Abstract
OBJECTIVE
Unlike visceral adipose tissue (VAT), the association between subcutaneous adipose tissue (SAT) and obesity-related morbidity is controversial. In patients with type 2 diabetes, we assessed whether this variability can be explained by a putative favorable, distinct association between abdominal superficial SAT (SSAT) (absolute amount or its proportion) and cardiometabolic parameters.
RESEARCH DESIGN AND METHODS
We performed abdominal magnetic resonance imaging (MRI) in 73 patients with diabetes (mean age 58 years, 83% were men) and cross-sectionally analyzed fat distribution at S1-L5, L5-L4, and L3-L2 levels. Patients completed food frequency questionnaires, and subgroups had 24-h ambulatory blood pressure monitoring and 24-h ambulatory electrocardiography.
RESULTS
Women had higher %SSAT (37 vs. 23% in men; P < 0.001) despite a similar mean waist circumference. Fasting plasma glucose (P = 0.046) and HbA1c (P = 0.006) were both lower with increased tertile of absolute SSAT. In regression models adjusted for age, waist circumference, and classes of medical treatments used in this patient population, increased %SSAT was significantly associated with decreased HbA1c (β = −0.317; P = 0.013), decreased daytime ambulatory blood pressure (β = −0.426; P = 0.008), and increased HDL cholesterol (β = 0.257; P = 0.042). In contrast, increased percent of deep SAT (DSAT) was associated with increased HbA1c (β = 0.266; P = 0.040) and poorer heart rate variability parameters (P = 0.030). Although total fat and energy intake were not correlated with fat tissue distribution, increased intake of trans fat tended to be associated with total SAT (r = 0.228; P = 0.05) and DSAT (r = 0.20; P = 0.093), but not with SSAT.
CONCLUSIONS
Abdominal SAT is composed of two subdepots that associate differently with cardiometabolic parameters. Higher absolute and relative distribution of fat in abdominal SSAT may signify beneficial cardiometabolic effects in patients with type 2 diabetes.
Intra-abdominal visceral adipose tissue (VAT), which is linked to cardiometabolic risk (1–3), differs anatomically and functionally from subcutaneous adipose tissue (SAT) (4). In addition, abdominal SAT is further separable into two distinct subcompartments by the fascia superficialis: the superficial SAT (SSAT) and the deep SAT (DSAT) (5). Whereas the abdominal SSAT subdepot is organized into tightly packed lobules, the lobules in the DSAT subdepot are larger, more irregular, and less well organized, and may represent an intermediate tissue organization between SSAT and VAT (4).
An increased proportion of VAT (6) is frequently reported to be associated with type 2 diabetes and lower heart rate variability, an indicator for autonomic neuropathy, and therefore increased cardiovascular risk (7). In contrast with VAT (1–3), there is less consensus regarding the association between abdominal (8) and peripheral SAT with disease risk, and both negative and positive associations have been reported (9–11). Recent studies suggest that abdominal DSAT may be associated with disease parameters, much like VAT, particularly insulin resistance (5,12,13). Yet, the association between peripheral and abdominal SAT and cardiometabolic risk is weak, if not “paradoxical” (9,14), and it is still unknown whether absolute or relative amount of abdominal SSAT is responsible for the putative “protective effects” of abdominal SAT reported in some of these studies.
RESEARCH DESIGN AND METHODS
Study population
As part of baseline measurements in the 2-year Cardiovascular, Diabetes, and Ethanol (CASCADE) randomized controlled trial, a cross-sectional analysis was performed in a subgroup of 73 men and women, aged 41–73 years, with type 2 diabetes (defined as fasting plasma glucose [FPG] >126, HbA1c >6.5, physician diagnosis, or evidence of purchase of oral hypoglycemic medications) who underwent magnetic resonance imaging (MRI) of the abdomen. Persons were excluded if they were smokers, pregnant, lactating, or using an insulin pump or injecting insulin more than twice per day; had evidence of severe diabetes complications (e.g., proliferative retinopathy or advanced renal disease); had autonomic neuropathy manifested as postural hypertension or hypoglycemia unawareness; had a fasting serum triglyceride level >400 mg/dL or a serum creatinine level of ≥2 mg/dL (177 μmol/L); had liver dysfunction (greater than twice the upper limit of normal of alanine aminotransferase and aspartate aminotransferase levels); had active cancer or received chemotherapy in the last 3 years; or were participating in another trial. The study protocol was approved by the Soroka University Medical Center Medical Ethics Board and Helsinki Committee. All participants provided written informed consent.
