Abstract
This functional magnetic resonance imaging (fMRI) study examined medial prefrontal cortex (mPFC) activity as young and older participants rated an unknown young and older person, and themselves, on personality characteristics. For both young and older participants, there was greater activation in ventral mPFC (anterior cingulate) when they made judgments about own-age than other-age individuals. Additionally, across target age and participant age, there was greater activity in a more anterior region of ventral mPFC (largely medial frontal gyrus, anterior cingulate) when participants rated others than when they rated themselves. We discuss potential interpretations of these findings in the context of previous results suggesting functional specificity of subregions of ventral mPFC.
Keywords: medial prefrontal cortex, own-age effects, self-referential processing, similarity, ambiguity
People pay greater attention to, and have better memory for, own-age than other-age faces (Anastasi & Rhodes, 2005; Baeckman, 1991; Ebner & Johnson, in press; Harrison & Hole, 2009; He, Ebner, & Johnson, in press). One possibility is that this “own-age bias” is related to greater self-relevance of own-age as compared to other-age individuals. If so, functional magnetic resonance imaging (fMRI) should identify brain regions associated with self-referential processing that are more active when people think about own-age than other-age individuals.
Activity in medial prefrontal cortex (mPFC), including anterior cingulate cortex, is associated with a wide range of self-referential thinking (see Amodio & Frith, 2006; Northoff et al., 2006; Van Overwalle, 2009, for reviews). Furthermore, there is increasing evidence suggesting functional specificity within mPFC, in that subregions of mPFC are associated with different aspects or dimensions of self-relevant thinking (Amodio & Frith, 2006; Benoit, Gilbert, Volle, & Burgess, 2010; D’Argembeau et al., 2007; D’Argembeau, Xue, Lu, Van der Linden, & Bechara, 2008; Jenkins & Mitchell, in press; Johnson, Nolen-Hoeksema, Mitchell, & Levin, 2009; Johnson et al., 2006; Kelley et al., 2002; J. P. Mitchell, Banaji, & Macrae, 2005; Moran, Macrae, Heatherton, Wyland, & Kelley, 2006; Northoff et al., 2006; Ochsner et al., 2004, 2005; Packer & Cunningham, 2009). For example, ventral mPFC is more active when thinking about self and familiar or similar others, and dorsal mPFC is more active when thinking about unfamiliar or dissimilar others (Amodio & Frith, 2006; J. P. Mitchell, Macrae, & Banaji, 2006). In J. P. Mitchell et al. (2006), similarity was manipulated by asking participants to think about and rate themselves and two individuals (one described as having liberal views and one as having conservative views) on how they would feel about a range of opinions, likes and dislikes (e.g., to enjoy having a roommate from a different country; to drive a small car entirely for environmental reasons). The more liberal participants showed greater activity in ventral mPFC when thinking about the liberal than the conservative target, and the less liberal participants showed the reverse. In addition, the more liberal participants showed greater activity in dorsal mPFC when thinking about the conservative target than the liberal target, and the less liberal participants showed the reverse.
Like young adults, older adults show mPFC activity during self-referential processing (Gutchess, Kensinger, & Schacter, 2007; K. J. Mitchell et al., 2009). But no study, to our knowledge, has investigated age-group differences in mPFC when participants think about young versus older individuals. We adapted the paradigm used by J. P. Mitchell et al. (2006) to explore this question. Young and older participants were asked to make judgments about personality characteristics of an unknown young and an unknown older adult individual, as well as about themselves. If age, like political orientation, induces a sense of similarity with another person, then we would expect greater activity in ventral mPFC when participants think about an individual similar (versus dissimilar) to themselves in age and greater activity in dorsal mPFC when they think about an individual dissimilar (versus similar) to themselves in age. Such findings would support the hypothesis that there is a functional ventral/dorsal subdivision of mPFC related to similarity to the self.
In addition, we compared activity in mPFC when participants thought about others, regardless of age, to when they thought about themselves. Previous studies suggest that the amount of activity in ventral mPFC is greater when thinking about oneself than thinking about others, both similar and dissimilar to the self (e.g., in political views, self > similar other > dissimilar other; J. P. Mitchell et al., 2006). But, there is also evidence that activity in ventral mPFC increases when evaluative judgments must be made under ambiguous conditions (Jenkins & Mitchell, 2010) or when decisions must be made under greater uncertainty (Stern, Gonzales, Welsh, & Taylor, 2010). In our study, participants were not given any information about the young and older target individuals, other than their pictures. Greater activity in ventral mPFC when thinking about oneself than others would suggest that similarity is the more important factor in determining activity in ventral mPFC, whereas greater activity when thinking about others than oneself would suggest that uncertainty is the more important factor (presumably there is more uncertainty about the personality characteristics of an unknown other than one’s self).
