Abstract
Most cases of catheter-related bloodstream infections (CRBSIs) involve colonization of microorganisms on catheter surfaces where they eventually become embedded in a biofilm. Fungal biofilm formation is studied using a number of techniques, involving the use of a wide variety of substrates and growth conditions. In vitro techniques involving use of confocal scanning laser/scanning electron microscopy, metabolic activity assay, dry weight measurements and antifungal susceptibility assays are increasingly used by investigators to quantify and evaluate biofilm morphology. However, there are not many in vivo models used to validate biofilm-associated infections. In this protocol, we describe clinically relevant rabbit model of C. albicans biofilm-associated catheter infection to evaluate the morphology, topography and architecture of fungal biofilms. The methods described here can be completed in a typical laboratory setting. Evaluation of the formation of fungal biofilms on catheters in vivo, their analysis using scanning electron microscopy (SEM) and quantitative catheter culture (QCC) and treatment of biofilms using antimicrobial lock therapy can be completed using the described methods in ∼20–25 d. This model has utility in evaluating the efficacy of lock solutions. In addition, it is a useful approach for characterizing/comparing the formation of biofilms by wild-type and isogenic mutants including clinical isolates in vivo. This model can also be used for testing different biomaterials.
Key words: biofilm, antimicobial lock therapy, rabbit model, catheter, Candida albicans
Introduction
Candida species are the fourth most common cause of nosocomial bloodstream infections.1 Of these nosocomial infections, most are device-related, particularly central venous catheter (CVCs)-related, with up to 25–30% attributable mortality. Most cases of catheter-related bloodstream infections (CRBSIs) involve colonization of microorganisms on catheter surfaces where they eventually become embedded in a biofilm.2–5 Biofilms are defined as structured community of microorganisms surrounded by self-produced extracellular matrix and is adherent to an inert or living surface.3 These biofilms are resistant to most antimicrobials. Other organisms most commonly associated with CVCs-biofilms are Staphylococcus aureus, coagulase-negative staphylococci and aerobic Gram-negative bacilli, and C. albicans.6 Treatment guidelines for infections related to intravascular catheters suggest removal of the affected devices.6,7 However, removal of CVCs is not always easy or feasible (e.g., for patients with coagulopathy or limited vascular access) and is associated with healthcare expenses and complications due to catheter removal. Therefore, biofilm-associated CRBSIs represent an important problem, underscoring the need to find novel clinically relevant ways to prevent and treat such infections.
In vitro fungal biofilm formation is studied using a number of techniques which suggested the possible strategy for the salvage of CVCs infected with C. albicans biofilms. But the clinical relevance of the biofilms formed on catheters has been studied by only few scientists. Our CVC-associated C. albicans rabbit model was the first clinically relevant animal model that evaluated the effectiveness of an antifungal agent [liposomal amphotericin B (lipoAmB)] as a lock therapy8 for the treatment of biofilms formed intraluminally in the catheters. In this study, we used quantitative catheter culture (QCC) and scanning electron microscopy (SEM) to study the effect of antifungals. QCC results showed zero colony forming units (CFUs) for the catheters treated with liposomal amphotericin B compared with untreated controls and the fluconazole treated groups. SEM revealed abundant biofilm in the control and fluconazole groups, while the liposomal amphotericin B group was cleared.8 A rat model of CVC-associated C. albicans biofilm was introduced by Andes et al. who characterized in vivo C. albicans biofilm development. They used time-course quantitative culture and demonstrated a progressive increase in the burden of viable cells for the first 24 h of development. Fluorescence and scanning electron microscopy revealed a bilayered architecture.9
Quantification of in vivo catheter biofilms is commonly performed using QCC method. In our studies, we optimized and used the QCC assay to quantify biofilms formed on catheters in vivo. By using sterile technique, catheters are removed and cut into 4 cm long pieces; one half, i.e., 2 cm of these pieces are placed in 10 mL sterile saline for QCC and the other half is used for SEM analyses. The catheter pieces for QCC are sonicated in sterile saline, vortexed, serially diluted and 1 mL aliquots are plated onto Sabouraud dextrose agar supplemented with chloramphenicol and gentamicin. The plates are incubated for 48 h and the CFUs are counted. Based on prior clinical studies, catheters growing 100 CFU or more of C. albicans are considered infected.6,8
Morphology and architecture of biofilms are visualized using different microscopic techniques. Confocal microscopy analysis allows visualization of 3-dimensional architecture of biofilm and the appearance of extracellular matrix during biofilm formation. Scanning electron microscopy enables evaluation of detailed surface topography and morphology at high magnification, but involves degradation of the native hydrated structural features of biofilm due to the fixation and dehydration steps performed during sample preparation. Andes et al. used SEM and confocal scanning laser microscopy to reveal a bilayered architecture of CVC-associated in vivo biofilms.9 In our study, we used SEM to observe biofilms formed on catheters in vivo.
