Abstract
Rationale
The ability of the adult heart to generate new myocytes after injury is not established.
Objective
Our purpose was to determine if the adult heart has the capacity to generate new myocytes after injury, and to gain insight into their source.
Methods and Results
Cardiac injury was induced in the adult feline heart by infusing Isoproterenol (ISO) for 10 days via minipumps and then animals were allowed to recover for 7 or 28 days. Cardiac function was measured with echocardiography and proliferative cells were identified by nuclear incorporation of 5-bromodeoxyuridine (BrdU; 7 day minipump infusion). BrdU was infused for 7 days before euthanasia at Days 10, 17, and 38 or during injury and animals euthanized at Day 38. ISO caused reduction in cardiac function with evidence of myocyte loss from necrosis. During this injury phase there was a significant increase in the number of proliferative cells in the atria and ventricle, but there was no increase in BrdU+ myocytes. cKit+ cardiac progenitor cells were BrdU labeled during injury. During the first seven days of recovery there was a significant reduction in cellular proliferation (BrdU incorporation) but a significant increase in BrdU+ myocytes. There was modest improvement in cardiac structure and function during recovery. At day 38, overall cell proliferation was not different than control but increased numbers of BrdU+ myocytes were found when BrdU was infused during injury.
Conclusions
These studies suggest that ISO injury activates cardiac progenitor cells that can differentiate into new myocytes during cardiac repair.
Keywords: Cardiac regeneration, catecholamine injury, cardiac progenitor cells
Introduction
Adult cardiac myocytes are terminally differentiated and are not thought to be capable of reentering the cell cycle and dividing1. Therefore, the adult heart has been viewed as a terminally differentiated organ, with no capacity for myocyte renewal with aging2. However, recent studies have suggested that new myocyte formation can occur in the adult heart with normal aging3, 4 and in response to pathological stress5–7. These new myocytes appear to be derived from a cardiac myocyte precursor (progenitor) cell that resides within the myocardium8, 9.
One type of cardiac precursor cell can be identified by the surface expression of the cKit receptor and the absence of the hematopoietic marker CD458. These cKit+/CD45− cells have been shown8 to have cardiogenic potential, both in-vitro and in-vivo. There are numerous varieties of putative cardiac precursor cells10–12, but their roles in the normal physiology of the heart, or their ability and capacity for cardiac repair after injury is still not well known, especially in large animals and humans13. This topic is clearly of clinical relevance since human heart disease that culminates in cardiac performance deficits is largely the result of the loss of contractile cardiocytes, such as after a myocardial infarction14, 15.
One reason why the adult heart is not thought to be capable of generating new myocytes is that it does not have the capacity to repair itself and recover normal structure and function after an injury that reduces myocyte number, such as a myocardial infarction (MI)7, 14, 15. MI induced myocyte death produces pump function defects that require persistent activation of neurohumoral reflex systems to maintain systemic blood pressure16–18. Often the dysfunctional state degenerates into a syndrome called congestive heart failure, a leading cause of death in the US.
The fact that myocyte loss is fundamentally related to the induction and progression of CHF has led to animal studies to test putative cardiac precursor cells for their ability to improve cardiac function8, 11, 19, 20. The success of some of these animal studies led to a number of clinical trials that have had mixed results21. At present the mechanism for the modest improvement in cardiac performance shown in human cell therapy trials is unclear. Possibilities include the generation of new, fully functional myocytes that differentiate from the injected stem cells and integrate with the parent myocardium22, 23 and/or improved survival or performance of the myocardium through a paracrine effect mediated by the injected cells24, 25. Another possibility is that the injected cells activate resident progenitor cells which are then responsible for enhanced endogenous cardiac repair25–27. However, little is known about the ability of the adult mammalian heart to repair itself after injury.
The present study examined the idea that the adult heart of a large mammal has the ability to repair itself after injury by generating new cardiac myocytes. Injury was induced by infusion of isoproterenol (ISO) for 10 days. ISO caused diffuse myocardial injury throughout the heart, allowing comparison of endogenous repair in the atria and the ventricle. We infused BrdU at various times during injury and recovery to identify proliferative cells. Our results show that during ISO injury c-kit+/CD45− cells in the atria and ventricles proliferate but few new myocytes were formed. During recovery from ISO injury, BrdU+ myocyte nuclei were observed and these myocytes appear to have been derived from resident cardiac precursors that were activated during the injury phase.
Methods
Isoproterenol (Catecholamine) Induced Cardiomyopathy
Isoproterenol (ISO) induced cardiomyopathy was produced in adult felines at 5–6 months of age. These animals were divided into three groups and sacrificed at Day 10 (injury), Day 17 (early recovery), or Day 38 (late recovery) following ISO-filled minipump implantation at Baseline (Day 0) and removal at Day 10. Full details can be found in the on-line methods.
All other techniques used in this study have been described in previous studies and details can be found in the on-line methods.
Results
Chronic ISO infusion causes depressed systolic and diastolic function and chamber dilation
Catecholamines increase Ca2+ influx and the contractility of cardiac myocytes, but if catecholamine exposure is persistent and excessive it can induce myocyte apoptosis and necrosis, cardiac hypertrophy, replacement fibrosis, depressed cardiac pump function and premature death28–30. In the present study, we developed an isoproterenol (ISO)-induced cardiac injury model in the feline heart so that we could explore the role of new myocyte formation in endogenous cardiac repair in an animal model with human type physiological properties.
Cardiac structure and function were measured with ECHO during and after 10 days of ISO injury. BrdU incorporation into proliferating cells was also measured to determine if either injury or recovery from injury involved an endogenous cardiac repair process with the generation of new cardiac myocytes. Supplemental Figure I details the experimental protocol for induction of ISO injury, the 7 day periods when BrdU containing minipumps were inserted, and the timing of terminal studies.
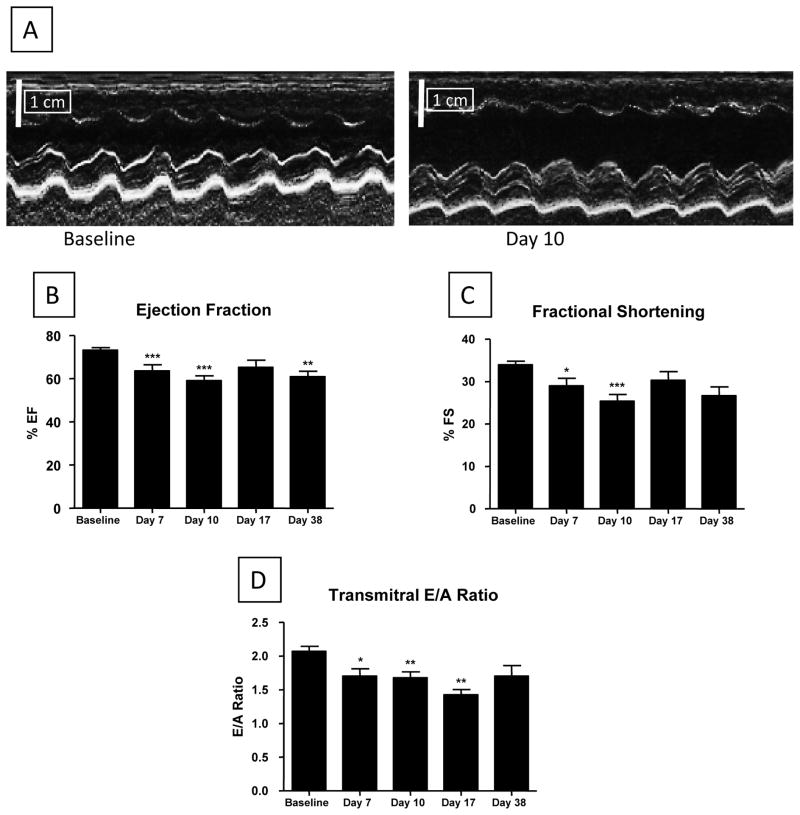
Cardiac structure and function were measured with ECHO at baseline, during injury (Day 7), at the end of the injury phase (Day 10), during early recovery (Day 17), and during late recovery (Day 38) (Figure 1). ISO initially enhanced cardiac function (not shown) but caused significant depression of left ventricular ejection fraction (EF) by Day 7 (65.1 ± 2.8, p<0.001) and Day 10 (59.1 ± 2.1, p<0.001) compared with control (74.2 ± 1.1), with similar changes in fractional shortening (Figures 1B/C). There were also significant changes in the transmitral E/A ratio, suggesting the presence of abnormal diastolic function. These results are consistent with previous studies showing that chronic ISO treatment leads to reduced cardiac function and cardiac hypertrophy31–33. The dose of ISO used in our study caused significant myocyte injury, as evidenced by increases in Troponin I (a cardiac contractile protein) in the blood (Supplemental Figure II). Troponin I levels peaked at 3 days and rapidly returned to control levels after removal of ISO minipumps. The increase in circulating myocyte contractile proteins suggests that ISO injury involves necrotic myocyte death.
