Abstract
MitoNEET is a small mitochondrial protein that has been identified recently as a target for the thiazolidinedione (TZD) class of diabetes drugs. MitoNEET also binds a unique 3His/1Cys-ligated [2Fe2S2] cluster. Here we use protein film voltammetry (PFV) as a means to probe the redox properties of MitoNEET and demonstrate the direct impact of TZD drug binding upon the redox chemistry of the FeS cluster. Upon binding TZDs the midpoint potential at pH 7 is lowered by over 100 mV, shifting from ~ 0 mV to −100 mV. In contrast, a His87Cys mutant negates the ability of TZDs to affect the midpoint potential, suggesting a model of drug binding where His87 is critical to communication with the FeS center of MitoNEET.
While the thiazolidinedione (TZD)1 drugs have been useful in the treatment of type 2 diabetes, their primary mode of action has been attributed to activation of peroxisome proliferator-activated receptor γ (PPARγ) (1–3). Recent appreciation of PPARγ-independent modes of TZD action (4) spurred the discovery of MitoNEET, a mitochondrial protein, through a cross-linking study with a photoactive form of the TZD drug pioglitazone (Figure 1) (5). Intriguingly, MitoNEET bears a [2Fe-2S] cluster (6; 7), and the cardiac mitochondria of mice lacking MitoNEET display dramatic decreases in respiratory function (6). The typical association of FeS proteins with redox events in biology, the growing appreciation of the interplay between mitochondrial dysfunction and type 2 diabetes (8) as well as the observation of oxidative stress in diabetic patients (9), led us to posit that MitoNEET may possess redox chemistry that is an important component of mitochondrial function, and that TZD drug binding may stabilize normal function of MitoNEET in diabetes. Here we describe the redox chemistry of the mitoNEET [2Fe-2S] cluster for the first time, demonstrate that TZD binding directly impacts the mitoNEET reduction potential, and show that TZD binding stabilizes the oxidized state of the wild-type protein.
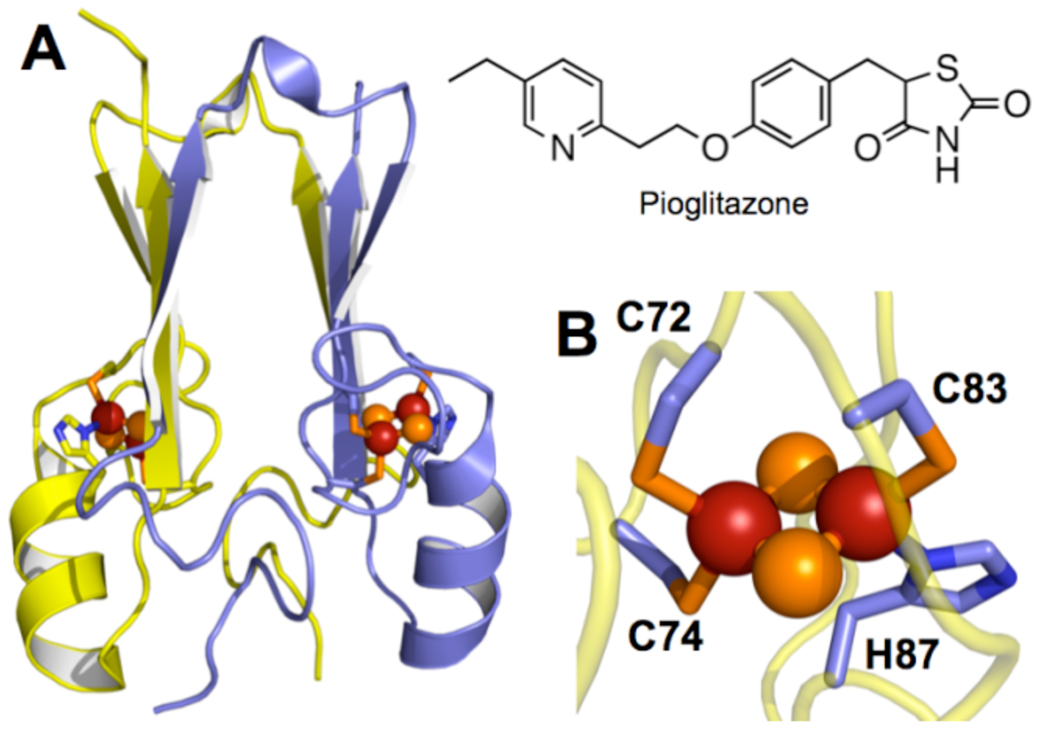
Figure 1.