Susceptibility of Candida biofilms formed on catheters in vivo to locked antimicrobial agents is most commonly determined by assessing cell viability as measured by CFUs.
In this protocol, we describe our rabbit model and provide detailed step-by-step procedure for in vivo catheter placement, inoculation of catheter to form biofilm, catheter removal, quantitative catheter culture of biofilms formed, and SEM of catheter segments to evaluate biofilm surface topography. In addition, we provide a description of how this model can be used to evaluate the efficacy of antifungal lock therapy. The methods described in this protocol can be completed in a typical laboratory setting with minimum involvement of software. Evaluation of the formation of fungal biofilms on catheters in vivo, their analysis using SEM and QCC and treatment of biofilms using antimicrobial lock therapy can be completed using the described methods in ∼20–25 d.
Materials
Reagents.
General reagents.
Phosphate-Buffered Saline 1X without calcium and magnesium (PBS; Cellgro Mediatech Inc., cat. no. 21-040-CM).
MilliQ water (using Millipore filtration apparatus). If MilliQ water is not available, deionized or double distilled water can also be used.
Reagents for catheter placement, inoculation and removal in rabbits.
Female New Zealand White rabbits weighing 2.5 to 3.0 kg (Covance Inc.). Candida albicans M61 (obtained from a central venous catheter tip of a patient with catheter-associated candidiasis). In addition, this model can also be utilized for characterizing/comparing the formation of biofilms by other wild type and isogenic mutant strains and also other clinical isolates.
Sabouraud Dextrose broth (SDB; Difco Laboratories, cat. no. 238230).
Ketaset (Ketamine HCl Injection 100 mg/mL, Fort Dodge Animal Health).
Anased Injection® (Xylazine 100 mg/mL, Akorn, Inc.).
1 mL syringe with 27½ gauge needle (BD Syringe, Becton Dickinson, cat. no. 309623).
3 mL syringe (BD Syringe, Becton Dickinson, cat. no. 309585).
22 gauge 1 inch needles (Becton Dickinson, cat. no. 305155).
22 gauge 1½ inch needles (Becton Dickinson, cat. no. 305159).
25 gauge 5/8 inch needles (Becton Dickinson, cat. no. 305122).
70% ethyl alcohol (Decon Labs, Inc., cat. no. 8601).
Silastic tubing 0.04-in. internal diameter and 0.085-in. external diameter (Dow Corning).
Polyethylene cuff (PE 240, Becton Dickinson).
Heparin (Abott Laboratories, North Chicago, IL).
0.9% Sodium Chloride Injection, USP (Hospira).
Normal Saline.
Betadine Scrub (Purdue Products L.P.).
Betadine Solution (Purdue Products L.P.).
Reagents for rabbit euthanasia.
Euthasol (Virbac AH, Inc.).
Quantitative catheter culture reagents.
Sabouraud Dextrose agar (SDA; Becton Dickinson, cat. no. 211584).
Chloramphenicol (Fisher Scientific, cat. no. BP904).
Gentamicin Sulfate Salt (Sigma-Aldrich, cat. no. G3632).
Scanning electron microscopy reagents.
8% Glutaraldehyde Solution (GA; Electron Microscopy Sciences, cat. no. 16020) !CAUTION Toxic (irritant, allergen, carcinogen).
Sodium Cacodylate (Electron Microscopy Sciences, cat. no. 12300) !CAUTION Toxic.
Uranyl acetate (Electron Microscopy Sciences, cat. no. 22400) !CAUTION Highly Toxic. This reagent is radioactive and should be stored covered by lead sheathing. Lead and uranyl are toxic, avoid contact with skin and eye.
Tannic acid (Sigma-Aldrich, cat. no. 0125) !CAUTION Toxic (irritant).
Osmium Tetroxide 4% aqueous solution (OsO4; Electron Microscopy Sciences, cat. no. 20816-12-0) !CAUTION Highly Toxic.
Ethyl Alcohol 200 Proof, Absolute (Pharmco Products Inc., cat. no. 111 USP 200 CSGL).
Antifungal lock experiment and catheter treatment reagents.
Liposomal Amphotericin B obtained from Fujisawa Healthcare, Inc., and Gilead Sciences.
Fluconazole (Pfizer Pharmaceuticals Group).
Equipment.
Ultrasonicator (Bransonic 1510; Branson Ultrasonics Corp.).
Autoclave (Amsco 3021).
Spectrophotometer (Spectronic Genesys 5).
Lazy-L Spreaders (Fisher Scientific, cat no. SPR-L-S10).