Figure 1. Cardiac function in the ISO injured heart.
Echocardiography (ECHO) was performed at Baseline, Day 7 (during injury), Day 10 (end of injury phase), Day 17 (early recovery) and Day 38 (late recovery). Representative ECHO data is shown in (A). B–D: Cardiac systolic and diastolic function was increasingly depressed from Baseline through Day 10 (N=19) and showed modest improvement after removal (at Day 10) of ISO minipumps by Day 17 (N=12) and at Day 38 (N=6). The transmitral E to A ratio was significantly depressed, indicating diastolic stiffening of the heart at Days 7 (N=15), 10 (N=17) and 17 (N=9), with modest recovery at Day 38 (N=6). *P<0.05; **P<0.01; ***P<0.001 versus Baseline. Data are presented as mean ± SEM.
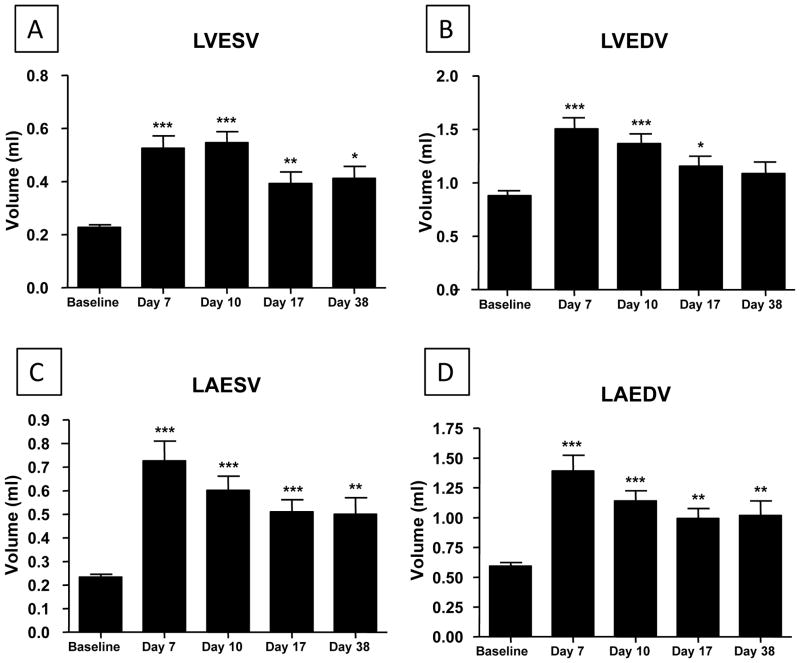
ISO injury caused significant dilation of the left ventricle and atrium (Figure 2). After removal of ISO containing minipumps there was only modest recovery of systolic and diastolic function over the next four weeks (Figure 1). Atrial and ventricular chamber volumes showed some recovery toward control levels after the removal of ISO minipumps (Figure 2). These results show that continuous ISO infusion for 10 days results in depressed systolic and diastolic function, myocyte injury and chamber enlargement. Upon removal of ISO the heart appears to have some capacity for endogenous structural and functional repair.
Figure 2. Left ventricular (A/B) and left atrial (C/D) end-diastolic and end-systolic volumes increase after ISO injury.
ISO caused significant dilatation of the left atrium and ventricle. Left ventricular and atrial end-systolic and diastolic volumes increased significantly from Baseline through Day 10 (N=19) with moderate recovery at Day 17 (N=12; LAESV, LAEDV N=11) and Day 38 (N=6). *P<0.05; **P<0.01; ***P<0.001 versus Baseline. Data are presented as mean ± SEM.
ISO-Induced Cardiac Injury is Associated with Cardiac and Myocyte Hypertrophy
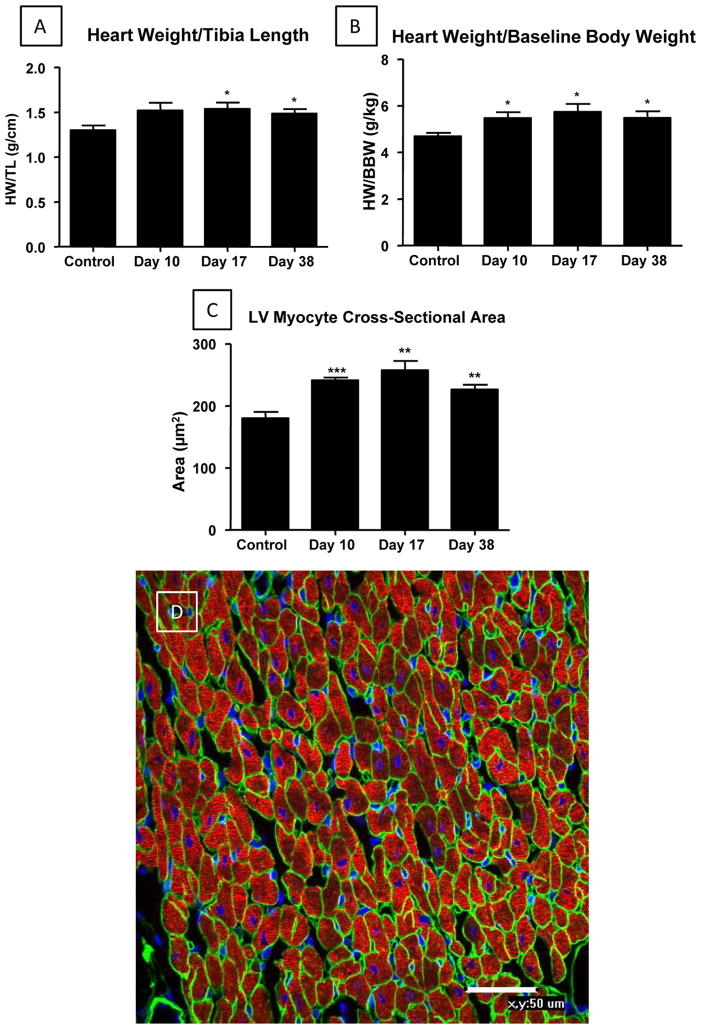
Control and ISO-injured hearts were studied at the end of the injury phase, Day 10, and during recovery phases at Days 17 and 38 (Figure 3). Hearts were weighed and then perfusion fixed for histological analyses (see below). Gross heart weight was normalized by tibia length (Figure 3A) and pre-ISO body weight (Figure 3B). The heart weight to tibia length ratio was increased at Day 10 (1.52 ± 0.09), and significantly increased at Day 17 (1.54 ± 0.07, p<0.05) and Day 38 (1.49 ± 0.05, p<0.05) compared with age matched control hearts (1.3 ± 0.05) (Figure 3A). The heart weight to body weight ratio was also significantly increased at Day 10 (5.47 ± 0.25, p<0.05), Day 17 (5.74 ± 0.34, p<0.05) and Day 38 (5.49 ± 0.28, p<0.05) compared with control hearts (4.69 ± 0.15) (Figure 3B). To determine if increases in heart weight involved cardiac myocyte hypertrophy, the cross-sectional area of left ventricular myocytes was measured. Myocytes cut in cross-section, as determined by laminin and cardiac actin staining (Figure 3C/D) were analyzed. Myocyte cross-sectional area was significantly increased at Day 10 (241.0 ± 4.6 μm2, p<0.001), Day 17 (257.6 ± 15.0 μm2, p<0.01) and Day 38 (226.5 ± 7.48 μm2, p<0.01) compared with control heart tissue (180.2 ± 10.2 μm2). These results show that ISO-induced injury leads to myocyte hypertrophy. ECHO analysis of cardiac wall thickness also documented cardiac hypertrophy after ISO injury (Supplemental Figure III).
