Abstract
Rationale
Abnormal behavior of the cardiac ryanodine receptor (RyR2) has been linked to cardiac arrhythmias and heart failure (HF) after Myocardial infarction (MI). It has been proposed that protein kinase A (PKA) hyperphosphorylation of the RyR2 at a single residue, Ser-2808 is a critical mediator of RyR dysfunction, depressed cardiac performance and HF after MI.
Objectives
We used a mouse model (RyRS2808A) in which PKA hyperphosphorylation of the RyR2 at Ser-2808 is prevented to determine whether loss of PKA phosphorylation at this site averts post MI cardiac pump dysfunction.
Methods and Results
MI was induced in WT and S2808A mice. Myocyte and cardiac function were compared in WT and S2808A animals before and after MI. The effects of the PKA activator Isoproterenol (Iso) on L-type Ca2+ current (ICaL), contractions and [Ca2+]I transients were also measured. Both WT and S2808A mice had depressed pump function after MI and were no differences between groups. MI size was also identical in both groups. L type Ca2+ current, contractions, Ca2+ transients and SR Ca2+ load were also not significantly different in WT versus S2808A myocytes either before or after MI. Iso effects on Ca2+ current, contraction, Ca2+ transients and SR Ca2+ load were identical in WT and S2808A myocytes before and after MI at both low and high concentrations.
Conclusions
These results strongly support the idea that PKA phosphorylation of RyR-S2808 is irrelevant to the development of cardiac dysfunction after MI, at least in the mice used in this study.
Keywords: Myocardial infarction, ryanodine receptor, heart failure, PKA hyperphosphorylation
Introduction
Myocardial infarction (MI)1 is a major cause of left ventricular dysfunction (LVD) that leads to heart failure (HF) and arrhythmogenic sudden cardiac death2. The development of HF after MI involves remodeling at the molecular, cellular and organ levels and is characterized by ventricular dilatation, elevated systolic wall stress and poor cardiac pump function. Dysregulated intracellular Ca2+ handling plays a major role in the pathophysiology of contractile, electrophysiological and structural abnormalities that evolve after MI3. There are alterations in the abundance and phosphorylation state of almost all Ca2+ regulatory proteins in the failing heart4–5. Collectively these changes produce alterations in diastolic and systolic Ca2+ and diminished responses to sympathetic regulatory stimulation6–7. There is some evidence, primarily from the Marks laboratory8–9 that hyperphosphorylation of the Ca2+ release channel (Ryanodine receptor; RyR2) plays a significant independent role in the altered Ca2+ regulation of the heart after MI. This idea was tested in the current study.
Cardiac myocyte contraction occurs when the action potential activates voltage-gated L-type Ca2+ channels (LTCCs)10 in the cell membrane and its invaginations, the T-tubules. A small amount of Ca2+ influx through these LTCCs triggers the release of much larger amount of Ca2+ from the sarcoplasmic reticulum (SR) via activation of RyR2. This process is termed Ca2+-induced Ca2+ release (CICR) and RyR2s are essential for this process.
LTCCs in T-tubules are in close proximity to the junctional SR that contains RyRs, and together they form a “couplon”11, where CICR takes place. SR Ca2+ release within a couplon is regenerative and occurs when one or more LTCCs open, to raise local junctional cleft [Ca2+], to promote binding to RyRs, to cause their opening. The resultant flux of SR Ca2+ into the junctional space further elevates cleft Ca2+ and this activates other nearby RyRs to induce locally regenerative Ca2+ release12. In normal hearts, all couplons within a cell appear to be activated with each heart beat11 and it is the sum of the Ca2+ release from all couplons that produces the systolic Ca2+ transient that activates the contractile apparatus13. Relaxation occurs via Ca2+ reuptake into the SR via the SR Ca2+ ATPase (SERCa) and Ca2+ extrusion via the Na/Ca exchanger14–15.
Length independent changes in the force of cardiac contraction (contractility) are critical to normal cardiac function and are largely brought about by altering the amplitude and duration of the systolic [Ca2+] transient. Contractility is efficiently regulated in the normal heart and this primarily results from activation of adrenergic signaling pathways through protein kinase A (PKA). Ca2+ regulatory PKA targets include the LTCC (to increase Ca2+ current and SR Ca2+ load), phospholamban (to increase the activity of SERCa, promote more rapid removal of Ca2+ from the cytoplasm and to help increase SR Ca2+ loading16 and RyR (to increase Ca2+ dependent opening)16–17. The roles of PKA mediated LTCC and SERCa regulation in the normal control of cardiac contractility are widely accepted18–19. However, the independent role of PKA mediated regulation of RyR2 activity as a major regulator of cardiac contractile strength is not broadly supported in the literature20. This effect is predicted from the fact that increasing the Ca2+ dependent opening probability of RyR2 should not increase the number of couplons that release stored Ca2+, since all couplons participate in normal EC coupling21–23.
In cardiac disease, the contractile demands on the heart are persistently increased, and chronic activity of the sympathetic nervous system is needed to maintain cardiac output24. This heightened hyperadrenergic state of the stressed heart is thought to contribute to depression of contractility reserve and cardiac arrhythmias25. Many studies have shown that deranged Ca2+ handling in heart failure involves altered SERCa activity and reduced LTCC abundance with increased activity6, 26–27. The Marks laboratory has proposed that abnormal PKA mediated phosphorylation of RyR2 (at Serine 2808) is centrally involved in defective Ca2+ handling in HF9,28. PKA mediated hyperphosphorylation of RyR at S2808 is proposed to increase RyR Ca2+-dependent opening (leak), leading to depletion of SR Ca2+ stores9. Studies by the Marks group have shown that PKA mediated hyperphosphorylation of RyR2 at serine-2808 is a critical mediator of normal cardiac contractility regulation29–30 and cardiac dysfunction after MI8. These ideas were explored in experiments with genetically modified mice in which Ser-2808 is mutated to alanine (S2808A), to eliminate PKA-mediated phosphorylation29. In previous studies we31 and others32 were unable to show any alterations in sympathetic regulation of cardiac or myocyte function in RyR S2808A mice. Given the critical nature of this topic we studied if prevention of RyR2 phosphorylation after MI preserved cardiac function and reduced premature death, consistent with the development of cardiac dysfunction after MI being dependent on PKA phosphorylation of a single amino acid on RyR2.