Cell scrapers (Fisher Scientific, cat. no. 353086).
Scalpels (Personna Medical, cat. no. 73-0110).
Tweezers (Durmont tweezers; Electron Microscopic Sciences, cat. no. 72800-D).
Filter sterilization assembly (Fisher Scientific, cat. no. SCGPU11RE).
Glass microanalysis filter holders (Millipore, cat. no. XX10 025 00).
Durapore 0.22 µm membrane filters (Millipore, cat. no. GVWP02500).
50-mL polypropylene centrifuge tubes (Fisher Scientific, cat. no. 05-539-8).
BD Falcon Tissue Culture Plates (12-well; 6.0 mL) (BD Biosciences, cat. no. 353043).
Fisherbrand* Petri Dishes with Clear Lids (100 mm × 15 mm, Fisher Scientific, cat. no. 08-757-12).
Fisherbrand* Media-Miser* Dishes (60 mm × 15 mm small Petri dishes (Fisher Scientific, cat. no. 08-757-13A; SciEd # S67961).
Microcentrifuge tubes (Fisher Scientific, cat. no. 02-682-550).
Rocker (Bellco Glass Inc., cat. no. 7740-10000, S/N UCRB-522).
Incubator (Forma Scientific, Model # 3154, S/N 30860-652).
Scanning electron microscopy (SEM) specimen mount stubs (Electron Microscopy Sciences, cat. no. 75230).
Pelco tabs, SEM adhesive tape (Ted Pella, Inc., cat. no. 16084-1).
Glass slides (Fisher Scientific, cat. no. 12-550-34).
Glass coverslips (Corning, cat. no. 2865-22).
Parafilm (Fisher Scientific, cat. no. 13-374-10).
Water purification apparatus (MilliQ, Millipore, cat no. QGARDOOD2).
Scanning electron microscope (model XL3C ESEM Philips microscope).
Sputter coating machine.
Reagent Set Up
Chloramphenicol.
Prior to autoclaving, add 0.125 g/L media and mix well. Then prepare the media as per manufacturer's instructions.
Gentamicin.
Prior to autoclaving, add 0.03 g/L media and mixed well. Then prepare the media as per manufacturer's instructions.
Both antibiotics are added to the media prior to autoclaving. Gentamicin and chloramphenicol have been shown to be heat stable according to the product description sheet for Gentamicin from Sigma Aldrich and chloramphenicol from BD Diagnostic systems. Also, QC tests have been done to ensure the activity of the antibiotics post-autoclaving.
Heparinized saline.
One milliliter of heparin, 1,000 units/mL is drawn up and injected into a vial containing 9 mL of 0.9% Sodium Chloride Inj.
Liposomal amphotericin B.
Reconstitute liposomal amphotericin B in sterile water and dilute with 5% sterile dextrose.
Fluconazole.
Reconstitute and dilute with sterile water.
C. albicans inoculum.
From the frozen stock, sub-culture C. albicans cells in SD broth, wash cells three times with saline and place appropriate volume of Candida cell suspension in saline to obtain a cell density of 107 cells in 300 µL inoculum. This is the primary inoculum used for catheter inoculation.
Female New Zealand white rabbits.
Weighing 2.5 to 3.0 kg (Covance Inc.).
Rabbit restraint.
!CAUTION Rabbits can be unpredictable; while they are usually timid they are easily upset. Caution should be taken whenever handling rabbits. Lift the rabbit firmly by the scruff with the head pointing away from your body, use your other hand to support the rabbit's hind end. Craddle the rabbit to your body. If the rabbit struggles, place it on a solid surface and calm it by shielding the rabbit's eyes by tucking its head into the crook of your arm, this should keep the animal calm. When done, keep a firm grip on the scruff and gently release the rabbit into the correctly marked cage.
Blood culture.
Blood cultures from the catheter are sent to the Microbiology department in University Hospitals of Cleveland and processed with the BacT/Alert® 3D Microbial Detection System (BioMe'rieux, Inc., Durham, NC) to confirm the presence of yeast.
Sabouraud dextrose broth (SDB).
Dissolve 30 g of SD powder in 1 L of MilliQ water. Autoclave the resulting suspension at 121°C for 15 min. Cool to room temperature on the bench.
Sabouraud dextrose agar (SDA).
Suspend 65.0 g of the powder in 1 L of MilliQ water. Mix thoroughly. Heat with frequent agitation and boil for 1 min to completely dissolve the powder. Autoclave at 121°C for 15 min. Cool to about 50°C in a water bath and pour into sterile Petri dishes. Allow agar to solidify. Place the plates in plastic sleeves and refrigerate until use. Bring back to room temperature before use. !CAUTION Avoid overheating during autoclaving. Be careful while pouring the liquefied agar into Petri dishes, avoiding bubble formation.