(A) Overall fold of the MitoNEET soluble domain (2QH7.pdb), which interacts with TZD drugs like pioglitazone. (B) Cluster organization with inner iron ligated by Cys72 and Cys74, and the outer iron by Cys83 and His87.
MitoNEET is a small ~17kD protein localized to the cytosolic face of the outer mitochondrial membrane by a single transmembrane helix (6). X-ray crystallographic analyses of the soluble portion of the protein (Figure 1A) have demonstrated that mitoNEET forms dimers with one [2Fe-2S] cluster per monomer, and a cluster-cluster distance of ~14 Å (10–12). The cluster is uniquely ligated by three Cys residues and one His, a novel coordination environment for [2Fe-2S] clusters (Figure 1B), differing from the all-Cys ligation of ferredoxins and the two-Cys/two-His ligation seen in Rieske centers (13).
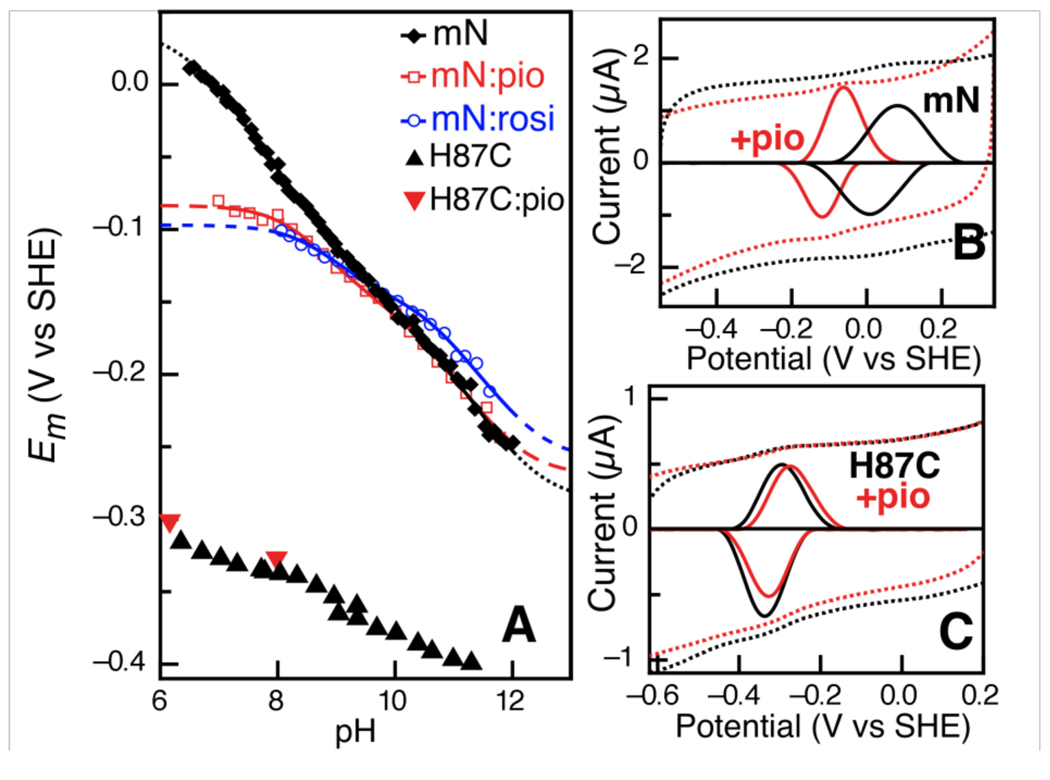
We have used protein film voltammetry (PFV) to interrogate the [2Fe-2S] cluster TZD-free and –bound states of the crystallographically characterized, soluble form of mitoNEET (Figure 1A). Baseline-subtracted oxidative and reductive voltammograms (Figure 2B) were averaged to yield midpoint potentials (Em) over a pH range from 6 to 12 (Figure 2A). A midpoint potential of approximately 0 mV vs. SHE was observed at pH 7.0 (Em,7) for the wild-type protein. The pH dependence of the MitoNEET potential is modeled well assuming a one-electron process that is coupled to two distinct protonation events, similar to Rieske FeS proteins. In the case of Rieske proteins a region of steep pH dependence (−120mV/pH) is observed due to the relative values of pKox1,2 and pKred1,2 (14). Here the relative placements of pKa values (pKox1 < pKred1 < pKox2 < pKred2) results in more shallow slope, as shown in Table 1. (An alternate 1:1 model gives reasonable agreement as well, and is given as Figure S1 for comparison; pKa values for both models are collected in Table S1). Finally, we note that the mitoNEET direct electrochemistry data are also in good agreement with solution redox titrations (Figure S2).