Method
Participants
Participants were healthy college students (n = 12 [6 females], M age = 21.7 years [SD = 2.1; range = 19–26]) and healthy and active, independently living older adults (n = 12 [4 females], M age = 69.9 years [SD = 6.7; range = 62–85]). Participants reported being in good health, with no history of stroke, heart disease, or primary degenerative neurological disorder, and were right-handed, native English speakers. They all had normal, or corrected to normal, vision and none were taking psychotropic medications. Young and older participants did not differ on self-ratings of physical or emotional health (scale 1–5, with 5 = excellent), when asked how they were feeling the day of the scan (Physical: MYoung Participants= 4.2 [SD = 0.7], MOlder Participants= 4.5 [SD = 0.5]; Emotional: MYoung Participants= 4.1 [SD = 0.9], MOlder Participants= 4.3 [SD = 0.5]). There were no age-group differences in an abbreviated version of the verbal subscale of the Wechsler Adult Intelligence Scale (WAIS; Wechsler, 1987; MYoung Participants= 22.5 [SD = 5.2], MOlder Participants= 21.4 [SD = 6.0]; max possible = 30) or education level (reported in years, 12 = high school diploma; MYoung Participants= 14.8 [SD = 1.5], MOlder Participants= 15.7 [SD = 2.7]) (all p’s > .05). Older participants scored high on the Folstein Mini Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975; M = 28.3 [SD = 1.5]; max possible = 30). All participants were compensated for participation. The Human Investigation Committee of Yale University Medical School approved the protocol; informed consent was obtained from all participants.
Design and Stimuli
The design was a mixed 2 (Participant Age Group: young, older) X 3 (Target Type: Young, Older, Me) factorial design, with Participant Age as a between-subjects factor and Target Type as a within-subjects factor. Participants saw three types of stimuli: A picture of a young person, a picture of an older person, and a black silhouette superimposed with the word ‘ME.’ On each trial they also saw a personality statement and rated how much they thought the statement applied to the person shown or to themselves.
Across the experiment, stimuli were six pictures of young faces and six of older faces, all with neutral expressions, from the standardized and validated FACES database (Ebner, Riediger, & Lindenberger, 2010). Pictures were full-color frontal head shots on grey backgrounds (see Figure 1 for samples; FACES item numbers of the twelve stimuli used across the experiment were 5, 8, 15, 60, 69, 89, 98, 102, 131, 133, 153, 182). There were no age-group differences in attractiveness, distinctiveness, or facial expression of these faces as rated by an independent sample of young and older adults. Each participant saw only two pictures of their own gender, one young and one older. Particular faces were counterbalanced across participants. Consistent with the procedure of J. P. Mitchell et al. (2006), intermixed between face trials, a gender-neutral black silhouette superimposed with the word ‘ME’ in white letters appeared on a grey background. In addition, 150 personality statements adapted from the Revised NEO Personality Inventory (NEO-PI-R; Costa & McCrae, 1992) were used, divided into three sets of 50 statements (see Table 1 for examples). Each participant saw one set of 50 statements (10 of each NEO-PI-R personality trait); each statement appeared once for each target picture (Young, Older, Me). The three sets were counterbalanced across participants. Trials were pseudo-randomly presented with not more than two pictures of the same face or the ‘ME’ picture in succession and not more than two statements referring to the same personality trait in sequence. Stimulus presentation and response collection were controlled using E-Prime (Schneider, Eschman, & Zuccolotto, 2002).
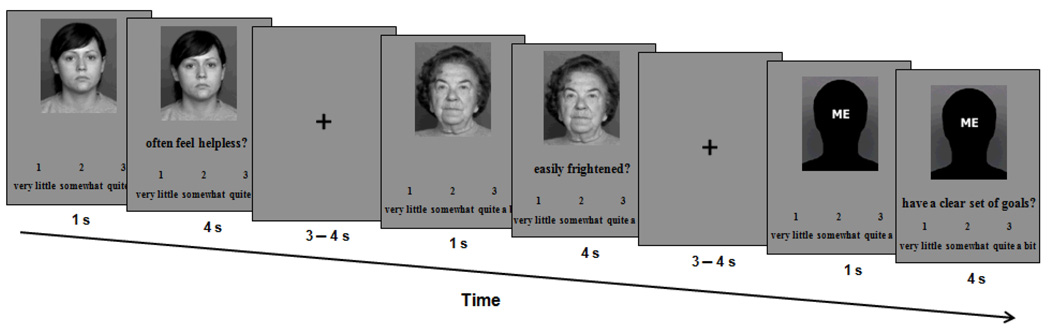
Figure 1.
Trial event timing for the Personality Rating Task (female version). Faces shown are sample faces from the FACES database; see text for list of numbers of faces actually used in the experiment. Pictures were presented in color.