2% glutaraldehyde.
Mix 10 mL of 8% glutaraldehyde with 10 mL of sterile MilliQ water. !CAUTION Glutaraldehyde is toxic. Handle it with care.
Sodium cacodylate (0.2 M).
Dissolve 21.4 g sodium cacodylate in 500 mL MilliQ water. Adjust pH to 7.4 with HCl (6 N) and autoclave the solution for 15 min. !CAUTION Sodium cacodylate is toxic. Handle it with care.
SEM wash buffer (0.1 M sodium cacodylate).
Add 100 mL of 0.2 M sodium cacodylate to 100 mL of autoclaved MilliQ water to make 0.1 M sodium cacodylate. 1% (aqueous) uranyl acetate. Dissolve 1 g uranyl acetate in 100 mL MilliQ water, filter through filter sterilizing assembly using 0.22 µm filter in dark. !CAUTION Avoid light exposure and immediately transfer to 4°C.
1% (aqueous) tannic acid. Dissolve 1 g tannic acid in 100 mL MilliQ water, filtersterilize using a filtration assembly fitted with a 0.22 µm diameter (pore size) filter. !CAUTION: Avoid light exposure, store at room temperature after preparation.
1% OsO4 solution in 0.1 M sodium cacodylate.
Mix 10 mL of 4% OsO4 with 10 mL of sterile MilliQ water, and 10 mL of 0.2 M sodium cacodylate. Final working solution is 1% OsO4 in 0.1 M sodium cacodylate.
Ethanol working solutions.
Prepare 25%, 50%, 75%, 95% and 100% ethanol in autoclaved MilliQ water by making up 25 mL, 50 mL, 75 mL, 95 mL ethanol to 100 mL, respectively and 100% is used pure with no water added to it. !CAUTION Keep away from flame. The solutions should be stored in tightly capped bottles to prevent evaporation.
Procedures
(1) Rabbit model.
Catheter placement, inoculation and removal (Timing: 20–25 d) (This protocol is adapted from Schinabeck et al.).
Catheter placement (Timing: Day 8, after acclimatizing rabbits 0–7 d). (a) Preparation of the rabbit for surgery.
Acclimatize rabbits for 7 d prior to surgery.
Anesthetize rabbits intramuscularly with ketamine 70 mg/kg and xylazine 7 mg/kg. !CAUTION There can be anesthetic complications including decreased respiration and heart rate, which may lead to death. Proper animal restraining techniques are important for proper dosing and in minimizing the stress on the animal. Before attempting injections one should be competent in rabbit handling.
Shave the right cervical, shoulder and scapular regions with electric clippers.
Wash the shaved area with betadine soap followed by isopropyl alcohol and betadine solution.
Place the rabbit on the surgery table in dorsal recumbency, with the scrubbed area covered in gauze.
Make a “pillow” of sterile gauze and place under the neck for support.
Tape forearms and jaw loosely to the table.
(b) Operative procedures.
Cover the animal with sterile drape leaving only the incision site open. Make 1–2 cm incision in the right anterolateral cervical region exposing the external jugular vein.
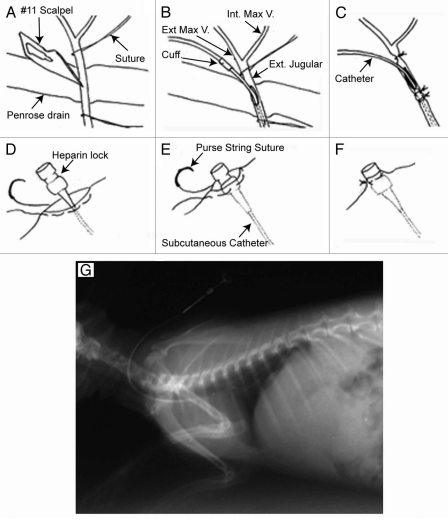
Free a segment of the external jugular vein, just distal to the bifurcation of the internal and external maxillary veins from subcutaneous fat and place a 18 × 1/4 in. Penrose drain (Medline Industries, Inc.) under the vein (Fig. 1A).
Place two segments of 3-0 vicryl suture (Ethicon Inc.) proximally and distally to the Penrose drain.
Flush the catheter with sterile saline and clamp it with a hemostat.
Using a #11 scalpel blade, make an incision in the isolated segment of vein which controls the bleeding with upward traction of the Penrose drain. !CAUTION To avoid ripping the vein into two pieces, when incising the vein, do not transect more than 1/3 of the way through. There can be some surgical complications; i.e., excessive bleeding, tearing blood vessels, nerve damage and death, care should be taken to avoid it.
