chain | gi 124106312 | 54827.9 | 7.9 | 0.0008907 | 0.33 | 0.51 | 1.84 | 1.87 | 2.55 | 0.55 | 0.53 | Signal transduction | Membrane |
| 106 | CDK5RAP2 | CDK5 regulatory associated prtoein 2 | gi 109476582 | 208169 | 5.27 | 0.001 | 1.47 | 0.85 | 1.70 | 2.22 | 6.94 | 2.21 | 1.67 | tRNA modification | Cytoplasm |
| 115, 123 | SERPINAL1 | Alpha-1-antiproteinase | gi 112889 | 46277.6 | 5.7 | 0.272 | 11.10 | 6.35 | 22.27 | 10.54 | 10.96 | 8.36 | 18.61 | Acute phase | Extracellular |
| 149 | PUS1 | Pseudouridine synthase 1 | gi 149063707 | 44463.4 | 8.1 | 0.000911 | 6.34 | 1.67 | 0.78 | 6.50 | 4.39 | 1.16 | 1.17 | tRNA processing | Mitochondria |
| 153 | MYL2 | Myosin, light polypeptide 2 | gi 149067749 | 17669.7 | 4.96 | 4.491E-05 | 9.21 | 3.03 | 27.07 | 38.86 | 10.34 | 2.37 | 2.66 | Muscle contraction | Cytosol |
| 177 | A1M | Alpha-1-macroglobulin precursor | gi 21955142 | 168422 | 6.46 | 2.312E-10 | 2.88 | 1.84 | 3.71 | 28.06 | 1.60 | 2.77 | 2.87 | Protein binding | Extracellular |
| 181 | SERPINA3L | Serine protease inhibitor A3L | gi 2507387 | 46419.1 | 5.48 | 0.007 | 1.83 | 3.57 | 3.99 | 17.08 | 1.90 | 7.53 | 3.22 | Inhibitory protein | Extracellular |
| 184, 257, 328 | CFH | Complement inhibitory factor H | gi 15485713 | 144814 | 6.52 | 0.001 | 3.69 | 9.51 | 4.54 | 2.46 | 5.85 | 6.15 | 3.32 | Immune response | Cytoplasm |
| 207, 555 | CCHL1A1 | Calcium channel alpha-1 subunit | gi 1184038 | 13893.9 | 6.52 | 0.0001206 | 15.79 | 10.78 | 13.23 | 13.97 | 9.12 | 11.32 | 18.65 | Transport | Membrane |
| 215 | GPT | Glutamic pyruvic transaminase 1 | gi 149066073 | 50475.5 | 6.63 | 2.201E-08 | 3.47 | 1.20 | 1.73 | 26.17 | 1.97 | 3.23 | 1.98 | Gluconeogenesis | Cytoplasm |
| 266 | CEP350 | Centrosome-associated protein 350 | gi 18027304 | 166810 | 8.63 | 0.012 | 0.56 | 0.22 | 0.75 | 0.56 | 0.54 | 1.43 | 1.22 | Cytoskeleton | Cytoplasm |
| 299 | UMPS | Uridine monophosphate synthetase | gi 149060638 | 33345.2 | 6.02 | 1.753E-05 | 2.05 | 1.45 | 1.92 | 0.66 | 1.17 | 1.17 | 2.01 | Metabolism | Cytoplasm |
| 315 | ITIH4 | Inter-alpha-inhibitor H4 heavy chain | gi 126722991 | 103862 | 5.82 | 1.561E-05 | 1.93 | 2.95 | 6.24 | 7.00 | 8.23 | 5.19 | 3.92 | Metabolism | Extracellular |
| 334 | ZNF689 | Zinc finger, HIT 6 | gi 157818873 | 53374.8 | 5.51 | 4.73E-08 | 26.07 | 0.59 | 0.61 | 1.00 | 0.71 | 1.05 | 0.45 | Transcription | Nucleus |
| 341 | Nmag_2782 | Na+/Ca+ antiporter | gi 8825638 | 11217.6 | 8.91 | 5.217E-06 | 1.87 | 1.50 | 0.59 | 1.10 | 1.10 | 1.44 | 6.59 | Transport | Membrane |
| 355, 359 | PLG | Plasminogen | gi 16758216 | 93213.9 | 6.79 | 1.998E-05 | 3.15 | 4.34 | 2.99 | 3.15 | 2.20 | 3.29 | 3.45 | Tissue remodeling | Extracellular |
| 382, 385, 378, 572, 578, 595 | CFB | Complement factor B | gi 149027999 | 83735.6 | 6.05 | 1.675E-10 | 5.91 | 43.78 | 33.76 | 30.02 | 3.29 | 23.45 | 5.94 | Immune response | Extracellular |
| 389 | GSN | Gelsolin | gi 149038928 | 86314.2 | 5.76 | 6.661E-14 | 6.51 | 70.05 | 37.55 | 38.06 | 4.32 | 44.17 | 8.39 | Cytoskeleton | Cytosol |
| 430 | SCD2 | Stearyl-CoA desaturase 2 | gi 1763027 | 3479.7 | 9.9 | 2.661E-09 | 11.28 | 40.97 | 22.40 | 9.29 | 8.21 | 13.65 | 11.38 | Fatty acid synthesis | Membrane |
| 434, 678 | COG1 | Component of golgi complex 1 | gi 149054700 | 72098.8 | 8.06 | 1.465E-08 | 29.10 | 110.50 | 55.14 | 34.52 | 24.63 | 43.20 | 30.09 | Transport | Membrane |
| 439 | C4BPA | C4b-binding protein alpha | gi 2493792 | 64277.8 | 7.06 | 9.889E-09 | 4.21 | 1.72 | 4.04 | 13.64 | 14.99 | 1.36 | 7.56 | Innate immunity | Extracellular |
| 452 | AFM | Afamin, Albumin-binding | gi 60688254 | 54205.3 | 5.8 | 5.923E-09 | 25.61 | 13.68 | 18.92 | 17.57 | 10.01 | 59.65 | 55.78 | Transport | Extracellular |
| 464 | IGH-6 | Immunoglobulin heavy chain 6 | gi 62201965 | 69059.5 | 5.69 | 4.813E-05 | 5.89 | 7.22 | 3.89 | 3.22 | 6.65 | 4.66 | 7.97 | Antigen binding | N/A |
| 479 | IGHM | Ig mu chain C region | gi 111977 | 38189.1 | 6.72 | 1.038E-11 | 2.72 | 21.09 | 23.96 | 10.08 | 32.56 | 8.65 | 14.62 | Immune response | Membrane |
| 493 | ZFP637 | Zinc finger protein 637 | gi 201027426 | 31712.4 | 9.5 | 6.464E-07 | 3.75 | 14.49 | 9.82 | 5.45 | 13.46 | 17.41 | 12.88 | Protein binding | Intracellular |
| 515 | CESIC/ES2 | Carboxylesterase | gi 468766 | 59196.9 | 5.51 | 5.223E-07 | 17.08 | 3.47 | 6.87 | 8.05 | 7.78 | 24.22 | 19.57 | Metabolism | Endoplasmic reticulum |
| 524 | ERCC4 | DNA repair endonuclease | gi 109487684 | 110025 | 9.2 | 9.067E-09 | 120.80 | 32.57 | 53.74 | 55.47 | 21.96 | 68.54 | 30.55 | DNA repair | Nucleus |
| 551, 587, 908 | C3 | Complement C3 | gi 158138561 | 187746 | 6.06 | 1.64E-11 | 7.046 | 146.9 | 88.04 | 46.76 | 54.1 | 32.88 | 31.97 | Inflammation | Extracellular |
| 559 | HPX | Hemopexin | gi 122065203 | 52059.6 | 7.58 | 0.167 | 26.14 | 16.11 | 24.39 | 38.23 | 27.58 | 17.91 | 22.98 | Heme scavenging | Extracellular |
| 571 | GC | Group specific component | gi 51260133 | 55079.6 | 5.65 | 9.36E-12 | 37.07 | 11.96 | 7.84 | 74.44 | 38.63 | 3.75 | 24.61 | Vitamin D binding | Extracellular |
| 576, 620 | Ac2–248 | SERPIN-like protein | gi 32527753 | 67191 | 6.85 | 1.098E-07 | 10.86 | 14.95 | 18.08 | 16.62 | 16.66 | 2.38 | 11.23 | Inflammation response | Extracellular |
| 586, 614 | SERPINC1 | Serine/cysteine peptidase inhibitor | gi 58865630 | 52714 | 6.18 | 3.838E-06 | 63.42 | 48.70 | 50.44 | 57.02 | 38.00 | 132.20 | 75.38 | Blood clotting | Extracellular |
| 592 | DNASE1L1 | Deoxyribonuclease 1 | gi 149029874 | 32437 | 6.31 | 1.664E-07 | 21.16 | 20.04 | 15.61 | 12.37 | 31.55 | 11.70 | 17.68 | DNA catabolism | Endoplasmic reticulum |
