Abstract
Human activities have greatly reduced the amount of the earth's area available to wild species. As the area they have left declines, so will their rates of speciation. This loss of speciation will occur for two reasons: species with larger geographical ranges speciate faster; and loss of area drives up extinction rates, thus reducing the number of species available for speciation. Theory predicts steady states in species diversity, and fossils suggest that these have typified life for most of the past 500 million years. Modern and fossil evidence indicates that, at the scale of the whole earth and its major biogeographical provinces, those steady states respond linearly, or nearly so, to available area. Hence, a loss of x% of area will produce a loss of about x% of species. Local samples of habitats merely echo the diversity available in the whole province of which they are a part. So, conservation tactics that rely on remnant patches to preserve diversity cannot succeed for long. Instead, diversity will decay to a depauperate steady state in two phases. The first will involve deterministic extinctions, reflecting the loss of all areas in which a species can ordinarily sustain its demographics. The second will be stochastic, reflecting accidents brought on by global warming, new diseases, and commingling the species of the separate bio-provinces. A new kind of conservation effort, reconciliation ecology, can avoid this decay. Reconciliation ecology discovers how to modify and diversify anthropogenic habitats so that they harbor a wide variety of species. It develops management techniques that allow humans to share their geographical range with wild species.
Einstein pointed out that the essence of science consists in connecting state variables to their derivatives. This is true of the state variable called species diversity and the derivative called speciation rate. We must study them together. And no examination can succeed if it does not pay at least passing attention to the other derivative involved—the extinction rate. What determines the number of species alive in a biological province, such as the Neotropics? Answer: The cumulative difference between the creative process of speciation and the destructive process of extinction.
In this paper, I will restrict my attention to the species level of biodiversity (although I do not thus mean to imply that no other level is worthy or interesting). Furthermore, I will use an old-fashioned definition for species—i.e., a collection of organisms that can exchange genes. I know that this definition does not deal adequately with bacteria or interspecies hybrids or jumping genes. But it will lead us to principles that will illuminate the status of an important portion of diversity. And, perhaps, successfully using it may guide us into the world of the multimyriads of species whose diversities we all too often ignore.
Further restricting myself, I will focus on diversity at the grand scale, that of the entire biogeographical province. A biological province is a self-contained region whose species originate entirely by speciation within the region (1). Scales smaller than a province get their species through some form of dispersal, echoing provincial diversity (2). Most probably, every real province has obtained a small proportion of its species by immigration, so the definition constitutes a mathematical ideal. But percentages in excess of 90% are often found, and that should be enough to allow speciation dynamics to dominate the rate processes of a region.
Speciation results from a number of processes, in all of which species are nurseries for other species. Ultimately, that is why the future of speciation is bound up inextricably with the future of species diversity itself. But the connection is even more interesting than that. Both rates—speciation and extinction—depend in part on the geographical range sizes of species. Larger ranges tend to increase the speciation rates and decrease the extinction rates of otherwise similar species. That gives us a parameter to examine—the area of the biogeographical province available to life. Other things being equal, the larger the provincial area, the larger the average species range and speciation rate and the smaller the extinction rate.
What leads evolutionary ecology to its conclusions about range size and the components of diversity dynamics?
Larger ranges offer larger targets for geographical isolating barriers. The formation of geographical isolates begins the sequence of events leading to allopatric speciation, probably the source of most new species. Thus, a range that is more readily subdivided by geographical barriers will spawn more isolated populations per unit time and have a higher rate of speciation.
Widespread species also have more genetic variability than narrowly distributed species (3). This may also contribute to the rate of speciation.
Larger ranges contain species with more subpopulations of their metapopulation (4). Ecologists believe that a complex metapopulation offers some protection from extinction. Although this question continues to be addressed (5), the basic idea seems straightforward and intuitive. When a metapopulation unit becomes extinct, recolonization may occur from surviving units (6). Each unit may survive or become extinct independently of the others. So, the more units, the smaller the probability that all will vanish simultaneously. (Notice that I am not concerned here with the usual question: How does increasing fragmentation of a species range effect extinction probability? Instead, I assume a constant degree of fragmentation and focus on how more fragments alter extinction rate.)
Larger ranges are more difficult to contain entirely in the area covered by any climatic change or anomaly. Any finite climatic change (such as global warming) or anomaly (such as a severe storm) will affect a finite amount of the earth's surface. If the change covers a species' entire range, and degrades all its appropriate habitat, the species will vanish. And if the anomaly destroys all of the individuals that live within its swath, the species will also vanish. Thus, the larger the area covered by a species' range, the less chance that range will suffer permanent or temporary obliteration. Again, a larger area diminishes extinction rates.
Larger ranges produce species with greater total population sizes. And, larger populations may have both lower extinction (7) and higher speciation rates. Many conservation biologists take its effect on extinction largely for granted. They see it as the outcome of the improbability of the simultaneous, stochastic demise of an entire large population. Much work modifies that theory substantially without, however, challenging the relationship (8–12). In addition, evidence from islands supports it (13). Yet, a recent investigation suggests that the literature needs substantial revision to achieve a reliable estimate of how long such extinctions take (14). It concludes that such extinctions may take much less time than we once thought.