Insert the catheter into the vein caudally 4 cm, up to the cuff, (Fig. 1B) placing the catheter tip in the right anterior vena cava as demonstrated in the venogram in Figure 1G. !CAUTION Caution should be taken when moving the rabbit to lateral recumbency to avoid dislodging the catheter.
Tie the proximal and distal ligatures and withdraw blood to test catheter patency (Fig. 1C). !CAUTION Needles stick and can be dangerous; never recap needles.
Flush the catheter with heparinized saline.
Passing a hemostat cephalad through a 1.0 cm incision in the intracapsular region to the external jugular vein incision site creates a subcutaneous tunnel.
Use the hemostat to pull the catheter through the subcutaneous tunnel, cut the excess catheter, and place an 18 gauge Luer Stub Adaptor (Becton Dickinson) and sterile Heparin Lock device (Medex) on the proximal end.
Test the catheter patency again by withdrawing blood and flushing with heparinized saline.
Bury the Luer hub in the subcutaneous tract and use a purse string suture to attach the heparin lock device flush to the skin (Fig. 1D–F).
Figure 1.
Surgical placement of the intravenous catheter. (A–C) Catheter insertion into the external jugular vein; (D–F) attachment of the heparin lock device to skin (G) postoperative venogram of catheter placement. Adapted from Schinabeck.8
(c) Clean up.
Dress the rabbit in an orthopedic stockinette (Courier) sleeve with arm holes cut.
Wrap the rabbit loosely in blankets, place in a heated recovery cage and monitor every 30 min.
When the rabbit is able to sit sternally, return it to its cage.
Catheter inoculation (Timing: Day 0, Table 1).
Table 1.
Number of days post-infection
| Days post-infection | |||||||||||
| Animals arrive and acclimatize for up to 1 week | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Surgery/inoculate | Biofilm develops | Seven days of treatment | Sacrifice/take samples | ||||||||
Candida albicans isolate M61, obtained from a central venous catheter tip of patient with catheter-associated candidiasis, used for inoculating catheters.
From the frozen stock, sub-culture C. albicans cells and place appropriate volume of Candida cell suspension in saline to obtain a cell density of 107 cells in 300 µL inoculum. This is the primary inoculum used for inoculating catheters.
Lock lumen of catheters with 300 µL of 1 × 107 CFU of C. albicans and allow it to dwell for 24 h.
Remove the inoculum and flush the catheters daily with 300 µL of heparinized saline (100 U).
Obtain 5 mL blood sample on day 3 from the catheter and submit for blood culture to confirm the presence of yeast.
Catheter removal (Timing: Day 10 postinfection, Table 1).
Anesthetize rabbits (as described above) after ten days post infection.
Obtain blood cultures through the catheter and peripherally via cardiac puncture.
Euthanize the animals as described below under Rabbit Euthanasia section.
Remove the catheter by using sterile technique and divide it into proximal (subcutaneous tunnel) and distal (intravenous) 4 cm segments.
Divide each segment into half (2 cm) lengthwise for QCC and examination of biofilms by SEM.
(2) Quantitative catheter.
Place 2 cm catheter segments (above) in 10 mL sterile saline.
Sonicate specimens at 40,000 Hz using ultrasonicator for 12 min at 4 min intervals and then vortex for 15 sec.
Serially dilute specimens with saline prior to plating 1 mL aliquots into SDA supplemented with chloramphenicol and gentamicin. Do this step in triplicate plates.
Incubate the plates for 48 h at 37°C.
Count the colony forming units (CFUs).
(3) Scanning electron microscopy (SEM) (SEM method is adapted from Chandra et al.5).
(a) System fixation.
Add 2 mL of 2% glutaraldehyde to wells of 12-well tissue culture plates containing 2 cm catheter segments.
Cover plates with aluminum foil.
-
Incubate at 4°C for 2 h.
PAUSE POINT These samples can be stored in 2% glutaraldehyde overnight or longer if sealed (with Parafilm) and kept at 4°C, in the dark.
Using a glass pipette, aspirate the glutaraldehyde solution.
Add 2 mL of 0.1 M sodium cacodylate buffer, and let stand for 10 min. Remove the buffer by aspiration using a pipette.
Repeat above step twice (total three washes over 30 min.).
-
Incubate washed catheter segments with 2 mL of 1% OsO4 for 1 h at 4°C.
PAUSE POINT Samples can be stored for 3–4 h.
Repeat washing steps with sodium cacodylate buffer as described above (for 3 total washes).
Add 2 mL of sterile MilliQ water, let stand for 5 min, and aspirate the liquid.
Repeat the above step.
To the wells containing the catheter segment above, add 2 mL of 1% tannic acid and incubate for 30 min in the dark at room temperature.