| 604 | APOH | Beta-2-glycoprotein 1 | gi 57528174 | 39743.2 | 8.58 | 6.515E-06 | 18.54 | 10.78 | 22.81 | 17.48 | 23.66 | 67.12 | 26.76 | Transport | Cell surface |
| 606 | GC | Vitamin D binding protein | gi 203927 | 55089.6 | 5.65 | 0.006 | 15.31 | 5.92 | 9.60 | 9.50 | 5.58 | 11.27 | 10.14 | Transport | Extracellular |
| 627 | MAP1 | LMW T-kininogen I | gi 205085 | 48757 | 6.29 | 1.263E-07 | 108.90 | 20.28 | 30.23 | 45.81 | 54.85 | 44.92 | 38.20 | Acute-phase response | Extracellular |
| 638 | LOC501738 | Immune activating receptor | gi 264681503 | 24023.2 | 9.41 | 1.901E-05 | 3.11 | 4.73 | 4.42 | 5.82 | 5.92 | 4.69 | 12.14 | Receptor activity | N/A |
| 649 | SYNE2 | Nesprin-2 like | gi 109478368 | 442636 | 5.29 | 1.843E-08 | 52.04 | 10.43 | 40.69 | 33.92 | 37.11 | 26.48 | 25.27 | Cytoskeleton | Nuclear |
| 651 | DSTN | Destrin | gi 75991707 | 18806.7 | 8.19 | 3.884E-10 | 23.61 | 109.00 | 94.03 | 176.80 | 57.85 | 37.12 | 51.53 | Actin binding | Cytoplasm |
| 655 | DLGAP2 | Disks large-associated protein 2 | gi 16758774 | 111349 | 6.82 | 6.158E-10 | 48.96 | 280.60 | 240.60 | 254.70 | 149.20 | 151.30 | 122.60 | Synapse transmission | Membrane |
| 672 | SERPINF | Serine/cysteine peptidase inhibitor | gi 29293811 | 46493.2 | 6.04 | 3.611E-05 | 9.02 | 3.59 | 7.34 | 3.45 | 17.65 | 23.29 | 10.52 | Inhibitory protein | Extracellular |
| 676 | rCG33447 | Hypothetical | gi 149052919 | 20157.4 | 9.14 | 1.667E-05 | 13.09 | 19.5 | 14.69 | 49.61 | 42.59 | 24.67 | 17.95 | N/A | N/A |
| 691 | APOA4 | Apolipoprotein A-IV | gi 114008 | 44428.7 | 5.12 | 1.996E-06 | 46.70 | 42.11 | 49.99 | 39.11 | 16.29 | 65.41 | 14.52 | Lipid binding | Extracellular |
| 860 | KRT10 | Keratin, type I | gi 57012436 | 56698.6 | 5.1 | 4.594E-08 | 6.32 | 2.94 | 3.06 | 3.63 | 5.79 | 2.64 | 20.82 | Protein binding | Intermediate filament |
| 966 | Hypothetical | CMRF-35-like -7 | gi 109511428 | 9215.8 | 7.93 | 3.917E-09 | 12.66 | 76.58 | 53.33 | 87.53 | 59.29 | 49.99 | 28.90 | Immunity | Membrane |
| 897 | MYO5A | Myosin Va | gi 149019170 | 96579.8 | 9.48 | 5.875E-06 | 24.00 | 9.21 | 4.82 | 16.65 | 31.94 | 11.65 | 19.24 | Myosin | Cytoplasm |
| 715 | SERPINA1 | Proteinase inhibitor | gi 930263 | 22867.8 | 6.06 | 2.851E-07 | 6.35 | 12.36 | 10.83 | 24.11 | 32.20 | 30.96 | 12.93 | Protein binding | Extracellular |
| 724 | ALB | Serum albumin | gi 158138568 | 70709.9 | 6.09 | 5.902E-07 | 1.19 | 3.31 | 1.72 | 0.46 | 2.23 | 2.19 | 11.17 | Transport | Extracellular |
| 728 | SYMPK | Symplekin-C | gi 62531221 | 33433.1 | 4.97 | 1.601E-11 | 0.84 | 1.00 | 0.76 | 0.49 | 1.13 | 2.21 | 7.89 | Protein binding | Nucleus |
| 733 | DECR2 | 2,4-dienoyl-CoA reductase | gi 25282441 | 31614.4 | 8.51 | 0.0006324 | 1.71 | 1.90 | 2.24 | 2.06 | 2.44 | 1.67 | 7.42 | Metabolism | Peroxisome |
| 740 | PRPH | Peripherin | gi 166063971 | 54063.5 | 5.32 | 0.0001771 | 14.90 | 5.56 | 7.81 | 4.46 | 7.33 | 4.02 | 13.03 | Cytoskeleton | Intermediate filament |
| 745 | PFKFB1 | 6-phosphofructo-2-kinase | gi 77020248 | 55301 | 6.78 | 3.474E-08 | 8.56 | 4.94 | 4.51 | 4.17 | 3.21 | 2.30 | 8.93 | Metabolism | Cytoplasm |
| 749 | MYO5B | Myosin-Vb | gi 8393817 | 215241 | 6.53 | 6.828E-10 | 5.53 | 9.51 | 4.71 | 2.88 | 1.70 | 2.46 | 19.91 | Myosin complex | Intracellular |
| 766 | SCG2 | Secretogranin 2 | gi 149016236 | 61578.7 | 4.69 | 0.000699 | 3.94 | 5.60 | 3.20 | 3.16 | 6.32 | 3.79 | 14.46 | Inflammation | Extracellular |
| 779, 909 | VIM | Vimentin | gi 149021116 | 4131.2 | 9.75 | 2.984E-09 | 1.52 | 6.85 | 5.45 | 36.40 | 6.88 | 3.08 | 7.28 | Cell integrity | Cytoplasm |
| 796 | Hypothetical protein | gi 109468251 | 53156.7 | 8.32 | 1.702E-07 | 10.25 | 8.42 | 9.97 | 7.48 | 8.33 | 5.11 | 11.64 | N/A | N/A | |
| 814 | RBM15B | RNA binding motif 15B | gi 109483938 | 96223.1 | 9.85 | 4.256E-07 | 1.65 | 1.41 | 0.60 | 0.63 | 1.56 | 3.61 | 13.27 | RNA splicing | Nucleoplasm |
| 817 | BRPF1 | Bromodomain/PHD finger- protein 1 | gi 109472470 | 151416 | 8.55 | 0.024 | 10.16 | 21.83 | 12.14 | 8.66 | 29.59 | 25.15 | 31.35 | Transcription | Cytoplasm |
| 820, 1081 | BFAR | Bifunctional apoptosis regulator | gi 61557021 | 53617.2 | 6.44 | 0.006 | 4.77 | 3.19 | 4.59 | 31.17 | 2.20 | 13.38 | 5.61 | Apoptosis | Membrane |
| 827 | GNAT | Guanine binding protein alpha | gi 84662745 | 42416.4 | 5.48 | 4.658E-09 | 11.89 | 15.94 | 17.91 | 3.82 | 17.80 | 10.78 | 12.84 | N/A | Cytoplasm |
| 832 | PSMB | Proteasome beta-type subunit RN3 | gi 9653292 | 15426.5 | 6.82 | 0.014 | 2.47 | 3.16 | 6.23 | 1.86 | 7.55 | 3.47 | 8.94 | Protein catabolism | Cytoplasm |
| 867 | RAI14 | Ankycorbin | gi 58865464 | 106502 | 5.64 | 0.0002925 | 4.34 | 5.72 | 2.56 | 2.86 | 3.00 | 2.41 | 12.07 | N/A | Cytoplasm |
| 881 | CHD4 | Chromodomain helicase DNA binding protein 4 | gi 149049419 | 111042 | 5.43 | 2.167E-10 | 11.78 | 5.61 | 13.92 | 17.58 | 6.68 | 28.89 | 8.62 | Transcription | Nucleus |
| 900 | rCG25416 | Transferrin region | gi 149018747 | 66991.6 | 6.41 | 9.426E-08 | 20.46 | 9.26 | 25.94 | 22.29 | 44.00 | 7.06 | 18.89 | N/A | N/A |
| 906 | APOE | Apolipoprotein E | gi 149056721 | 27354.1 | 7.93 | 0.004 | 43.97 | 56.73 | 49.40 | 26.45 | 87.74 | 74.42 | 57.50 | Oxidative stress response | Chylomicron |
| 979 | rCG25357 | Hypothetical | gi 149018900 | 3285.6 | 9.5 | 2.003E-07 | 20.05 | 55.99 | 59.46 | 45.31 | 41.38 | 31.72 | 31.72 | N/A | N/A |
| 992 | APOA1 | Apolipoprotein A-I | gi 2145143 | 29869.1 | 5.51 | 8.784E-07 | 15.55 | 41.44 | 34.51 | 32.12 | 57.94 | 38.11 | 39.05 | Transport | Membrane |
| 1019 | ZNHIT6 | Hypothetical protein FLJ20729 | gi 149026162 | 37686.2 | 9.39 | 1.376E-07 | 10.86 | 1.29 | 1.39 | 0.64 | 0.23 | 0.29 | 3.12 | Protein binding | Pre-snoRNP complex |
Subcellular Location and Functional Characterization