No one denies that population size influences speciation rate. However, the direction of its effect is in doubt. At one time, many evolutionists, led by Ernst Mayr (15), believed that small isolated populations provide the crucible for evolution. They believed that getting speciation started is a matter of breaking up coadapted complexes of genes in geographical isolates. If that is correct, small populations would speed up speciation by enhancing statistical sampling accidents. But an alternative view exists. Called “centrifugal speciation” (16), it claims that large populations speed up speciation. Centrifugal speciation also begins with geographical separation of sister populations. But after separation, the larger isolates—not the smaller ones—do the changing. The small ones remain as evolutionary relicts.
According to the centrifugal model of speciation, even the small population sizes of small areas work against evolution. Some theoretical evidence from population genetics supports the idea that small populations cannot evolve quickly (17). So does some (but not all) evidence from real populations (18, 19). And today we know that large populations foster novel gene functions (17). Certainly, genetic variability correlates positively with the total population size of species (3).
We should not allow our unfinished scientific debates to distract us from noticing what we have already learned. Both centrifugal speciation and Mayrian speciation are allopatric modes and emphasize the importance of isolate formation. Isolate formation should proceed faster in larger areas. Finally, no biogeographical patterns suggest that “small” is more productive. Instead, small is a recipe for choking off the speciation process.
The fossil record supports the conclusion that extinction rate and geographical range are inversely related over long periods (20–23). It does not however test the independent significances of the various separate theoretical influences that I mentioned above. The relative importance of these factors remains unknown. Moreover, no one has yet tested the relationship of speciation rate to range size in the fossil record. Although more restricted in time scale, studies of modern areas do support both the correlation of speciation rate with area (24), and the inverse correlation of extinction rate with area (1).
Interprovincial Patterns in Steady States of Species Diversity
The influence of diversity on average species' range size produces a negative feedback system in which species diversity is self-regulating (1). The difference between the speciation and extinction rates of a province ought to approach zero. The net result is a steady-state value of diversity at which new species evolve as fast as established ones become extinct (1).
The steady state of such systems might be only theoretical. The environmental background within which it must be achieved might be too fickle and variable for life ever to attain it. Or its rates could be so slow that it has never been consummated. But paleontological evidence strongly suggests that diversities of regions are often not very far from such steady states (1, 25–27). Alroy's analysis of Cenozoic mammals in North America goes even farther (27). He shows that the dynamic elements of a steady state have been present and active during this time. As diversity fluctuated, speciation rate and extinction rate responded, keeping diversity within narrow bounds. In addition, the two rates intersected over a diversity that did not change significantly during the 65 million year (my) period. Finally, although the per-taxon extinction rate did not vary significantly with diversity, the per-taxon speciation rate did, declining with increases in S and implicating variations in speciation rate as the most responsive component of the system's dynamic stability.
Certainly, periods following a mass extinction are exceptions to the rule of steady states, and so are periods following any major increase in the value of the steady state. During both types of periods, diversity tends to rise more or less monotonically. Nevertheless, during the Phanerozoic Eon, the Earth has experienced only five mass extinctions and a similar number of major increases in steady state (e.g., invasion of the land, invasion of the muddy sea floor, radiation of angiosperms). Each such event appears to have generated no more than 10 my of response in species diversity. Most of the rest of the Phanerozoic Eon is a record of long plateaus interspersed by quick episodes of extinction and recovery (28, 29). Thus, I estimate that species diversity has spent at least 90% of the past 500 my near some steady state. (It would not surprise me to learn that the true proportion is closer to 95% or even 98%.)
The Three Scales of Species–Area Relationships
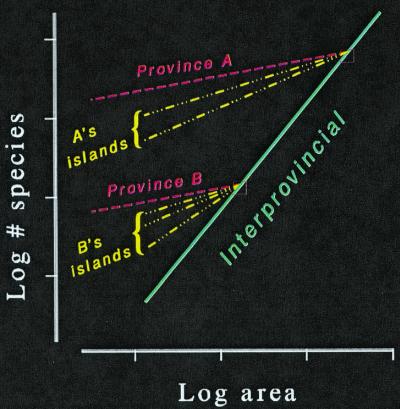
The theory of provincial diversity predicts that larger provinces will have higher steady state diversities. And they do. But in the power equation that describes species–area curves, their z-values hover near unity (30). Thus, species–area curves among provinces—quite unlike those of islands or of subsamples of a single province—exhibit a lack of curvature in an arithmetic coordinate space.
Other scales of the species–area relationship exist (31). Among islands of an archipelago, species diversity is governed by the dynamics of immigrations to islands—often a slow process, but rarely as slow as speciation. Thus, they have more species than would an equivalent-sized province. This reduces the z-values among islands of an archipelago; they vary between 0.25 and 0.55. Among sample areas within a biogeographical province, species diversity is governed both by the sample of habitats in the area and by rates of local dispersal to sink populations. Such rates are quite high compared with those of immigration. Consequently, they have more species than would an equivalent-sized island. Having more species further reduces the z-values of areas within a single province compared with those of islands; they vary between 0.1 and 0.2. In summary, we see a multiscale picture of species–area curves with regularly varying z-values (Fig. 1).
Figure 1.
The three biological scales of species–area curves. Interprovincial curves have z-values near unity; archipelagic curves have z-values that vary from about 0.25 to 0.55; intraprovincial curves have the shallowest logarithmic slopes: their z-values vary from about 0.1 to 0.2. After Rosenzweig (1): Fig. 9.11.