Table 1.
Example personality statements
| apprehensive about the future |
| keep a cool head in emergencies |
| work slowly but steadily |
| have often been leader of groups |
| try to forgive and forget insults |
| known for generosity |
| try to do jobs carefully |
| always able to get organized |
| empathize with feelings of others |
| often experience strong emotions |
Note. Across the experiment, 150 personality statements were presented. Fifty statements were presented to each participant; selection of statements was counterbalanced across participants.
Procedure
Figure 1 shows the Personality Rating Task (female version) and event timing. On each trial, participants first saw a target picture (Young, Older, Me) with the rating scale below it. After 1 second, a statement appeared on the screen between the picture and the scale for 4 seconds, followed by a crosshair for an average of 3.5 seconds (jittered: 3.00, 3.25, 3.50, 3.75, or 4.00 seconds). Trials were pseudo-randomly presented, and displaying the face alone before the statement allowed participants to orient their thinking to the appropriate person before making their rating about the particular personality statement. Participants were asked to infer each person’s personality as accurately as possible from the picture, and to rate each statement (1 = very little, 2 = somewhat, 3 = quite a bit) with respect to how much they thought the statement would apply to the person or, on Me trials, to themselves. Participants indicated their ratings by pressing one of three buttons on the response pad they held in their right hand using their index (1), middle (2), or ring (3) finger. They were told not to dwell on the item but to give a spontaneous response to the statement as soon as they had made their decision and while the picture was still present. Ratings and response latencies were collected.
Before participants entered the scanner, they practiced the task for 10 trials with target individuals and personality statements that were different from those used during the scan session. Instructions were clarified, if necessary. In the scanner, there were 3 runs of 50 trials each, for a total of 150 trials, resulting in 50 trials per participant per target type (Young, Older, Me).
About five minutes after the scan, in a separate testing room, participants rated how generally similar they thought each of the two persons they had seen during the scan was to them on a scale ranging from 1 (very dissimilar) to 7 (very similar). They also responded to a short demographic questionnaire including items on physical and emotional health, and completed the verbal subscale of the WAIS. Older participants also were administered the MMSE.
Imaging Details
Images were acquired using a 1.5T Siemens Sonata scanner at Yale’s Magnetic Resonance Research Center. After anatomical localizer scans, functional images were acquired with a single-shot echoplanar gradient-echo pulse sequence (TR=2000 ms, TE=35 ms, flip angle=80 degrees, FOV=240mm). The 24 oblique axial slices were 3.8 mm thick with an in-plane resolution of 3.75 X 3.75 mm; they were aligned with the AC-PC line. Each run began with 4 discarded acquisitions to allow tissue to reach steady state magnetization, and was followed by an approximately 1 minute rest interval.
fMRI Analyses
Data were analyzed using Statistical Parametric Mapping (SPM5; Wellcome Department of Imaging Neuroscience). Pre-processing included slice timing correction, motion correction, co-registration of functional images to the participant’s anatomical scan, spatial normalization and smoothing (9mm full-width half maximum [FWHM] Gaussian kernel). Spatial normalization used a study-specific template brain composed of the average of the young and older participants’ T1 anatomical images (the detailed procedure is available from the authors). Functional images were re-sampled to 3mm isotropic voxels at the normalization stage.
Standard whole-brain general linear model (GLM) analyses were conducted. First-level, single-subject statistics were modeled by convolving each trial with the SPM canonical hemodynamic response function to create a regressor for each condition (Target Type: Young, Older, Me). Parameter estimates (beta images) of activity for each condition and each participant were then entered into a second-level group whole-brain random-effects analysis using a mixed 2 (Participant Age Group) X 3 (Target Type) ANOVA, with Participant Age Group as a between-subjects factor and Target Type as a within-subjects factor. From within this group model, contrasts were conducted: (1) to examine interactions between Participant Age Group (Young, Older) and Target Type (Young, Older), and (2) to compare Other trials (collapsed across Young and Older targets) versus Me trials across all participants. To ensure both an acceptable Type I error rate and a reasonable balance between Type I and Type II errors, both contrasts were conducted at the threshold of 10 contiguous voxels each significant at p < .005 (Lieberman & Cunningham, 2009; see also Forman et al., 1995).
For each region of activation in mPFC identified by either of the two contrasts examined (i.e., Own-age targets > Other-age targets for participant age groups separately, and Other targets > Me across all participants, respectively), beta values were extracted for each participant from a 5mm sphere around the local maximum and averaged to produce a single value for each target type. These values are depicted in the bar graphs of Figures 2 and 3. In the fashion of follow-up F- and t-tests in ANOVA, subsequent statistical comparisons of these values were conducted outside SPM to aid interpretation of the activations.