In the present study we examined if the absence of PKA phosphorylation of RyR at Ser-2808 (RyRS2808A mice) reduced the damaging effects of MI on cardiac structure and function. Cardiac performance was compared before and after MI in wild type (WT) and RyRS2808A mice. Isoproterenol (Iso) effects on L-type Ca2+ current (ICaL), contractions and [Ca2+]I transients were also measured. Our results showed that both WT and S2808A mice have depressed pump function after MI but we could not show a difference between groups. MI size was identical in both groups. L type Ca2+ current, contractions and [Ca2+]I transients were also not significantly different in WT versus S2808A mice myocytes either before or after MI. Iso effects on Ca2+ current, contraction, and Ca2+ transients were identical in WT and S2808A myocytes before and after MI at both low and high concentrations. These results strongly support the idea that PKA phosphorylation of RyR-S2808 plays little or no role in the development of cardiac dysfunction after MI in these mice.
Methods
All methods have been described in detail in previous reports and are described in detail in the on-line supplement. Briefly, myocardial infarction was induced in wild type and S2808A mice by permanent occlusion of the left anterior descending coronary artery33. Cardiac function was measured with echocardiography. At sacrifice hearts were removed and used for functional studies (isovolumic hearts and isolated myocytes), tissue was used for Western or biochemical analyses, or hearts were fixed and processed for histological studies.
Results
Cardiac Function (in vivo) After MI
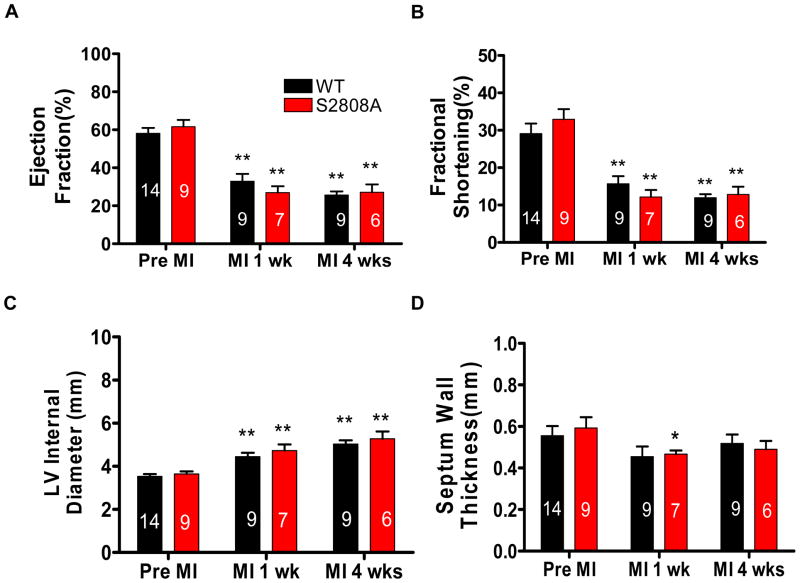
MI was induced by permanent ligation of the LAD. Before MI, there was no significant difference in cardiac function or structure between WT and RyR-S2808A mice. Ejection fraction (EF), fractional shortening (FS), left ventricle internal diameter (LVID), septum wall thickness (SWT) were not different in WT and RyR-S2808A mice (Figure 1). These data are consistent with those we have previously reported31. One week after MI, both groups of mice had significant decreases of EF (pre-MI vs. 1 week post MI, WT: 58.14±2.81 vs. 32.87±3.88; S2808A: 61.59±3.57 vs. 26.90±3.36) and fractional shortening (pre-MI vs. 1 week post MI, WT: 29.08±2.72 vs. 15.68±2.05; S2808A: 32.92±2.73 vs. 12.13±1.90) (Figure 1A and B), but there was no significant difference between WT and S2808A mice. 4 weeks post MI, EF and FS were still depressed and there were no differences between WT and S2808A hearts (WT vs. S2808A, EF: 25.65±1.90 vs. 27.14±4.07; FS: 12.82±2.08 vs. 11.94±0.93). There were significant changes in ventricular geometry and wall thickness after AMI. The LV was dilated in all hearts and chamber size was significantly increased in the first week and 4 weeks after MI (Figure 1C). Septum wall thickness was decreased significantly in the first week after MI in both groups and became stable at the end of 4 weeks (Figure 1D). There were no significant differences in geometry or wall thickness between WT and S2808A mice after MI.
Figure 1. Cardiac structure and function are not different in WT and S2808A animals before or after MI.
Ejection fraction (A), fractional shortening (B) LV internal diameter (C) and septum wall thickness (D) were measured with Echocardiography before and 1,4 weeks after MI. * p< 0.05 and ** p<0.01 between Pre-MI and Post-MI within groups.
Survival and Cardiac Phenotype after MI
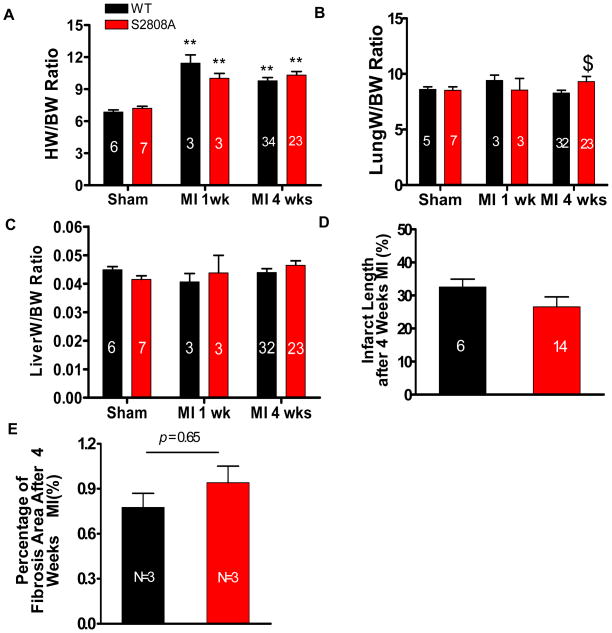
Animals were studied for 4 weeks after MI surgery. There was no significant difference in survival between WT and S2808A mice (87% of all animals survived the 4 week study interval). Heart weight was significantly greater in MI mice compared with sham mice (Figure 2A), but there was no difference between control and S2808A hearts. Lung weight was also significantly increased after MI in S2808A mice, suggesting that more severe heart failure. Liver weight was slightly increased in S2808A mice but there was no significant difference between the groups 4 weeks after MI (Figure 2B and C). These data show that after MI both WT and S2808A mice had cardiac dysfunction with signs of acute heart failure. We were unable to show any significant benefit in survival, structure or function in S2808A versus WT mice.