No mathematical theory explains or predicts the fact that diversity among provinces should be approximately a linear function of area. Yet, several lines of evidence bolster our confidence that the interprovincial z-value is close to unity. First, the linear pattern also exists deep in the fossil record, as we can see from examining some unusually good data about seed plants in the northern hemisphere.
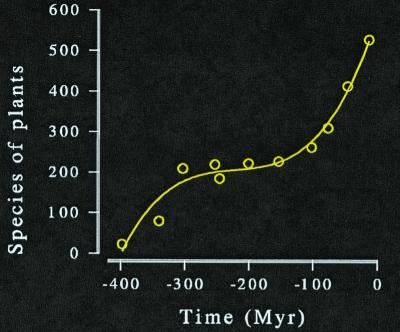
Tiffney and Niklas (32) compiled the numbers of seed plant species known from 11 periods during the past 408 my. The last of them (mid-Miocene) ended some 11.3 my ago. These periods have two characteristics. We know their fossils rather well and we also know the extent to which shallow seas flooded the land during their tenure. The 11 periods have durations from 3.1 to 40 my, and cover a total of 225.6 my. (The rest of the 408 my is too poorly known for reliable analysis.)
Displayed against time, these data indicate a substantial rise in species diversity (Fig. 2). The most thorough analyses would also note the long period of little change (ca. −330 my to −140 my) and infer the dominance of a steady state during that interval, at least. But area also has a significant effect on these data. The multiple linear regression:
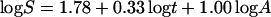 |
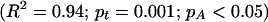 |
where t is the amount of time since −408 my.
Figure 2.
Species of known fossils of seed plants in the northern hemisphere.
The most remarkable feature of that equation is the coefficient of area. It is unity, just as it is (approximately) for regressions of modern provincial diversities.
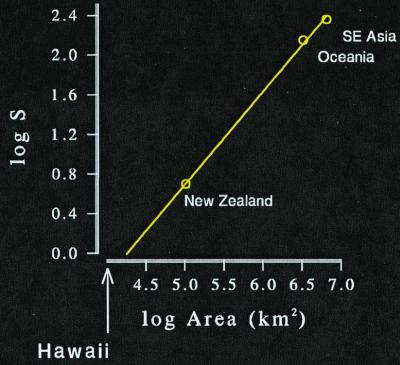
The linear response of diversity to area among provinces appears to explain the total absence of certain groups of native species on small continents, such as ponerine and cerapachyine ants on Hawaii. Two studies give us a picture of the diversity of these subfamilies in tropical southeast Asia, Oceania, and New Zealand (33, 34). These fall nearly on a straight line in arithmetic space (logS = −4 + 0.94 logA) and enable us to estimate the area of a province that would have a single species (Fig. 3). It is 18,354 km2. Hawaii, having only 10,378 km2, falls short.
Figure 3.
The interprovincial species–area relationship for cerypachine and ponerine ants extrapolated to the area of a province that would sustain a single native species. That area is 18,354 km2. Hawaii has only 10,378 km2 and has no native species of these ants.
A similar problem faces bracken-eating insects (35). None live in Hawaii, although the fern does grow there, occupying a range of roughly 1,500 km2. Based on the species–area curve of such insects in five other provinces, I estimate that one bracken-eating insect species cannot be sustained on less than 10,361 km2. Obviously, with so few data, such an estimate can give only the order of magnitude of the surface required. However, this is one order greater than what is available.
Finally, echo patterns of species diversity provide additional confidence that we understand the scale differences among species–area relationships. Echo patterns relate local diversity to regional diversity. (In ideal case, regional diversity is equivalent to the diversity of a biogeographical province, although the ideal is rarely met in published examples of the phenomenon.) Based on the scale differences of Fig. 1, theory predicts near-linear Echo patterns (2). Most often, that is what we see (36).
Diversity in Shrunken Natural Provinces
Ecologists question the amount of the earth's nonglaciated terrestrial surface that people now use. Myers et al. (37) find that some 88% of the world's richest habitats have been taken. Huston (38) gives a figure of 95% for the world's nonglaciated habitats; Vitousek et al. (39) estimate only 40 or 50%. Some of these differences arise from disparate definitions of use, and they are all honest attempts to deal with a somewhat amorphous quantity. But no ecologist would disagree with the qualitative judgment that we are taking a lot.
What does the pervasive impact of civilization do to wild species? It shrinks the area of their province that they can use for themselves. Will that reduce their diversity? Many careful ecologists are not so sure. To explain their doubt, they point to two real phenomena—biotic reserves and diversity hot spots—and the hope associated with combining them.
Biotic reserves constitute a collection of relictual habitats scattered all over the world. They vary greatly in size, but all exist to preserve a remnant of a once vast network of Edens. As an ensemble, they are hyperdispersed in biotic space. That is to say, they form a deliberately varied collection. We spend our limited resources to save the dwindling last bits of long-leaf pine forest or tall-grass prairie or Arizona riparian bottomland. We delay reserving that which is presently common. Later, I will explain that this approach has indeed given us precious time, although it cannot forever avert the evil decree.