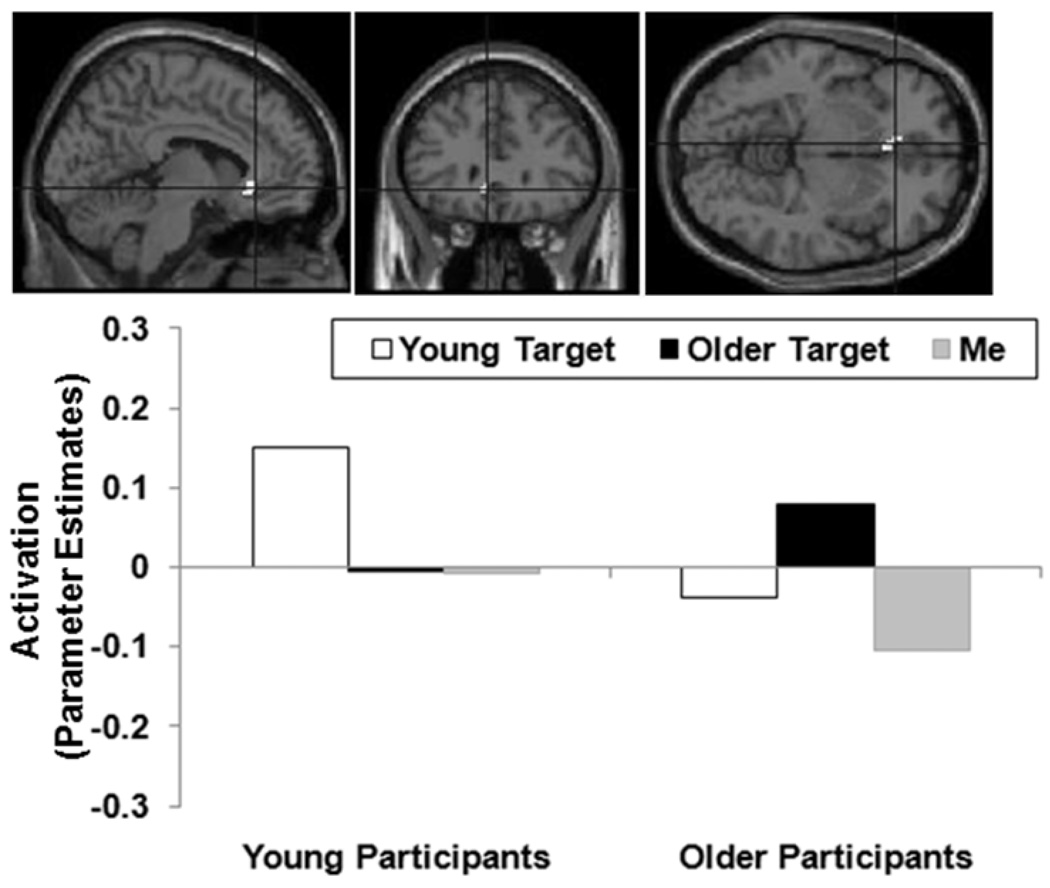
Figure 2.
A region of ventral mPFC (anterior cingulate; BA 24; MNI: x = −6, y = 30, z = −6; cluster size 11 voxels; maximum F-value for the cluster 12.95) showing a Participant Age Group × Target Type interaction. The region of activation represents the F-map of the contrast; it is displayed on the standard reference brain in SPM. The crosshair indicates the peak voxel (local maximum) within the region of activation. Bar graphs show the mean parameter estimates (beta values) separately for age groups and target types; betas for this region of activation identified by the contrast Own-age targets > Other-age targets were extracted for each individual from a 5mm sphere around the local maximum and averaged to produce a single value for each target type.