MRI acquisition and image analysis
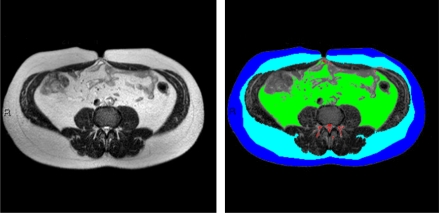
MRI scans of the abdomen were performed using a 1.5 Tesla machine (Intera, Philips Medical Systems, Best, the Netherlands) using a body coil. Subjects were examined in the supine position with arms positioned parallel along the lateral sides of the body. MRI scans demonstrating fat in the different compartments were assessed using a MATLAB-based in-house program (Fig. 1). Fat tissues of specific anatomical landmarks were quantified. The MRI scan allows visualizing the fascia superficialis as a fine black line. To divide SSAT from DSAT, we drew a continuous line over the fascia superficialis. After quantification, fat tissues were divided into color-coded groups: superficial subcutaneous fat = dark blue; deep subcutaneous fat = light blue; VAT = green; perimuscular fat (fat surrounding and within the latissimus dorsi and diaphragm) = purple; and nonclassified fat (fat surrounding the vertebrae and fat depots unrelated to any of the groups listed above) = red. Selecting the specified fat mass area was performed using a semiautomatic method (i.e., connected pixels) or various manual tools, such as rectangle/circle/polygon or free hand for fine adjustments and corrections if needed. Quantification of the fat mass regions included the area of each fat type and the proportion (percentage) of the total area of all fat types. To obtain absolute measurements in metric units, a scaling procedure was applied before the segmentation to determine real pixel dimensions. Finally, in accordance with other studies (15), we calculated the fat distribution using the mean of the three slices: S1L5, L5L4, and L3L2. Perimuscular and the nonclassified fat tissues, totaling a negligible fraction of total abdominal fat, were omitted from our analysis. All multivariate analyses were performed twice, using the absolute abdominal fat tissue distribution (SSAT, DSAT, VAT) or the relative (in percent) of each depot from total abdominal adipose tissue (TAAT) (SSAT%, DSAT%, VAT%).
Figure 1.
MRI imaging of abdominal fat tissues compartments. The subcutaneous fascial plane was delineated using the computer interface semiautomatic method, where initially an intensity-based automatic segmentation was generated and presented followed by semimanual fine tuning. The area of each compartment was quantitated separately. Fat tissues of specific anatomical landmarks were quantified and divided into color-coded groups as follows: dark blue, superficial subcutaneous fat; light blue, deep subcutaneous fat; green, visceral adipose tissue; and red, nonclassified fat—fat surrounding the vertebrae and fat depots that were unrelated to each of the groups listed above. (A high-quality digital representation of this figure is available in the online issue.)
Clinical parameters
Anthropometric measures were evaluated as clinical estimates of whole-body adipose tissue compartments. Participants were weighed without shoes to the nearest 0.1 kg. Height was measured using a wall-mounted stadiometer to the nearest millimeter for determination of BMI. Waist circumference was measured halfway between the last rib and the iliac crest by the qualified study nurse, with the same type of measuring tape. Mean blood pressure from two measures was recorded after resting, with the use of an automated system (Datascop Acutorr 4, SOMA Technology, Inc., Bloomfield, CT). Blood samples were drawn after at least an 8-h fast, and current use of all medication was recorded. To assess blood pressure and heart rate variability, 24-h ambulatory blood pressure monitoring (OSCAR 2 oscillometric SunTech Medical Model 222, Morrisville, NC) and 24-h ambulatory electrocardiography (ECG) (LIFECARD-CF, Delmar Reynolds Medical Ltd., Hertfordshire, UK) were performed in a substudy group of our population (n = 31 and n = 37, respectively). Heart rate variability parameters included time domain variables (16): the mean duration of the time interval between two R waves (RR), graphically presented in the form of an RR interval tachogram; the SD of all normal RR (SDNN); the mean of all the 5-min SDs of NN (normal RR) intervals during the 24-h period; the root mean square successive difference, calculates the square root of the mean of the squared differences between successive NN intervals over 24 h; and the 24-h triangular index, the integral of the density distribution (i.e., the number of all NN intervals) divided by the maximum of the density distribution, which is more influenced by the lower than the higher frequencies. From the 24-h blood pressure monitor, we calculated the average systolic and diastolic blood pressure during the day (average of records between 6 a.m. and 10 p.m.) and night (average of records between 10 p.m. and 6 a.m.). All medications in current use were recorded and classified.
Dietary assessment
We evaluated dietary assessment by a validated food-frequency questionnaire (17) that included 127 food items and 3 portion-size pictures for 17 items. Electronic questionnaires ensured completeness of the data by prompting the participant when a question was not answered.