What is the apparent significance of hot spots of diversity? They tantalize us with the promise that we can minimize the amount of reservation needed to prevent a mass extinction. For example, about 44% of all vascular plant species and 35% of all species of mammals, birds, reptiles, and amphibians live confined to only about 1.4% of its land in 25 hot spots (37). Clearly, this 1.4% is critical. If it goes, so do all those species. But, it is very tempting to reverse that argument: Let us save the 1.4%. Perhaps, then, we will save all those species now living in it. That is the combination of reservation and hot spots that offers hope to some conservation biologists.
Unfortunately, the interprovincial pattern of diversity questions that hope. It underscores the fact that diversity is the outcome of dynamical processes, whereas hot spots constitute a static view of diversity. The differences among the scales of species–area relationships and their z-values provide a dynamic basis for predicting what will happen to species diversity and to speciation in the future.
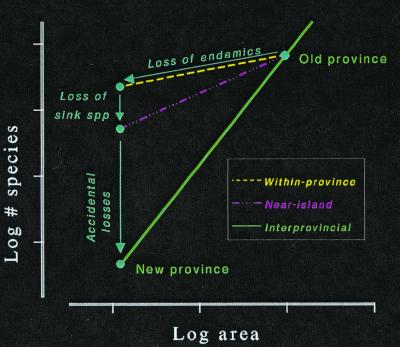
Following a reduction in province size, the three species–area relationships predict a mass extinction in three phases (Fig. 4). First, we lose the endemics—those species whose habitat gets entirely expropriated (40). These extinctions will be deterministic and virtually instantaneous. Second, we lose the sink species—those which get restricted to marginal habitats (i.e., habitats in which their death rates exceed their birth rates). These extinctions will also be deterministic (41, 42); however, they will take a while because some individuals of sink species not only survive, but may also reproduce. Third, we lose not particular species, but the diversity in excess of the new steady state. These extinctions will be stochastic, and predicting their rate—even their average rate to an order of magnitude—may not be possible as I will soon point out. Nevertheless, it is possible to say that the diversity lost because of these extinctions will be deterministically and irreplaceably lost—after all, the predicted loss constitutes however many species it takes to restore a steady-state diversity to the earth.
Figure 4.
The three phases of mass extinction that follow a severe reduction in area. Endemic extinctions occur because species lose all their habitat. Sink species disappear because all their source populations lose their habitats. Accidental extinctions remove species that suffer a run of bad luck despite being able to sustain their populations during an average generation.
Stages I and II: The Deterministic Extinctions
The coefficients of species–area equations at smaller scales permit us to estimate the proportions of species that are endemics or sink species. The prediction that we lose the sum of these is the well known and often cited equation of island biogeography:
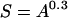 |
where S and A are proportions of diversity and natural area that remain. I plot this relationship in Fig. 5.
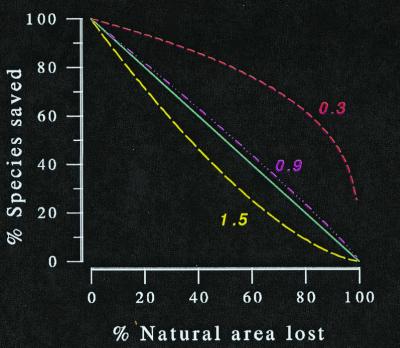
Figure 5.
Declining area reduces the proportion of species maintained at steady state. The amount of the reduction depends on the z-value. This figure shows the result for z-values of 1.5 (– – –), 1 (—), 0.9 (- ⋅ ⋅ ⋅ -), and 0.3 (- - -). Assuming the latter, which is a typical archipelagic z, we would predict the least severe losses. But data show that interprovincial z-values are close to unity.
Some impatient types have complained that these predicted extinctions have not happened. In some cases, no doubt, their analyses have been flawed (43). But another factor has been at work. Conservation's two strategies have stayed the imposition of the island equation, and may even have reduced its severity. Focusing on preserving the habitats most likely to vanish entirely, we considerably reduced the likelihood that any species would lose everything, including its sink habitats. That transferred some extinction of endemics to the category of extinction of sink species. And, although the island equation assumes so, we have not lost natural area randomly. Instead, we diversified our biotic reserves, making them a stratified sample of our habitats and the species they harbor. Although we still have no analytical theory to predict the power coefficient of the island equation, a stratified sample has to have preserved more habitats and their species than a random sample would have. That is the good news.
Other news is more upsetting. Extinction, like speciation, is a dynamical process. Extinction, like speciation, takes time (44). Relaxation to island equilibrium is likely to take a number of human generations (see ref. 37 and references 34–43 therein).
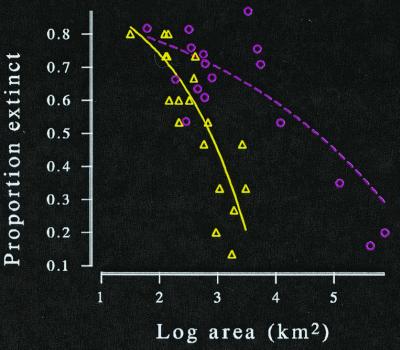
We know that the past 10,000 years have not sufficed to allow mammals on newly formed islands to attain new steady states (e.g., Fig. 6). Lizard species diversity in the wheatbelt reserves of Western Australia has declined to a z-value of 0.26, but has yet to reach the value of 0.36 found among the state's true islands (Fig. 7). And on new islands in the Sea of Cortez, lizard species diversity continued to decline for at least 12,000 years (45). We must not be too impatient.