Figure 2. MI Causes cardiac remodeling and fibrosis in WT and S2808A Hearts.
A–C. Heart weight (HW), lung weight (LW) and Liver weight (LiverW) normalized to body weight (BW) (HW/BW, LW/BW, LiverW/BW) in sham- and MI-operated mice after 1 and 4 weeks surgery. D. Infarct length 4 weeks after MI. E. Fibrotic area (blue) in remote zones of trichrome-stained cardiac histological sections was measured 4 weeks after MI. $ P <0.05 between WT and S2808A mice. **p<0.01 between sham and MI
Infarct Length and Fibrosis were not Different in WT and S2808A Mice
Infarct length was measured 4 weeks after MI. There was no significant difference in infarct length between WT and S2808A mice (WT vs. S2808A: 32.55±2.38% vs. 26.55±3.03%) (Figure 2D). Representative examples of infarcted cardiac tissue and fixed heart tissue sections are shown in Online figure IA–C. We also measured fibrosis in noninfarcted (remote) zones of infarcted hearts. The collagen content in the remote zone was not significantly different in WT and S2808A infarcted hearts (Figure 2E). These data show that WT and S2808A mice had similar infarct size and fibrotic remodeling after MI.
Western Blot Analysis
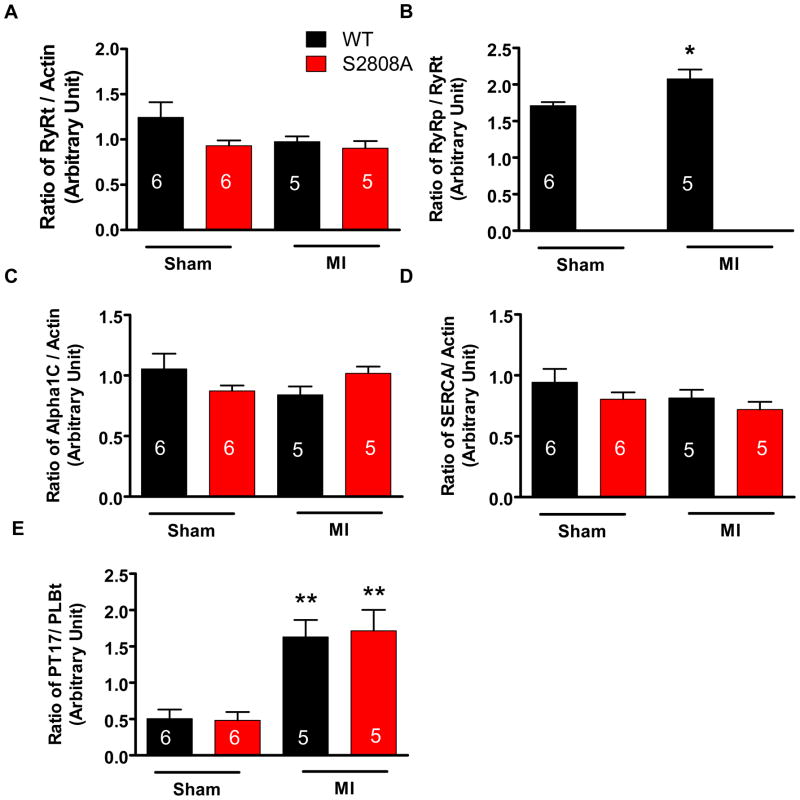
If PKA-mediated RyR-S2808 phosphorylation plays a critical role in either the normal physiology of the mouse heart or in the progression of cardiac dysfunction after MI, then eliminating this site would be expected to induce adaptive alterations in other Ca2+ regulatory proteins, as shown in other studies34. Ca2+ regulatory proteins including the L-type Ca2+ channel α1C subunit, Phospholamban, total and phosphorylated at Thr-17 (PLBt, PT17), RyR2 total protein (RyRt) and RyR2 phosphorylated at S2808 (RyRp) were measured with Western analysis in sham and MI mice (representative blots are shown in Online Figure II). Average data are shown in Figure 3. There was no significant difference in the abundance of any of these Ca2+ regulatory proteins between WT and S2808A mice either in sham or MI groups (Figure 3). There was a significant increase of PLB phosphorylation at Threonine17 in both WT and S2808A hearts after MI but there were no differences between the groups. An antibody against phosphorylated RyR-Ser2808 did not react to RyR-S2808A samples. RyR Ser2808 phosphorylation was significant in sham hearts and increased after MI (Online Figure II and Figure III). These results show that there are no significant differences in Ca2+ regulatory protein abundance or phosphorylation either before or after MI in WT versus S2808A hearts. These results are inconsistent with a significant role for Ser2808 phosphorylation in the normal physiology of the mouse heart or in the deranged structure and function after MI.
Figure 3. Western analysis of Ca2+ regulatory proteins in WT and S2808A mice 4 weeks after MI.
Total RyR (RyRt), S2808 phosphorylated RyR (RyRp), LTCC alpha1C, SERCa2, and phosphorylated Phospholamban (PT17) are shown. The phosphorylated forms of RyR2 and PT17 increased in MI hearts. *p<0.05 and **p<0.01 vs sham.