Figure 2.
(A) Comparison of wild-type mitoNEET (mN) pH dependence of midpoint potentials in the TZD-free (black diamonds) and pioglitazone-, rosiglitazone-bound states (red squares and blue circles, respectively). Additional H87C mutant data (black triangles) and pioglitazone treated H87C (red triangles). (B) Raw and baseline-subtracted data for mN alone (B, black dotted and solid lines, respectively), and in the presence of pioglitazone (B, red). (C) Analogous data for the H87C mutant in the absence and presence of the TZD drug.
Table 1.
Comparison of the reduction potentials and pKa values of mitoNEET (with TZDs) and three Rieske proteins characterized by Zu and co-workers (11).
| Protein | pKox1 | pKox2 | pKred1 | pKred2 | Eacid (mV) | Reference |
|---|---|---|---|---|---|---|
| mN | 6.5±0.1 | 10.1±0.1 | 9.5±0.1 | >12.5 | 40 ±4 | This work |
| mN:pio | 8.2±0.1 | 10.0±0.2 | 9.3±0.2 | >12 | −80±3 | This work |
| mN:rosi | 8.7±0.1 | 10.6±0.2 | 9.3±0.2 | >12 | −100±3 | This work |
| RsRp | 7.6±0.1 | 9.6±0.1 | 12.4±0.4 | 12.4±0.4 | 308±3 | 11 |
| TtRp | 7.85±0.15 | 9.65±0.12 | 12.5±0.5 | 12.5±0.5 | 161±4 | 11 |
| BphF | 9.8±0.2 | 11.5±0.4 | 13.3±0.8 | 13.3±0.8 | −135±5 | 11 |
Using PFV analyses we have interrogated the connection between redox chemistry of mitoNEET and TZD drug binding. Data from both the pioglitazone-(Figure 2B) and rosiglitazone-bound forms of mitoNEET yield pH dependent Em values shown in Figure 2A (red and blue). Both TZD-bound forms of mitoNEET display similar changes: in both cases the reduction potential is lowered at pH values near neutral, and the decrease in potential can be ascribed to an increase of pKox1 that occurs upon drug binding (from 6.5 to ~8.5, Table 1).The shift in pKox1 results in the decrease of Em,7 by greater than 100mV for the TZD bound forms of mitoNEET, where other perturbations to the pH dependence curve upon drug binding are minor.