The differentially expressed protein data sets (Table I) were submitted to Ingenuity Pathway Analysis (IPA) and UniProt for identifying cellular localization and function analysis. As expected for plasma, >65% of the differentially expressed proteins were extracellular (supplemental Fig. S5A). Detection of the increased release of proteins belonging to cellular compartments, including the cell surface (3%), cytoplasm (6%), endoplasmic reticulum (2%), endosome (3%), membrane (13%), and nucleus (8%) in chagasic plasma (supplemental Fig. S5A), indicated that T. cruzi-induced cellular events and cell injury/cell death, and the resultant release of intracellular proteins, at least partially, contribute to disease-specific differential plasma proteomes. Following a functional analysis of T. cruzi-stimulated proteins, we found a maximal number of the proteins belonged to the acute response/inflammation category (66%) (supplemental Fig. S5B), of which >50% returned to control levels in the plasma of PBN-treated/infected rats (supplemental Fig. S5C). Associated network analysis allowed us to classify the differentially expressed proteins into three networks (Table II), with the maximal number assigned to the antigen presentation, humoral immune response and inflammatory response networks (19 proteins, supplemental Table S2, supplemental Fig. S6A), followed by the lipid metabolism, molecular transport, small molecular biochemistry networks (17 proteins, supplemental Table S3, supplemental Fig. S6B), and, finally, the cellular development, free radical scavenging, molecular transport networks (14 proteins, supplemental Fig. S6C). In addition, diseases and disorders analysis by IPA also associated maximal number of differentially expressed proteins to the acute phase response signaling pathway (13 proteins, supplemental Table S4, supplemental Fig. S7A). Further IPA analysis of identified proteins to determine the correlation with heart diseases provided evidence that the differential plasma proteome was indicative of myocardial infarction, contractile dysfunction, hypertrophy and fibrosis, inflammation and tissue damage, and pulmonary embolism and hypertension in chagasic rats (supplemental Table S5, supplemental Fig. S7B).
Table II. IPA network analysis of differentially expressed plasma proteins in Chagasic rats. Sprague Dawley rats were infected with T. cruzi and treated with phenyl-α-tert-butyl nitrone (PBN) and benznidazole (BZ) as detailed in Materials and Methods. Plasma samples were collected at day 25 (acutely infected) and 150 (chronically infected) post-infection, and subjected to 2D-gel electrophoresis/mass spectrometry for identification of the differentially expressed proteins. All differentially expressed proteins (listed in Table I) were uploaded into Ingenuity Pathway Analysis (IPA) to interpret datasets in the context of biological processes and function, and pathway and molecular networks. Presented are the top three networks with a score [-log(p value)] of 25 or greater to which maximal number of the differentially expressed proteins identified in chagasic plasma (bolded) were associated with. Focus molecules are the number of differentially expressed plasma proteins associated with individual network.
| ID | Molecules in network | Score | Focus molecules | Top functions |
|---|---|---|---|---|
| 1 | Actin, ALB, APOA1, APOA4, APOE, APOH, C3, CFB, CFH, CFHR1, CFP, CHD4, Cytokeratin, DSTN, FGB, Fibrinogen, GSN, HPX, IgG, KRT1, KRT3, KRT4, KRT10, KRT12, KRT13, KRT23, KRT6B, PLG, SERPINF, SERPINF1, SETX, TF, TMPRSS6, TRY6, VIM | 41 | 19 | Antigen Presentation, Humoral Immune Response, Inflammatory Response |
| 2 | AFM, APP, BRPF3, C4BPA, CACNA1D, COL25A1, DLGAP2, GAB1/2, GC, GRB2, heparin, INSR, KCNMA1, KNG1 (includes EG:16644), Met dimer, MUG1, MYLPF, MYO5A, MYO5B, MYO5C, PFKFB2, PIK3AP1, PIK3R1, PLA2G2D, PLA2G2E, PRPH, PZP, RAI14, SCG2, SERPINC1, SLC23A1, SNX8, VPS13A, YWHAZ, ZNF32 | 36 | 17 | Lipid Metabolism, Molecular Transport, Small Molecule Biochemistry |
| 3 | ATG4C, BFAR, C1ORF25, CASP8, CEBPB, CEP350, CIDEC, CRAT, DSCR3, ESR1, GNAQ, GPT, HNF4A, IDH3A, ITIH4, LGMN, MINA, MYC, PEPD, PPARG, PUS1, PUS3, SCD2, SERPINA1, SLC25A19, SQRDL (includes EG:58472), SRPRB,SYMPK, TMEM176A, TMEM176B, TRUB2, TTC37,UMPS, YME1L1, ZNHIT6 | 29 | 14 | Cellular Development, Free Radical Scavenging, Molecular Transport |
In prior investigations in our lab, we have shown that PBN, an antioxidant, controlled the pathologic oxidative tissue injury and mitochondrial dysfunction induced by T. cruzi, and cotreatment of rats with PBN and anti-parasite drug (BZ) was most effective in preserving the cardiac function in chronically infected chagasic hearts (18, 23). The plasma proteome profile of chagasic rats showed that the recovery of heart function in PBN- and PBN/BZ-treated chagasic rats was associated with control of expression of the inflammation- and tissue remodeling-related proteins (supplemental Fig. S5C). Simultaneously, the plasma detection of other proteins was increased by PBN (e.g. SERPNA1, KRT1, SRPRB, Igh-1a, SroTP, TF, FGB, CDKSRAP2, Itih4 and PLG) and BZ (e.g. IDH3A, ALI3, MUG1, CFH, CEP350, COG1, AFM, ZFP37, ES2, ERCC4, SERPINC1, APOH, SPI-1, APOA4, SYMPK, RBM15B, and CHD4) treatment of infected rats. These data, along with those presented in supplemental Figs. S5–S7, suggested that acute response and inflammation play an important role in Chagas disease development, and recovery of heart function by PBN/BZ treatment was reflected by the normalization of the plasma profile of immune and tissue remodeling responses in infected rats.