LTCC Current (ICa,L) in S2808A Mice After MI
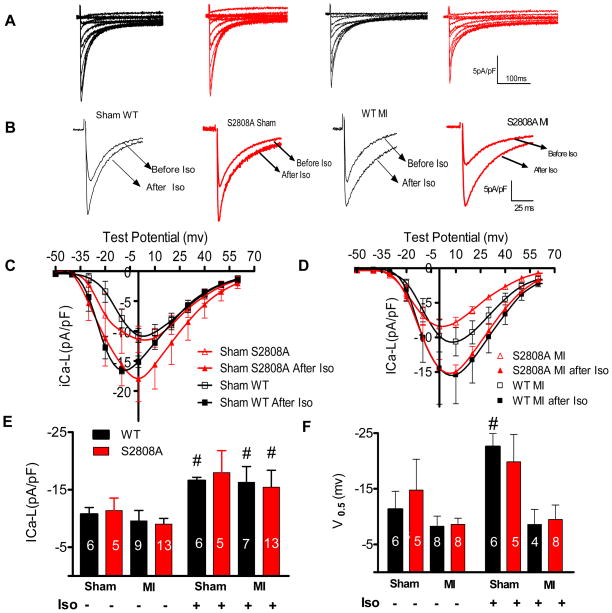
Elimination of a critical element in normal EC coupling or in the regulation of the contractile response to stress would be expected to have a major effect on baseline cardiac function. If not, as we have shown in this and other studies31, then significant adaptation of related molecules would be needed to offset the defective or lost protein. An example would be the conditional NCX knock out mouse34 that survives with adaptive reduction in the L-type Ca2+ current density. If phosphorylation of RyR at Ser-2808 is critical to the regulation of EC coupling in health and disease, then its loss should either induce a significant basal phenotype (which it did not) or there should be adaptive changes in other EC coupling proteins. The major protein that would be expected to change would be the L-type Ca2+ channel, with a known essential role in EC coupling. Therefore, we measured ICa-L density and responsiveness to catecholamines. Peak ICa,L density was not significantly different in WT and S2808A myocytes after sham operations (WT Sham: −10.71±1.78 pA/pF, n=6; S2808A Sham: −11.35±2.18 pA/pF, n=5). Isoproterenol (Iso) increased ICa,L similarly in both WT and S2808A myocytes (sham WT after Iso: 16.55±0.58 pA/pF n=5; sham S2808A after Iso: 17.95± 3.82 pA/pF, n=5)(Figure 4A–C). Iso also caused similar hyperpolarizing shifts (a signature of PKA phosphorylation effect) in the voltage dependence of ICa,L activation in sham control and S2808A myocytes (Online figure III A). After MI, there was also no difference in peak ICa,L in WT and S2808A myocytes ( WT MI: −11.76±2.1pA/pF, n=7; S2808A MI: −9.00 ±0.98 pA/pF, n=7). Iso increased ICa,L significantlyin both WT (−16.2±2.7 pA/pF, n=7) and S2808A (−15.2±3.0 pA/pF, n =7) myocytes, but again no significant difference was detected between groups (Figure 4A, B and D). The voltage dependence of activation of ICa,L in S2808A myocytes was not significantly different than control myocytes either before or after Iso treatment (Online figure III B). Collectively, these data show that there are no significant changes in LTCC density or PKA regulation in RyR-S2808A mice either before or after MI.
Figure 4. L-type Ca2+ current (ICa,L) in Sham and Post-MI myocytes.
A. Representative ICa,L in sham or post-MI myocytes from WT and S2808A hearts. B. Representative ICa,L after 1umol/L isoproterenol in sham or post-MI myocytes from WT and S2808A hearts. C–D. Current-voltage relationships in sham and MI myocytes +/− Iso. E–F. Peak ICa,L and Voltage dependence of ICa,L activation in sham or post-MI myocytes +/− Iso from WT and S2808A hearts. # p<0.05 between +/− Iso.
Contractions and [Ca2+]i Transients in WT and S2808A Mice before and after MI
Twitch contractions and [Ca2+]i transients were studied at 0.5 and 2 Hz in the absence and presence of Iso (representative data are shown in Online Figures IV and V). Low and high concentrations of Iso were tested, since others have suggested that differences in catecholamine responsiveness of WT and S2808A myocytes are only observed at low concentrations29. The rationale for this is unclear since the hyperphosphorylation hypothesis predicts that differences between WT and S2808A animals should be most robust when WT RyR is hyperphosphorylated. Nevertheless we compared the effects of lower [Iso] on WT and RyR-S2808A animals and myocytes
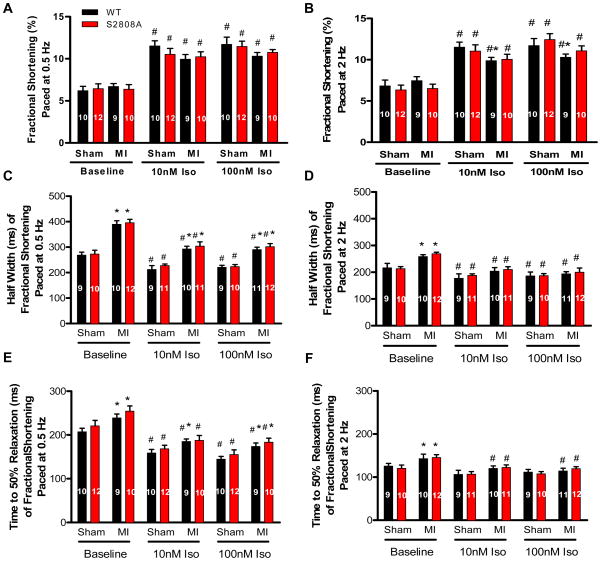
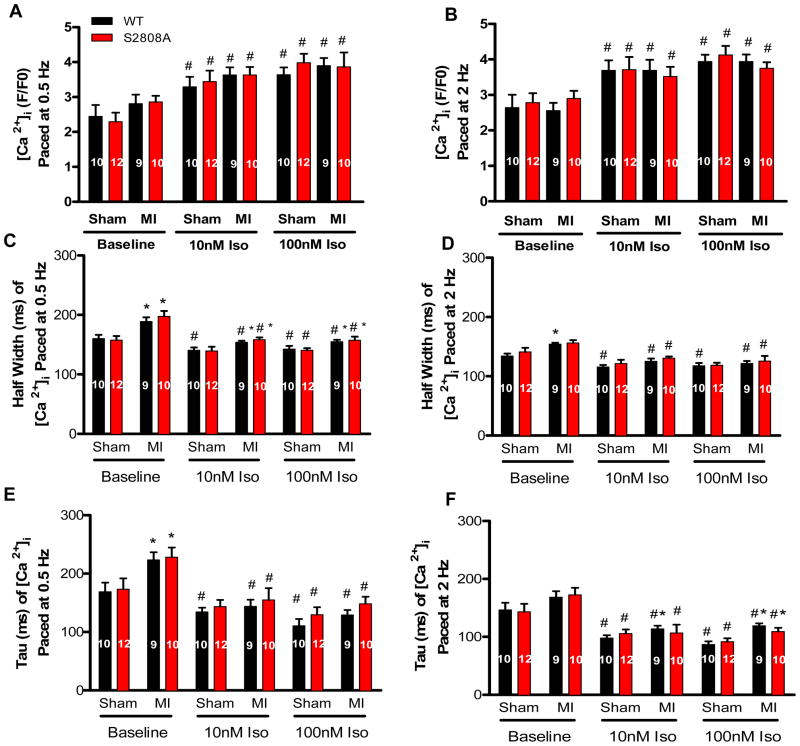