Statistical analysis
We divided our study population across tertiles of SSAT (range 5,052–26,836 mm2) and used both absolute and proportional fat distribution for the analysis to account for both subdepot adiposity and interdepot distribution, respectively. Fat distribution was calculated by dividing each fat depot by TAAT, creating three new variables: SSAT%, DSAT%, and VAT%. ANOVA linear test was used to evaluate the characteristics of the study population across SSAT tertiles. We performed multivariate linear regression models, adjusted for age and waist circumference, to evaluate associations among SSAT, anthropometric measures, diet, blood biomarkers, 24-h ambulatory blood pressure monitoring, and 24-h ambulatory ECG recordings. Models including cardiovascular outcomes (24-h ambulatory blood pressure monitoring and 24-h ambulatory ECG recordings) were further adjusted, one at a time, for FPG or HbA1c. We further performed similar models adjusted one at a time to the various classes of medical treatments used in this patient population (insulin, oral hypoglycemic medications, antihypertensive medications, and lipid-lowering medications) and performed the same models, stratified by sex. The Statistical Package for the Social Sciences (version 19, SPSS Inc., Chicago, IL) was used for all statistical analyses. P < 0.05 denoted statistical significance. P value was not adjusted for multiple testing. Values reported are means ± SDs unless otherwise stated. Multiple linear regression results are reported with the parameter estimate and P value for each variable.
RESULTS
Fat distribution, blood biomarkers, and diet
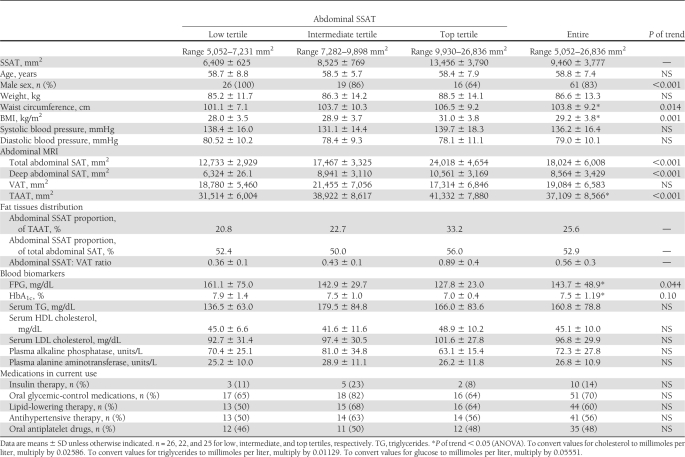
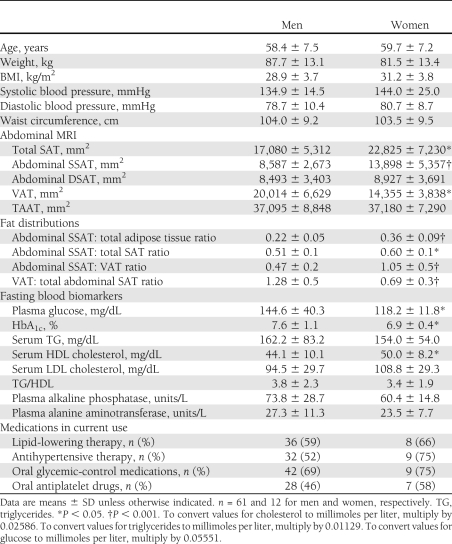
The baseline characteristics of the participants in the entire study group and acrosstertiles of SSAT are shown in Table 1. Mean HbA1c was 7.5 ± 1.1%, and 10 (14%) patients were taking one daily dose of insulin. The oral hypoglycemic medications used by the patients included sulfonylureas (11 patients,15%), dipeptidyl peptidase-4 inhibitor (9 patients, 12%), and metformin (31 patients, 42%). None of the patients were taking thiazolidinediones. The mean fat tissues distribution was as follows: SSAT 26%, DSAT 23%, and VAT 51%. SSAT positively correlated with DSAT (r = 0.389, P < 0.001) and TAAT (r = 0.461, P < 0.001). SSAT, DSAT, and TAAT correlated positively and significantly with waist circumference (SSAT: r = 0.313, P = 0.009, DSAT: r = 0.276, P = 0.023, TAAT: r = 0.449, P < 0.001) and BMI (SSAT: r = 0.490, P < 0.001, DSAT: r = 0.327, P = 0.005, TAAT r = 0.508, P < 0.001). VAT correlated positively and significantly with waist circumference and weight (r = 0.330, P = 0.006; r = 0.346, P = 0.003, respectively). Medical treatment, including insulin therapy and antihypertensive, lipid-lowering, antiplatelet, and oral hypoglycemic medications, was similarly distributed across SSAT tertiles (Table 1).
