Abstract
OBJECTIVE
In young-onset diabetes, insulin therapy status is a rough marker of diabetes type. We describe the mortality experience of a low-income, predominantly minority population with diabetes diagnosed before age 30 years, stratified by insulin therapy.
RESEARCH DESIGN AND METHODS
A total of 1,098 adults aged 40–79 years (median 49) diagnosed with diabetes before age 30 years and 49,914 without diabetes were recruited from community health centers. Individuals with diabetes were categorized by insulin therapy at baseline: group A, insulin therapy only; group B, insulin therapy and an oral hypoglycemic agent; and group C, no insulin therapy. Cox models were used to compute hazard ratios (HRs) and 95% CI for cause-specific mortality based on both underlying and contributing causes of death from death certificates.
RESULTS
During follow-up (mean 3.9 years), 15.0, 12.5, and 7.3% of groups A, B, and C, respectively, and 4.6% without diabetes died. Compared with individuals without diabetes, HRs (CI) for all-cause mortality were 4.3 (3.4–5.6), 4.2 (2.8–6.3), and 2.0 (1.4–2.8) in groups A, B, and C, respectively. The leading cause of death was renal failure (end-stage renal disease [ESRD]) in group A, ESRD and coronary artery disease (CAD) in group B, and CAD in group C and individuals without diabetes. HRs for these conditions were at least twice as high as the HRs for all-cause mortality, reaching 17.3 (10.2–29.3), 17.9 (8.3–38.7), and 5.1 (2.3–11.7) in groups A, B, and C, respectively, for ESRD.
CONCLUSIONS
Excess mortality persists among people with young-onset diabetes of long duration, with ESRD and CAD as the leading contributors to mortality.
The recent rise in obesity and diabetes among adolescents and young adults from predominantly low socioeconomic status (SES) and minority backgrounds has been well noted, with the diagnostic challenges it presents in distinguishing between type 1 and type 2 diabetes (1–4). However, little data are available on long-term mortality outcomes in this population. Whether it be type 1, type 1.5, or type 2 diabetes, the early age of onset of the disease heralds an early age of onset of late end-organ complications and very early mortality. Quantifying the level of excess mortality in this population and identifying specific causes of death may help in developing strategies to increase the survival of individuals with young-onset diabetes.
Age at diabetes diagnosis has long been used in the classification into type 1 or type 2 diabetes, with age 30 years being a commonly used cutoff point, particularly in epidemiological studies (5). Insulin therapy is a marker of poor β-cell function and thus is commonly used, along with age at diabetes diagnosis, in the clinical classification of individuals with diabetes. We therefore sought to describe the contribution of major complications in diabetes to mortality in a low SES, predominantly minority population with diabetes diagnosed before the age of 30 years, grouped into three different groups based on therapy at study baseline: 1) treated only with insulin, 2) treated with insulin and an oral hypoglycemic agent, and 3) not treated with insulin. The mortality experience of individuals in these three groups was compared with that of a comparison group without diabetes drawn from the same source population. To our knowledge, this is the first study to describe cause-specific mortality in young-onset diabetes by insulin therapy status.
RESEARCH DESIGN AND METHODS
The Southern Community Cohort Study (SCCS) is a population-based study designed to investigate causes of health disparities in the incidence and mortality of cancer and other chronic diseases. Details of the rationale, study design, and methods have been described previously (6). In brief, between 2002 and 2008, 64,096 participants aged 40–79 years were recruited from community health centers from 12 states in the southeastern U.S. Community health centers are government-funded health care facilities offering basic health care services to the medically underserved. Of the 64,096 participants, 15% were recruited in 2002, 20% in 2003, 18% in 2004, 17% in 2005, 14% in 2006, 13% in 2007, and 3% in 2008. All study procedures were approved by the Institutional Review Boards of Vanderbilt University and Meharry Medical College.