There were no significant differences in the amplitude of contraction (FS) or the [Ca2+]i transients between WT or S2808A myocytes (0.5 and 2 Hz) either before or after MI. Low and high [Iso] caused identical changes in contraction and [Ca2+]i transients in both groups under all conditions tested (Figures 5 and 6). Low [Iso] (10 nM) caused robust increases in contraction and [Ca2+]i transients in all myocytes and there were no differences between WT and S2808A myocytes at any tested pacing rate or with or without MI. High [Iso] did not increase the amplitude of contraction or the [Ca2+]i transient beyond that produced by 10 nM Iso in either group under any conditions tested. These results do not support the hypothesis that the loss of PKA mediated phosphorylation of RyR2 at S2808 has any effect on baseline cardiac myocyte function in the absence or presence of low or high [Iso].
Figure 5. Low and high [Iso] had identical effects on contractions of WT and S2808A myocytes from sham and MI hearts.
A–B. Myocyte contractions −/+ Iso in sham and post-MI myocytes. C–D. Average Half Width (ms) of fractional shortening −/+ Iso in WT and S2808A myocytes from sham or post-MI hearts. E–F. Average Time to 50% Relaxation (ms) of fractional shortening −/+ Iso in WT and S2808A myocytes from sham or post-MI hearts. * P<0.05 sham versus MI, # P<0.05 between −/+ Iso. . 10 and 100 nMol/L Iso were used in this study.
Figure 6. Low and high [Iso] had identical effects on Ca2+ transients of WT and S2808A myocytes from sham and MI hearts.
A–B. Myocyte peak Ca2+ transients −/+ Iso in sham and post-MI myocytes. C–D. Half-width of Ca2+ transients −/+ Iso in myocytes from sham or post-MI hearts. E–F. Average Tau of Ca2+ transients −/+ Iso in myocytes from sham or post-MI hearts. *P<0.05 between sham and MI, # P<0.05 between −/+ Iso.
MI induced changes (half width and tau) in the duration of contractions and [Ca2+]i transients (Figures 5 and 6 and Online Figures IV and V). These changes were identical in WT and S2808A mice. Slowing and prolongation of contraction after MI likely results from changes in a host of processes, but changes in phosphorylation of RyR2 at S2808A does not appear to be a central element of this functional remodeling.
Low and high [Iso] was also tested in-vivo (Online Figure VI) and we could not define a difference in effects in WT and S2808A animals. These results are similar to those we have shown previously in isolated hearts from these animals31. Collectively these results suggest that PKA mediated phosphorylation of RyR-S2808 is not critical modulator of myocyte function in health or disease, at least under our conditions.
SR Ca2+ load in WT and S2808A Myocytes
SR Ca2+ load is often decreased in myocytes isolated from failing hearts35 and this is thought to be, at least in part, due to an abnormal ‘leak” of Ca2+ from the SR. PKA mediated hyperphosphorylation of RyR at S2808 has been proposed as the mechanism that leads to SR ‘leak” and a reduction in SR Ca2+ stores in hyperadrenergic states36, but other studies suggest that CaMKII mediated RyR phosphorylation at an alternative site is the cause of this ‘leak’37. In the present experiments, SR Ca2+ load was measured as the caffeine releasable SR Ca2+ transient (Figure 7). The effects of Iso on SR Ca2+ load were determined in sham and MI myocytes +/− Iso.
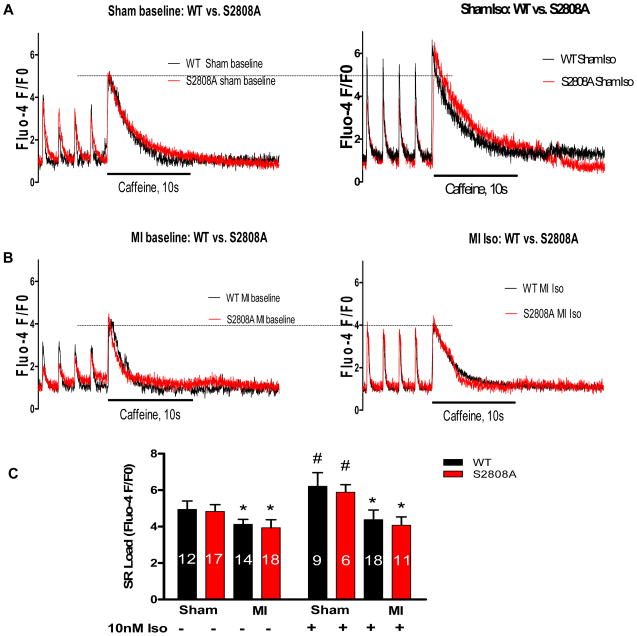
Figure 7. Sarcoplasmic Reticulum (SR) Ca2+ content is identical in WT and S2808A myocytes from Sham and MI hearts.