Table 1.
Distribution of fat depots, biomarkers, and clinical parameters across tertiles of absolute abdominal SSAT among patients with type 2 diabetes
FPG (P of trend = 0.046, P = 0.073 between extreme tertiles) and HbA1c (P of trend = 0.006, P = 0.011 between extreme tertiles) were both lower with increased tertile of SSAT. SSAT was inversely correlated with HbA1C (r = −0.262, P = 0.027), whereas VAT (r = 0.240, P = 0.042) and TAAT positively correlated with fasting triglycerides (r = 0.278, P = 0.018).
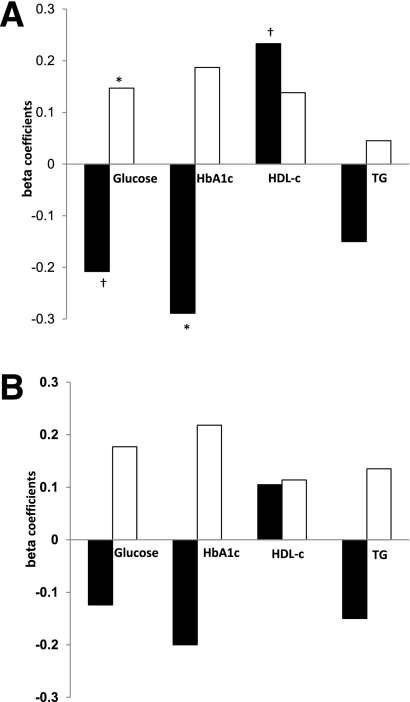
In a regression model (Fig. 2A), adjusted for age and waist circumference, increased absolute SSAT was associated with decreased HbA1c (β = −0.289, P = 0.017) and tended to be associated with decreased fasting glucose (β = −0.208, 0.084). Further adjustment of the model to medical treatment classes (one at a time: insulin therapy and antihypertensive, lipid-lowering, antiplatelet, and oral hypoglycemic medications) did not significantly attenuate these associations (data not shown). Total energy intake, total dietary fat, saturated fat, and unsaturated fat content were not associated with fat tissue mass (data not shown). However, increased total dietary intake of trans fatty acids tended to be positively correlated with total SAT (r = 0.228, P = 0.054) and DSAT (r = 0.200, P = 0.093). Collectively, the absolute area (mass) of abdominal SSAT seemed to be associated with more favorable glycemic and cardiovascular parameters, unlike DSAT or VAT.
Figure 2.
Association of metabolic parameters and cardiovascular parameters with absolute abdominal fat tissues in patients with type 2 diabetes, stratified by sex. A: Entire group. B: Men only. Multivariate model adjusted for age and waist circumference. SSAT (black bar). DSAT (white bar). Numbers represent β standardized coefficient: the amount and direction by which absolute abdominal fat tissues change (mm2) for each unit change in the metabolic parameters, while accounting for the other variables in the model. TG, triglycerides (mg/day). *P < 0.05. †P < 0.1.
Proportional fat tissue distribution
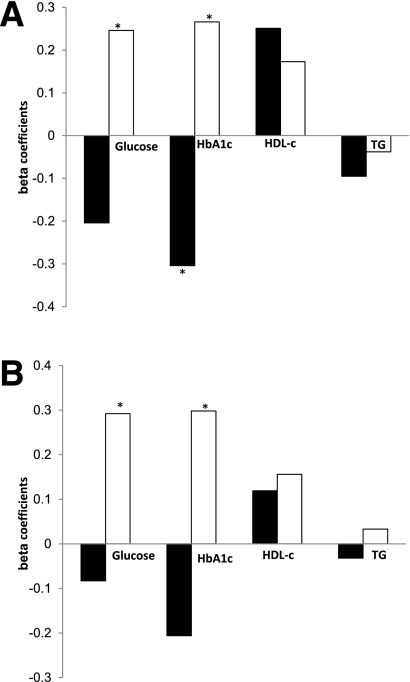
We next assessed whether the relative adipose tissue distribution, i.e., the percentage of fat in a certain abdominal (sub)-depot, was associated with clinical parameters. The percentage of SSAT from TAAT (%SSAT) was associated with lower HbA1c in regression models adjusted for age and waist circumference (β = −0.304, P = 0.017), consistent with the finding using the absolute SSAT area mentioned above. When we added medical treatment to the model (insulin therapy and antihypertensive, lipid-lowering, antiplatelet, and oral hypoglycemic medications), the association remained significant. Conversely, increased DSAT was associated with increased levels of HbA1c (β = 0.266, P = 0.039) and fasting glucose (β = 0.246, P = 0.054). Increased %SSAT was associated with increased HDL cholesterol (β = 0.251, P = 0.047) (Fig. 3A).