Add 2 mL of sterile MilliQ water, let stand for 10 min., and aspirate the liquid.
Repeat the above wash step twice.
-
Add 2 mL of 1% uranyl acetate and place the plate in the dark for 1 h.
PAUSE POINT Samples can be stored overnight or longer at 4°C if sealed (with Parafilm) and is in the dark (cover with aluminum foil).
Add 2 mL of sterile MilliQ water, let stand for 5 min, and aspirate the liquid.
Repeat the above wash step once.
(b) Sample dehydration.
Add 25% ethanol to sample, leave for 15 min.
Aspirate to remove ethanol.
Add 50% ethanol to sample, leave for 15 min.
Aspirate to remove ethanol.
Add 75% ethanol to sample, leave for 15 min.
Aspirate to remove ethanol.
Add 95% ethanol to sample, leave for 15 min.
Aspirate to remove ethanol.
Add 100% ethanol to sample, leave for 15 min.
Aspirate to remove ethanol.
Add 100% ethanol to sample, leave for 15 min.
Aspirate to remove ethanol.
-
Add 100% ethanol to sample, leave for 15 min.
PAUSE POINT Samples can be stored for up to 4 weeks in 100% ethanol at 4°C, sealed with Parafilm (to ensure the ethanol is not evaporated), and under dark (cover with aluminum foil).
(c) Mounting on SEM stubs.
Stick round black SEM adhesive tape on specimen mounting stubs.
Use sterile tweezers to remove the substrate (catheter segments) containing dehydrated cells from 12-well plates (from step above) and place on mounting stubs prepared above. !CAUTION Be careful to place the catheters on the stubs, making sure that catheters with C. albicans biofilms remains exposed to the top.
Press gently to ensure that the biofilm- containing substrate is firmly stuck to the adhesive tape.
Place the stub in stub-holder and then transfer to a vacuum desiccator. !CAUTION Use appropriate tweezers to handle the stubs since they can easily slip off and fall.
Seal the desiccator under vacuum, and store at room temperature for 24 h. CRITICAL STEP Proper vacuum desiccation is critical to ensure complete evaporation of ethanol and to prevent rehydration of the sample. Presence of even the smallest amount of water due to rehydration from the atmosphere will cause interference with subsequent steps.
(d) Sputter coating and microscopic observation.
Sputter coat the stubs with Au/Pd (60/40) for 30 sec using the sputter coating machine. !CAUTION Care should be taken not to exceed the sputter coating step for more than 30 s, since exceeding this time may impart excess charge on the samples, and result in burnt areas when observed under the microscope.
Transfer the stub to the sample holding platform in the scanning electron microscope. !CAUTION Care should be taken while placing the stubs in the holding platform, to avoid dropping them.
Examine the biofilms under the electron microscope following the unitspecific instructions. !CAUTION We observe samples under ESEM (model XL3C Philips microscope) using voltage of 15 kV and spot size of 4. These settings may need to be optimized for different microscopes.
Scan through the specimen surface and select representative areas to record.
Acquire images at different magnifications. !CAUTION When recording images at high magnifications (e.g., 5,000X), it is important to acquire them quickly since the intense electron source at these magnifications can result in overexposure (“burning”) and poor quality images.
(4) Antifungal lock and catheter treatment (Timing: Day 3–9).
(a) Antifungals.
Liposomal amphotericin B and Fluconazole are used in this protocol.
(b) Catheter treatment.
Flush catheter with 300 µL of heparinized saline (100 U) on a daily basis for the first 3 d of the study.
On day 3 post infection, draw 5 mL blood through each catheter for culture.
To determine the efficacy of antifungal lock therapy, randomize the rabbits into three groups, each consisting of seven animals (we estimate that seven animals will be needed per group to detect a reduction of 300 CFUs at the 5% α error level with 80% power).
Flush group I catheters daily with 300 µL of heparinized saline (100 U).
Lock catheter lumens of animals in group II with 300 µL solution containing (1) 3 mg of liposomal amphotericin B, (2) 100 U of heparin and (3) 5% dextrose solution (Since dextrose solution is used for reconstituting lipoAmB, we use it as a blank).
Lock catheter lumens of rabbits in group III with 300 µL solution containing (1) 3 mg of fluconazole (2) 100 U of heparin and (3) sterile normal saline. !CAUTION The concentrations of drugs used in the study was guided by dosages in prior case reports, it is necessary to check the dosage from published reports before using it for the study so that the amount of animals needed for the study does not exceed too much and also the concentration of the drug used is not harmful for the animals. The fluconazole used in this study required a 10 min incubation in a hot water bath to dissolve completely.
Lock antifungal solutions for 8 h per day for 7 d. !CAUTION In other experiments, 4 h periods are also used, depending on the drug the time for lock treatment may vary.