After obtaining informed consent, participants completed an ∼1-h in-person interview that collected data on medical history, lifestyle, and demographic factors. If a participant answered “yes” to the question, “Has a doctor ever told you that you have had diabetes or high blood sugar?”, they were asked questions about age at diabetes diagnosis and medications currently taken to treat the disease. Women were specifically asked not to include gestational diabetes in their reporting. Of the 64,096 participants, there were 339 individuals with missing data on diabetes status who were excluded. A total of 13,843 individuals reported having a physician diagnosis of diabetes, of whom 1,149 were diagnosed before the age of 30 years (hereafter called “young-onset”). Among those diagnosed with young-onset diabetes, 400 reported using insulin as the only hypoglycemic agent at the time of enrollment into the SCCS and formed group A, 192 reported using insulin plus an additional hypoglycemic agent and formed group B, and 506 reported not being on insulin therapy (315 of whom were using an oral hypoglycemic agent) and formed group C. An additional 51 with young-onset diabetes were excluded from analysis because they reported not knowing whether they were using any diabetes medication (n = 1) or had missing information on use of diabetes medication (n = 50). Those reporting no history of physician-diagnosed diabetes at enrollment into the SCCS formed our reference population (n = 49,914).
Mortality status and underlying and contributing causes of death were determined from linkages of the SCCS population with the National Death Index database, with mortality censored on 31 December 2008. Because data relying only on the underlying causes of death listed on death certificates may substantially underestimate the impact of diabetes-related complications on total mortality in individuals with diabetes, we used both the underlying and contributing causes listed on the death certificates in determining cause-specific mortality (7,8). The specific causes of death investigated were coronary artery disease (CAD), myocardial infarction (not mutually exclusive with CAD), heart failure, renal failure, respiratory failure/arrest, sepsis, and hypoglycemia/ketoacidosis. The ICD-10 codes listed as either the underlying or contributing cause of death used to define the above disease classifications can be found in Supplementary Table 1.
General linear models were used to test for differences in continuous variables across the comparison groups, and χ2 tests were used to test for differences in categorical data. Cox proportional hazards modeling, using age as the timescale, was used to determine hazard ratios (HRs) and their 95% CIs for all-cause and cause-specific mortality by diabetes group relative to individuals without diabetes. Because mortality outcomes in diabetes may vary by race (9–11), the multivariate model included a term for race (self-reported, African American, white, other) in addition to terms for diabetes group, sex, education (less than high school vs. high school graduate or greater), annual household income (less than $15,000 vs. $15,000 or more), and smoking (former, current, never). Because of sample size limitations in other racial/ethnic groups, effect modification by race was assessed, and race-specific analyses were conducted among African Americans and whites only. In a second set of analyses, the effect of duration of diabetes was also assessed, with a value of zero being assigned to individuals without diabetes. The proportional hazards assumption was checked by plotting the log of the negative log of the survival function against log time; no major violations were observed. The criterion for statistical significance was a two-tailed P value of <0.10 for multiplicative interaction and <0.05 otherwise. Statistical analyses were conducted using SAS version 9.2 (Cary, NC).
RESULTS
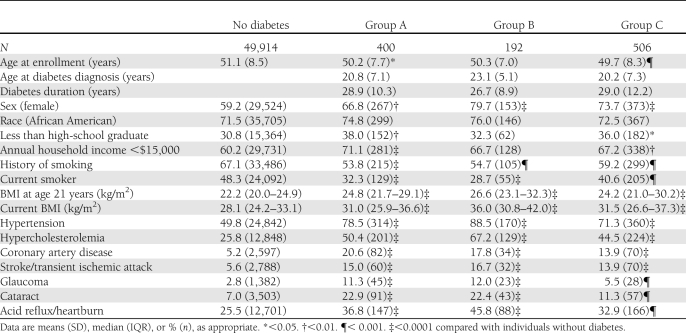
Table 1 compares the characteristics of the study participants by diabetes group. Participants were similar in age at study enrollment (median 49 years). Compared with individuals without diabetes, people with young-onset diabetes were more likely to be female; had a higher BMI at age 21 years and at study enrollment; were more likely to have had a history of hypertension, hypercholesterolemia, CAD, stroke or transient ischemic attack, glaucoma, cataracts, and acid reflux; and less likely to have a history of smoking or to be current smokers.
Table 1.
Baseline characteristics of the SCCS participants by diabetes group
During an average of 3.9 years of follow-up, 4.6% of people without diabetes died. Among people with young-onset diabetes, 15.0, 12.5, and 7.3% in groups A, B, and C, respectively, died. Table 2 presents the number of events and contribution of each outcome to total mortality. In individuals without diabetes, CAD was the leading contributor to mortality, documented in 16% of all deaths. CAD was also among the leading contributors to mortality in people with diabetes, accounting for 23–29% of deaths. Renal failure contributed to slightly more deaths than CAD in group A (insulin therapy only), while it contributed equally with CAD to mortality in group B (insulin therapy plus an oral hypoglycemic agent). There were 31 cases where hypoglycemia or acidosis/ketoacidosis was listed on death certificates. Of these 31, 24 occurred in individuals without known diabetes at baseline, 3 occurred in group A, 1 occurred in group B, and 3 occurred in group C.