Figure 3.
Association of metabolic and cardiovascular parameters with abdominal fat tissues distribution in patients with type 2 diabetes, stratified by sex. A: Entire group. B: Men only. Multivariate model, adjusted for age and waist circumference. SSAT% (black bar). DSAT% (white bar). Numbers represent β standardized coefficient: the amount and direction by which proportional abdominal fat tissues change (%) for each unit change in the metabolic parameters, while accounting for the other variables in the model. TG, triglycerides (mg/day). *P < 0.05.
Of the 12 women recruited, 11 were postmenopausal, none of whom were taking hormone replacement therapy. Although men and women had similar waist circumferences (104 vs. 103 cm, P = 0.902), TAAT (37,095 vs. 37,108 mm2, P = 0.975), and medical treatment, women had higher %SSAT than did men and twice the ratio of SSAT to VAT (Table 2). They had lower fasting glucose (P < 0.001) and lower HbA1c (P = 0.002), and tended to have higher HDL cholesterol (P = 0.076). Men had a higher VAT to SAT ratio, whereas %DSAT was similar between men and women. To verify that the association between SSAT and favorable cardiometabolic parameters did not simply reflect intrinsic differences between genders, we stratified the regression models by sex (Figs. 2B and 3B). In a subgroup of the men only, similar opposite associations among %SSAT, %DSAT, and cardiometabolic parameters were observed (Fig. 3B). In addition, in the men-only subgroup, %DSAT was significantly associated with higher glucose (β = 0.292, P = 0.040) and HbA1c (β = 0.298, P = 0.038).
Table 2.
Abdominal fat tissue distribution, biomarkers, and clinical parameters in men and women with type 2 diabetes
Fat distribution, 24-h ambulatory blood pressure monitoring, and 24-h ambulatory ECG
We further found associations among the absolute abdominal subdepot fat (mass), proportional abdominal subdepot fat area, blood pressure, and heart rate variability parameters: Both absolute (r = −0.381, P = 0.050) and proportional DSAT (r = −0.428, P = 0.026) negatively correlated with SDNN. In regression models, adjusted for age and waist circumference, higher absolute DSAT (β = −0.425, P = 0.034) and higher proportional DSAT (β = −0.431 P = 0.029) were associated with lower SDNN. Proportional DSAT was significantly associated with decreased 24-h triangular index (β = −0.417, P = 0.030), suggesting decreased heart rate variability with increased fat distribution to the DSAT depot. However, further adjustment to oral hypoglycemic medications, but not to insulin therapy, antihypertensive, lipid-lowering, or antiplatelet drugs, attenuated the association between DSAT and markers of heart rate variability (24-h triangular index: β = −0.249, P = 0.228, SDNN: β = −0.294, P = 0.186). Because hyperglycemia is a key risk factor for autonomic neuropathy, we further adjusted the models, one at a time, for HbA1c or FPG. The association between both absolute and proportional DSAT and heart rate variability remained negative, but the significance was attenuated. When adjusted for HbA1c, higher absolute (β = −0.355, P = 0.093) and proportional (β = −0.401 P = 0.073) DSAT were associated with lower SDNN. Proportional DSAT was negatively associated with decreased 24-h triangular index (β = −0.406, P = 0.063). When adjusted for glucose, higher absolute (β = −0.278 P = 0.144) and proportional (β = −0.322 P = 0.122) DSAT were not associated with lower SDNN. In contrast, opposite trends were observed with absolute and proportional abdominal SSAT: Higher absolute (β = −0.329, P = 0.043) and proportional SSAT (β = −0.425, P = 0.008) were associated with lower mean daytime diastolic blood pressure. When medical treatment was added to the model (insulin therapy, antihypertensive, lipid-lowering, antiplatelet, or oral hypoglycemic medications), the association remained significant. Increased proportional SSAT tended to positively correlate with increased square root of the mean of the squared differences between successive NN intervals over 24 h (r = 0.367, P = 0.060). When HbA1c was added to the model, the association between absolute SSAT and mean daytime diastolic blood pressure was attenuated (β = −0.284, P = 0.072), although the association between proportional SSAT and mean daytime diastolic blood pressure remained significant (β = −0.379, P = 0.008). When adjusted for glucose, the association between both absolute (β = −0.329, P = 0.043) and proportional (β = −0.444, P = 0.006) SSAT and mean daytime diastolic blood pressure remained significant.