Caffeine-induced SR Ca2+ release was measured in WT and S2808A myocytes +/− Iso (10 nM) from sham or MI mice. A–B. Examples of Ca2+ transients elicited by 0.5Hz of electrical field stimulation (the first four traces) followed by Ca2+ transients induced by caffeine (the fifth large trace; the peak indicates SR Ca2+ content). C. Average amplitudes of caffeine induced Ca2+ transients −/+ Iso in WT and S2808A myocytes. *p<0.05 between sham and MI and # p<0.05 between −/+ Iso.
There were no significant differences in SR Ca2+ load or the decay rate of these transients (not shown) between WT and S2808A myocytes under control conditions (Figure 7C). Iso caused a significant increase in SR Ca2+ load in both WT and S2808A myocytes (Figure 7C) and the extent or rate of decay (not shown) were not different.
MI caused a significant reduction in SR Ca2+ load in both WT and S2808A myocytes and there were no differences between groups (Figure 7C). Iso had very small effects on SR Ca2+ load in MI myocytes, and again there were no differences between groups (Figure 7C). These results do not support the hypothesis that hyperphosphorylation of RyR at S2808 is critical to the SR Ca2+ leak that develops after MI.
Discussion
This study evaluated the hypothesis that PKA – mediated phosphorylation of RyR-S2808 is critical for the normal regulation of cardiac contractility and RyR hyperphosphorylation is critical for the cardiac dysfunction that develops after MI. Although the RyR hyperphosphorylation hypothesis has been supported by some previous studies8, the topic is still controversial38–39 and many critical issues have not been resolved.
In the normal heart, contractility is primarily regulated by the sympathetic nervous system (SNS). To increase myocyte contractility sympathetic signaling cascades activate protein kinase A (PKA). The PKA target proteins that become phosphorylated include the LTCC, PLB, RyR, and troponin C. Increased myocyte contractility involves an increased amplitude and reduced duration of the systolic [Ca2+] transient. The contribution of increased Ca2+ influx through the phosphorylated LTCC complex and increased Ca2+ uptake by the SR after PLB phosphorylation are well described in many laboratories40. The idea that PKA mediated phosphorylation of RyR plays a critical role in the regulation of cardiac contractility is less well accepted and is not supported by modeling studies41. Studies with a S2808A mouse from the Marks group support a significant role for RyR2 phosphorylation at S2808 in the normal regulation of cardiac contractility29–30 and in the response of the heart to pathological stress8. The present results with a similar mouse are inconsistent with these previous reports.
MI reduces functional cardiac mass and a hyper-adrenergic state is required to maintain cardiac pump function. The persistent hyperadrenergic state eventually contributes to abnormal myocyte function42 and death43 which precipitates heart failure. The question posed here was if preventing phosphorylation of RyR2 at a single amino acid is sufficient to reduce adverse functional and structural remodeling after MI. Our results suggest that eliminating PKA-mediated phosphorylation at RyRS2808 has no impact on remodeling after MI, at odds with results of studies discussed below.
A number of reports8,17,30,44 strongly support the hypothesis that PKA mediated phosphorylation of RyR at S2808 causes the RyR-associated protein FKBP12.6 (also called Calstabin) to dissociate from RyR and that this increases the Ca2+ dependent opening of RyR. Hyperphosphorylation of RyR2 by PKA has been proposed as the cause of RyR “leak” that leads to reduced SR content and contractility in HF8. However, there is equally compelling data suggesting that PKA-mediated phosphorylation of RyR at S2808 has little or no role in the regulation of myocyte function in the normal or diseased heart31–32, 38. There are studies showing that there are no appreciable differences in the level of phosphorylation of RyR2 and basal channel activity between failing and non-failing canine hearts38. S2808A animals with pressure overload did not have better function than WT animals32. In addition we have previously suggested that phosphorylation of RyR2 at Ser-2808 is not involved in the adrenergic regulation of normal cardiac contractility31. These data are consistent with a detailed modeling study from the Bers group23 that predicts that increasing RyR Ca2+-mediated opening should have very little effect on contractility. In addition others have shown that CaMKII-mediated phosphorylation of RyR at S2814 rather than PKA mediated phosphorylation of S2808 is responsible for regulation of RyR function45 and that PKA phosphorylation of RyR does not induce significant displacement of FKBP from RyR246. The results we report in the present investigation are consistent with those that have been unable to document any significant role for RyRS2808 phosphorylation in health and disease.
We were unable to find any significant difference in any Ca2+ regulatory process in S2808A mice. Our logic for looking at other molecules involved in EC coupling and the regulation of cardiac contractility in S2808A mice was that since everyone agrees that S2808A mice have no basal phenotype, either S2808 phosphorylation is unimportant or there are adaptive changes in other proteins involved in the regulation of myocyte Ca. An example of this in mouse models is the conditional cardiac Na/Ca2+ exchanger (NCX) KO that was expected to die after eliminating the major Ca2+ efflux mechanism in the normal heart, but did not34. It was shown that these mice were able to live with a large reduction in Ca2+ efflux by an adaptive reduction in Ca2+ influx through the L-type Ca2+ channel34. If RyR-S2808 phosphorylation is a critical component of the physiological regulation of cardiac contractile function then related adaptations would be expected since there is no basal phenotype. We were unable to detect any change in abundance, phosphorylation or function of any Ca2+ regulatory protein involved in Ca2+ entry or efflux and SR Ca2+ uptake, storage and release.