Figure 3. ISO injury caused cardiac and myocyte hypertrophy.
A/B: Cardiac hypertrophy was present by Day 17. D: An example of a section of normal heart is shown. This section was stained for cardiac actin (Red), DAPI (Nucleus; Blue) and laminin (Cell Perimeters; Green). Myocyte cross-sectional area was measured with ImageJ Software (NIH). Myocytes cut in cross section, with centrally located nuclei, were chosen for study. C: Approximately 300 cells were analyzed from each heart. Control-Day 10 (N=7); Day 17 (N=6); Day 38 (N=6). HW/TL and HW/BW: Control (N=7); Day 10 (N=10); Day 17-Day 38 (N=6). *P<0.05; **P<0.01; ***P<0.001 versus Control. Data are presented as mean ± SEM.
Chronic ISO exposure induces replacement fibrosis
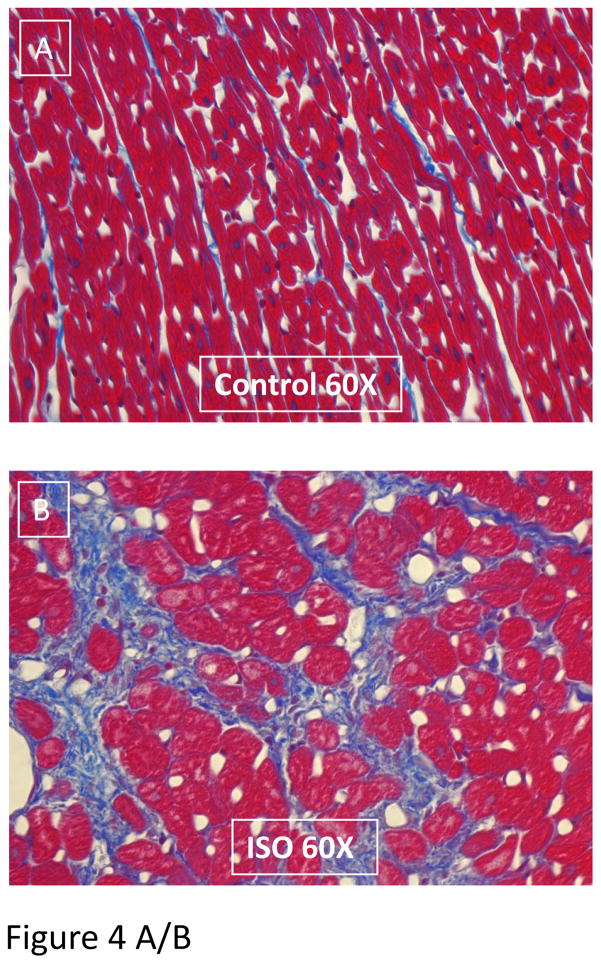
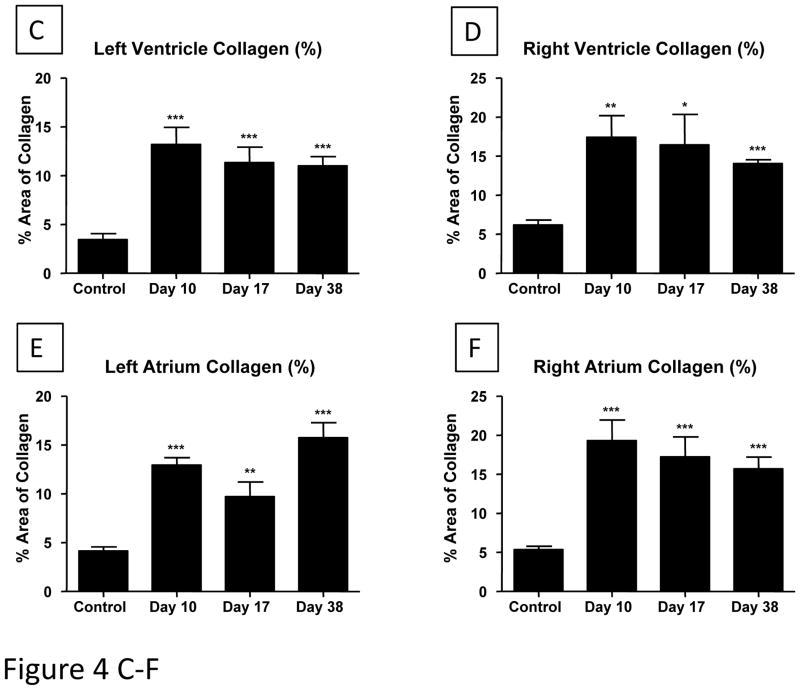
The collagen content (and overall tissue structure) of control and ISO-injured hearts was determined in tissue sections (Figure 4 and Supplemental Figure 4). The collagen content was increased in all regions of the heart (Figure 4A and B) and there were small areas of focal injury with replacement fibrosis (Supplemental Figure IV). The collagen staining in the left ventricle was significantly increased at Day 10 (13.19 ± 1.74%, p<0.001), Day 17 (11.33 ± 1.59%, p<0.001) and Day 38 (11.02 ± 0.94%, p<0.001) when compared to control tissue (3.46 ± 0.61%) (Figure 4C). Similar amounts of replacement fibrosis were found for the right ventricle, left and right atrium (Figure 4D–F).
Figure 4. A/B. Representative LV tissue sections of control (A) and ISO damaged (B) hearts are shown.
Cell loss, myocyte hypertrophy and replacement fibrosis can be seen in ISO-treated heart tissue. Collagen (Blue); cardiac tissue (Red). The percent blue area was quantified. The collagen content of the ventricles (C/D) and atria (E/F) increase after ISO injury: Masson’s Trichrome stained slides were used to measure changes in collagen content of all cardiac chambers. The assessment and analysis of the data was carried out in a blinded fashion. All chambers had significant increases in collagen content after ISO injury (Day 10), with small reductions during the next few weeks. Control-Day 10 (N=7); Day 17-Day 38 (N=6). *P<0.05; **P<0.01; ***P<0.001 versus Control. Data are presented as mean ± SEM.
ISO injury causes depressed myocyte contractility
Myocytes were studied at the end of the injury phase (Day 10) to determine if depressed global cardiac function was associated with reduction in the density and ISO responsiveness of the L-type Ca2+ current (ICa-L) and depression of myocyte contraction and systolic Ca2+ transients. ICa-L was significantly smaller than control in myocytes from ISO injured hearts and showed almost no response to bath application of ISO (Supplemental Figure VA/B and Supplemental Figure V I/J). Myocyte contractions (Supplemental Figure VC-E) and Ca2+ transients (Supplemental Figure VF-H) were significantly smaller in myocytes from ISO-injured hearts versus controls and these myocytes had blunted responses to applied catecholamines. These changes in myocyte contractility and Ca2+ handling are similar to those we have found previously in feline models of pressure overload induced cardiac dysfunction34 and in failing human ventricular myocytes35, 36. These results show that our ISO-induced cardiac injury model causes alterations in myocyte Ca2+ handling reminiscent of those found in other forms of cardiac disease.
BrdU+ myocyte nuclei increase in the left atrium, but only after removal of ISO minipumps
Histological analysis of left atrial tissue was performed at the end of the injury phase (day 10) and during spontaneous recovery at Days 17 and 38. BrdU was infused for 7 days before euthanasia, except in one group of animals where it was infused during the injury phase (pulse) and then animals were euthanized at Day 38 (chase) (Supplemental Figure I).