Table 2.
All-cause and cause-specific mortality in individuals diagnosed before the age of 30 years compared with individuals never diagnosed with diabetes
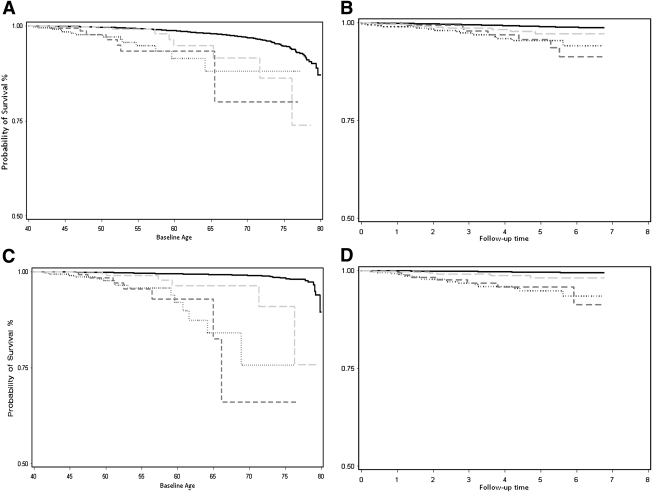
Age-adjusted all-cause mortality rates per 1,000 person-years were 44.7, 36.8, 20.9, and 11.6 in groups A, B, and C and in individuals without diabetes, respectively. HRs for all-cause and cause-specific mortality for each diabetes group compared with those without diabetes are presented in Table 2 (fully delineated models are presented in Supplementary Tables 2A and 2B). In these multivariable analyses, individuals in groups A and B were at a fourfold increased risk for all-cause mortality compared with individuals without diabetes. People in group C had a doubled all-cause mortality risk compared with people without diabetes. The HRs for the specific causes of death evaluated (namely CAD, myocardial infarction, heart failure, renal failure, sepsis, and respiratory failure) all tended to be higher than the all-cause HR within each of the three diabetes groups. For CAD specifically, mortality risk was sevenfold, ninefold, and threefold greater in groups A, B, and C, respectively, compared with individuals without diabetes. The relative excess was even more pronounced for renal failure, with HRs being 17-fold, 18-fold, and 5-fold greater in groups A, B, and C, respectively, compared with individuals without diabetes. CAD and renal failure were the two leading contributors to mortality in people with diabetes. Figure 1A and B graphically depicts the patterns for CAD mortality, and Figure 1C and D graphically depicts patterns for renal failure mortality among the diabetes groups by age and follow-up time, respectively.
Figure 1.
Survival curves by age and follow-up time. A: CAD mortality by diabetes status for a given age (years). B: Kaplan-Meier curves of CAD mortality by diabetes status. Follow-up time is in years. C: Renal failure mortality by diabetes status for a given age (years). D: Kaplan-Meier curves of renal failure mortality by diabetes. Follow-up time is in years. No diabetes, solid line. Group A, dotted line. Group B, dark grey dashed line. Group C, light grey dashed line. Group A, diagnosed before the age of 30 years and on insulin therapy only; group B, diagnosed before the age of 30 years and on insulin therapy plus an oral hypoglycemic agent; group C, diagnosed before the age of 30 years but not on insulin therapy.
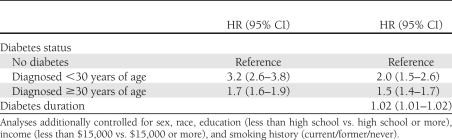
Duration of diabetes was not significantly related to all-cause mortality, but mortality from renal failure increased with rising duration (HR 1.03, 95% CI 1.00–1.07, P = 0.03) (Table 2). Thus, when duration was controlled for, the high HR for renal failure associated with groups A, B, and C were each reduced by nearly two-thirds (Table 2). Duration of diabetes was not significantly related to any of the other diabetes outcomes. Finally, in analyses comparing individuals diagnosed before age 30 years, individuals diagnosed at or after age 30 years, and individuals without diabetes, people diagnosed before age 30 years were at nearly twice the mortality risk as individuals diagnosed at or after age 30 years and more than three times the risk of individuals without diabetes (Table 3). When further controlled for diabetes duration, which was significantly associated with mortality in these analyses, the excess risks compared with individuals without diabetes were attenuated, but the higher mortality among people with young onset versus later onset persisted.