Carbonyl Proteome Profile in Chagas Disease
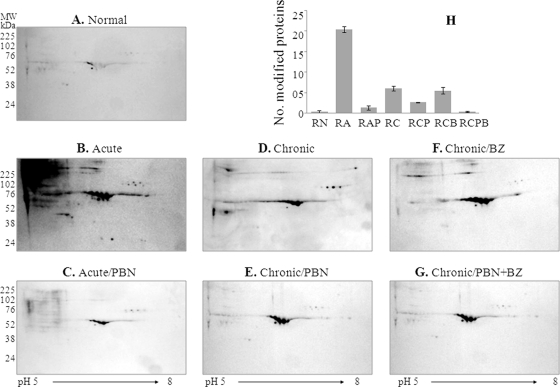
We performed Western blotting to identify the plasma proteins that are sensitive to oxidative stress during Chagas disease. For this, protein-loaded one-dimension IPG strips were incubated with DNPH to derivatize the carbonyl proteins, and after second-dimension gel electrophoresis, immuno-blotting was carried out by using anti-DNP antibody. Shown in Figs. 3A–3G are the representative images of plasma carbonyl-proteome profiles in acute and chronic chagasic conditions, and the impact of PBN and BZ treatment on disease-related carbonyl proteomes. The data from MALDI-TOF-MS/MS analysis of the modified proteins are presented in supplemental Table S1. Overall, we observed that 115, 28, 34, 15, 31, and 12 protein spots were carbonylated in RA, RAP, RC, RCP, RCB, and RCPB groups (Fig. 3H). These data clearly showed that plasma proteins were exposed to acute oxidative stress (Fig. 3B), and their oxidation was significantly diminished by PBN treatment (Fig. 3C). At the chronic infection stage, the total number of carbonyl-proteins was less than that noted in the acute stage; however, it is important to note that PBN treatment (Figs. 3E and 3G), but not BZ treatment (Fig. 3F), ameliorated the protein carbonylation in chronic chagasic plasma. We also found through comparative analysis of signal densities from SYPRO Ruby-stained gels (Fig. 1) and WB images (Fig. 3) that some proteins in the plasma of infected rodents (e.g. spot 115) were decreased in expression, but increased in carbonylation, whereas others (e.g. spot # 184, 328, 355, 385, 389, and 766) were increased in expression as well as carbonylation (compare Table I and supplemental Table S1). Upon PBN treatment, carbonylation of several of the proteins was ameliorated, either partially (e.g. spot # 355, 389, 592, 604, and 606) or completely (e.g. spot # 73, 77, 115, 123, 153, 184, 207, 299, 328, 359, 385, 733, and 766). Nine proteins (spot # 299, 328, 385, 733, 766, 796, 906, 908, and 948) were distinctly modified in acute plasma, and 15 proteins (spot # 73, 77, 115, 123, 153, 184, 207, 355, 359, 389, 592, 604, 606, 614, and 1081) were modified throughout the course of infection and disease development (supplemental Table S1). IPA analysis for cellular location revealed 56% of the proteins oxidatively modified in an infected host were extracellular (supplemental Fig. S8A). IPA analysis of biological response networks revealed a majority of the modified proteins (42%) represented host immunity to infection (i.e. acute phase response, complement activation and inflammatory response, response to cytokine stimulus), followed by protein transport (19%), tissue remodeling and metabolism (10%), and DNA catabolic process and myosin complex function (5% each) (supplemental Fig. S8B). These data suggested that oxidative stress affects the expression level (and possibly function) of the proteins associated with inflammation and protein transport during T. cruzi infection.
Fig. 3.
Protein carbonyls detected in plasma of T. cruzi-infected rats. Depleted plasma samples from normal and T. cruzi-infected rats (±PBN/BZ) were subjected to first-dimension isoelectric focusing on 11-cm, linear pH 5–8 immboilized pH gradient (IPG) strips. Strips were incubated with dinitrophenylhydrazine (DNPH) to derivatize carbonyl proteins, and second-dimension separation was carried out by SDS-PAGE on 8–10% gradient gels. (A–G) Shown are the representative images of Western blotting with anti-DNP antibody. H, The bar graph shows average number of carbonylated protein spots identified in the plasma of each experimental group. Abbreviations are as in Fig. 2. Carbonyl proteins were identified by mass spectrometry (listed in supplemental Table S1).
Validation of Differentially Expressed Proteins in Chagas Disease
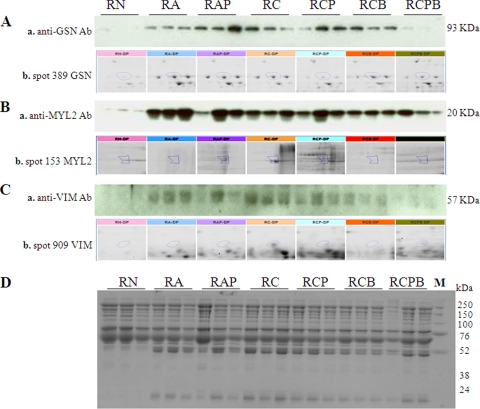
To validate the T. cruzi-induced differential expression of plasma proteins identified by 2D-GE/mass spectrometry, we chose to perform WB with protein-specific antibody. Plasma proteins were resolved by SDS-PAGE, and probed with antibodies to detect the expression level of GSN, MYL2 and VIM (Fig. 4). Anti-GSN, anti-MYL2 and anti-VIM antibodies exhibited strong reactivity for 93 kDa, 20 kDa, and 57 kDa bands, respectively, in acute and chronic plasma (Figs. 4A–4C). The enhanced plasma levels of GSN, MYL2 and VIM in chronically infected rats returned to normal control level by PBN/BZ treatment of infected rats. Coomassie staining of the gels showed equal loading of all protein samples (Fig. 4D). These data verified the identity of GSN, MYL2 and VIM in chagasic plasma and validated the identification of proteins by mass spectrometry.
Fig. 4.
Western blotting confirmed the differential expression profile of selected proteins in chagasic rat plasma. (A–C) Whole plasma from normal and infected rats (±PBN/BZ) were resolved by SDS gel electrophoresis, and membranes were probed using anti-gelsolin (Aa), anti-MYL2 (Ba) and anti-vimentin (Ca) antibodies. The expanded view of the corresponding spot for gelsolin, MYL2 and vimentin from 2D-gels is presented in panels Ab, Bb, and Cb, respectively. D, Coomassie-stained gel image demonstrates equal loading of all samples.
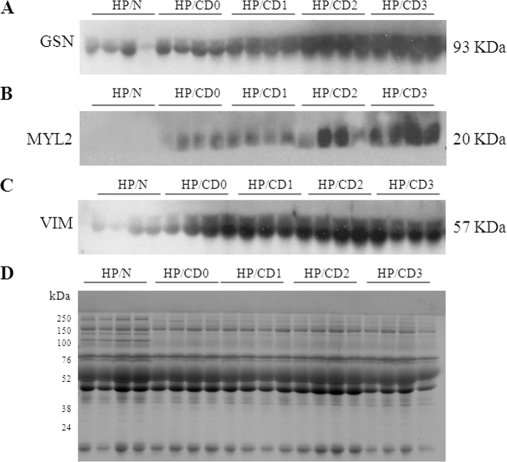
To investigate if the selected proteins exhibit disease-specific expression patterns in human patients and are useful biomarkers of the Chagas disease state, we resolved the plasma proteins from clinically characterized chagasic patients (n = 10/group) and performed WB as above. Criteria for characterization of seropositive chagasic subjects exhibiting variable degrees of cardiac disease (CD0-CD3), seronegative cardiomyopathy patients of other etiologies; and seronegative, healthy individuals exhibiting no history or clinical symptom of cardiac disease have been described previously (25). Representative data from four samples/group is presented in Fig. 5. We detected low levels of GSN and VIM and none of MYL2 in the plasma of normal healthy controls. In comparison, GSN, MYL2, and VIM were significantly increased in plasma from chagasic patients (Fig. 5A–C). The detection of GSN, MYL2, and VIM in plasma from chagasic patients correlated directly with the disease stage, with a moderate increase in patients at the CD0/CD1 stage, whereas a maximal increase was noted in patients at the CD2/CD3 stage (Figs. 5A–C). Seronegative patients with cardiac symptoms because of other etiologies exhibited a marginal, but not statistically significant, increase in GSN, and no increase in MYL2 and VIM levels in sera when compared with normal controls (data not shown). These data validate the experimental results (Fig. 4) and suggest that the plasma levels of GSN, MYL2, and VIM are useful indicators of disease and disease severity in human chagasic patients.
Fig. 5.
Expression profile of GSN, MYL2 and VIM is altered in chagasic human patients. Human plasma samples were obtained from patients graded according to Chagas disease (CD) severity as CD0, CD1, CD2, and CD3. Samples from normal (N) subjects were utilized as controls. Plasma samples were resolved by SDS-PAGE and Western blotting performed by using anti-gelsolin (A), anti-MYL2 (B), and anti-vimentin (C) antibodies. D, Coomassie-stained gel image demonstrates the equal loading of all samples.