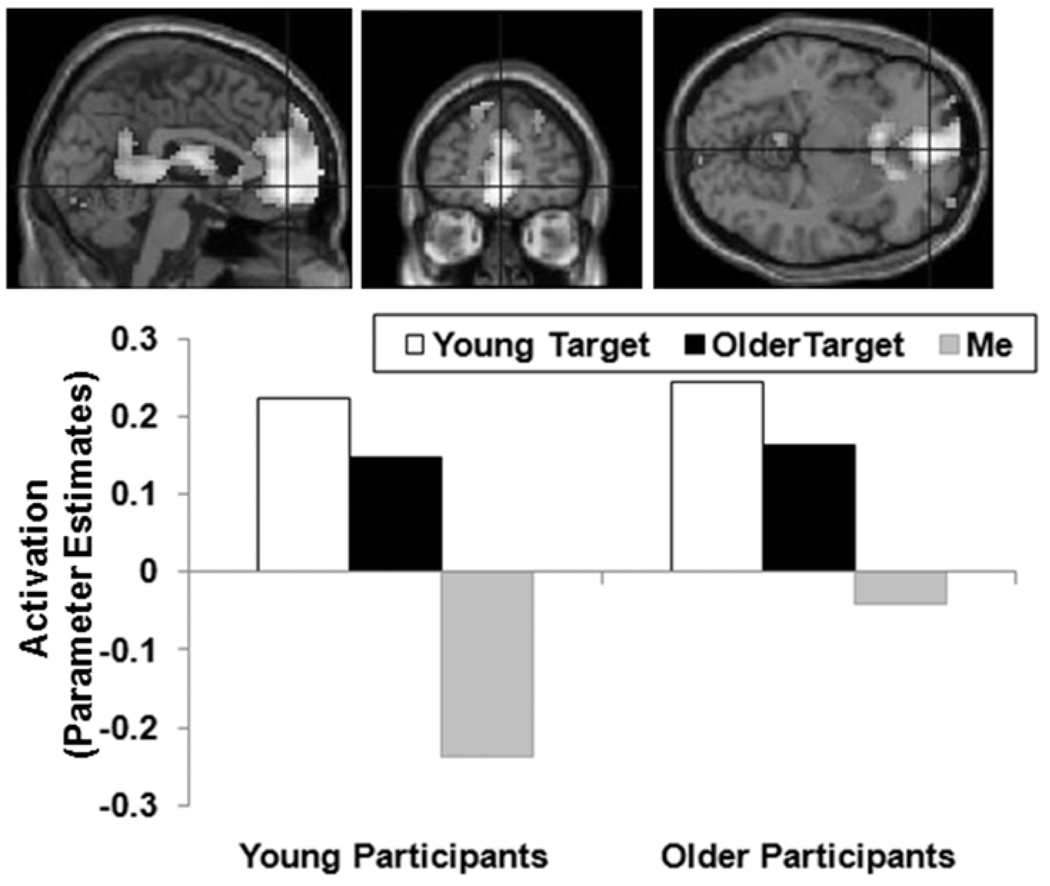
Figure 3.
An anterior area of ventral mPFC (medial frontal gyrus extending into anterior cingulate and superior frontal gyrus; BA 10, 9, 32; MNI: x = 0, y = 51, z = −6; cluster size 2348 voxels; maximum t-value for the cluster 6.68) showing Other targets > Me in young and older participants. The region of activation represents the t-map of the contrast; it is displayed on the standard reference brain in SPM. The crosshair indicates the peak voxel (local maximum) within the region of activation. Bar graphs show the mean parameter estimates (beta values) separately for age groups and target types; betas for this region of activation identified by the contrast Other targets > Me across participant age groups were extracted for each individual from a 5mm sphere around the local maximum and averaged to produce a single value for each target type.
Montreal Neurological Institute (MNI) coordinates are reported. Anatomical localization used the Talairach Daemon (Lancaster et al., 2000) on coordinates transformed using icbm2tal (http://www.brainmap.org/icbm2tal/), and labels were confirmed visually using the Talairach and Tournoux (1988) atlas.
Results
Behavioral Results
Compliance with the rating task in the scanner was high, with a button press on 99% of the trials. A mixed 2 (Participant Age Group) X 3 (Target Type) repeated-measures analysis of variance (ANOVA) on mean ratings of personality statements (see Table 2) showed a main effect for Participant Age Group (F(1, 22) = 4.33, p < .05, ηp2 = .16), with young participants indicating greater endorsement of the presented personality statements than older participants for all target types (i.e., Young, Older, or Me). It also showed a main effect for Target Type, Wilks’ λ = 0.80, F(2, 21) = 19.88, p < .001, ηp2 = .65, with greater endorsement of personality traits when participants evaluated Me than Young targets and when participants evaluated Young targets than Older targets (all ps < .005). The interaction was not significant (p > .10).
Table 2.
Means and standard deviations (SD) for ratings of and response time (ms) to personality statements of young and older participants
| Rating Mean (SD) |
Response Time Mean (SD) |
|||
|---|---|---|---|---|
| Young Participants |
Older Participants |
Young Participants |
Older Participants |
|
| Young Target | 2.06 (0.16) | 1.98 (0.21) | 2445 (361) | 2706 (365) |
| Older Target | 1.89 (0.15) | 1.82 (0.17) | 2376 (302) | 2738 (422) |
| Me | 2.22 (0.14) | 2.15 (0.17) | 2259 (376) | 2711 (469) |
In addition, mean response times for ratings (see Table 2) showed a main effect for Participant Age Group, F(1, 22) = 5.55, p < .05, ηp2 = .20, with young participants responding faster than older participants. In addition to a marginal main effect for Target Type, Wilks’ λ = 0.80, F(2, 21) = 2.69, p = .09, ηp2 = .20, there was a marginal Participant Age Group × Target Type interaction, Wilks’ λ = 0.77, F(2, 21) = 3.06, p = .07, ηp2 = .23, that arose because young participants responded faster to Me trials than to Older targets and faster to Older targets than to Young targets (all ps < .05), whereas older participants did not differ in their response times to the different target types (all ps > .10).