Table 3.
Risk of mortality in early- and later-onset diabetes compared with individuals without diabetes
CONCLUSIONS
Until recently, diabetes observed in children and young adults was almost exclusively type 1 diabetes, and young age (e.g., <30 years) at diagnosis was commonly used to differentiate between the two major forms of diabetes, particularly for research purposes. However, the rise in obesity and the parallel rise in diabetes in adolescents and young adults have presented a diagnostic challenge for clinicians, since the clinical and blood chemistry features of the two major forms of diabetes, type 1 and type 2, often overlap in this young population (2,4,12). Diabetes in this age-group is still almost exclusively type 1 diabetes in whites (13), and considering race, in addition to obesity and family history of type 2 diabetes, is recommended when classifying diabetes type when diagnosis is indeterminate based on other clinical or chemical features (3). It has been suggested that there now may be considerable misclassification of both types of diabetes at younger ages (4,14,15). The overlapping clinical and laboratory features of both type 1 and type 2 diabetes in many youth has led to discussion of a hybrid form of diabetes, which has been variously dubbed type 1.5 diabetes, double diabetes, hybrid diabetes, or latent autoimmune diabetes in youth (2,4,13).
The more aggressive clinical course in these youth than observed in individuals diagnosed with type 2 diabetes in older adulthood suggests a much earlier onset of end-organ complications and mortality. However, with few exceptions (16,17), studies have not investigated end-organ complications or mortality among young-onset type 1.5 and 2 diabetes. Pavkov et al. (16) investigated mortality in Pima Indians with type 2 diabetes diagnosed before the age of 20 years and found that renal disease was the leading cause of death in that population. In Japanese youth with type 2 diabetes, atherosclerotic vascular disease was the leading cause of death (18). Published data for type 1.5 are largely nonexistent to date. Our findings herein provide some of the first data on long-term overall and cause-specific mortality risk after age 40 years in three different clinical young-onset diabetes groups compared with people without diabetes.
Although we could not cleanly divide this population into type 1, type 2, type 1.5, or atypical diabetes, we have shown a fourfold increase in all-cause mortality in our insulin-treated population diagnosed with diabetes before age 30 years, rates that are consistent with other type 1 diabetes populations in Western nations (19–21). The twofold increased all-cause mortality risk observed in our young-onset population not treated with insulin is also in line with the twofold increased all-cause mortality risk observed in traditional type 2 diabetes populations, i.e., individuals diagnosed in middle-age and beyond (22,23).
The high excess mortality due to renal disease in individuals diagnosed with diabetes before the age of 30 years is well established (7,20,24,25). We have shown that even in a low SES largely African American population where end-stage renal disease risk is already elevated, renal disease mortality increased 17-fold among people treated with insulin compared with people without diabetes. The impact of renal disease in type 1 diabetes was recently highlighted in a report from the Pittsburgh Epidemiology of Diabetes Complications Study showing that, although the mortality rate overall was six times higher than in the general population, in the absence of any underlying renal disease, mortality in type 1 diabetes was comparable to that observed in the general population (25).
In a recent report from the Allegheny County diabetes registry, CAD was the leading cause of death in both blacks and whites with type 1 diabetes, although renal failure also played a major contributory role (9). In our population, CAD was the leading contributor to mortality in individuals not on insulin therapy (group C), contributed equally with renal failure to mortality in individuals on both insulin therapy and an oral hypoglycemic agent, and contributed slightly less than renal failure to mortality in individuals treated with insulin only (group A). Our results suggest that in low-income largely minority patients with long duration of insulin-treated young-onset diabetes, excess mortality due to CAD is high (seven- to ninefold higher than in people without diabetes) and appears to compete with renal disease as a contributor to mortality.