Figure 6.
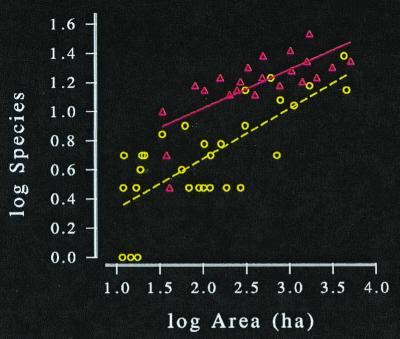
Mammal diversity on Sunda Islands (circles) and southwestern U.S. mountaintops (triangles). These isolates formed about 10,000 years ago at the end of the Pleistocene. In both archipelagos, larger islands have experienced proportionately less extinction. After Rosenzweig's (1) figure 6.5.
Figure 7.
Lizard species diversity in the 22 isolated reserves of Western Australia's wheatbelt (triangles) and its marine islands (circles). The reserve species area curve shows some decline from what would be expected in pre-European times as its z-value is 0.26. But it has not yet reached the value of 0.36 found among the true islands.
Stage III: The Stochastic Extinctions
A true island reaches its steady state as a result of the balance between immigration and extinction. But our shrunken natural world will see no immigrations. Species that become extinct cannot immigrate from the past to recolonize the world of the future. So, even after our new world reaches its levels of island-like diversity, its diversity will continue to diminish.
Relaxation below the island-like steady state will come from inflated extinction rates. But the species of our shrunken island world will all begin with at least one source population. They will all have enough habitat to keep them going in perpetuity—provided that nothing ever changes in those habitats. But something always changes. So, species will vanish simply because they lose one roll of the dice. Global warming may push their remaining habitats out of all reserves and into cornfields or the sea (46). New parasites and diseases will appear to take their toll. A series of bad weather events may be too much for some dwindled populations.
The Depauperate Steady State
Accidents that eradicate successful species have always accompanied life. However, in ordinary times, life has replaced such losses by speciation. Not this time. This time the loss of area will have also depressed the speciation rate curve. Constricted geographic ranges will have fewer isolates. Many species will be restricted to a single reserve with no chance of further allopatric speciation. The loss of ecological theater will change the evolutionary play. New speciations will not be able to keep up with the losses.
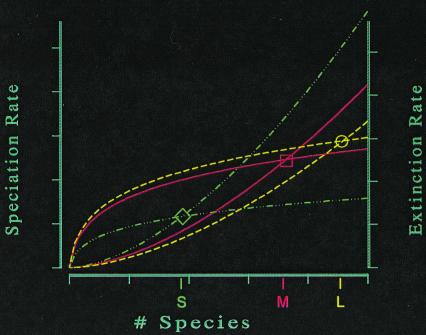
Yes, eventually, a balance will be restored. But before that happens, the total extinction rate must decline to the level of the total speciation rate (Fig. 8). Thus, at the steady state of the future, when life is replacing its losses by speciation, it will not build back the diversity it now enjoys. The new balance will occur with life decimated of its richness.
Figure 8.
Diversity dynamics of three provinces of different area: S (small, - ⋅ ⋅ ⋅ -); M (medium, —); and L (large, - - -). Extinction rates have positive second derivatives; speciation rates have negative second derivatives. The steady state of each province is indicated at the point where speciation and extinction balance (diamond, small; box, medium; circle, large).
Like any evolutionarily independent province, our future world must seek its steady state along the interprovincial species–area curve. The z-value of this curve is approximately unity, so our losses of species should be approximately linear (Fig. 5). Lose 10% of the natural world's surface and we save about 90% of its species. Lose 95% and save only 5% of the species. Diversity in provinces appears to have been following such a law for hundreds of millions of years. We have no evidence to indicate that law has been repealed.
This prediction is much more severe a decree than the usual proclamation of doom. The usual bad news predicts the loss of about half the world's species. But such rosy predictions rest on the z-value of island species–area curves, which is only about 0.3.
How long does it take to restore a steady state in species diversity? Perhaps a very long time—although, in truth, we do not know. One thing we can say. The process of re-achieving a steady state after this biotic crisis will not resemble any previous recovery from a mass extinction.
First, as we have seen, life will not recover anything like its previous levels of diversity.
Second, some taxa will vanish forever. The new world's area will fall below the threshold they require for any speciation (24), and so it will be unable to sustain even one of these species, just as Hawaii cannot sustain even one species of native insect that specializes in eating bracken, or even one ant of certain subfamilies (see above).
Third, previous mass extinctions were a violent interruption and perturbation of steady-state diversities. Afterward, background conditions returned and life recovered its steady states through the action of speciation. In this case, however, the mass extinctions represent a gradual relaxation to a new steady state dictated by the shrunken area available to nature. Thus, recovery of steady-state dynamics will occur as soon as the mass extinction is over—i.e., after complete relaxation. The trajectory of extinctions—not the trajectory of originations—will determine how long the process will take.