Upon completion of each daily treatment, remove antifungal lock solution and flush catheters with 300 µL of heparinized saline.
After 7 d of antifungal lock therapy, anesthetize animals and obtain blood through the catheter and via a cardiac puncture.
Euthanize animals as described below.
Remove catheters under sterile conditions perform QCC and SEM as described above.
(5) Rabbit euthanasia (Timing: Day 10).
(a) Preparation of drug.
Animals are given a standard dose of 3 mL Euthasol as determined by the Animal Resource Center (about 390 mg/kg).
When drawing up the working solution of drug the rim of the plunger in the syringe should be even with the mark for the appropriate amount.
After drawing up, put the appropriate size needle on the end of the syringe.
(b) Administration of the drug.
Remove the rabbit from its housing and restrain.
Once the rabbit is comfortably restrained, give IM anesthesia as described above.
Place rabbit back in its housing until fully anesthetized.
Place rabbit on right side lateral recumbency and wet target area with alcohol.
Feel for strongest point of heartbeat with thumb.
Insert needle, pulling back on the syringe the whole time.
Once blood is observed stop advancing needle and draw up appropriate amount of blood if sample is required.
While leaving needle in place, direct euthanasia needle along side.
Pull back on plunger to check for blood to be sure needle is in the heart.
Remove blood collection syringe and inject euthanasia solution. !CAUTION Do not remove syringe until heart stops beating in case additional euthanasia solution is needed.
When dosing is complete remove the needle and discard the needle and syringe in a biohazard sharps container.
Confirm death by verifying absence of respiration, cardiac function, corneal reflex, muscle tone and mucus membrane color.
!CAUTION Do not remove catheters until death of the rabbit is confirmed.
When finished, place rabbit in two bio-hazard plastic bags.
Place animal in the freezer in the post-mortem room for disposal by the Animal Resource Center. !CAUTION Ketamine and Euthasol are drugs under control of the Drug Enforcement Agency (DEA) and all usage must be recorded in a drug log book. Controlled drugs must be stored in a double-locked cabinet in a secure area. Be careful to avoid accidental needle sticks; never recap needles.
(c) Clean up. (e) If done in a biosafety cabinet, wipe thoroughly with Clidox-soaked paper towels
(f) Wash hands after handling animals.
Anticipated Results
In this protocol, using the first clinically relevant rabbit model, it will be possible to evaluate C. albicans biofilm associated with catheter infection and study their detailed treatment strategies. The quantitative catheter culture technique described in this protocol will allow quantification of biofilms formed on catheters in vivo. Scanning electron microscopy method described above will allow evaluation of gross biofilm morphology and surface topography during catheter infection and when it is treated with the drug. Finally, using the antifungal lock therapy methodology described here, investigators can determine whether formed biofilms are susceptible or resistant to an antifungal agent (Table 2) and how these biofilm infections can be controlled.
Table 2.
Quantitative catheter culture (log CFU s, Mean ± SD) of proximal and distal segments obtained from catheters inserted in rabbits
| Treatment | Proximal | Distal |
| Untreated | 1.95 ± 1.06 | 1.73 ± 1.29 |
| L-AmB | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Fluconazole | 0.68 ± 0.98 | 0.45 ± 0.58 |
Adapted from Schinabeck. L-AmB = liposomal amphotericin B.
In Figure 1, we describe a rabbit model with catheter C. albicans biofilm and show the surgical placement of the intravenous catheter and attachment of the lock device to skin. (A to C) Catheter insertion into the external jugular vein; (D to F) attachment of the heparin lock device to skin and (G) postoperative venogram of catheter placement. Steps outlined in Section 1 are followed to obtain this figure.
In Figure 2, we show mature biofilm formation using our catheter in vivo model. Scanning electron micrographs of C. albicans biofilms adherent to the intraluminal surface of catheters showing biofilm architecture at 7 d postinfection. These images are obtained using SEM methods as described in Section 3.
Figure 2.