fMRI Results
The primary interest of this study was whether areas of mPFC showed differential activation in young and older participants for Young and Older targets. Figure 2 shows a ventral area of mPFC (anterior cingulate; BA 24; MNI x = −6, y = 30, z = −6) that demonstrated a Participant Age Group × Target Type interaction (p < .005): Activity for both participant age groups was greater when evaluating own-age than other-age targets (p = .02 and p =.07, respectively, for young and older participants). In addition, for young participants, activity in this area was greater for Young targets than Me targets (p = .06), but was not different for Older targets than Me targets (p > .10). Older participants, in contrast, showed greater activity for Older targets than Me targets (p = .002), but no difference between Young targets and Me targets (p > .10).
A second set of contrasts compared activation when rating both other target types (i.e., collapsing across Young and Older targets) versus Me targets (Figure 3). A more anterior area of ventral mPFC (medial frontal gyrus extending into anterior cingulate and superior frontal gyrus; BA 10, 9, 32; MNI: x = 0, y = 51, z = −6) showed greater activity during Other trials than Me trials for both young and older participants (both ps < .005). No medial frontal areas in this contrast showed a Participant Age Group effect (even when the threshold was lowered to p < .01). In addition, no area of mPFC showed greater activity when contrasting Me versus Other targets.
No other mPFC areas were identified in either of these contrasts. A table of all regions of activation identified in these contrasts is available from the authors.
Discussion
In the present fMRI study, both young and older adults showed greater activity in ventral mPFC (anterior cingulate; Figure 2) when rating personality characteristics of own-age as compared to other-age individuals. This is consistent with previous suggestions that ventral mPFC is more active when thinking about similar than dissimilar others (Amodio & Frith, 2006; J. P. Mitchell et al., 2006; Van Overwalle, 2009). That is, one possible explanation for the greater activation in ventral mPFC when evaluating own-age than other-age individuals in the current study is that participants perceive themselves to be more similar to own-age than other-age individuals. Consistent with this, the post-scan reports showed that young participants perceived greater similarity with the Young targets (M = 4.50, SD = 1.57) than the Older targets (M = 2.50, SD = 1.17; t(11) = 3.83, p = .003). Older participants, however, reported equal similarity with the Older targets (M = 2.67, SD = 1.78) and the Young targets (M = 2.75, SD = 1.71; t(11) = 0.12, p = .91). It is possible that older adults are reluctant to explicitly admit similarity with other older adults (e.g., due to a negative aging stereotype; Gluth, Ebner, & Schmiedek, 2010; Hummert, Garstka, O’Brien, Greenwald, & Mellott, 2002). The idea that this region reflects perceived similarity also receives support from a positive correlation, r = .53, p < .01, across participant age groups, between participants’ perceived similarity to own-age as compared to other-age targets and BOLD response to own-age as compared to other-age targets in this region of ventral mPFC (Figure 2).1 Consistent with the analysis of mean ratings, when the age groups were analyzed separately, the correlation was only significant in young participants, r = .58, p < .05 (for older participants: r = .24, p > .10).
If this area of ventral mPFC is related to similarity, one might expect activity to be greatest for Me trials than for either young or older participants, which was not the case. It is possible that presentation of only a black silhouette superimposed with the word ‘ME’ somehow reduced engagement of self-referential processing during Me trials. This seems unlikely, however, given that J. P. Mitchell et al. (2006) found greater ventral mPFC activity (MNI: 18, 57, 9) for Me than Other targets using a silhouette for Me trials (and pictures for Other trials). Another possibility is that the greater activation in this particular area of ventral mPFC (Figure 2) when evaluating own-age than other-age individuals reflects valenced evaluative processing (Cunningham, Raye, & Johnson, 2004), or intuitive feelings about value (Ochsner et al., 2005; see also Lebreton, Jorge, Michel, Thirion, & Pessiglione, 2009). Cunningham et al. identified a region (MNI: 0, 28, −8) very similar to the one in our study (MNI: −6, 30, −6) when participants made good-bad (as compared to abstract-concrete) judgments about socially relevant concepts such as “abortion”, “welfare”, and “happiness.” Asking participants to make judgments about unknown others may engage more evaluative processing than making judgments about the self, as attitudes about the self may be more readily available. Furthermore, evaluations may be more affectively laden when referring to members of one’s own age group than those of other age groups, consistent with a role for ventral anterior cingulate in affective processing (Bush, Luu, & Posner, 2000; see also Amodio & Frith, 2006).