We can determine an upper limit to relaxation time by realizing that, as a dynamic process, its time scale should resemble its flux rates. Most of the time speciation–extinction flux is glacial. Estimates of background rates come from both the fossil record and modern phylogenetic reconstructions. Mammal species appear to turn over on a scale of 105 yr. Shelly marine invertebrates on a scale of 106 yr. But these are normal background rates.
Human pressure may greatly accelerate the relaxation process by increasing accidental extinction rates. Various human activities suggest this. We increasingly commingle evolutionarily separate provincial biotas, creating the New Pangaea and introducing native species to predatory and competitive threats from exotics (47). We rapidly transport novel diseases and parasites around the world. We simplify biotic temporal regimes (for example by limiting disturbances such as fire). And we are warming the globe. The National Research Council (44) implicates exotic species or lack of adequate disturbance as the root cause in endangering a significant proportion of threatened U.S. species. But global warming may constitute the worst threat of all: by altering the basic abiotic conditions of reserves, it can destroy their ability to do much of their job. When the earth was covered with contiguous tracts of natural habitat, species could track such changes, moving to keep up with the shifts in location of their favored habitats and so avoiding extinction (48–50). But today, with natural habitats restricted to patches of reserves, this is not possible. Meanwhile, we show little sign of abandoning the destruction of habitat that brings deterministic extinction to species.
So, how long could it take to achieve a world so poor in species that its speciation rates once again counterbalance its accidental extinctions? Perhaps a million years, perhaps a hundred.
Reconciliation Ecology
Not all species are losing ground to us. Some live with us and prosper. In German, they are known as kulturfolger—culture followers. One might dream that these kulturfolger would speciate rapidly and take up the slack. But the population isolates of kulturfolger cannot make much evolutionary headway. To do so would be to adapt to local circumstances, and that adaptation requires time. Thus, it requires that the isolates face some stability in the environmental challenges we cast at them. But Technological Man does not allow stable intervals; we constantly change our habitats and exert new pressures on the kulturfolger. They may not disappear, but they are also unlikely to radiate into a wide variety of new species.
Can we find a way to change all this? In the felicitous metaphor of Norman Myers, can we find a way to save Darwin's Genie and the treasures it has given us? We can. But not if we accept today's dominant strategy of conservation biology. For historical reasons, conservation biology has become mired in an attitude of confrontation: The green forces of nature versus the green forces of money. Conservation divides the world into pristine habitats and ruined habitats. It tries to save and restore the former while preventing further loss.
Let us ignore the fact that all of us must live in one world, accepting both its economic environment and its ecology. Let us also ignore the fact that if we view nature as an embattled victim, we are ultimately consigning her to defeat at the hands of the superior forces of human population pressure, human guile, and human greed. Let us instead concentrate on the science.
Science insists that area is an intrinsic property of natural ecosystems. To maintain their diversity, they must have their area. Thus, conservation biology has to address itself to the habitats in which human beings live, work, and play. Conservation biology has to learn how to share anthropogenic habitats with wild species. It needs to discover how to modify and diversify those habitats so that they harbor a wide variety of species. I call this sort of conservation biology reconciliation ecology.
Reconciliation ecology seeks techniques of management that turn more species into kulturfolger, giving them back their geographical ranges without taking away ours. It takes advantage of the fact that the earth remains as large as ever, but that we have devised new habitats in which most species cannot function at all. If we meet wild species half way, many will adapt to our world. They will spread in it and reestablish their potential for speciation and their resistance to extinction.
A growing number of examples demonstrates that reconciliation ecology can work. Countryside biogeography is showing that some styles of land use are already compatible with the needs of many species (51–53). Projects devoted to particular species are bringing them back in anthropogenic, economically productive habitats (54–56). Other projects focus on developing and managing habitats (57–60). The examples I cite are but a small sample of those I have collected over the past 3 years.
One project created a single patch of salt marsh at a critical point in the migratory flyway of perhaps a third of all of the bird individuals in Europe and western Asia. In doing so, it saved at least a fraction of the 257 species that use that flyway (61). Until 30 years ago, a nearby 12-km2 natural salt marsh had done this job. But the natural salt marsh was totally destroyed by resort development. Today's patch of salt marsh little resembles its predecessor or any natural habitat. It is carefully built up, contoured, and planted on a refuse dump. And it is regularly irrigated with treated, nutrient-rich sewage water.
Another project set out to save a long-leaf pine forest and its endangered species such as the red-cockaded woodpecker (62). Through novel, carefully studied, and continuous management in a large, important Air Force base, it has multiplied—by two orders of magnitude—the area covered by a sustainable long-leaf pine forest. Meanwhile, residential, recreational, timbering, and all military uses continue. This is not restoration. Never before has there been a long-leaf pine forest like this one.
As the millennium changes, the media engorge on visions of the future. I have yet to hear one that does not involve technology. My own vision of the year 2100 is fundamentally different. We will have the technology, but we will be using it in magnificent surroundings that help to bring us peace and fulfillment, and to discharge our responsibilities to nature. I see my great-grandchildren opening their doors on a world of wonders.
Perhaps this vision is mere fantasy. Perhaps it is my way to cope with the unthinkable, because, indeed, the alternative is too disheartening to contemplate. Evidence indicates that we cannot preserve the large scale at the tiny scale (63). Area constitutes a basic inherent property of every biome, a property crucial to the dynamical functioning of its components. So it is an oxymoron to imagine a pristine biome that retains only 2 or 5 or even 10% of its original size.