Mature in vivo C. albicans biofilm formation during rabbit model development. Scanning electron micrographs of C. albicans biofilms adherent to the intraluminal surface of catheters showing biofilm architecture at 7 d post-infection (A) magnification, 1,500x (B) magnification, 6,500x. There was no difference in biofilms at 3 d post-infection (data not shown). Adapted from Schinabeck et al.8
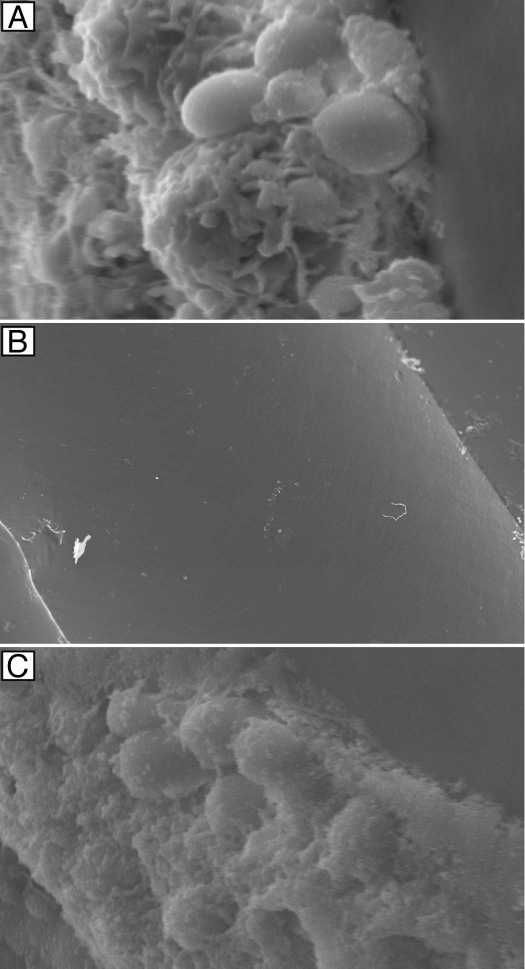
In Figure 3, we used the antifungal lock therapy to show the effectiveness of liposomal amphotericin B on C. albicans catheter associated biofilms. Scanning electron micrographs of intraluminal catheter surfaces following 7 d of therapy with heparinized saline (A), liposomal amphotericin B (B) and fluconazole (C) are shown. These images clearly demonstrate that antifungal lock therapy with liposomal amphotericin B is an effective treatment strategy against biofilms.
Figure 3.
Effectiveness of antifungal lock therapy. Scanning electron micrographs of intraluminal catheter surfaces following 7 d of therapy with heparinized saline (magnification, 5,000x) (A), liposomal amphotericin B (magnification, 121x) (B) and fluconazole (magnification, 3,500x) (C) are shown. Adapted from Schinabeck et al.8
Problem Handling.
| Problem | Possible reason | Solution |
| Catheter Placement, Inoculation Removal | ||
| Rabbit Restraint: Rabbits can be unpredictable | Rabbits are usually timid and are easily upset. | Lift the rabbit firmly by the scruff with the head pointing away from your body, use your other hand to support the rabbit's hind end. Cradle the rabbit to your body. If the rabbit struggles, place it on a solid surface and calm it by shielding the rabbit's eyes by tucking its head into the crook of your arm; this should keep the animal calm. When done, keep a firm grip on the scruff and gently release the rabbit in to the correctly marked cage. |
| Scanning Electron Microscopy (Adapted from Chandra et al.5) | ||
| Catheter segments too thick and Candida biofilms too dense | Catheter segments not cut flat and uniform and there is overproduction of Candida biofilm on the segments. | Focus on areas where biofilms are spread out or on edges of the segment, and catheter segments are flat Evaluate cells at earlier time point. |
| “Burnt” images | Sputter-coating step too long Exposing the cells to electron beam for extended period | Check sputter coating setting. Take images quickly. Use lower magnification to scan the image, then capture image rapidly at higher magnification. |
| Blurry/out of focus | Insufficient sputter coating Instrument setting not correct |
Re-sputter coat sample. Ensure the instrument is focusing correctly. Make sure this problem is not user-dependent. |
Acknowledgments
Studies in this protocol are funded by NIH/NIDCR grant (RO1 DE017846) to M.A.G. and NIH R21 grant (AI074077 and EY021303) to P.K.M.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Passerini L, Lam K, Costerton JW, King EG. Biofilms on indwelling vascular catheters. Crit Care Med. 1992;20:665–673. doi: 10.1097/00003246-199205000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, et al. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res. 2001;80:903–908. doi: 10.1177/00220345010800031101. [DOI] [PubMed] [Google Scholar]
- 4.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans—development, architecture and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-94.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra J, Mukherjee PK, Ghannoum MA. In vitro growth and analysis of Candida biofilms. Nat Protoc. 2008;3:1909–1924. doi: 10.1038/nprot.2008.192. [DOI] [PubMed] [Google Scholar]
- 6.Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249–1272. doi: 10.1086/320001. [DOI] [PubMed] [Google Scholar]
- 7.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schinabeck MK, Long LA, Hossain MA, Chandra J, Mukherjee PK, Mohamed S, et al. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob Agents Chemother. 2004;48:1727–1732. doi: 10.1128/AAC.48.5.1727-32.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun. 2004;72:6023–6031. doi: 10.1128/IAI.72.10.6023-31.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]