We were unable to detect any beneficial effect of loss of RyRS2808 phosphorylation after MI. Importantly we were able to show reduced SR Ca2+ loading in MI myocytes, but there were similar reductions in WT and S2808A myocytes. These results support the idea that alterations in SR Ca2+ uptake, storage and release are involved in post MI functional remodeling, but do not support a role for hyperphosphorylation of RyR S2808 in these changes.
It is difficult to find common ground with those studies that have reported such strong evidence for the RyRS2808 hyperphosphorylation hypothesis8. A recent report29 suggested that the reason for the differences between those studies finding and not finding different catecholamine effects in WT versus S2808A mice is related to the fact that studies not detecting differences used ISO concentrations that were too high. Interestingly there was already data in the literature using low [Iso] that did not support this notion. A previous study31 showed that exposing WT and RyR2808A isovolumic hearts to 10 nM Iso caused identical increases in contractility in both groups while when a 10 fold higher concentration (100 nM) of Iso was used in the recent studies from the Marks laboratory, reduced Iso effects were seen in S2808A hearts. Because of this concentration issue we tested the effects of lower [Iso] on isolated myocyte function and could not find any difference in effects in WT and S2808A cells. Therefore, our present and previous results do not support the idea that low doses of catecholamines are required to demonstrate differences in cardiac function related to RyR-S2808 phosphorylation. We are at a loss to find an explanation for the differences between our data and those studies documenting a critical role for this single amino acid in the regulation of cardiac function in health and disease. One potential explanation for the differences in the literature could be that the two sets of S2808A mice have a different genetic background.
In summary, our results do not support a major role of S2808 PKA phosphorylation on myocyte contractility regulation in health and disease.
Supplementary Material
Novelty and Significance.
What is known?
After myocardial infarction (MI) sympathetic regulatory pathways are activated (with heightened activity of protein kinase A; PKA) to increase the contractility of surviving myocytes and thereby maintain cardiac pump performance.
Some studies propose that PKA-mediated hyperphosphorylation of a single serine (S2808) on the ryanodine receptor (RyR; responsible for Ca2+ release from the sarcoplasmic reticulum (SR)) is a critical determinant of myocyte contractile and electrical dysfunction after MI.
Other studies have suggested that phosphorylation of RyRS2808 is not an essential regulator of RyR function in health and disease.
What new information does this article contribute?
Preventing phosphorylation of RyR at S2808 (with an alanine substitution; RyRS2808A) does not induce a significant change in cardiac function or in the contractile response to adrenergic agonists, at either low or high concentrations.
MI induced similar reductions in cardiac function and myocyte adrenergic responsiveness in wild type and RyRS2808A mice.
Changes in Ca2+ regulatory processes after MI, including a reduction in SR Ca2+ stores, were similar in wild type and RyRS2808A mice, documenting the lack of functional benefit of preventing PKA phosphorylation of S2808 after MI.
MI causes regional cellular death in the heart and results in depressed cardiac pump function. Heightened adrenergic signaling with activation of PKA helps maintain cardiac function after MI but eventually cause myocyte dysfunction. Some investigators have suggested that PKA mediated phosphorylation of RyR at S2808 is critical to the normal regulation of cardiac contractile function and that hyperphosphorylation of S2808 is responsible for SR disturbances that reduce contractility and promote arrhythmias in disease, making hyperphosphorylation of S2808 a therapeutic target. The results of our study show that RyRS2808 phosphorylation increases after MI and that SR Ca2+ stores are reduced, consistent with SR Ca2+ “leak” hypothesis. However, preventing RyRS2808 phosphorylation had no effect on adrenergic regulation of myocyte or heart function in the normal heart, and it did not prevent depressed function after MI, or reductions in SR Ca2+ loading after MI. Our results show that, at least under the experimental conditions used, PKA mediated phosphorylation of RyR at S2808 is not a critical regulator of cardiac contractile function in the normal heart and that it might not be the mechanism that reduces SR Ca2+ loading and myocyte contractility reserve after MI.
Acknowledgments
Sources of Funding
Supported by NIH HL089312, HL033921, HL091799 to SRH
Non-standard Abbreviations and Acronyms
- MI
Myocardial Infarction
- HF
Heart Failure
- CHF
Congestive Heart Failure
- SR
Sarcoplasmic Reticulum
- LAD
Left Anterior Descending Coronary Artery
- LVID
Left Ventricle Internal Diameter
- PWT
Posterior Wall Thickness
- LVDP
Left Ventricular Developed Pressure
- ECHO
Echocardiography
- Iso
Isoproterenol
- RyR
Ryanodine receptor
- ICaL
L-type Ca2+ current
Footnotes
Disclosures
None
References
- 1.Abbate A, Bussani R, Amin MS, Vetrovec GW, Baldi A. Acute myocardial infarction and heart failure: Role of apoptosis. Int J Biochem Cell Biol. 2006;38:1834–1840. doi: 10.1016/j.biocel.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Vetter R, Rehfeld U, Reissfelder C, Weiss W, Wagner KD, Gunther J, Hammes A, Tschope C, Dillmann W, Paul M. Transgenic overexpression of the sarcoplasmic reticulum ca2+atpase improves reticular ca2+ handling in normal and diabetic rat hearts. FASEB J. 2002;16:1657–1659. doi: 10.1096/fj.01-1019fje. [DOI] [PubMed] [Google Scholar]
- 3.Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: Altered excitation-contraction coupling. Circulation. 2001;104:688–693. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- 4.Kubo H, Margulies KB, Piacentino V, 3rd, Gaughan JP, Houser SR. Patients with end-stage congestive heart failure treated with beta-adrenergic receptor antagonists have improved ventricular myocyte calcium regulatory protein abundance. Circulation. 2001;104:1012–1018. doi: 10.1161/hc3401.095073. [DOI] [PubMed] [Google Scholar]