In particular, area helps set the provincial diversity of a mature province. In turn, provincial diversity determines local diversity—local diversity can only echo provincial diversity. Thus, if the area available to native species remains very low or declines even farther, the loss of speciation rate will prevent even our biotic preserves from maintaining their diversities for very long.
For an evolutionary ecologist, the year 2100 lies in plain sight, a short trip into the future. At the end of this path, we could find a splendid set of human environments, clean and brimming with a richness of life that will invigorate and restore our spirits. But this will happen only if we take advantage of the ecological opportunities we still have.
Acknowledgments
Thanks to Norman Myers and Andrew Knoll for their vision. Useful suggestions came from Andrew Knoll, John Alroy, Harald Beck-King, an anonymous reviewer, and discussions at the colloquium itself.
Abbreviation
- my
million years
Footnotes
This paper was presented at the National Academy of Sciences colloquium, “The Future of Evolution,” held March 16–20, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Rosenzweig M L. Species Diversity in Space and Time. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 2.Rosenzweig M L, Ziv Y. Ecography. 1999;22:614–628. [Google Scholar]
- 3.Mitton J B. Selection in Natural Populations. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 4.Hanski I, Gilpin M E, editors. Metapopulation Dynamics: Ecology, Genetics and Evolution. New York: Academic; 1997. [Google Scholar]
- 5.Harrison S. In: Biodiversity Dynamics: Turnover of Populations, Taxa, and Communities. McKinney M L, Drake J A, editors. New York: Columbia Univ. Press; 1998. [Google Scholar]
- 6.Allen J C, Schaffer W M, Rosko D J. Nature (London) 1993;364:229–232. doi: 10.1038/364229a0. [DOI] [PubMed] [Google Scholar]
- 7.Maurer B A, Nott M P. In: Biodiversity Dynamics: Turnover of Populations, Taxa, and Communities. McKinney M L, Drake J A, editors. New York: Columbia Univ. Press; 1998. pp. 31–50. [Google Scholar]
- 8.Leigh E G. J Theor Biol. 1981;90:213–239. doi: 10.1016/0022-5193(81)90044-8. [DOI] [PubMed] [Google Scholar]
- 9.Goodman D. In: Viable Populations for Conservation. Soule M E, editor. Cambridge, U.K.: Cambridge Univ. Press; 1987. pp. 11–34. [Google Scholar]
- 10.Gilpin M. J Theor Biol. 1992;154:1–8. [Google Scholar]
- 11.Lande R. Am Nat. 1993;142:911–927. doi: 10.1086/285580. [DOI] [PubMed] [Google Scholar]
- 12.Lynch M, Conery J, Bürger R. Am Nat. 1995;146:489–518. [Google Scholar]
- 13.Pimm S L, Jones H L, Diamond J. Am Nat. 1988;132:757–785. [Google Scholar]
- 14.Belovsky G E, Mellison C, Larson C, van Zandt P A. Science. 1999;286:1175–1177. doi: 10.1126/science.286.5442.1175. [DOI] [PubMed] [Google Scholar]
- 15.Mayr E. In: Evolution as a Process. Huxley J S, Hardy A C, Ford E B, editors. London: Allen and Unwin; 1954. pp. 157–180. [Google Scholar]
- 16.Brown W L., Jr Q Rev Biol. 1957;32:247–277. [Google Scholar]
- 17.Walsh J B. Genetics. 1995;139:421–428. doi: 10.1093/genetics/139.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenbaum I F, Baker R J, Ramsey P R. Evolution. 1978;32:646–654. doi: 10.1111/j.1558-5646.1978.tb04609.x. [DOI] [PubMed] [Google Scholar]
- 19.Templeton A R, Robertson R J, Brisson J, Strasburg J. Proc Natl Acad Sci USA. 2001;98:5426–5432. doi: 10.1073/pnas.091093098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen T A. Paleobiology. 1980;6:193–207. [Google Scholar]
- 21.Jablonski D. Science. 1986;231:129–133. doi: 10.1126/science.231.4734.129. [DOI] [PubMed] [Google Scholar]
- 22.Jackson J B C. Am Nat. 1974;108:541–560. [Google Scholar]
- 23.McKinney M L. In: The Biology of Rarity. Kunin W E, Gaston K J, editors. London: Chapman & Hall; 1997. pp. 110–129. [Google Scholar]
- 24.Losos J B. Philos Trans R Soc London B. 1996;351:847–854. [Google Scholar]
- 25.Boucot A J. Evolution and Extinction Rate Controls. Amsterdam: Elsevier Scientific Publications; 1975. [Google Scholar]
- 26.Miller A I, Foote M. Paleobiology. 1996;22:304–309. doi: 10.1666/0094-8373-22.2.304. [DOI] [PubMed] [Google Scholar]
- 27.Alroy J. In: Biodiversity Dynamics: Turnover of Populations, Taxa and Communities. McKinney M L, Drake J A, editors. New York: Columbia Univ. Press; 1998. pp. 232–287. [Google Scholar]