When the models were stratified by sex, we found that the men-only subgroup also exhibited similar associations among abdominal subdepot fat (mass and proportion), blood pressure, and heart rate variability parameters. Higher absolute DSAT (β = −0.452, P = 0.014) and proportional DSAT (β = −0.535 P = 0.009) were associated with lower SDNN. Higher absolute DSAT (β = −0.486, P = 0.007) and proportional DSAT (β = −0.557, P = 0.005) were associated with decreased 24-h triangular index. Further adjustment to oral hypoglycemic medications, but not to insulin therapy, antihypertensive, lipid-lowering, or antiplatelet drugs, attenuated the association between proportional DSAT and markers of heart rate variability (24-h triangular index: β = −0.439, P = 0.075, SDNN: β = −0.443, P = 0.074) and between absolute markers of heart rate variability (24-h triangular index: β = −0.408, P = 0.073, SDNN: β = −0.408, P = 0.072).
CONCLUSIONS
In this study of patients with type 2 diabetes, we observed a distinct association between both the absolute (representing “sub-depot adiposity”) and the relative amounts (representing interdepot distribution) of abdominal SSAT and markers of more favorable glycemic control and cardiovascular function as determined by higher heart rate variability and lower blood pressure. After adjusting for age and waist circumference, higher relative distribution of abdominal fat in SSAT was correlated with improved glycemic control (HbA1c and fasting glucose) and better indicators of cardiovascular health (lower blood pressure and higher heart rate variability). Conversely, DSAT correlated with higher heart rate and lower heart rate variability, both indicators of autonomic neuropathy (18), and therefore indicators for increased cardiovascular risk. Of note, controlling for markers of glycemic control attenuated the negative association between DSAT and cardiovascular end point, but not the favorable association with SSAT. In terms of diet, increased intake of trans fat tended to be associated with total abdominal SAT and DSAT, but not with SSAT. Because most studies support the more “adverse metabolic role” of intraabdominal/VAT, whether SAT is simply “less pathogenic” than VAT or exerts direct or indirect “protective effects” on cardiovascular and metabolic morbidity is still controversial (6,9). Furthermore, even more uncertainty exists as to the functional differences and risk associated with the DSAT or SSAT subdepots in persons with type 2 diabetes (19). Our results suggest a favorable distinct association between abdominal SSAT subdepot and cardiometabolic health in type 2 diabetes.
Our study has several limitations. This is a cross-sectional design that may suggest associations but not clear cause–effect protective relationships between abdominal SSAT and cardiometabolic parameters. Because we did not measure peripheral (lower body) SAT, our results refer to abdominal SSAT only. Our sample size limits the statistical power, although we still could identify significant differences between parameters. A significant proportion of our patients received treatment with medications that may modify the levels of risk factors and directly affect glycemic control parameters. Although we have made an attempt to adjust our results to the use of these drugs, doses were not assessed. Finally, we had a lower proportion of women, but nevertheless we could identify significant differences between the sexes. The strengths of our study include the specific group with type 2 diabetes, the high MRI quality imaging, the comprehensive 24-h ambulatory blood pressure and 24-h ambulatory ECG measurements as cardiometabolic measurements, the medication follow-up, and the dietary assessment.
We found that abdominal SSAT correlated with improved glycemic control and indicators of cardiovascular risk. The SSAT depot may be less lipolytic than VAT, or even DSAT, and so improved insulin sensitivity of SSAT may favor accumulation of excess energy in this depot. In this regard, higher deposition of excess calories in the SSAT is a consequence, not the cause, of improved metabolic function. Conversely, it is plausible that the abdominal SSAT fat mass may be a unique abdominal fat subdepot that has protective effects on glycemic control and cardiovascular function. This is reminiscent of a finding by some, but not all, studies that suggest peripheral SAT might be less “pathogenic” than VAT. Currently, two hypotheses have been put forward to explain the difference between peripheral SAT and VAT: The “portal theory” (6,20) implicates a direct mechanism whereby VAT is more pathogenic because its venous blood drainage is directly via the portal vein to the liver. The “ectopic fat hypothesis” (6) suggests an indirect mechanism whereby increased energy storage in peripheral SAT exerts a protective effect by decreasing fat deposition in the liver, muscle, and heart. Because abdominal DSAT exhibits an intermediate phenotype between VAT and abdominal SSAT in various functions tested (lipolysis, adipocytokine profile) (4), it is possible that these theories can underlie the unique positive association between abdominal SSAT and cardiovascular and metabolic health. Although it may be possible that peripheral SAT differs significantly from abdominal SAT, and some studies have indicated the potential pathogenic role of increased abdominal (total) SAT as opposed to peripheral SAT (9–11), further studies are required to fully understand the distinct role of the SSAT subdepot. It is tempting to speculate that discrepancies in the literature among studies assessing associations between abdominal SAT and morbidity were confined by differences in the SSAT or SSAT/DSAT distribution.