Day 10: End of the injury phase
A small % of nonmyocyte nuclei were BrdU+ and very few myocyte nuclei were BrdU+ in uninjured control tissues (Figure 5A/D/E). ISO-induced injury was associated with significant increases in total BrdU+ nuclei at Day 10 (17.03 ± 2.74%, p<0.001) compared with control (1.51 ± 0.37%). BrdU+ nuclei were abundant (Figure 5B/D/E), and almost all of these labeled cells were nonmyocytes. There was no significant increase in BrdU+ myocyte nuclei in the LA during the ISO injury phase and the % of BrdU+ myocyte nuclei was very low (0.0177 ± 0.0129% compared with 0.0187 ± 0.0089% in controls). These studies show that ISO injury causes an increase in the number of proliferative nonmyocytes in the left atrium, with no detectable increase in the number of newly formed atrial myocytes, at least as defined by BrdU incorporation.
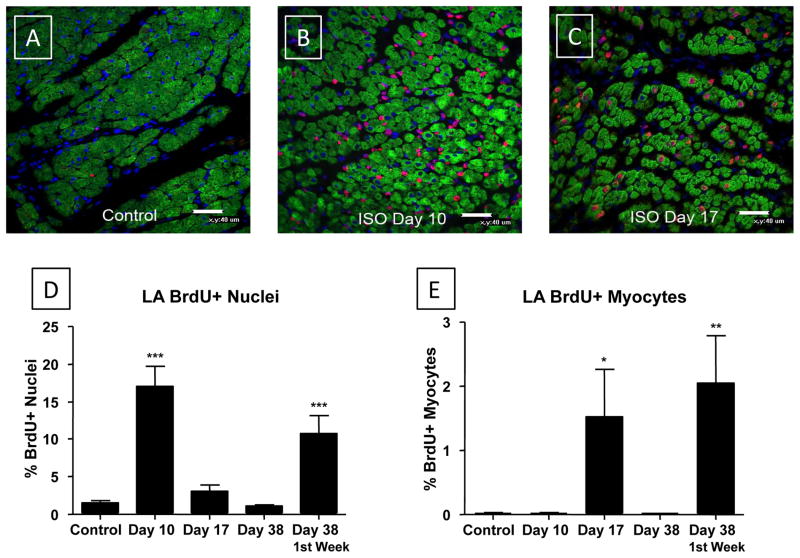
Figure 5. ISO injury increases the number of BrdU+ non-myocytes during injury and the number BrdU+ myocytes in the atria after injury.
BrdU was infused for 7 days before euthanasia, except in pulse-chase studies where it was infused during injury (Pulse) and animals were euthanized at Day 38 (Chase). BrdU (Red); α-Actin (Green); DA PI (Blue). Few BrdU+ nuclei were observed in normal atria and most of these were non-myocyte nuclei (A/D/E). ISO induced injury caused a large increase in the number of BrdU+ nuclei (Day 10; B) but almost all of the BrdU+ nuclei were non-myocytes (D/E). The total number of BrdU+ nuclei decreased rapidly after removal of ISO minipumps, at Day 17 (C/D) and Day 38 (D). The number of BrdU+ myocyte nuclei increased at Day 17 (C/E) but was back to control levels by Day 38 (E). BrdU+ non-myocytes labeled during the injury phase (Pulse) persisted in the heart for 4 weeks (Day 38; Chase) and the largest number of BrdU+ myocytes were found when labeling was done at this time period (E). *P<0.05; **P<0.01; ***P<0.001 versus Control. Data are presented as mean ± SEM.
Day 17: 7 Days After Removal of ISO Minipumps
At Day 17, (BrdU infused from Day 10 to Day 17) there was a significant decrease in the number of total BrdU+ nuclei (3.13 ± 0.75%) versus that observed at Day 10 and the total number of BrdU+ nuclei had returned to almost control levels (Figure 5C/D). However, there was a significant (80-fold) increase in the number of BrdU+ LA myocyte nuclei (1.53 ± 0.73%, p<0.05) and BrdU+ myocyte nuclei were easily identified in every tissue section (Figure 5C). These results show that after removal of ISO the proliferation of most non-myocytes in the left atria decreased rapidly while the number of BrdU+ myocytes increases. This increase in BrdU+ myocytes was associated with improved cardiac performance (see above).
Day 38: 28 Days after Removal of ISO Minipumps
Animals euthanized at Day 38 had BrdU minipumps inserted either during cardiac injury (pulse-chase studies) or for 7 days prior to euthanasia (Figure 5E). ISO-injured animals that received BrdU minipumps one week prior to euthanasia at Day 38 did not have increased numbers of BrdU+ myocyte and non-myocyte nuclei versus controls. These results suggest that by Day 38 any endogenous cardiac repair that does not involve non-myocyte or myocyte proliferation had been largely completed.
In animals in which BrdU minipumps were implanted during the ISO injury phase (pulse), the number of total BrdU+ LA nuclei (10.70 ± 2.44%, p<0.001) and BrdU+ LA myocyte nuclei (2.045 ± 0.746%, p<0.01) at Day 38 (chase) were significantly greater than in controls. These results show that non-myocytes that proliferated during the ISO injury phase were still present in the heart after repair is completed. Our results with BrdU infusion during the ISO injury phase and heart explant at Day 10 (above) showed that only non-myocytes are proliferative at this time. Here we show that identical BrdU infusion during injury (and then pumps are removed) resulted in a significant increase in the number of BrdU+ atrial myocytes at Day 38, suggesting that these BrdU+ myocytes were derived from BrdU+ non-myocytes that were labeled during the injury phase.
BrdU+ myocyte nuclei also increase in the left ventricle, but again, only after removal of ISO minipumps
Histological evaluation of left ventricular tissue (Figure 6) was performed as described above for left atrial samples.
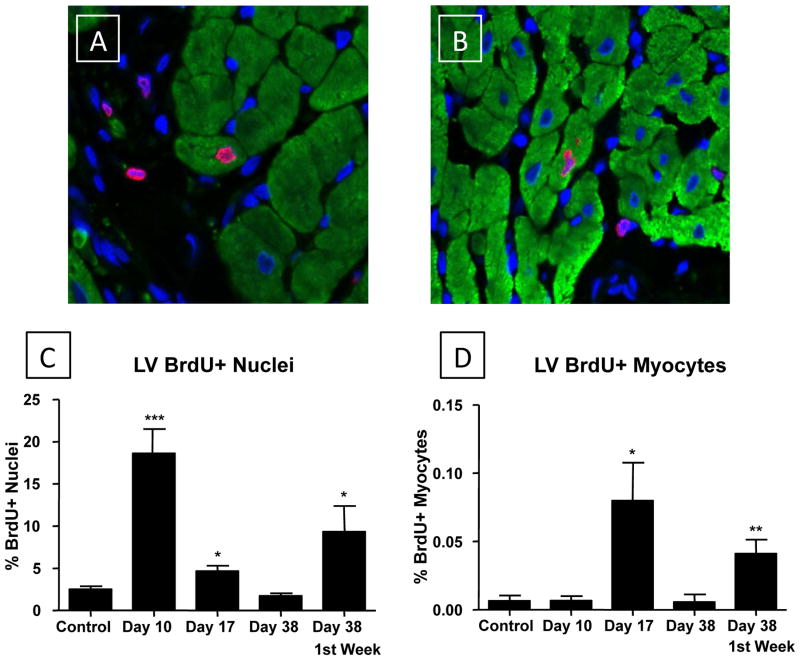
Figure 6. ISO injury increases the number of BrdU+ non-myocyte and myocyte nuclei in the ventricle.
Experimental approaches were as described in Figure 6. Representative BrdU+ myocyte and non-myocyte nuclei (at Day 38) are shown in A and B. C: The number of BrdU+ nuclei was greatest during the injury phase (Day 10) and decreased rapidly afterwards. Nuclei labeled during injury (Pulse) were still present 4 weeks later (Day 38; Chase). D: The number of BrdU+ myocytes increased during the first week after injury (Day 17) and returned to control levels by Day 38. BrdU infusion during injury (Pulse), when few Brdu+ myocytes were found, resulted in BrdU labeling of myocyte nuclei at Day 38 (Chase). *P<0.05; **P<0.01; ***P<0.001 versus Control. Data are presented as mean ± SEM.