We also observed greater activity in a larger, more anterior, region of ventral mPFC (medial frontal gyrus extending into anterior cingulate and superior frontal gyrus) when participants rated personality characteristics of others (i.e., both Young and Older targets) than when they rated themselves (Figure 3). This appears inconsistent with other studies finding self > other in regions of ventral mPFC (Benoit et al., 2010; D’Argembeau et al., 2007; Gutchess et al., 2007; Heatherton et al., 2006; J. P. Mitchell et al., 2006). However, previous studies used either vignettes to familiarize participants with the political views of to-be-evaluated targets before the rating task (J. P. Mitchell et al., 2006) or presented familiar individuals as to-be-evaluated targets (e.g., friend, Albert Einstein; Benoit et al., 2010; Gutchess et al., 2007; Heatherton et al., 2006). Thus participants presumably had particular individuals in mind. In the present study, participants saw only a picture of each of the to-be-rated individuals without any additional information and thus had to infer from the picture or speculate about the targets’ personality characteristics.
This difference among studies in what participants know about the to-be-evaluated targets makes it likely that somewhat different processes were involved, as represented in the recruitment of different subregions of mPFC. That is, whereas in previous studies with known others, responses may have been made based on more explicit person knowledge, in the present study’s “minimal information paradigm” participants had to make inferences under uncertain or ambiguous conditions. In line with this interpretation, a similar region of anterior ventral mPFC was found to be more active when participants made judgments about ambiguous versus unambiguous mental states of protagonists in scenarios (MNI: −8, 50, −2; Jenkins & Mitchell, 2010). Also, when participants made judgments about which activities are associated with which gender, a just slightly less ventral mPFC region (MNI: −4, 54, 6) showed greater activation for stereotypic (e.g., maintaining the car) as compared to non-stereotypic (e.g., using a cell phone) activities (Quadflieg et al., 2008). Thus, one possibility consistent with these various findings is that under conditions of uncertainty, such as making evaluative judgments about personality traits of unknown others with minimal prior information, both young and older adults rely largely on stereotypes. Furthermore, in the present study, as they rated the targets, participants likely developed an impression of each of the two targets online and over time (as opposed to information available prior to ratings, as in other studies). Supporting this suggestion, Schiller, Freeman, Mitchell, Uleman, and Phelps (2009) found an anterior area of ventral mPFC (MNI: −1, 44, 1) to be active during impression formation.
Another possibility is raised by the findings of Ruby and Decety (2004) that a similar region of anterior ventral mPFC (MNI −8, 64, −8) was involved in third-person versus first-person perspective taking (according to your mother > according to you). Our participants may have attempted to take the target persons’ perspective in order to infer their personality characteristics (but see, Ames, Jenkins, Banaji, & Mitchell, 2008; D’Argembeau et al., 2007; Ochsner et al., 2005, for counter-evidence).
It is important to note that although many studies investigating the role of mPFC in self-referential processing focus on areas of mPFC where self > other, several also report areas of mPFC where other > self (Benoit et al., 2010; Ochsner et al., 2005, Experiment 2), as in the current study. As discussed above, procedures vary widely across studies (e.g., evaluation of trait adjectives versus complex personality characteristics, attitudes, preferences, or beliefs; level of closeness and/or similarity of, and amount of information about, to-be-evaluated targets; time available for decision making). A more systematic investigation of conditions under which one finds self > other versus other > self, and in which subregions of mPFC, is necessary. Nevertheless, the present study’s findings, together with existing literature, suggest several, perhaps interrelated, hypotheses: That evaluation of personality traits of unknown others may be different from evaluation of known others, and, as compared to evaluating oneself, may be characterized by uncertainty and reliance on stereotypes, possibly involving processes of impression formation and perhaps perspective taking. Disentangling such factors (e.g., affect, similarity, closeness, self-relevance, ambiguity), and their relation to self-referential processing, and to specific subregions of mPFC, will advance our understanding of the functional specificity of mPFC.
In sum, whereas previous studies have emphasized potential differences in function between ventral and dorsal regions of mPFC, the results of the present study suggest that there are functionally separable regions within ventral mPFC, for both young and older participants. One hypothesis is that during evaluation of unknown others, activity in one area of ventral mPFC is related to similarity to self or affective evaluative processing, whereas activity in a more anterior area is related to ambiguity/uncertainty, perspective taking and/or stereotyping. Further clarifying such differentiation of functions in ventral mPFC, and identifying patterns of intact and disrupted functioning associated with different populations and contexts, is an important goal for social cognitive and affective neuroscience.