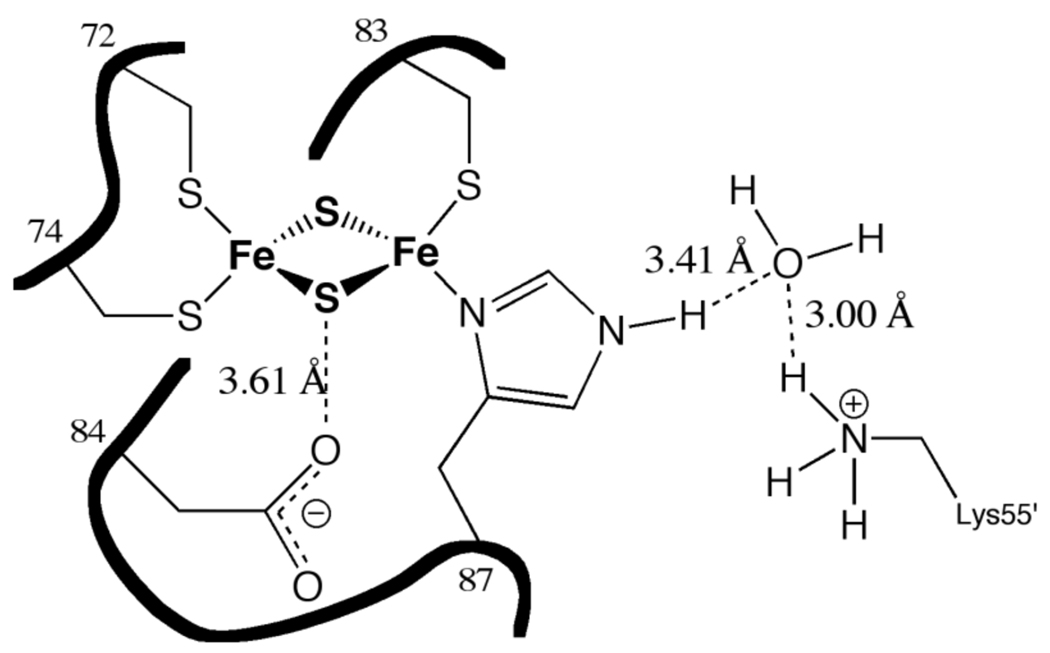
Presently available crystallographic analyses yield promising possibilities for the identities of sites of protonation and drug binding. In analogy to Rieske FeS proteins (14), it is most likely that the site of redox-linked protonation is ε-N of the cluster ligand His87. However, the mitoNEET [2Fe-2S] cluster environment does display other differences with Rieske proteins, including a lysine residue positioned within ~4Å of the His87 ε-N. A conserved bridging water molecule could theoretically create a hydrogen bond network between His87 and Lys55 (Figure 3), providing an alternative arrangement that leads to proton coupling at His87.
Figure 3.
Schematic of the [2Fe-2S] cluster of mitoNEET surrounded by ligating residues Cys72, Cys74, Cys83, and His87 and important ionizable residues Asp84 and Lys55’. Potential hydrogen bonds are represented by dashed, distances as dotted line. All distances are averages between non-hydrogen atoms from the mitoNEET pdb files 2QH7, 2R13, and 2QD0.
While articulating the details of the mechanism of proton coupling will remain a future challenge, it is clear that His87 is of critical importance. Replacement of His87 with Cys results in a dramatic decrease in the midpoint potential by over 300 mV (Figure 2C) to value of −320 mV at pH 7, suggestive of 2Fe ferredoxins (16), The overall pH dependence becomes much more nondescript with an apparent linear slope of −15 to −20 mV/pH unit (also mimicked by solution redox titrations, Figure S1). Most importantly, upon treatment of the H87C mutant with pioglitazone a nominal change in potential is observed, a positive shift of +5 mV. Taken together, the H87C mutant data suggest that a significant component of TZD drug interaction with mitoNEET occurs via His87, resulting in the modulation of the redox-linked pKox, and therefore, the midpoint potential. Notably, this interaction may be through direct hydrogen-bonding or van der Waals interactions, or might be through a more indirect, allosteric mechanism of binding to another site on MitoNEET.