Day 10: End of the injury phase
In control LV samples a small % of the nuclei were BrdU+, and almost all of these nuclei were in non-myocytes. BrdU+ myocyte nuclei were rarely observed in control hearts of animals of this age (Figure 6C/D). As we observed in the study of atrial tissue, ISO injury was associated with significant increases in total BrdU+ nuclei at Day 10 (18.6 ± 2.9%, p<0.001) compared with control (2.5 ± 0.4%) LV tissue. The increase was almost exclusively in non-myocytes. There was no detectable increase in BrdU+ myocyte nuclei in the LV following ISO treatment (0.0066 ± 0.0034%) compared with controls (0.0066 ± 0.0038%).
Day 17: 7 Days after Removal of ISO Minipumps
At Day 17 (BrdU infused from Day 10 to Day 17) there was a significant decrease in the number of total BrdU+ nuclei (4.67 ± 0.63%) versus that observed at Day 10. The % of BrdU+ nuclei was slightly greater than observed in control hearts. However, we found a significant (10-fold) increase in the number of BrdU+ LV myocyte nuclei (0.08 ± 0.028%, p<0.05) (Figure 6A/B and Supplemental Figure VI). However, the % of BrdU+ LV myocyte nuclei was more than 10-fold lower than we observed in the atria (see above). These results show that there is no detectable new myocyte formation during the ISO injury phase, but there is a significant increase in the number of proliferative, small, non-myocytes during this time period. After removal of ISO the proliferation of non-myocytes decreases toward control levels but the number of BrdU+ myocytes increase, consistent with new myocyte formation in association with improved ventricular performance.
Day 38: 28 Days after Removal of ISO Minipumps
ISO-injured animals that received BrdU minipumps one week prior to euthanasia at Day 38 had the same % of BrdU+ myocyte and non-myocyte nuclei that were observed in control tissues (Figure 6C/D). These results are consistent with our observations in the atria and show that aspects of cardiac repair after ISO injury that involved enhanced proliferation of cells within the myocardium has returned to control levels within weeks after injury.
In animals with BrdU minipumps implanted during the ISO injury phase and then removed, the number of total BrdU+ nuclei (9.35 ± 3.04%, p<0.05) and BrdU+ myocyte nuclei (0.04 ± 0.01%, p<0.01) at Day 38 were significantly greater than in controls (Figure 6C/D). These results show that many of the BrdU labeled non-myocyte nuclei are still present after repair of ISO-induced injury. More importantly, these data suggest that the BrdU+ myocytes found at Day 38, were derived from BrdU+ cardiac precursors that were BrdU labeled during the injury phase. Our collective results show that the adult feline heart has some capacity to generate new myocytes after injury. We also show that significantly more brightly stained BrdU+ myocytes are found in the atria than in the ventricle after ISO-induced injury.
“Dimly” BrdU+ myocytes in pulse-chase experiments
Our pulse chase studies show that infusion of BrdU during ISO injury labeled nonmyocytes which appear to differentiate into new myocytes after removal of ISO (and BrdU) at Day 10. If these newly formed myocytes are transiently proliferative, then in these pulse chase studies we would expect to see myocytes containing low levels (dimly labeled) of BrdU, since the label would be diluted with each cell division. All the results reported above were from analysis of “brightly” labeled cells. An analysis of “dimly” labeled cells was performed in LV samples from 3 hearts from each of our time points (Day 10, Day 17, Day 38, and Day 38 pulse chase). No significant numbers of “dimly” BrdU+ nuclei were found in any of the hearts in which BrdU was infused for 7 days prior to sacrifice. Only “brightly” labeled myocytes and nonmyocytes were observed. However, a significant number (12.6 +/− 6.3%) of LV myocytes had low levels of BrdU labeling (Supplemental Figure VII) in the pulse-chase group. Similar results were found in the left atria (data not shown). These results are consistent with the idea that some of the new myocytes derived from BrdU+ nonmyocyte precursor cells proliferate transiently before exiting the cell cycle.
cKit+ and CD45+ Cells in the Heart Proliferate with Cardiac Injury
Our most provocative finding is that we detected BrdU+ myocytes in the atria and ventricle, weeks after BrdU labeling of non-myocytes during the injury phase. The nature and source of these proliferative cells is not clear. They could be derived from cells that normally reside within the heart8, 9, 37, or from cells that enter the heart from the blood during injury6, 10, 20, 38. While a variety of precursor cells with the ability to form new cardiovascular tissue have been identified8, 11, 39, we studied those cardiac progenitor cells expressing the stem cell receptor cKit (CD117) that did not express markers of the hematopoietic lineage (cKit+/CD45−)8. We also studied hematopoietic cells40 that expressed both cKit and CD45 (leukocyte common antigen) (cKit+/CD45+)6, 41. We analyzed cardiac tissue sections from control, injury (Day 10), early (Day 17), and late recovery (Day 38) for cKit, CD45, and BrdU incorporation to determine if cKit+ or CD45+ cells were proliferative during ISO injury.
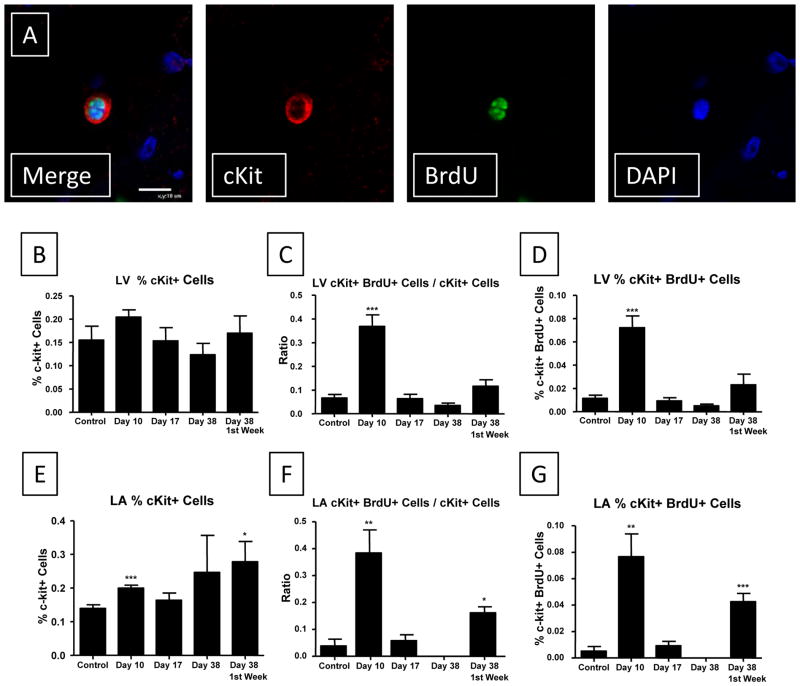
In the left ventricle and the left atrium, cKit cells (Figure 7A) were a minor fraction of the total cell population (Figure 7B/E). There was a small increase the % of cKit+ cells in the LV at Day 10 but this difference failed to reach statistical significance. In the left ventricle there was a significant increase (6-fold) in the percent of cKit+ cells that were BrdU+ (0.0722 ± 0.01%, p<0.001) during the ISO injury period (Day 10) compared with control (0.0115 ± 0.0027%) (Figure 7 and Supplemental Figure VIII).
Figure 7. cKit+ cells in the heart proliferate during ISO induced injury.
A: Representative cKit staining of a proliferative cell is shown. B: The % of cKit cells in the ventricle did not change during the time course of our study. C: A significant increase in the ratio of proliferative cKit cells to non-proliferative cKit cells was observed during the injury phase. D: cKit+/BrdU+ cells are a very small % of the cells in the ventricle, but this % increases during ISO injury. E–G: Similar results were observed in the left atrial sections. *P<0.05; **P<0.01; ***P<0.001 versus Control. Data are presented as mean ± SEM.
The % of CD45+ cells in the heart was also very small and did not change at any of the times periods we examined. There was a smaller (than cKit cells) increase (2-fold) in the percent of CD45+/BrdU+ nuclei at Day 10 (0.0193 ± 0.0018%, p<0.01) compared with control (0.0081 ± 0.0018%) (Supplemental Figures VIII–X).