Acknowledgments
This research was conducted at Yale University and supported by National Institute on Aging Grant R37AG009253 awarded to MKJ and German Research Foundation Research Grant DFG EB 436/1-1 to NCE. The authors wish to thank Hedy Sarofin, Chief Magnetic Resonance Technologist, for assistance in collecting the imaging data, Kathleen Muller for assistance in behavioral data collection, Jenny-Kathinka Krueger for assistance in data preprocessing, and the Memory and Cognition Lab for various discussions of the data reported in this paper.
Footnotes
There was no correlation between perceived similarity and activation in the more anterior region of ventral mPFC that showed Other targets > Me (Figure 3).
References
- Ames DL, Jenkins AC, Banaji MR, Mitchell JP. Taking another person’s perspective increases self-referential neural processing. Psychological Science. 2008;19:642–644. doi: 10.1111/j.1467-9280.2008.02135.x. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of the minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anastasi JS, Rhodes MG. Evidence for an own-age bias in face recognition. North American Journal of Psychology. 2005;8:237–253. [Google Scholar]
- Baeckman L. Recognition memory across the adult life span: The role of prior knowledge. Memory & Cognition. 1991;19:63–71. doi: 10.3758/bf03198496. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Volle E, Burgess PW. When I think about me and simulate you: Medial rostral prefrontal cortex and self-referential processes. NeuroImage. 2010;50:1340–1349. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Normal personality assessment in clinical practice: The NEO personality inventory. Psychological Assessment. 1992;4:5–13. [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: fMRI correlates of valence, emotional intensity, and control in the processing of attitudes. Journal of Cognitive Neuroscience. 2004;16:1717–1729. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Xue G, Lu Z-L, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. NeuroImage. 2008;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Johnson MK. Age-group differences in interference from young and older emotional faces. Cognition & Emotion. doi: 10.1080/02699930903128395. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Riediger M, Lindenberger U. FACES—A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behavior Research Methods. 2010;42:351–362. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, Hugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gluth S, Ebner NC, Schmiedek F. Attitudes toward younger and older adults: The German Aging Semantic Differential. International Journal of Behavioral Development. 2010;34:147–158. [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience. 2007;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Harrison V, Hole GJ. Evidence for a contact-based explanation of the own-age bias in face recognition. Psychonomic Bulletin & Review. 2009;16:264–269. doi: 10.3758/PBR.16.2.264. [DOI] [PubMed] [Google Scholar]
- He Y, Ebner NC, Johnson MK. What predicts the own-age bias in face recognition memory? Social Cognition. doi: 10.1521/soco.2011.29.1.97. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummert ML, Garstka TA, O’Brien LT, Greenwald AG, Mellott DS. Using the implicit association test to measure age differences in implicit social cognitions. Psychology and Aging. 2002;17:482–495. doi: 10.1037//0882-7974.17.3.482. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Social Neuroscience. doi: 10.1080/17470919.2010.507948. (in press). [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Mentalizing under uncertainty: Dissociated neural responses to ambiguous and unambiguous mental state inferences. Cerebral Cortex. 2010;20:404–410. doi: 10.1093/cercor/bhp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection, and depression. Social Cognitive and Affective Neuroscience. 2009;4:313–327. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: Evidence from functional neuroimaging. Neuron. 2009;64:431–439. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Ebner NC, Tubridy SM, Frankel H, Johnson MK. Age-group differences in medial cortex activity associated with thinking about self-relevant agendas. Psychology and Aging. 2009;24:438–449. doi: 10.1037/a0015181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain: A meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JDE, Kihlstrom JF, D’Esposito M. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Packer DJ, Cunningham WA. Neural correlates of reflection on goals states: The role of regulatory focus and temporal distance. Social Neuroscience. 2009;4:412–425. doi: 10.1080/17470910902750186. [DOI] [PubMed] [Google Scholar]
- Quadflieg S, Turk DJ, Waiter GD, Mitchell JP, Jenkins AC, Macrae CN. Exploring the neural correlates of social stereotyping. Journal of Cognitive Neuroscience. 2008;21:1560–1570. doi: 10.1162/jocn.2009.21091. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience. 2004;16:988–999. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nature Neuroscience. 2009;12:508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime Reference Guide. Pittsburgh: Psychology Software Tools Inc.; 2002. [Google Scholar]
- Stern ER, Gonzales R, Welsh RC, Taylor SF. Updating beliefs for a decision: Neural correlates of uncertainty and underconfidence. Journal of Neuroscience. 2010;30:8032–8041. doi: 10.1523/JNEUROSCI.4729-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain -3-dimensional proportional system: An approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Van Overwalle F. Social cognition and the brain: A meta-analysis. Human Brain Mapping. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Revised manual. New York: Psychological Corporation; 1987. [Google Scholar]