DISCUSSION
In this study, we developed a comprehensive plasma proteome profile during T. cruzi infection and disease development in a rodent model of Chagas disease. Parasite persistence and oxidative damage in the heart are known to be of pathological significance during Chagas disease (reviewed (14, 15)). We treated the infected rats with an anti-parasite drug (BZ) and/or antioxidant (PBN) to examine whether the beneficial effects of these treatments in controlling parasite- and oxidative stress-induced pathology, respectively (18, 23), are reflected in a plasma proteome profile of chagasic rats. We identified 92 proteins that were differentially expressed or oxidized in chagasic plasma. Functional analysis allocated a majority of these proteins to inflammation/immunity and lipid metabolism categories and to molecular pathways associated with cardiovascular dysfunction, i.e. myocardial infarction, hypertrophy, and fibrosis, and pulmonary embolism and hypertension in chagasic rats and to curative pathways (immune regulation and cardiac remodeling) in infected rats treated with PBN and/or BZ. The 2D-GE results were validated by Western blotting, and we demonstrated that the disease-associated increased expression of GSN and VIM, and release of cardiac MYL2 in the plasma of chagasic rats was controlled by PBN/BZ treatment. Increased plasma levels of GSN, MYL2 and VIM were directly correlated with the severity of cardiac disease in human chagasic patients. To the best of our knowledge, this is the first study demonstrating that the plasma oxidative and inflammatory response profile and plasma detection of cardiac proteins parallel the pathologic events contributing to Chagas disease development; furthermore, we believe the findings have a potential utility in diagnosing disease severity and designing suitable therapy for management of human chagasic patients.
Inflammation/Immune Response
IPA is a highly curated and comprehensive software used for the integration of proteins into networks and pathways with biological meaning (32). Network analysis of the plasma proteome profile of chagasic rats identified three major subnetworks linked to host response to T. cruzi infection and disease development (Table II). The maximal numbers of the differentially expressed plasma proteins (19 proteins) in chagasic rats were associated with acute phase response signaling, antigen presentation and inflammatory response (Table II and supplemental Tables S2, S4; supplemental Figs. S6A and S7A), known to be of pathological significance in Chagas disease (33–35). Following functional analysis of the inflammation-associated proteins, 12 proteins (APOA1, APOE, C3, CFB, CFH, FGB, GSN, KRT10, PLG, SCG2, SERPINA1, and SERPINC1) were identified as being involved in immune cell trafficking and cell movement of leukocytes, granulocytes, phagocytes, neutrophils, and dendritic and antigen presenting cells (supplemental Table S2). Some of the differentially expressed proteins in inflammation category were associated with activation (APOE, C3, CFH, GC, and PLG), chemotaxis (C3, SCG2, SERPINA1, SERPINC1), and infiltration of leukocytes (APOA1, APOE, C3, PLG, KRT10) and neutrophils (C3, CFB, CFH, PLG) (supplemental Table S2). Up-regulation of APOE, APOA1, APOH, GC, and PLG in chagasic plasma was indicative of activation, binding and accumulation of macrophages in the disease state. It is important to note that of the 19 differentially expressed inflammation-associated proteins, 11 proteins (APOE, C3, CFB, CFH, GSN, KRT10, SCG2, SERPINA1, SERPINC1, GC, and PLG) were carbonylated (supplemental Table S1) in chagasic plasma. PBN treatment prevented the oxidative modification of five of these proteins (i.e. CFB, CFH, SERPRINA1, SERPINC1, and PLG). Interestingly, PBN treatment also regulated the expression of several inflammation-associated proteins, including APOH, APOE, CFH, PLG, and SCG2 in acutely infected rats and APOH, GC, GSN, and PLG in chronically infected rats; whereas the expression level of CFB, GSN, C3, and SERPINC1 was partly regulated by PBN in infected rats (Table I, Fig. 2 and supplemental Fig. S2). Other proteins (APOE, C3, KRT3, and SCG2) were exposed to oxidation because of T. cruzi-induced acute oxidative stress, but were normalized in expression and oxidation during the chronic phase. Treatment of rats with anti-parasite drug (BZ) was not effective in preventing protein carbonylation (supplemental Table S1). These observations allow us to propose that oxidative stress plays an important role in modulating the host immune response against T. cruzi. Other studies have demonstrated that ROS elicit inflammatory cytokines (e.g. TNF-α, IFN-γ, IL-1β) in cardiomyocytes infected by T. cruzi (36, 37). Inflammatory pathology was controlled in chronically infected experimental animals and human patients by enhancing the antioxidant status, which was also beneficial in preserving the cardiac function during Chagas disease (18, 23, 38, 39). Our recent observations indicate that the mitochondrial release of ROS because of electron transport chain dysfunction and enhanced release of electrons to molecular oxygen is the primary source of oxidative stress in the heart (21). Further studies will be required to delineate the significance of mitochondrial ROS and classical ROS producers, i.e. NADPH oxidase and myeloperoxidase, known to play an important role in parasite control during the acute phase of infection (33, 40, 41), in elicitation of inflammatory responses during Chagas disease.
Lipid Metabolism
Seventeen of the differentially expressed proteins in chagasic plasma, i.e. AFM, C4BPA, CACNA1D, DLGAP2, GC, KNG1, MUG1, MYLPF, MYO5A, MYO5B, PRPH, PZP, RAI14, and SCG2, were allotted to the lipid metabolism/molecular transport/small molecule biochemistry category (Table II, supplemental Fig. S6B) and functionally linked by IPA network analysis to lipid, fatty acid and carbohydrate metabolism (supplemental Table S3). A majority of the proteins in this category, i.e. ALB, APOA1, APOA4, APOE, APOH, C3, GC, GNAQ, MYO5A, PLG, SCD2, SERPINA1, SERPINC1, and VIM, were linked to the synthesis, metabolism, transport, and modification of lipids and fatty acids, and to uptake and release or efflux of lipids, eicosanoids, and cholesterol. PBN/BZ-treated/infected rats exhibited normal expression level of 13 of the lipid/fatty acid metabolism-related proteins that were otherwise differentially expressed in chagasic rats (Table I). These data, to the best of our knowledge, provide the first indication that lipid/fatty acid metabolism is dysregulated and of pathologic significance in Chagas disease. The observation of increased expression of CEP350 in chagasic rat plasma provides clues to the pathomechanism involved in altered lipid/fatty acid metabolism during Chagas disease. CEP350 is a large centrosome-associated protein with a CAP-Gly domain typically found in cytoskeleton-associated proteins (42, 43). CEP350 interacts with other centrosomal proteins (e.g. FGFR1) and has been implicated in the mechanisms underlying microtubule anchoring and organization at the centrosome (42). Interestingly, CEP350 is also shown to alter the activity and subcellular compartmentalization of members of the peroxisome proliferator-activated receptors family (PPARα, PPARβ/δ, and PPARγ) (43) that heterodimerize with retinoid X receptors (RXRs) to function as transcription factors, and play essential roles in the regulation of cell differentiation and lipid/fatty acid metabolism (44, 45). Besides CEP350, SERPINs and GPT that were up-regulated in chagasic plasma and function in inflammatory response/tissue remodeling and amino acid metabolism, respectively, also belong to the network of proteins regulated by PPARs (46, 47). We propose that PPARs' retention in the centrosome by CEP350 or by alteration of the transactivation complex activity contributes to the dysregulation of lipid/fatty acid metabolism and inflammation and tissue remodeling responses during Chagas disease; however, this must be validated in future studies.
Cardiovascular Disease-Associated Proteins
Twenty-four of the differentially expressed proteins, i.e. AFM, ALB, APOA1, APOA4, APOE, APOH, C3, CFB, CFH, FGB, GC, GNAQ, GSN, HPX, ITIH4, KNG1, MYO5A, PLG, SCD2, SCG2, SERPINA1, SERPINC1, SERPINF1, and VIM, were linked to cardiovascular function, skeletal and muscular disorders, and cardiovascular diseases (Table II, supplemental Table S5, supplemental Fig. S7B). Of these, nine proteins (APOA1, APOE, APOH, C3, KNG1, PLG, SCG2, SERPINC1, and SERPINF1) play a functional role in the proliferation of endothelial cells, whereas several others correlate with angiogenesis (APOE, APOH, PLG, SCG2, SERPINC1, and SERPINF1), thrombosis or thromboembolism (APOE, APOH, CFB, GNAQ, PLG, and SERPINC1), myocardial ischemia and infarction (ALB, C3, FGB, GSN, PLG, APOA1, APOE, PLG, and SERPINC1), and atherosclerosis (APOA1, APOA4, APOE, and PLG) (supplemental Table S5) (48). The latter findings indicate that endothelial cell dysfunction plays a role in the progression of Chagas disease and demonstrate that the plasma proteome profile is a useful indicator of clinical disease status, to be validated in future studies by profiling the plasma proteins in human chagasic patients.