To our knowledge, no long-term mortality data are available specifically for type 1.5 or type 2 young-onset diabetes. Combined therapy with both insulin and oral hypoglycemic agents has been suggested for obese individuals with the classic features of both type 1 and type 2 diabetes, so our group B likely contains a sizeable percentage of patients who would fall in this intermediate type 1.5 category. Although all participants in group B and 62% of participants in group C were using at least one oral hypoglycemic agent, 31 and 14%, respectively, were using thiazolidinediones. Noteworthy is the 11-fold increase in heart failure risk among people in group B, whose usage of thiazolidinediones was more than twice as high as in group C, where heart failure mortality risk was increased twofold. Recently, increased heart failure risk was associated with thiazolidinediones, particularly rosiglitazone (26). The risk of heart failure with thiazolidinedione use appears to be exacerbated when combined with insulin (27), as also suggested by our data. We also observed an excess heart failure mortality risk in our population treated with insulin only (group A), although not as severe as in group B. Standardized mortality ratios from the Diabetes U.K. study of over 23,000 insulin-treated diabetes subjects diagnosed before the age of 30 years suggests heart failure mortality is also increased in type 1 diabetes (28), which our data seem to support.
A few reports have noted an increased frequency of respiratory failure on death certificates of individuals with diabetes (8), although this has not been observed by others (7). Respiratory failure mortality in individuals with diabetes may be due to complications of infection, renal failure, or ketoacidosis; however, in the 22 cases where respiratory failure contributed to mortality in our diabetes population, only seven co-occurred with renal failure or sepsis, two co-occurred with CAD, and none co-occurred with acute complications (data not depicted). A meta-analysis of studies of pulmonary function in diabetes revealed a modest reduction in pulmonary function in both type 1 and type 2 diabetes (29), although the relationship appeared to be stronger for type 2 diabetes. However, reduced pulmonary elastic recoil (30), lung volume, and impaired diffusing capacity (30,31) were observed in type 1 diabetes. Respiratory failure mortality may also be due to autonomic neuropathy (32,33) or polyneuropathy (34), perhaps partially via reduced pulmonary function, and this link between neuropathy and respiratory control has been hypothesized to play a role in the dead in bed syndrome observed in type 1 diabetes (35). A central disorder in respiratory control in diabetic autonomic neuropathy has been hypothesized (36), and although we did not have data on autonomic neuropathy, it has been suggested that cardiorespiratory arrest is a distinctive “feature of diabetic autonomic neuropathy” and plays a role in the high mortality of autonomic neuropathy in type 1 diabetes (33). In our population, individuals treated with insulin were seven to nine times more likely to die of respiratory failure as an underlying or contributing cause of death than individuals without diabetes.
Duration of diabetes, for a given age, was not significantly related to all-cause mortality in our main analyses. However, after controlling for diabetes duration, excess risk due to renal failure was reduced by two-thirds; nevertheless, insulin-treated individuals with young-onset diabetes in our population were still at a more than fivefold excess renal failure mortality risk compared with individuals without diabetes, even after the length of time with diabetes had been controlled for. The impact of duration of diabetes was also highlighted by our results showing that, for a given age at study enrollment, the differences in all-cause mortality between individuals diagnosed before age 30 years versus at a later age were attenuated after control for diabetes duration.
Our study has several limitations to be considered. First, all baseline data were self-reported. Importantly, diabetes status was based on self-reported physician diagnosis of diabetes and self-reported age at diagnosis. Like most studies of this type where diabetes was diagnosed several decades before study entry, we were unable to confirm age at diagnosis. We did not have data on age at initiation of antihyperglycemic therapy; however, all individuals in groups A and B and the majority of group C reported currently taking diabetes medications at entry into the SCCS, indicating they did in fact have the disease. In addition, separate validation efforts within the SCCS based on review of medical records and/or A1C levels for samples of patients (mainly those first diagnosed after age 30 years) confirmed over 95% of the self-reports (37). Inclusion into the study as a diabetes case required that diabetes had to be diagnosed before the participant was 30 years of age. Whereas some not reporting diabetes may have actually had undiagnosed young-onset diabetes, the minimum entry age into the SCCS was 40 years, and thus a subject would have to have had undiagnosed diabetes for a minimum of 10 years to have been misclassified as a noncase. It is possible that some young-onset cases were not diagnosed until after age 30 years, but such patients would have been excluded from our comparisons. It also must be kept in mind that our analyses were conditional on survival to at least age 40 years, the youngest eligible age for entry into the SCCS. Thus, our cases are not representative of all young-onset diabetes cases, a number of whom may have died before reaching age 40 years. Nevertheless, we believe our data are applicable to middle-aged individuals from low SES communities with long duration of young-onset diabetes where clinical features of both type 1 and type 2 diabetes may overlap. Finally, limitations regarding the use of death certificate data have been well described (7,20,38), and the presence of multiple end-organ complications is common in individuals with longstanding diabetes (39); for example, CAD is rare in the absence of underlying renal disease in individuals with young-onset diabetes, particularly type 1 (40). Thus, data relying only on the underlying causes of death listed on death certificates may substantially underestimate the impact of diabetes-related complications on total mortality in individuals with diabetes. By using both the underlying and contributing causes of death provided by the National Death Index, we have attempted to minimize some of these limitations and more accurately ascertain contributors to total mortality in individuals with young-onset diabetes.