To determine whether cKit+ or CD45+ cells in the left ventricle were more proliferative during the injury phase, we calculated the ratio of cKit+(or CD45+)/BrdU+ to cKit+ (or CD45) nuclei. There was a significant increase (5-fold) in the ratio of cKit+/BrdU+ to cKit+ nuclei at Day 10 (0.369 ± 0.048, p<0.001) compared with control (0.067 ± 0.015) (Figure 7 and Supplemental Figure VIII). This was much larger than the increase (2-fold) in the ratio of CD45+/BrdU+ to CD45+ nuclei at Day 10 (0.126 ± 0.014, p<0.01) compared with control (0.064 ± 0.009) (Supplemental Figures IX and X). The % of cKit+/BrdU+ cells was not different than control at Days 17 and 38 (with BrdU infused 7 days before euthanasia). Similar results were observed for CD45+ cells. These results show that while both cKit+ and CD45+ cells in the heart are proliferative during the injury phase their absolute number does not increase dramatically during either the injury or repair periods.
In the left atrium (Figure 7E–G), ISO injury was associated with a significant increase (14-fold) in the percent of cKit+/BrdU+ nuclei at Day 10 (0.0767 ± 0.0171%, p<0.01) compared with control (0.0052 ± 0.0034%) (Figure 7). The percent of CD45+/BrdU+ nuclei was somewhat increased at Day 10 (0.022 ± 0.0065%, NS; p<0.07) compared with control (0.0068 ± 0.0039%) (Supplemental Figures IX and X). We also analyzed the ratio of c-kit+/BrdU+ to cKit+ nuclei in the left atrium. There was a significant increase (10-fold) in the ratio of c-kit+/BrdU+ to cKit+ nuclei at Day 10 (0.384 ± 0.086, p<0.01) compared with control (0.038 ± 0.026). Similar to the left ventricle, this was a much larger increase than that seen for the increase (3-fold) in the ratio of CD45+/BrdU+ to CD45+ nuclei at Day 10 (0.130 ± 0.031, p<0.05) compared with control (0.043 ± 0.024), indicating a more active proliferative response by cKit+ progenitor cells following injury. These data show that the cKit+ (Figure 7) and CD45+ (Supplemental Figure IX) cell populations in the atria and ventricle proliferate following ISO injury and the magnitude of the proliferative response is greater in the atria.
In animals in which BrdU minipumps were implanted during the ISO injury phase and then removed at Day 10, with hearts explanted at Day 38, the percent of cKit+/BrdU+ left atrial nuclei (0.0426 ± 0.0063%, p<0.001) and the ratio of cKit+/BrdU+ to cKit+ nuclei (0.162 ± 0.022, p<0.05) was significantly greater than in controls (Figure 8). In comparison, ISO-injured animals that received BrdU minipumps one week prior to sacrifice at Day 38 did not have an increased percent of cKit+/BrdU+ left atrial nuclei or an increased ratio of cKit+/BrdU+ to cKit+ nuclei. These results suggest that a small group of the proliferative cKit+/BrdU+ cells that were labeled during the injury phase were still present at Day 38. Although a similar trend was seen in the left ventricle, these changes were not significant, again indicating a somewhat less robust regenerative response in the left ventricle versus the left atrium.
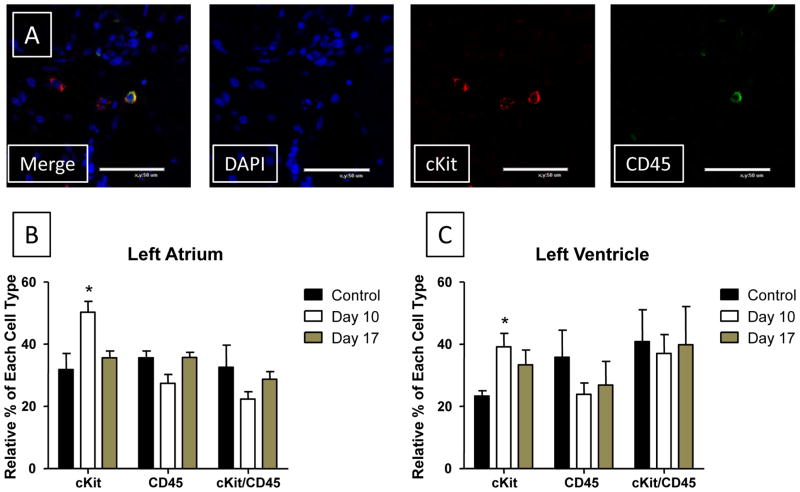
Figure 8. The % of cKit+/CD45- cells increases during ISO induced injury.
A: Representative staining of tissue for cKit, CD45 and DAPI. Three cKit cells are present and one of these was also positive for CD45. B/C: Tissue sections from Control, Day 10 ISO-treated and Day 17 ISO-treated hearts were co-stained with cKit and CD45. Cells that stained positive for cKit only, CD45 only and with both cKit and CD45 were each counted. The percent of each cell type present (cKit+ only, CD45+ only, or cKit+/CD45+) relative to the group as a whole is plotted for Atrial (B) and Ventricular (C) sections. cKit (Red); CD45 (Green); DAPI (Blue). Control (N=3) Day 10 (N=4); Day 17 (N=3). *P<0.05; **P<0.01; ***P<0.001 versus Control. Data are presented as mean ± SEM.
cKit+ Cells are both CD45+ and CD45−
Resident cardiac progenitor cells are thought to be cKit+/CD45−8, while cKit+ cells from the bone marrow are thought to be CD45+41. We showed above that BrdU incorporation into cKit+ cells following injury was much greater than in CD45+ cells, suggesting that most of the cKit+ cells we stained were not of hematopoietic origin. To further address this issue we co-stained tissue sections for cKit and CD45 (Figure 8). The relative percentages of cKit+/CD45− (called cKit), cKit−/CD45+ (called CD45), and cKit+/CD45+ cells were measured. All three varieties of cells were observed in both the left atrium and ventricle (Figure 8). The relative percent of cKit+/CD45− cells increased significantly in ISO-treated animals at Day 10 (LA: 50.28 ± 3.47, p<0.05; LV: 39.14 ± 4.35, p<0.05) and stayed elevated at Day 17 (LA: 35.56 ± 2.22, NS; LV: 33.33 ± 4.81, NS) compared with control heart tissue (LA: 31.85 ± 5.19; LV: 23.33 ± 1.67). No significant change in CD45+ cells at Day 10 (LA: 27.41 ± 2.82, NS; LV: 23.88 ± 3.65, NS) compared with control heart tissue (LA: 35.56 ± 2.22, NS; LV: 35.83 ± 8.70, NS) was found. There was no significant change (with a trend towards a decrease) in the number of both CD45+ and cKit+/CD45+ cells during the injury (Day 10) and early recovery phases (Day 17). These data show that ISO-injury selectively increased proliferation of small non-myocytes that are cKit+/CD45−, at a time when we saw little evidence for new myocyte formation. Our overall findings are consistent with the idea that ISO injury induces the proliferation of a cKit+/CD45− resident cardiac precursor cell that expands the progenitor cell pool and could be the source of the new cardiac myocytes that we observed during the recovery phase. Our data also suggests that this response is more robust in the atria than in the ventricle.
Discussion
Cardiac dysfunction resulting from the death of cardiac myocytes is a major health problem and a leading cause of heart failure42. Many previous studies have documented that the adult cardiac myocyte is terminally differentiated with no apparent ability to reenter the cell cycle and divide1, 2. Therefore, pathological insults that reduce myocyte number decrease contractile mass and increase the contractile stress on surviving myocytes. Since adult myocytes cannot divide, the principle mechanism for restoring contractile mass in the face of pathological stress is thought to be myocyte hypertrophy rather than myocyte regeneration2, 4, 15. However, recent findings support the idea that the adult heart contains a population of resident progenitor cells with some capacity for new myocyte generation4, 7, 8. Animal studies in which these putative cardiac precursor cells are injected into the damaged heart have shown enhanced cardiac function8, 19. These findings have fueled a number of clinical trials that test the ability of different adult stem cells to restore cardiac function in patients with damaged hearts21. It is difficult to interpret these clinical studies with our limited understanding of the capacity of the adult heart to generate new myocytes in normal or pathological states. Our study suggests that cardiac myocyte regeneration can take place in the adult heart of large mammals with injury caused by ISO infusion and that resident cardiac precursor cells might be the source of these new myocytes.