Biomarkers of Chagas Disease
Our ultimate goal in this study was to identify the diagnostic biomarkers of Chagas disease. Western blot analysis using antibodies specific to GSN, MYL2, and VIM validated the 2D-GE plasma profile of chagasic rats and demonstrated that GSN, MYL2, and VIM were indeed increased in the plasma of chagasic rats (Fig. 4). We noted a direct correlation in plasma levels of GSN, MYL2, and VIM and disease severity in human chagasic patients (Fig. 5). Further, PBN/BZ-mediated control of cardiac pathology and preservation of heart contractile function in chagasic rats (18, 23) was associated with normalized plasma levels of GSN, MYL2 and VIM similar to that noted in normal controls (Fig. 4). Importantly, we detected no significant increase in plasma levels of GSN, MYL2, and VIM in cardiomyopathy patients of other etiologies. These findings indicate the importance of GSN, MYL2, and VIM as protein biomarkers of Chagas disease, and provide an impetus for a large-scale, preclinical study of plasma from human patients to establish the potential utility of GSN, MYL2, and VIM in diagnosing the severity of Chagas disease in human patients. The results of such a study could lead to procedures to identify the patients at risk of developing clinical symptoms of Chagas disease, and validate the curative efficacy of currently used and newly developed therapies for treatment and management of chagasic patients.
Pathologic Significance of GSN, MYL2, and VIM in Cardiovascular Diseases
Besides their importance as diagnostic markers in Chagas disease, as observed in this study, GSN, MYL2, and VIM play a significant role in heart disease, and, therefore, merit further discussion. GSN is an actin-binding protein, and a member of the gelsolin/villin super family (49), located intracellularly (cytosol, mitochondria) and extracellularly (blood, plasma) (50). It is a key regulator of actin filament assembly and disassembly and is involved in maintaining cell structure and motility (49). Increased GSN expression is associated with interstitial fibrosis and inflammation (51), likely because of the GSN-mediated destabilization of cytoskeleton and increased movement of platelets and immune infiltrate, and GSN−/− mice are shown to develop decreased pulmonary fibrosis and inflammation (51). In the heart, GSN is shown to catalyze the disassembly and degradation of myocardial proteins (52), and its increased expression is detected in failing human hearts (52). It has been suggested that GSN interacts with hypoxia inducible factor 1 (HIF1A), a master transcriptional regulator of the cellular and systemic responses to hypoxia, that is known to play an essential role in the pathophysiology of ischemic cardiovascular disease (53). A patent (WO/2005/112970) asserts that GSN, through its ability to inhibit motility of trypomastigotes in blood, will be useful in controlling blood parasitemia and T. cruzi infection. Future studies will be required to investigate the pathological significance of GSN in chagasic heart disease and to validate the utility of GSN as a therapy against T. cruzi.
MYL2 (myosin regulatory light chain 2) is a cardiac-specific protein. MYL2 dimerizes with cardiac myosin beta (or slow) heavy chain, and its phosphorylation by Ca+ triggers cardiac contractions. Mutations in MYL2 or abnormalities in MYL2 expression are associated with cardiomyopathy (54), heart failure (55), and left ventricular hypertrophy and familial hypertrophy (56, 57). Alterations in the expression of MYL2 in chagasic hearts (58) and isolated cardiomyocytes infected by T. cruzi (59) have been reported, and it is suggested that T. cruzi-induced immunoglobulin G autoantibodies and delayed type hypersensitivity to cardiac myosin contribute to disease pathogenesis (60, 61). To the best of our knowledge, our study provides the first evidence that the plasma release of MYL2 is linked to disease severity in chagasic patients and indicative of the extent of cardiac muscle injury during Chagas disease development.
VIM is a member of the intermediate filament network, and it is primarily expressed by mesenchymal cells and found in connective tissue. Along with microtubules and actin microfilaments, VIM plays an important role in maintaining cell shape, integrity of the cytoplasm, and stabilizing cytoskeletal interactions (62). VIM is also shown to be localized in the carotid artery and heart valves and serves as a target antigen of peripheral and heart-infiltrating T cells during valvular disease (63). Increased detection of VIM in the heart is indicative of occurrence of a fibrotic process, as infiltrating fibroblasts replace damaged cardiomyocytes in disease conditions and has been identified by proteomic inventory of myocardial proteins in patients with Chagas disease (64). Results obtained through IPA analysis indicated that VIM modulates NOS2 and is indirectly linked to IL-1β and TNF-α expression in the disease state. Future studies will be required to investigate the mechanisms linked to the increased secretion of VIM in chagasic plasma and evaluate whether VIM regulates the host immune response and immune pathology during T. cruzi infection and Chagas disease development.
In summary, we have demonstrated that depletion of high-abundance plasma proteins enhanced the protein discovery of low-abundance proteins by 2D-GE. We found that pathological events, i.e. persistent inflammation and oxidative stress, associated with Chagas heart disease, and the beneficial effects of antioxidant and anti-parasite therapies in preserving the cardiac function, were reflected in the plasma protein profile of experimentally infected rodents. Our proteomic studies provide the first indication that lipid/fatty acid metabolism is dysregulated and of pathologic significance in Chagas disease. Importantly, we have identified three protein biomarkers (GSN, MYL2, and VIM) that have potential utility in diagnosing the severity of Chagas disease, identifying the patients at risk of developing clinical symptoms of Chagas disease, and validating the curative efficacy of currently used and newly developed therapies for treatment and management of chagasic patients.
Acknowledgments
We thank the Biomedical Resource Facility (BRF) Mass Spectrometry Laboratory at the University of Texas Medical Branch at Galveston for mass spectrometric analysis and Ms. Mardelle Susman for editing the manuscript.
Footnotes
* This work was supported in part by grants (AI054578, HL088230 and HL094802) from the National Institute of Allergy and Infectious Diseases and the National Heart Lung Blood Institute of the National Institutes of Health to NJG.
 This article contains supplemental Figs. S1 to S8 and Tables S1 to S5.
This article contains supplemental Figs. S1 to S8 and Tables S1 to S5.
1 The abbreviations used are:
- PBN
- Phenyl-α-tert-butyl nitrone
- 2D-GE
- Two-dimensional gel electrophoresis
- BZ
- Benznidazole
- DNPH
- 2,4-dinitrophenylhydrazine
- GSN
- Gelsolin
- IPA
- Ingenuity pathways analysis
- IPG
- Immboilized pH gradient
- MYL2
- Myosin light chain polypeptide 2, cardiac specific
- RA
- Acutely infected rats
- RAP
- Acutely infected/PBN-treated rats
- RC
- Chronically infected rats
- RCB
- Chronically infected/BZ-treated rats
- RCP
- Chronically infected/PBN-treated rats
- RCPB
- Chronically infected/PBN+BZ-treated rats
- RN
- Normal rats
- ROS
- Reactive oxygen species
- VIM
- Vimentin.