Nutritional habits may also affect fat distribution. In our study, increased trans fatty acids consumption tended to be associated with increased total abdominal SAT and DSAT, but not SSAT. Limited evidence has suggested that increased dietary intake of trans fatty acids may increase fat accumulation and abdominal circumference (21,22). Although these studies emphasize the increase in VAT, our findings suggest that DSAT also may be increased by excessive intake of trans fatty acids.
Women in our study had a higher proportion of abdominal SSAT, whereas men had a higher intra-abdominal fat to subcutaneous fat ratio. The proportion of DSAT was similar in men and women. Similar findings have been shown by other cohorts (12,23,24). Fat is distributed in a sexual dimorphic manner, beyond the differences between upper body (more in men) versus lower body locations (more in women). For a given amount of intra-abdominal fat, women, who are relatively protected from cardiometabolic morbidity, at least before menopause (6), possess up to twice as much subcutaneous fat as men (25,26). Of note, although sexual dimorphism in fat distribution usually tends to diminish with postmenopausal state, we observed such changes in our cohort despite the fact that most (11/12) women were postmenopausal. This finding may complement a prior report that postmenopausal women who possess a higher proportion of adipose tissue located in the total midthigh depot had a more favorable metabolic profile (25). Although studies have reported an association between hormone replacement therapy and cardiovascular health (27,28), none of the women in our study were on such therapy. Future studies will unravel whether increased abdominal SSAT and its seemingly favorable metabolic and cardiovascular correlates diminish with years into menopause, or have other determinants, possibly unique to persons with diabetes.
Finally, it has been proposed that antidiabetic medications, particularly of the thiazolidinedione family, may exert their therapeutic effect at least partially by inducing redistribution of fat from “pathogenic” to “less-pathogenic” depots (e.g., from VAT to SAT) (29,30). However, none of the patients in our study were receiving thiazolidinedione therapy. Other hypoglycemic medications were distributed similarly among tertiles of SSAT and controlled for in the regression models.
In summary, the current study adds to the understanding of the pattern of abdominal fat distribution, including “sub-depots,” and its relation to glycemic control and cardiovascular risk in diabetic patients. Effective interventions that can alter abdominal fat distribution may help sort out whether fat distribution phenotypes are merely a reflection of obesity subphenotypes or can causally affect risk and severity of obesity-associated morbidities.
Acknowledgments
Grant to I.S. was provided by the European Foundation for the Study of Diabetes of the European Association for the Study of Diabetes for the study of the 2-year CASCADE randomized controlled trial.
No potential conflicts of interest relevant to this article were reported.
R.G. researched data, performed statistical analyses, and wrote the manuscript. I.S. performed data analysis and contributed to discussion. A.R. designed the study, performed data analysis, and wrote the manuscript. Y.G., E.S., Y.C., D.S., S.B.A., S.W., I.F.L., O.T.-.R., and B.S. researched data. I.H.-B. and Y.H. researched data, contributed to discussion, and reviewed and edited the manuscript. M.J.S. edited the manuscript and contributed to discussion. I.S. (CASCADE Principal Investigator) contributed to discussion and reviewed and edited the manuscript. I.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work is dedicated to Harel Segal, our fellow colleague, who died during the study at the age of 43 years. The authors thank the participants in the CASCADE randomized controlled trial for consistent cooperation; the following consultants and health care providers: Hassia Krakauer, Meir Aviv, Haim Strasler, Dr. Ziva Schwartz, Dr. Einat Sheiner, Dr. Dov Brickner, Rachel Marko, Esther Katorza, and Ilanit Asulin (from the Nuclear Research Center Negev); Dr. Tatiana Shuster, Sagit Kachlon, Yasmin Asuly, and Roman Tsirkin (from the Soroka University Medical Center); and Dr. Lena Novack and Dana Sarfaty (from The S. Daniel Abraham International Center for Health and Nutrition, Faculty of Health Sciences, Ben-Gurion University of the Negev).
Footnotes
Clinical trial reg. no. NCT00784433, clinicaltrials.gov.