Our experiments showed that ISO infusion caused cardiac injury with depressed systolic and diastolic function (Figure 1), dilation of the atria and ventricles (Figure 2), cardiac and myocyte hypertrophy (Figure 3), and replacement fibrosis (Figure 4). There was some improvement in these parameters after ISO removal, consistent with endogenous repair. These results suggest that ISO caused necrotic myocyte loss43, with reactive fibrosis and hypertrophy28. Myocytes studied at the end of the injury phase had many of the features of those in end-stage failing human hearts34–36; including reduced L-type Ca2+ channel currents with reduced ISO responsiveness, depressed myocyte contractions and Ca2+ transients and blunted ISO responsiveness (Supplemental Figure V). These results show that ISO causes diffuse cardiac injury and results in cardiac structural and functional remodeling, with substantial reactive myocyte hypertrophy. The major purpose of our study was to determine if the cardiac repair that follows this type of injury includes any myocyte regeneration.
ISO injury activates a cardiac precursor pool
Our experiments showed that during ISO injury there was a large increase in the number of proliferative (defined by BrdU incorporation) cells in the atria and ventricle. We were surprised that almost 20% of all atrial and ventricular nuclei were BrdU+ at the end of the ISO injury period (Figures 5 and 6). A major finding was that few BrdU+ myocyte nuclei were identified during this time period. This lack of BrdU+ myocyte nuclei during ISO injury reduced concern that BrdU was incorporating into myocytes needing to repair their DNA, which would have caused a significant increase in BrdU+ myocyte nuclei at the end of the injury phase (Day 10). This proliferative phase was clearly associated with ISO injury, because when BrdU was infused after removal of ISO, the rate of BrdU incorporation immediately decreased to levels close to those in normal hearts. We did not define the identity of all BrdU+ cells in the injured heart, but did examine the cKit+/CD45− cells thought to be resident cardiac precursors8 as well as the cKit+/CD45+ cells that are thought to be derived from the bone marrow via the blood stream 11, 19. The relative abundance of CD45+ cells did not increase substantially during injury, but both cKit+ and CD45+ cells were proliferative during ISO injury (Figure 7 and Supplemental Figures IX and X). The putative resident cardiac precursor cells (cKit+/CD45−) were the only cells that increased in relative abundance during ISO injury (Figure 9) and they were more proliferative than cKit+/CD45+ during the injury phase. These results show that ISO injury leads to proliferation of a pool of cells that are thought to have cardiogenic potential8, but no increase in new cardiac myocyte formation (defined by us as BrdU+ myocytes) was observed during this time period. These results are reminiscent of our observations in failing human hearts6 where we found an increase in cKit+ cells that were not repairing the damaged heart.
Cardiac repair includes new myocyte formation
Histological studies of cardiac tissue from hearts recovering from ISO injury clearly identified BrdU+ myocyte nuclei, but only under specific BrdU labeling conditions. Significant numbers of BrdU+ myocyte nuclei were found at Day 17 (7 days of recovery, with BrdU infused from Days 10–17). Importantly, BrdU+ non-myocyte labeling was only slightly greater than control, and significantly less than during injury with this labeling strategy. Significant numbers of BrdU+ myocyte nuclei were also found after 4 weeks (Day 38) of recovery but only in animals in which BrdU was infused during injury (Figure 5 and 6). The pulse-chase procedure also produced a significant number of dimly BrdU labeled cardiac myocytes, consistent with the idea that some of the new myocytes derived from BrdU labeled precursor cells were transiently proliferative. This putative proliferation would have had to be complete by our Day 31, since BrdU infusion from Days 31–38 failed to label a significant number of cardiac myocytes.
Are new cardiac myocytes derived from a cKit+/CD45− precursor pool?
Our results support the hypothesis that ISO injury induces proliferation of resident cardiac (cKit+/CD45−) progenitors, but these cells do not differentiate into new cardiac myocytes during injury (Supplemental Figure XI). Upon removal of ISO, these activated (BrdU+) cardiac precursors appear to differentiate into new cardiac myocytes. Our results suggest that new myocyte formation during recovery required a proliferative step, since myocyte BrdU labeling during Days 10–17 yielded a similar number of brightly labeled BrdU+ myocytes as observed at day 38, when BrdU was infused during ISO injury. BrdU infusion during Days 10–17 did not increase the BrdU labeling of the cKit+/CD45− pool (Figure 7), suggesting that cKit+ precursor cells were not proliferative at this time. Therefore, the simplest explanation of our composite results is that new myocytes are generated from a partially committed (to the cardiac lineage) cKit+ precursor cell that was activated (and proliferative) during the ISO-injury phase. During the recovery period these precursor cells appear to commit to the cardiac lineage to form “new” cardiac myocytes. At least some of these new myocytes proliferated one or more times to become brightly BrdU+ when BrdU was infused from Day 10 to 17. Some of the newly formed myocytes derived from BrdU+ cardiac precursors appear to be transiently proliferative (between days 10–38) producing a population of ‘dimly’ BrdU+ myocytes in our pulse-chase protocol. Only by varying the timing of BrdU infusion during injury and recovery could we reach these conclusions (Supplemental Figure XI).
Is there more regeneration in the atria than the ventricle?
Our results suggest that myocyte regeneration (as defined by BrdU+ myocytes) is more robust in the atria than the ventricle. The reasons for these differences are not clear and deserve additional study. Studies by others44 have shown a greater abundance and organized (into niches) environment for cKit+ cardiac precursor cells in the atria than the ventricle. If atrial tissue is a better source of cardiac precursors with great regenerative potential, then our findings suggest that cardiac precursors isolated and expanded from the atria might be a better source of cells for autologous cardiac cell therapy. Our study also suggests that the newly formed myocytes are transiently proliferative and this lowers their BrdU content, reducing the ability to detect BrdU in the myocyte nucleus if short term BrdU labeling is used. More reliable approaches to identify new myocytes and trace their lineage would help future studies.
Limitations
This study was performed with an ISO injury model rather than with a myocardial infarction (MI) model for a number of reasons. Substantial infarcts are not easily obtained in felines because of robust collateral circulation. We recognize that ISO injury has limitations, but it produces a reliable, reproducible, diffuse injury that can be rapidly terminated by removal of ISO minipumps. Therefore, it allowed us to have a defined injury phase and a rapid removal of the noxious stimulus. These characteristics turned out to be significant advantages in the present study.
We are cautious when BrdU is used to identify “new” myocytes because it could also be incorporated into myocyte DNA during repair or in myocytes with DNA synthesis without cytokinesis. Our composite findings are not consistent with these possibilities. We did not see any significant increases in BrdU incorporation (bright or dim) into myocytes during injury, we only observed brightly BrdU+ myocytes when we labeled during Days 10–17 or in pulse-chase studies. The only time we observed dimly BrdU+ myocytes was in our pulse chase studies, when BrdU was not infused during the times when BrdU+ myocytes were observed. These observations are most consistent with the conclusions stated. However, we cannot entirely rule out the caveats raised above.
In summary, our data shows that the adult heart has the ability to generate new myocytes after injury. The contribution of new myocytes to improved function of the ventricle cannot be determined with our approaches, and we may have underestimated the number of new myocytes with the intermittent BrdU labeling strategy employed. Our composite results are most consistent with the hypothesis that ISO injury caused proliferation and activation of the cKit+ cardiac myocyte precursor pool and that these cells differentiated into new cardiac myocytes that proliferate at least one time before they exit the cell cycle. Our results also suggest that there appears to be something about the environment of the ISO injured heart that blunts the differentiation of cardiac precursors into functional new myocytes. If these factors could be identified and eliminated then endogenous cardiac repair might be enhanced.