REFERENCES
- 1. World Health Organization (2006) Report of the Scientific Working Group on Chagas Disease, UNDP/World Bank/WHO, http://www.who.int/tdr/diseases/chagas/swg_chagas.pdf Accessed Nov. 10, 2011
- 2. World Health Organization (2010) Chagas disease: control and elimination. Report of the secretariat, UNDP/World Bank/WHO, http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_17-en.pdf Accessed Nov. 10, 2011
- 3. Bern C., Montgomery S. P. (2009) An estimate of the burden of Chagas disease in the United States. Clin. Infect. Dis. 49, e52–54 [DOI] [PubMed] [Google Scholar]
- 4. Inglessis I., Carrasco H. A., Añez N., Fuenmayor C., Parada H., Pacheco J. A., Carrasco H. R. (1998) Clinical, parasitological and histopathologic follow-up studies of acute Chagas patients treated with benznidazole. Arch. Inst. Cardiol. Mex. 68, 405–410 [PubMed] [Google Scholar]
- 5. Bastos C. J., Aras R., Mota G., Reis F., Dias J. P., de Jesus R. S., Freire M. S., de Araújo E. G., Prazeres J., Grassi M. F. (2010) Clinical outcomes of thirteen patients with acute Chagas disease acquired through oral transmission from two urban outbreaks in northeastern Brazil. PLoS Negl. Trop. Dis. 4, e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Urbina J. A. (2010) Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 115, 55–68 [DOI] [PubMed] [Google Scholar]
- 7. Maya J. D., Orellana M., Ferreira J., Kemmerling U., López-Muñoz R., Morello A. (2010) Chagas disease: Present status of pathogenic mechanisms and chemotherapy. Biol. Res. 43, 323–331 [PubMed] [Google Scholar]
- 8. Marin-Neto J. A., Cunha-Neto E., Maciel B. C., Simões M. V. (2007) Pathogenesis of chronic Chagas heart disease. Circulation 115, 1109–1123 [DOI] [PubMed] [Google Scholar]
- 9. Saravia S. G., Haberland A., Bartel S., Araujo R., Valda G., Reynaga D. D., Ramirez I. D., Borges A. C., Wallukat G., Schimke I. (2011) Cardiac troponin T measured with a highly sensitive assay for diagnosis and monitoring of heart injury in chronic Chagas disease. Arch. Pathol. Lab. Med. 135, 243–248 [DOI] [PubMed] [Google Scholar]
- 10. Rassi A., Jr., Rassi A., Little W. C., Xavier S. S., Rassi S. G., Rassi A. G., Rassi G. G., Hasslocher-Moreno A., Sousa A. S., Scanavacca M. I. (2006) Development and validation of a risk score for predicting death in Chagas' heart disease. N. Engl. J. Med. 355, 799–808 [DOI] [PubMed] [Google Scholar]
- 11. Rassi A., Jr., Rassi A., Marin-Neto J. A. (2010) Chagas disease. Lancet 375, 1388–1402 [DOI] [PubMed] [Google Scholar]
- 12. Sabatine M. S., Morrow D. A., de Lemos J. A., Gibson C. M., Murphy S. A., Rifai N., McCabe C., Antman E. M., Cannon C. P., Braunwald E. (2002) Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation 105, 1760–1763 [DOI] [PubMed] [Google Scholar]
- 13. Stanley B. A., Gundry R. L., Cotter R. J., Van Eyk J. E. (2004) Heart disease, clinical proteomics and mass spectrometry. Dis. Markers 20, 167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zacks M. A., Wen J. J., Vyatkina G., Bhatia V., Garg N. (2005) An overview of chagasic cardiomyopathy: pathogenic importance of oxidative stress. An. Acad. Bras. Cienc. 77, 695–715 [DOI] [PubMed] [Google Scholar]
- 15. Gupta S., Wen J. J., Garg N. J. (2009) Oxidative Stress in Chagas Disease. Interdiscip. Perspect. Infect. Dis. 2009, 190354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schijman A. G., Vigliano C. A., Viotti R. J., Burgos J. M., Brandariz S., Lococo B. E., Leze M. I., Armenti H. A., Levin M. J. (2004) Trypanosoma cruzi DNA in cardiac lesions of Argentinean patients with end-stage chronic chagas heart disease. Am. J. Trop. Med. Hyg. 70, 210–220 [PubMed] [Google Scholar]
- 17. da Silveira A. B., Arantes R. M., Vago A. R., Lemos E. M., Adad S. J., Correa-Oliveira R., D'Avila Reis D. (2005) Comparative study of the presence of Trypanosoma cruzi kDNA, inflammation and denervation in chagasic patients with and without megaesophagus. Parasitology 131, 627–634 [DOI] [PubMed] [Google Scholar]
- 18. Wen J. J., Bhatia V., Popov V. L., Garg N. J. (2006) Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas disease. Am. J. Pathol. 169, 1953–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen J. J., Vyatkina G., Garg N. (2004) Oxidative damage during chagasic cardiomyopathy development: Role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic. Biol. Med. 37, 1821–1833 [DOI] [PubMed] [Google Scholar]
- 20. Wen J. J., Dhiman M., Whorton E. B., Garg N. J. (2008) Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect. 10, 1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wen J. J., Garg N. J. (2008) Mitochondrial generation of reactive oxygen species is enhanced at the Q(o) site of the complex III in the myocardium of Trypanosoma cruzi-infected mice: beneficial effects of an antioxidant. J. Bioenerg. Biomembr. 40, 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen J. J., Garg N. J. (2010) Mitochondrial complex III defects contribute to inefficient respiration and ATP synthesis in the myocardium of Trypanosoma cruzi-infected mice. Antioxid. Redox. Signal. 12, 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wen J. J., Gupta S., Guan Z., Dhiman M., Condon D., Lui C., Garg N. J. (2010) Phenyl-alpha-tert-butyl-nitrone and benzonidazole treatment controlled the mitochondrial oxidative stress and evolution of cardiomyopathy in chronic chagasic rats. J. Am. Coll. Cardiol. 55, 2499–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhiman M., Nakayasu E. S., Madaiah Y. H., Reynolds B. K., Wen J. J., Almeida I. C., Garg N. J. (2008) Enhanced nitrosative stress during Trypanosoma cruzi infection causes nitrotyrosine modification of host proteins: implications in Chagas' disease. Am. J. Pathol. 173, 728–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wen J. J., Yachelini P. C., Sembaj A., Manzur R. E., Garg N. J. (2006) Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Rad. Biol. Med. 41, 270–276 [DOI] [PubMed] [Google Scholar]
- 26. de Oliveira T. B., Pedrosa R. C., Filho D. W. (2007) Oxidative stress in chronic cardiopathy associated with Chagas disease. Int. J. Cardiol. 116, 357–363 [DOI] [PubMed] [Google Scholar]
- 27. Floyd R. A., Hensley K., Forster M. J., Kelleher-Anderson J. A., Wood P. L. (2002) Nitrones as neuroprotectants and antiaging drugs. Ann. N.Y. Acad. Sci. 959, 321–329 [DOI] [PubMed] [Google Scholar]
- 28. Berger F., De Meulder B., Gaigneaux A., Depiereux S., Bareke E., Pierre M., De Hertogh B., Delorenzi M., Depiereux E. (2010) Functional analysis: evaluation of response intensities–tailoring ANOVA for lists of expression subsets. BMC Bioinformatics 11, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levine R. L., Garland D., Oliver C. N., Amici A., Climent I., Lenz A. G., Ahn B. W., Shaltiel S., Stadtman E. R. (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 186, 464–478 [DOI] [PubMed] [Google Scholar]
- 30. Wen J. J., Garg N. (2004) Oxidative modifications of mitochondrial respiratory complexes in response to the stress of Trypanosoma cruzi infection. Free Radic. Biol. Med. 37, 2072–2081 [DOI] [PubMed] [Google Scholar]
- 31. Yu H. L., Chertkow H. M., Bergman H., Schipper H. M. (2003) Aberrant profiles of native and oxidized glycoproteins in Alzheimer plasma. Proteomics 3, 2240–2248 [DOI] [PubMed] [Google Scholar]
- 32. Thomas S., Bonchev D. (2010) A survey of current software for network analysis in molecular biology. Hum. Genomics 4, 353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dhiman M., Estrada-Franco J. G., Pando J. M., Ramirez-Aguilar F. J., Spratt H., Vasquez-Corzo S., Perez-Molina G., Gallegos-Sandoval R., Moreno R., Garg N. J. (2009) Increased myeloperoxidase activity and protein nitration are indicators of inflammation in chagasic patients. Clin. Vaccine Immunol. 16, 660–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanowitz H. B., Machado F. S., Jelicks L. A., Shirani J., de Carvalho A. C., Spray D. C., Factor S. M., Kirchhoff L. V., Weiss L. M. (2009) Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease). Prog. Cardiovasc. Dis. 51, 524–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Junqueira C., Caetano B., Bartholomeu D. C., Melo M. B., Ropert C., Rodrigues M. M., Gazzinelli R. T. (2010) The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert Rev. Mol. Med. 12, e29. [DOI] [PubMed] [Google Scholar]