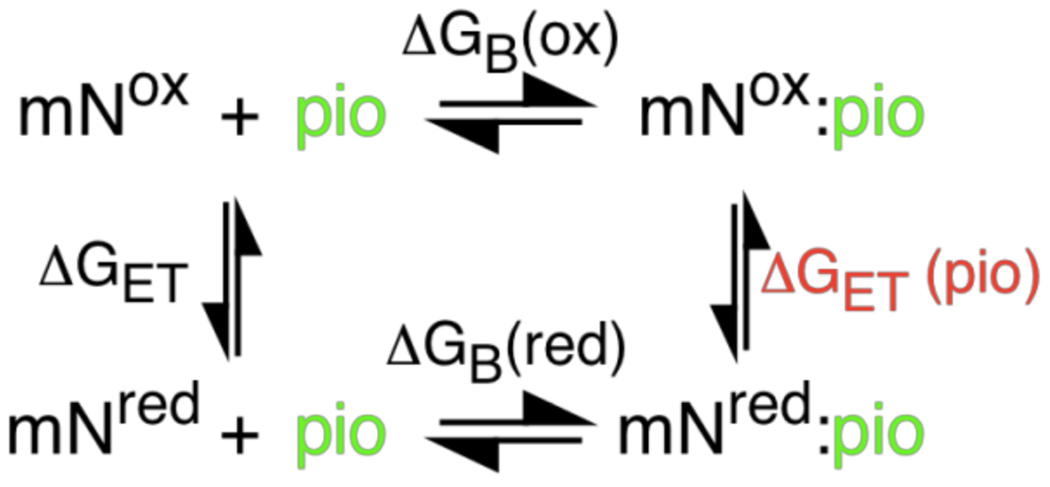
A clear challenge in this field is to understand TZD drug binding in a higher level of detail. Measuring association constants for TZD binding has not yet been attained due to the high insolubility of the drugs in aqueous buffers, and drug solubilization requires high concentrations of DMSO, which denature mitoNEET upon prolonged exposure, as required in equilibrium titrations. Here PFV has the benefit of probing relatively low concentrations of mitoNEET at an electrode (subpicomolar), such that the insolubility of the drug is not an issue. From the overall shift of the Em values, one can determine the ratio of relative affinities for the drug to the oxidized and reduced states of the protein based on the thermodynamic square shown in Figure 4. Using the Nernst equation we can find a ratio for the ΔG values for pioglitazone binding to the oxidized and the reduced states as illustrated in Figure 4, and thereby, the ratio of affinities (directly related to the free energies of binding). This estimate shows that the drug binds the oxidized state with a roughly 50 fold higher affinity than it does the reduced state.
Figure 4.
Thermodynamic Square relating free energies of binding of pioglitazone to mitoNEET in the oxidized and reduced states. ΔGET = -nFEm where n is the number of electrons, F is Faraday’s constant, and Em is the redox potential.
Collectively, our data may explain the role of the drug in alleviating oxidative stress: mitoNEET is most likely to be found in its reduced state under physiological conditions, based on the reducing environment of the cytosol (15) and the potential of ~ 0 mV at pH 7, the overall lowering of Em due to drug binding will make mitoNEET a better reducing agent, which may be of benefit under oxidative stress conditions. At the same time the enhanced TZD affinity for the oxidized form of mitoNEET reflects thermodynamic stabilization of the oxidized [2Fe-2S] cluster, when drug is bound. It has been appreciated that at pH values below 7 the [2Fe-2S] cluster of mitoNEET is labile, and that pioglitazone binding counteracts lability, as does a His87Cys mutation (9). Under oxidative stress conditions, cluster oxidation and subsequent lability may be a significant issue that TZD binding counteracts.
Here we have observed for the first time that two members of the TZD drug family similarly affect mitoNEET redox chemistry: pKox increases by ~2 units, resulting in a lowering of Em,7 by > 100mV. Thus, drug binding has a fundamental impact on the redox activity of mitoNEET. Previous spectroscopic evidence has demonstrated that mitoNEET can exist in both reduced and oxidized forms (7), and our data indicate facile electron transfer at an electrode can be achieved (k0 of 102 s−1, data not shown) and that TZD drug binding impacts the redox properties of the [2Fe-2S] cluster of mitoNEET, likely via His87. And while questions remain regarding mitoNEET function and the interplay between cluster lability and redox potential, it is clear that PFV can now serve as an exploitable tool in further assessing the impact of TZD drug binding in this emerging class of FeS proteins related to oxidative stress, mitochondrial function, and type 2 diabetes.
Supplementary Material
Footnotes
This work was supported by NIH Research Grants (SJE, GM072663 and PAJ, GM54038 and DK54441).