- 5.Yano M, Ikeda Y, Matsuzaki M. Altered intracellular ca2+ handling in heart failure. J Clin Invest. 2005;115:556–564. doi: 10.1172/JCI24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houser SR, Piacentino V, 3rd, Mattiello J, Weisser J, Gaughan JP. Functional properties of failing human ventricular myocytes. Trends Cardiovasc Med. 2000;10:101–107. doi: 10.1016/s1050-1738(00)00057-8. [DOI] [PubMed] [Google Scholar]
- 7.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 8.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel pka phosphorylation: A critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks AR. Ryanodine receptors/calcium release channels in heart failure and sudden cardiac death. J Mol Cell Cardiol. 2001;33:615–624. doi: 10.1006/jmcc.2000.1343. [DOI] [PubMed] [Google Scholar]
- 10.Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986;18:691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Bridge JH. Ca2+ sparks in rabbit ventricular myocytes evoked by action potentials: Involvement of clusters of l-type ca2+ channels. Circ Res. 2003;92:532–538. doi: 10.1161/01.RES.0000064175.70693.EC. [DOI] [PubMed] [Google Scholar]
- 12.Taur Y, Frishman WH. The cardiac ryanodine receptor (ryr2) and its role in heart disease. Cardiol Rev. 2005;13:142–146. doi: 10.1097/01.crd.0000128709.84812.86. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin i phosphorylation increases the rate of cardiac muscle relaxation. Circ Res. 1995;76:1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- 14.Barry WH, Bridge JH. Intracellular calcium homeostasis in cardiac myocytes. Circulation. 1993;87:1806–1815. doi: 10.1161/01.cir.87.6.1806. [DOI] [PubMed] [Google Scholar]
- 15.Kohomoto O, Levi AJ, Bridge JH. Relation between reverse sodium-calcium exchange and sarcoplasmic reticulum calcium release in guinea pig ventricular cells. Circ Res. 1994;74:550–554. doi: 10.1161/01.res.74.3.550. [DOI] [PubMed] [Google Scholar]
- 16.Xiao B, Tian X, Xie W, Jones PP, Cai S, Wang X, Jiang D, Kong H, Zhang L, Chen K, Walsh MP, Cheng H, Chen SR. Functional consequence of protein kinase a-dependent phosphorylation of the cardiac ryanodine receptor: Sensitization of store overload-induced ca2+ release. J Biol Chem. 2007;282:30256–30264. doi: 10.1074/jbc.M703510200. [DOI] [PubMed] [Google Scholar]
- 17.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. Pka phosphorylation dissociates fkbp12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 18.Marks AR. Intracellular calcium-release channels: Regulators of cell life and death. Am J Physiol. 1997;272:H597–605. doi: 10.1152/ajpheart.1997.272.2.H597. [DOI] [PubMed] [Google Scholar]
- 19.Marks AR. Cardiac intracellular calcium release channels: Role in heart failure. Circ Res. 2000;87:8–11. doi: 10.1161/01.res.87.1.8. [DOI] [PubMed] [Google Scholar]
- 20.Eschenhagen T. Is ryanodine receptor phosphorylation key to the fight or flight response and heart failure? J Clin Invest. 2010;120:4197–4203. doi: 10.1172/JCI45251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginsburg KS, Bers DM. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled sr ca2+ load and ca2+ current trigger. J Physiol. 2004;556:463–480. doi: 10.1113/jphysiol.2003.055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisner DA, Trafford AW. No role for the ryanodine receptor in regulating cardiac contraction? News Physiol Sci. 2000;15:275–279. doi: 10.1152/physiologyonline.2000.15.5.275. [DOI] [PubMed] [Google Scholar]
- 23.Shannon TR, Wang F, Bers DM. Regulation of cardiac sarcoplasmic reticulum ca release by luminal [ca] and altered gating assessed with a mathematical model. Biophys J. 2005;89:4096–4110. doi: 10.1529/biophysj.105.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Mann DL, Bristow MR. Mechanisms and models in heart failure: The biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 26.Hu ST, Liu GS, Shen YF, Wang YL, Tang Y, Yang YJ. Defective ca(2+) handling proteins regulation during heart failure. Physiol Res. 2011;60:27–37. doi: 10.33549/physiolres.931948. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Piacentino V, 3rd, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 28.Marks AR, Priori S, Memmi M, Kontula K, Laitinen PJ. Involvement of the cardiac ryanodine receptor/calcium release channel in catecholaminergic polymorphic ventricular tachycardia. J Cell Physiol. 2002;190:1–6. doi: 10.1002/jcp.10031. [DOI] [PubMed] [Google Scholar]
- 29.Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120:4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, Dura M, Chen BX, Marks AR. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonnell SM, Garcia-Rivas G, Scherman JA, Kubo H, Chen X, Valdivia H, Houser SR. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ Res. 2008;102:e65–72. doi: 10.1161/CIRCRESAHA.108.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase a phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 33.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of na+-ca2+ exchange: Cardiac-specific knockout of ncx1. Circ Res. 2004;95:604–611. doi: 10.1161/01.RES.0000142316.08250.68. [DOI] [PubMed] [Google Scholar]
- 35.Piacentino V, 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 36.Marks AR, Reiken S, Marx SO. Progression of heart failure: Is protein kinase a hyperphosphorylation of the ryanodine receptor a contributing factor? Circulation. 2002;105:272–275. [PubMed] [Google Scholar]
- 37.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 38.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase a phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- 40.Balaguru D, Haddock PS, Puglisi JL, Bers DM, Coetzee WA, Artman M. Role of the sarcoplasmic reticulum in contraction and relaxation of immature rabbit ventricular myocytes. J Mol Cell Cardiol. 1997;29:2747–2757. doi: 10.1006/jmcc.1997.0509. [DOI] [PubMed] [Google Scholar]
- 41.Puglisi JL, Wang F, Bers DM. Modeling the isolated cardiac myocyte. Prog Biophys Mol Biol. 2004;85:163–178. doi: 10.1016/j.pbiomolbio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 43.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The promise study research group. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 44.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. Fkbp12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 45.Huke S, Bers DM. Ryanodine receptor phosphorylation at serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun. 2008;376:80–85. doi: 10.1016/j.bbrc.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, Fruen BR, Bers DM. Kinetics of fkbp12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on ca sparks. Circ Res. 2010;106:1743–1752. doi: 10.1161/CIRCRESAHA.110.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.