- 28.Brett C E, Ivany L C, Schopf K M. Palaeogeogr Palaeoclimatol Palaeoecol. 1996;127:1–20. [Google Scholar]
- 29.Miller A I. Science. 1998;281:1157–1160. doi: 10.1126/science.281.5380.1157. [DOI] [PubMed] [Google Scholar]
- 30.Rosenzweig M L. In: Biodiversity Dynamics: Turnover of Populations, Taxa, and Communities. McKinney M L, Drake J A, editors. New York: Columbia Univ. Press; 1998. pp. 311–348. [Google Scholar]
- 31.Rosenzweig M L. In: Advanced Theoretical Ecology: Principles and Applications. McGlade J, editor. Vol. 80. Oxford: Blackwell Science; 1999. pp. 249–281. [Google Scholar]
- 32.Tiffney B H, Niklas K J. In: Causes of Evolution: A Paleontological Perspective. Ross R M, Allmon W P, editors. Chicago: Univ. of Chicago Press; 1990. pp. 76–102. [Google Scholar]
- 33.Wilson E O. Am Nat. 1961;95:169–193. [Google Scholar]
- 34.Wilson E O, Taylor R W. The Ants of Polynesia (Hymenoptera: Formicidae) Honolulu: Bernice P. Bishop Museum; 1967. [Google Scholar]
- 35.Lawton J H. In: Ecological Communities: Conceptual Issues and the Evidence. Strong D R, Simberloff D S, Abele L G, Thistle A B, editors. Princeton: Princeton Univ. Press; 1984. [Google Scholar]
- 36.Lawton J. Oikos. 1999;84:177–192. [Google Scholar]
- 37.Myers N, Mittermeier R A, Mittermeier C G, da Fonseca G A B, Kent J. Nature (London) 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 38.Huston M. Science. 1993;262:1676–1680. doi: 10.1126/science.262.5140.1676. [DOI] [PubMed] [Google Scholar]
- 39.Vitousek P, Mooney H, Lubchenco J, Melillo J. Science. 1997;277:494–499. [Google Scholar]
- 40.Harte J, Kinzig A. Oikos. 1997;80:417–427. [Google Scholar]
- 41.Patterson B D. Oikos. 1990;59:330–342. [Google Scholar]
- 42.Patterson B D, Atmar W. In: Isolated Vertebrate Communities in the Tropics; Proc. 4th Intern. Symp. Rheinwald G, editor. Vol. 46. Bonn. Zool. Monogr.; 2000. pp. 93–108. [Google Scholar]
- 43.Brooks T M, Pimm S L, Collar N J. Conserv Biol. 1997;11:382–394. [Google Scholar]
- 44.National Research Council. Science and the Endangered Species Act. Washington, DC: Natl. Acad. Press; 1995. [Google Scholar]
- 45.Wilcox B A. Science. 1978;199:996–998. doi: 10.1126/science.199.4332.996. [DOI] [PubMed] [Google Scholar]
- 46.Peters R L, Darling J D S. Bioscience. 1985;35:707–717. [Google Scholar]
- 47.Mooney H A, Cleland E E. Proc Natl Acad Sci USA. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brett C E. Palaios. 1998;13:241–262. [Google Scholar]
- 49.Coope G R. In: Organization of Communities Past and Present. Gee J H R, Giller P S, editors. Oxford: Blackwell Scientific; 1987. pp. 421–438. [Google Scholar]
- 50.Davis M B. In: Late-Quaternary Environments of the United States. Wright H E Jr, editor. Minneapolis: Univ. of Minnesota Press; 1983. pp. 166–181. [Google Scholar]
- 51.Daily G, Ehrlich P R, Sánchez-Azofeifa G A. Ecol Appl. 2001;11:1–13. [Google Scholar]
- 52.Vandermeer J, Perfecto I. Breakfast of Biodiversity. Oakland, CA: Food First; 1995. [Google Scholar]
- 53.Greenberg R, Bichier P, Sterling J. Biol Conserv. 1997;80:235–247. [Google Scholar]
- 54.Denton J S, Hitchings S P, Beebee T J C, Gent A. Conservation Biology. 1997;11:1329–1338. [Google Scholar]
- 55.Gaby R, McMahon M P, Mazzoti F J, Gillies W N, Wilcox J R. J Herpetol. 1985;19:189–198. [Google Scholar]
- 56.Yosef R, Grubb T C., Jr Auk. 1994;111:465–469. [Google Scholar]
- 57.Bormann F H, Balmori D, Geballe G T. Redesigning the American Lawn. New Haven, CT: Yale Univ. Press; 1993. [Google Scholar]
- 58.Esselink H, Geertsma M, Kuper J, Hustings F, van Berkel H. Proc West Found Vertebr Zool. 1995;6:287–293. [Google Scholar]
- 59.Edwards V M. Dealing in Diversity: America's Market for Nature Conservation. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 60.Western D. Proc Natl Acad Sci USA. 2001;98:5458–5465. doi: 10.1073/pnas.101093598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cherrington M. Earthwatch. 1999;May/June:39–47. [Google Scholar]
- 62.McWhite R W, Green D R, Petrick C J, Seiber S M, Hardesty J L. Natural Resources Management Plan, Eglin Air Force Base, Florida. Eglin Air Force Base, FL: U.S. Dept. of the Air Force; 1993. [Google Scholar]
- 63.Harrison S, Bruna E. Ecography. 1999;22:225–232. [Google Scholar]