Our grouping of our participants based on age at diagnosis and antihyperglycemic therapy was a categorization scheme to create groups enriched with participants with a diabetes type commonly seen recently in young-onset diabetes. We expect our group A (insulin only) to be enriched with type 1 diabetes, although we recognize that this group will also contain some insulin-treated type 2 diabetic patients. We expect our group C to be almost exclusively type 2 diabetes, while our group B will be made up mostly of insulin-treated type 2 diabetic patients with some latent autoimmune diabetes in youth and insulin-resistant type 1 diabetic patients. We only had antihyperglycemic therapy at study baseline, a limitation of our study, and some of the participants may have changed medications since diabetes diagnosis and thus our group classifications used to mimic diabetes types may not be entirely accurate. Nevertheless, while our study was not a study of medication effects, we did observe a fourfold increased mortality in insulin-treated participants relative to individuals without diabetes, and a twofold excess risk relative to individuals with diabetes but not treated with insulin, despite similar diabetes duration, consistent with that reported for individuals with type 1 diabetes (19,20) and type 2 diabetes (22,23), respectively. While it could be argued whether the increased mortality in individuals treated with insulin in our young-onset diabetes population is due to type 1 diabetes, to being treated with insulin, and/or to β-cell function regardless of diabetes etiology, this cannot be tested in our population. The question of insulin-related mortality, whether it is externally administered insulin or the hyperinsulinemia of insulin resistance, remains unanswered by our study.
In conclusion, we have documented cause-specific mortality in a low-SES, predominantly minority population with both insulin-treated and non–insulin-treated diabetes diagnosed before age 30 years. Given that this base population has seen a rapid rise of incident diabetes among youth and young adults in the past couple of decades, we believe our findings are of considerable importance. Excess mortality persists among individuals with young-onset diabetes of long duration, with a much greater excess among individuals characterized by a therapy more likely made up of individuals with type 1 or hybrid diabetes. End-stage renal disease and CAD remain the leading contributors to mortality.
Acknowledgments
The SCCS was supported by grant RO1 CA 092447 from the National Cancer Institute.
No potential conflicts of interest relevant to this article were reported.
B.N.C. wrote the manuscript and analyzed the data. M.E.M. reviewed the manuscript and contributed to the discussion. L.B.S. collected the data and reviewed and edited the manuscript for scientific content. W.J.B. collected the data, contributed to the methods and discussion, and reviewed and edited the manuscript for scientific content. B.N.C. takes full responsibility for all of the content of the manuscript.
The authors thank the participants of the SCCS for making this work possible.
Footnotes
The article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1385/-/DC1.