- 36. Gupta S., Bhatia V., Wen J. J., Wu Y., Huang M. H., Garg N. J. (2009) Trypanosoma cruzi infection disturbs mitochondrial membrane potential and ROS production rate in cardiomyocytes. Free Radic. Biol. Med. 47, 1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ba X., Gupta S., Davidson M., Garg N. J. (2010) Trypanosoma cruzi induces ROS-PARP-1-RelA pathway for up regulation of cytokine expression in cardiomyocytes. J. Biol. Chem. 285, 11596–11606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ribeiro C. M., Budni P., Pedrosa R. C., Farias M. S., Parisotto E. B., Dalmarco E. M., Fröde T. S., Oliveira-Silva D., Colepicolo P., Filho D. W. (2010) Antioxidant therapy attenuates oxidative insult caused by benzonidazole in chronic Chagas' heart disease. Int. J. Cardiol. 145, 27–33 [DOI] [PubMed] [Google Scholar]
- 39. Souza A. P., Jelicks L. A., Tanowitz H. B., Olivieri B. P., Medeiros M. M., Oliveira G. M., Pires A. R., Santos A. M., Araújo-Jorge T. C. (2010) The benefits of using selenium in the treatment of Chagas disease: prevention of right ventricle chamber dilatation and reversion of Trypanosoma cruzi-induced acute and chronic cardiomyopathy in mice. Mem. Inst. Oswaldo Cruz. 105, 746–751 [DOI] [PubMed] [Google Scholar]
- 40. Dhiman M., Garg N. J. (2010) Role of NADPH oxidase in progression of myocarditis during Trypanosoma cruzi infection and Chagas disease. Circulation submitted [Google Scholar]
- 41. Guiñazu N., Carrera-Silva E. A., Becerra M. C., Pellegrini A., Albesa I., Gea S. (2010) Induction of NADPH oxidase activity and reactive oxygen species production by a single Trypanosoma cruzi antigen. Int. J. Parasitol. 40, 1531–1538 [DOI] [PubMed] [Google Scholar]
- 42. Yan X., Habedanck R., Nigg E. A. (2006) A complex of two centrosomal proteins, CAP350 and FOP, cooperates with EB1 in microtubule anchoring. Mol. Biol. Cell 17, 634–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel H., Truant R., Rachubinski R. A., Capone J. P. (2005) Activity and subcellular compartmentalization of peroxisome proliferator-activated receptor alpha are altered by the centrosome-associated protein CAP350. J. Cell Sci. 118, 175–186 [DOI] [PubMed] [Google Scholar]
- 44. Qi C., Zhu Y., Reddy J. K. (2000) Peroxisome proliferator-activated receptors, coactivators, and downstream targets. Cell Biochem. Biophys. 32, 187–204 [DOI] [PubMed] [Google Scholar]
- 45. Szatmari I., Töröcsik D., Agostini M., Nagy T., Gurnell M., Barta E., Chatterjee K., Nagy L. (2007) PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood 110, 3271–3280 [DOI] [PubMed] [Google Scholar]
- 46. Carter J. C., Church F. C. (2009) Obesity and Breast Cancer: The roles of peroxisome proliferator-activated receptor-gamma and plasminogen activator inhibitor-1. PPAR Res. 2009, 345320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rakhshandehroo M., Knoch B., Muller M., Kersten S. (2010) Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010, 612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Diez D., Wheelock A. M., Goto S., Haeggström J. Z., Paulsson-Berne G., Hansson G. K., Hedin U., Gabrielsen A., Wheelock C. E. (2010) The use of network analyses for elucidating mechanisms in cardiovascular disease. Mol. Biosyst. 6, 289–304 [DOI] [PubMed] [Google Scholar]
- 49. Silacci P., Mazzolai L., Gauci C., Stergiopulos N., Yin H. L., Hayoz D. (2004) Gelsolin superfamily proteins: key regulators of cellular functions. Cell. Mol. Life Sci. 61, 2614–2623 [DOI] [PubMed] [Google Scholar]
- 50. Koya R. C., Fujita H., Shimizu S., Ohtsu M., Takimoto M., Tsujimoto Y., Kuzumaki N. (2000) Gelsolin inhibits apoptosis by blocking mitochondrial membrane potential loss and cytochrome c release. J. Biol. Chem. 275, 15343–15349 [DOI] [PubMed] [Google Scholar]
- 51. Oikonomou N., Thanasopoulou A., Tzouvelekis A., Harokopos V., Paparountas T., Nikitopoulou I., Witke W., Karameris A., Kotanidou A., Bouros D., Aidinis V. (2009) Gelsolin expression is necessary for the development of modelled pulmonary inflammation and fibrosis. Thorax 64, 467–475 [DOI] [PubMed] [Google Scholar]
- 52. Yang J., Moravec C. S., Sussman M. A., DiPaola N. R., Fu D., Hawthorn L., Mitchell C. A., Young J. B., Francis G. S., McCarthy P. M., Bond M. (2000) Decreased SLIM1 expression and increased gelsolin expression in failing human hearts measured by high-density oligonucleotide arrays. Circulation 102, 3046–3052 [DOI] [PubMed] [Google Scholar]
- 53. Li G. H., Shi Y., Chen Y., Sun M., Sader S., Maekawa Y., Arab S., Dawood F., Chen M., De Couto G., Liu Y., Fukuoka M., Yang S., Da Shi M., Kirshenbaum L. A., McCulloch C. A., Liu P. (2009) Gelsolin regulates cardiac remodeling after myocardial infarction through DNase I-mediated apoptosis. Circ. Res. 104, 896–904 [DOI] [PubMed] [Google Scholar]
- 54. Richard P., Charron P., Carrier L., Ledeuil C., Cheav T., Pichereau C., Benaiche A., Isnard R., Dubourg O., Burban M., Gueffet J. P., Millaire A., Desnos M., Schwartz K., Hainque B., Komajda M. (2003) Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107, 2227–2232 [DOI] [PubMed] [Google Scholar]
- 55. Poetter K., Jiang H., Hassanzadeh S., Master S. R., Chang A., Dalakas M. C., Rayment I., Sellers J. R., Fananapazir L., Epstein N. D. (1996) Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat. Genet. 13, 63–69 [DOI] [PubMed] [Google Scholar]
- 56. Flavigny J., Richard P., Isnard R., Carrier L., Charron P., Bonne G., Forissier J. F., Desnos M., Dubourg O., Komajda M., Schwartz K., Hainque B. (1998) Identification of two novel mutations in the ventricular regulatory myosin light chain gene (MYL2) associated with familial and classical forms of hypertrophic cardiomyopathy. J. Mol. Med. 76, 208–214 [DOI] [PubMed] [Google Scholar]
- 57. Kabaeva Z. T., Perrot A., Wolter B., Dietz R., Cardim N., Correia J. M., Schulte H. D., Aldashev A. A., Mirrakhimov M. M., Osterziel K. J. (2002) Systematic analysis of the regulatory and essential myosin light chain genes: genetic variants and mutations in hypertrophic cardiomyopathy. Eur. J. Hum. Genet. 10, 741–748 [DOI] [PubMed] [Google Scholar]
- 58. Cunha-Neto E., Dzau V. J., Allen P. D., Stamatiou D., Benvenutti L., Higuchi M. L., Koyama N. S., Silva J. S., Kalil J., Liew C. C. (2005) Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas' disease cardiomyopathy. Am. J. Pathol. 167, 305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goldenberg R. C., Iacobas D. A., Iacobas S., Rocha L. L., da Silva de Azevedo Fortes F., Vairo L., Nagajyothi F., Campos de Carvalho A. C., Tanowitz H. B., Spray D. C. (2009) Transcriptomic alterations in Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect. 11, 1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leon J. S., Engman D. M. (2001) Autoimmunity in Chagas heart disease. Int. J. Parasitol. 31, 555–561 [DOI] [PubMed] [Google Scholar]
- 61. Leon J. S., Daniels M. D., Toriello K. M., Wang K., Engman D. M. (2004) A cardiac myosin-specific autoimmune response is induced by immunization with Trypanosoma cruzi proteins. Infect. Immun. 72, 3410–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Katsumoto T., Mitsushima A., Kurimura T. (1990) The role of the vimentin intermediate filaments in rat 3Y1 cells elucidated by immunoelectron microscopy and computer-graphic reconstruction. Biol. Cell 68, 139–146 [DOI] [PubMed] [Google Scholar]
- 63. Faé K. C., Diefenbach da Silva D., Bilate A. M., Tanaka A. C., Pomerantzeff P. M., Kiss M. H., Silva C. A., Cunha-Neto E., Kalil J., Guilherme L. (2008) PDIA3, HSPA5 and vimentin, proteins identified by 2-DE in the valvular tissue, are the target antigens of peripheral and heart infiltrating T cells from chronic rheumatic heart disease patients. J. Autoimmun. 31, 136–141 [DOI] [PubMed] [Google Scholar]
- 64. Teixeira P. C., Iwai L. K., Kuramoto A. C., Honorato R., Fiorelli A., Stolf N., Kalil J., Cunha-Neto E. (2006) Proteomic inventory of myocardial proteins from patients with chronic Chagas' cardiomyopathy. Braz. J. Med. Biol. Res. 39, 1549–1562 [DOI] [PubMed] [Google Scholar]