References
- 1.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2006;2:367–373 [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39–48 [DOI] [PubMed] [Google Scholar]
- 3.Matsushita Y, Nakagawa T, Yamamoto S, et al. Associations of visceral and subcutaneous fat areas with the prevalence of metabolic risk factor clustering in 6,292 Japanese individuals: the Hitachi Health Study. Diabetes Care 2010;33:2117–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol 2007;36:220–225 [DOI] [PubMed] [Google Scholar]
- 5.Deschênes D, Couture P, Dupont P, Tchernof A. Subdivision of the subcutaneous adipose tissue compartment and lipid-lipoprotein levels in women. Obes Res 2003;11:469–476 [DOI] [PubMed] [Google Scholar]
- 6.Gallagher D, Kelley DE, Yim JE, et al. ; MRI Ancillary Study Group of the Look AHEAD Research Group Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr 2009;89:807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laakso M. Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med 2001;249:225–235 [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997;46:1579–1585 [DOI] [PubMed] [Google Scholar]
- 9.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 2009;32:1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bays HE, Fox KM, Grandy S; SHIELD Study Group Anthropometric measurements and diabetes mellitus: clues to the “pathogenic” and “protective” potential of adipose tissue. Metab Syndr Relat Disord 2010;8:307–315 [DOI] [PubMed] [Google Scholar]
- 11.Johnson JA, Fried SK, Pi-Sunyer FX, Albu JB. Impaired insulin action in subcutaneous adipocytes from women with visceral obesity. Am J Physiol Endocrinol Metab 2001;280:E40–E49 [DOI] [PubMed] [Google Scholar]
- 12.Koska J, Stefan N, Votruba SB, Smith SR, Krakoff J, Bunt JC. Distribution of subcutaneous fat predicts insulin action in obesity in sex-specific manner. Obesity (Silver Spring) 2008;16:2003–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 2000;278:E941–E948 [DOI] [PubMed] [Google Scholar]
- 14.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavorable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005;48:301–308 [DOI] [PubMed] [Google Scholar]
- 15.Thomas EL, Bell JD. Influence of undersampling on magnetic resonance imaging measurements of intra-abdominal adipose tissue. Int J Obes Relat Metab Disord 2003;27:211–218 [DOI] [PubMed] [Google Scholar]
- 16.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–1065 [PubMed] [Google Scholar]
- 17.Shai I, Shahar DR, Vardi H, Fraser D. Selection of food items for inclusion in a newly developed food-frequency questionnaire. Public Health Nutr 2004;7:745–749 [DOI] [PubMed] [Google Scholar]
- 18.Poanta L, Porojan M, Dumitrascu DL. Heart rate variability and diastolic dysfunction in patients with type 2 diabetes mellitus. Acta Diabetol 2011;48:191–196 [DOI] [PubMed]
- 19.Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2002;283:E1135–E1143 [DOI] [PubMed] [Google Scholar]
- 20.Björntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990;10:493–496 [PubMed] [Google Scholar]
- 21.Micha R, Mozaffarian D. Trans fatty acids: effects on cardiometabolic health and implications for policy. Prostaglandins Leukot Essent Fatty Acids 2008;79:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh-Banerjee P, Chu NF, Spiegelman D, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am J Clin Nutr 2003;78:719–727 [DOI] [PubMed] [Google Scholar]
- 23.Bertrais S, Balkau B, Vol S, et al. Relationships between abdominal body fat distribution and cardiovascular risk factors: an explanation for women’s healthier cardiovascular risk profile. The D.E.S.I.R. Study. Int J Obes Relat Metab Disord 1999;23:1085–1094 [DOI] [PubMed] [Google Scholar]
- 24.Rossi AP, Fantin F, Zamboni GA, et al. Predictors of ectopic fat accumulation in liver and pancreas in obese men and women. Obesity (Silver Spring) 2011;19:1747–1754 [DOI] [PubMed]
- 25.Westerbacka J, Cornér A, Tiikkainen M, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia 2004;47:1360–1369 [DOI] [PubMed] [Google Scholar]
- 26.Piché ME, Lapointe A, Weisnagel SJ, et al. Regional body fat distribution and metabolic profile in postmenopausal women. Metabolism 2008;57:1101–1107 [DOI] [PubMed] [Google Scholar]
- 27.Taylor HS, Manson JE. Update in hormone therapy use in menopause. J Clin Endocrinol Metab 2011;96:255–264 [DOI] [PubMed] [Google Scholar]
- 28.Harman SM, Vittinghoff E, Brinton EA, et al. Timing and duration of menopausal hormone treatment may affect cardiovascular outcomes. Am J Med 2011;124:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nam JS, Nam JY, Yoo JS, et al. The effect of rosiglitazone on insulin sensitivity and mid-thigh low-density muscle in patients with Type 2 diabetes. Diabet Med 2010;27:30–36 [DOI] [PubMed] [Google Scholar]
- 30.Virtanen KA, Hällsten K, Parkkola R, et al. Differential effects of rosiglitazone and metformin on adipose tissue distribution and glucose uptake in type 2 diabetic subjects. Diabetes 2003;52:283–290 [DOI] [PubMed] [Google Scholar]