Supplementary Material
Novelty and Significance.
What is known?
Cardiovascular diseases can induce poor cardiac performance by causing the death of cardiac muscle cells.
Adult cardiac myocytes are terminally differentiated and this limits the ability of the adult heart to increase myocyte number.
The adult heart contains a population of resident progenitor cells with some ability to form new cardiac tissue and these cells are likely to be involved in tissue repair.
What new information does this article contribute?
Catecholamine injury of the adult heart activates cKit+ cardiac progenitor cells but in the presence of high catecholamines these cells do not form new cardiac myocytes.
Reducing high catecholamine levels stimulates cardiac repair and new myocyte formation.
The source of these new myocytes appears to be nonmyocytes, potentially cKit+ precursors that were activated during the injury phase.
Cardiac diseases cause cardiac myocyte death and this can result in heart failure. Therapies for diseases that result from a reduction in myocyte number should include repopulation of the damaged heart with new myocytes. Since adult cardiac myocytes cannot reenter the cell cycle and divide to form new cardiac myocytes, cardiac regeneration is limited. However, recent studies suggest that the heart contains a small population of resident progenitor cells with the ability to form new cardiac tissue. The ability of these cells to repair the injured heart is not well understood, especially in large mammals. This study shows that catecholamine injury induces proliferation (BrdU labeling) of small nonmyocytes in the heart, including cells expressing the stem cell receptor cKit, but during the injury phase these cells do not differentiate into new myocytes. The major new finding of this study is that during cardiac repair the BrdU labeled nonmyocytes appear to differentiate into new cardiac myocytes. These results suggest that the adult heart has a finite capacity for endogenous repair. Future studies that define approaches to enhance the ability of the heart to more effectively repair itself after injury, could lead to novel therapies to prevent or reverse heart failure.
Acknowledgments
Source of Funding: These studies were supported by NIH grants HL089312, HL033921 and HL091799 to SRH and HL095322 to DA.
Abbreviations
- BrdU
5-bromodeoxyuridine
- cKit
Stem Cell Antigen
- ISO
Isoproterenol
- CD45
Protein tyrosine phosphatase, receptor type, C
- ECHO
Echocardiography
- EF
Ejection Fraction
- FS
Fractional Shortening
- LV
Left Ventricle
- LA
Left Atrium
- EDV
End Diastolic Volume
- ESV
End Systolic Volume
- ICa-L
L-type Ca2+ current
Footnotes
Disclosures: None.
Literature Cited
- 1.Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res. 1974;35(suppl II):17–26. [PubMed] [Google Scholar]
- 2.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D’Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, Del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 5.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 8.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 10.Laflamme MA, Myerson D, Saffitz JE, Murry CE. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 11.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood cd34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 12.Mayorga M, Finan A, Penn M. Pre-transplantation specification of stem cells to cardiac lineage for regeneration of cardiac tissue. Stem Cell Rev. 2009;5:51–60. doi: 10.1007/s12015-009-9050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D’Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz-Perez JT, Lee DC, Meyers SN, Davidson CJ, Bonow RO, Wu E. Determinants of myocardial salvage during acute myocardial infarction: Evaluation with a combined angiographic and cmr myocardial salvage index. JACC Cardiovasc Imaging. 2010;3:491–500. doi: 10.1016/j.jcmg.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 16.Zucker IH, Wang W, Brandle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis. 1995;37:397–414. doi: 10.1016/s0033-0620(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 17.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 18.Bristow MR, Kantrowitz NE, Ginsburg R, Fowler MB. Beta-adrenergic function in heart muscle disease and heart failure. J Mol Cell Cardiol. 1985;17 (Suppl 2):41–52. doi: 10.1016/0022-2828(85)90007-0. [DOI] [PubMed] [Google Scholar]
- 19.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leri A, Hosoda T, Rota M, Kajstura J, Anversa P. Myocardial regeneration by exogenous and endogenous progenitor cells. Drug Discov Today Dis Mech. 2007;4:197–203. doi: 10.1016/j.ddmec.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angert D, Houser SR. Stem cell therapy for heart failure. Curr Treat Options Cardiovasc Med. 2009;11:316–327. doi: 10.1007/s11936-009-0032-6. [DOI] [PubMed] [Google Scholar]
- 22.Gyongyosi M, Lang I, Dettke M, Beran G, Graf S, Sochor H, Nyolczas N, Charwat S, Hemetsberger R, Christ G, Edes I, Balogh L, Krause KT, Jaquet K, Kuck KH, Benedek I, Hintea T, Kiss R, Preda I, Kotevski V, Pejkov H, Zamini S, Khorsand A, Sodeck G, Kaider A, Maurer G, Glogar D. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: The mystar prospective, randomized study. Nat Clin Pract Cardiovasc Med. 2009;6:70–81. doi: 10.1038/ncpcardio1388. [DOI] [PubMed] [Google Scholar]
- 23.Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, Sorg RV, Kogler G, Wernet P, Muller HW, Kostering M. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: The iact study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 24.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghodsizad A, Niehaus M, Kogler G, Martin U, Wernet P, Bara C, Khaladj N, Loos A, Makoui M, Thiele J, Mengel M, Karck M, Klein HM, Haverich A, Ruhparwar A. Transplanted human cord blood-derived unrestricted somatic stem cells improve left-ventricular function and prevent left-ventricular dilation and scar formation after acute myocardial infarction. Heart. 2009;95:27–35. doi: 10.1136/hrt.2007.139329. [DOI] [PubMed] [Google Scholar]
- 26.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 27.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimm D, Elsner D, Schunkert H, Pfeifer M, Griese D, Bruckschlegel G, Muders F, Riegger GA, Kromer EP. Development of heart failure following isoproterenol administration in the rat: Role of the renin-angiotensin system. Cardiovasc Res. 1998;37:91–100. doi: 10.1016/s0008-6363(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 30.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–1637. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 31.Rona G, Chappel CI, Balazs T, Gaudry R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol. 1959;67:443–455. [PubMed] [Google Scholar]
- 32.Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: Pathophysiological aspects. Heart Fail Rev. 2007;12:66–86. doi: 10.1007/s10741-007-9007-4. [DOI] [PubMed] [Google Scholar]
- 33.Haft JI. Cardiovascular injury induced by sympathetic catecholamines. Prog Cardiovasc Dis. 1974;17:73–86. doi: 10.1016/0033-0620(74)90039-5. [DOI] [PubMed] [Google Scholar]
- 34.Harris DM, Mills GD, Chen X, Kubo H, Berretta RM, Votaw VS, Santana LF, Houser SR. Alterations in early action potential repolarization causes localized failure of sarcoplasmic reticulum ca2+ release. Circ Res. 2005;96:543–550. doi: 10.1161/01.RES.0000158966.58380.37. [DOI] [PubMed] [Google Scholar]
- 35.Piacentino V, 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Piacentino V, 3rd, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Wilson RM, Kubo H, Berretta RM, Harris DM, Zhang X, Jaleel N, MacDonnell SM, Bearzi C, Tillmanns J, Trofimova I, Hosoda T, Mosna F, Cribbs L, Leri A, Kajstura J, Anversa P, Houser SR. Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res. 2007;100:536–544. doi: 10.1161/01.RES.0000259560.39234.99. [DOI] [PubMed] [Google Scholar]
- 38.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 40.McNiece IK, Briddell RA. Stem cell factor. J Leukoc Biol. 1995;58:14–22. doi: 10.1002/jlb.58.1.14. [DOI] [PubMed] [Google Scholar]
- 41.Kubo H, Berretta RM, Jaleel N, Angert D, Houser SR. C-kit+ bone marrow stem cells differentiate into functional cardiac myocytes. Clin Transl Sci. 2009;2:26–32. doi: 10.1111/j.1752-8062.2008.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, Zhang H, Gao H, Kubo H, Berretta RM, Chen X, Houser SR. {beta}1-adrenergic receptor activation induces mouse cardiac myocyte death through both l-type calcium channel-dependent and -independent pathways. Am J Physiol Heart Circ Physiol. 2010;299:H322–331. doi: 10.1152/ajpheart.00392.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.