Abbreviations: TZD, thiazolidinedione; PFV, protein film voltammetry; mN, mitoNEET; pio, pioglitazone; rosi, rosiglitazone,; RsRp, Rhodobacter sphaeroides Rieske protein, TtRp, Thermus thermophilus Rieske protein; BphF, Burkholderia sp. Strain LB400 Rieske ferredoxin
SUPPORTING INFORMATION AVAILABLE
Materials and Methods, and Figures S1 and S2. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkinson WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high-affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR-gamma) J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 2.Semple RK, Chatterjee VKK, O'Rahilly S. PPAR gamma and human metabolic disease. J. Clin. Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tontonoz P, Spiegelman BM. Fat and beyond: The diverse biology of PPAR gamma. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 4.Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello Russo C. Receptor-independent actions of PPAR thiazolidinedione agonists: Is mitochondrial function the key? Biochem. Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Colca JR, McDonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, Lund ET, Mathews WR. Identification of a novel mitochondrial protein ("mitoNEET") cross-linked specifically by a thiazolidinedione photoprobe. Am. J. Physiol. Endocrinol. Metabol. 2004;286:E252–E260. doi: 10.1152/ajpendo.00424.2003. [DOI] [PubMed] [Google Scholar]
- 6.Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc. Natl. Acad. Sci. 2007;104:5318–5323. doi: 10.1073/pnas.0701078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiley SE, Paddock ML, Abresch EC, Gross L, van der Geer P, Nechushtai R, Murphy AN, Jennings PA, Dixon JE. The outer mitochondrial membrane protein mitoNEET contains a novel redox-active 2Fe-2S cluster. J. Biol. Chem. 2007;282:23745–23749. doi: 10.1074/jbc.C700107200. [DOI] [PubMed] [Google Scholar]
- 8.Lowell BB, Shulmanz GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 9.Mehta JL, Rasouli N, Sinha AK, Molavi B. Oxidative stress in diabetes: A mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int. J. Biochem. Cell Biol. 2006;38:794–803. doi: 10.1016/j.biocel.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Lin JZ, Zhou T, Ye KQ, Wang JF. Crystal structure of human mitoNEET reveals distinct groups of iron-sulfur proteins. Proc. Natl. Acad. Sci. 2007;104:14640–14645. doi: 10.1073/pnas.0702426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou XW, Liu RJ, Ross S, Smart EJ, Zhu HN, Gong WM. Crystallographic studies of human MitoNEET. J. Biol. Chem. 2007;282:33242–33246. doi: 10.1074/jbc.C700172200. [DOI] [PubMed] [Google Scholar]
- 12.Paddock ML, Wiley SE, Axelrod HL, Cohen AE, Roy M, Abresch EC, Capraro D, Murphy AN, Nechushtai R, Dixon JE, Jennings PA. MitoNEET is a uniquely folded 2Fe-2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc. Natl. Acad. Sci. 2007;104:14342–14347. doi: 10.1073/pnas.0707189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Link TA. In: Handbook of Metalloproteins. Messerschmidt A, Huber R, Poulos T, Wieghardt K, editors. vol. 1. New York: Wiley; 2001. pp. 518–531. [Google Scholar]
- 14.Zu YB, Couture MMJ, Kolling DRJ, Crofts AR, Eltis LD, Fee JA, Hirst J. Reduction Potentials of Rieske clusters: Importance of the coupling between oxidation state and histidine protonation state. Biochemistry. 2003;42:12400–12408. doi: 10.1021/bi0350957. [DOI] [PubMed] [Google Scholar]
- 15.Attene-Ramos MS, Kitiphongspattana K, Ishii-Schrade K, Gaskins HR. Temporal changes of multiple redox couples from proliferation to growth arrest in IEC-6 intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2005;289:C1220–C1228. doi: 10.1152/ajpcell.00164.2005. [DOI] [PubMed] [Google Scholar]
- 16.Zanetti G, Binda C, Aliverti A. In: Handbook of Metalloproteins. Messerschmidt A, Huber R, Poulos T, Wieghardt K, editors. vol. 1. New York: Wiley; 2001. pp. 532–542. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.