References
- 1.Dabelea D, Bell RA, D’Agostino RB, Jr, et al. ; Writing Group for the SEARCH for Diabetes in Youth Study Group Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 2.Gilliam LK, Brooks-Worrell BM, Palmer JP, Greenbaum CJ, Pihoker C. Autoimmunity and clinical course in children with type 1, type 2, and type 1.5 diabetes. J Autoimmun 2005;25:244–250 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes: 2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tfayli H, Arslanian S. Pathophysiology of type 2 diabetes mellitus in youth: the evolving chameleon. Arq Bras Endocrinol Metabol 2009;53:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahakyan K, Klein BEK, Lee KE, Myers CE, Klein R. The 25-year cumulative incidence of lower extremity amputations in people with type 1 diabetes. Diabetes Care 2011;34:649–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Signorello LB, Hargreaves MK, Blot WJ. The Southern Community Cohort Study: investigating health disparities. J Health Care Poor Underserved 2010;21(Suppl.):26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM; South Tees Diabetes Mortality Study Cause-specific mortality in a population with diabetes: South Tees Diabetes Mortality Study. Diabetes Care 2002;25:43–48 [DOI] [PubMed] [Google Scholar]
- 8.Dawson SI, Willis J, Florkowski CM, Scott RS. Cause-specific mortality in insulin-treated diabetic patients: a 20-year follow-up. Diabetes Res Clin Pract 2008;80:16–23 [DOI] [PubMed] [Google Scholar]
- 9.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes 2010;59:3216–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosnyak Z, Nishimura R, Hagan Hughes M, et al. Excess mortality in black compared with white patients with type 1 diabetes: an examination of underlying causes. Diabet Med 2005;22:1636–1641 [DOI] [PubMed] [Google Scholar]
- 11.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care 2003;26:2392–2399 [DOI] [PubMed] [Google Scholar]
- 12.Brooks-Worrell BM, Greenbaum CJ, Palmer JP, Pihoker C. Autoimmunity to islet proteins in children diagnosed with new-onset diabetes. J Clin Endocrinol Metab 2004;89:2222–2227 [DOI] [PubMed] [Google Scholar]
- 13.Pozzilli P, Guglielmi C, Pronina E, Petraikina E. Double or hybrid diabetes associated with an increase in type 1 and type 2 diabetes in children and youths. Pediatr Diabetes 2007;8(Suppl. 9):88–95 [DOI] [PubMed] [Google Scholar]
- 14.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000;136:664–672 [DOI] [PubMed] [Google Scholar]
- 15.Rhodes ET, Laffel LMB, Gonzalez TV, Ludwig DS. Accuracy of administrative coding for type 2 diabetes in children, adolescents, and young adults. Diabetes Care 2007;30:141–143 [DOI] [PubMed] [Google Scholar]
- 16.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 2006;296:421–426 [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama H, Okudaira M, Otani T, et al. Existence of early-onset NIDDM Japanese demonstrating severe diabetic complications. Diabetes Care 1997;20:844–847 [DOI] [PubMed] [Google Scholar]
- 18.Uchigata Y. Long-term outcome of type 2 diabetes in adolescence. In Type 2 Diabetes in Childhood and Adolescence: a Global Perspective, Slink M, Kaida K, Rosenbloom AL, Eds. Abingdon, England, Martin Dunitz, Taylor & Francis Group, 2003, p. 187–2109135953 [Google Scholar]
- 19.Secrest A, Orchard T. Dramatically increased mortality in women and African Americans with long-standing type 1 diabetes. Diabetes 2010;59(Suppl. 1):A87 [Google Scholar]
- 20.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006;49:298–305 [DOI] [PubMed] [Google Scholar]
- 21.Dahlquist G, Källén B. Mortality in childhood-onset type 1 diabetes: a population-based study. Diabetes Care 2005;28:2384–2387 [DOI] [PubMed] [Google Scholar]
- 22.Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabet Med 2006;23:516–521 [DOI] [PubMed] [Google Scholar]
- 23.Seshasai SR, Kaptoge S, Thompson A, et al. ; Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossing P, Hougaard P, Borch-Johnsen K, Parving HH. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ 1996;313:779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010;53:2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Home PD, Pocock SJ, Beck-Nielsen H, et al. ; RECORD Study Team Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373:2125–2135 [DOI] [PubMed] [Google Scholar]
- 27.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004;27:256–263 [DOI] [PubMed] [Google Scholar]
- 28.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–765 [DOI] [PubMed] [Google Scholar]
- 29.van den Borst B, Gosker HR, Zeegers MP, Schols AMWJ. Pulmonary function in diabetes: a metaanalysis. Chest 2010;138:393–406 [DOI] [PubMed] [Google Scholar]
- 30.Schuyler MR, Niewoehner DE, Inkley SR, Kohn R. Abnormal lung elasticity in juvenile diabetes mellitus. Am Rev Respir Dis 1976;113:37–41 [DOI] [PubMed] [Google Scholar]
- 31.Bell D, Collier A, Matthews DM, Cooksey EJ, McHardy GJ, Clarke BF. Are reduced lung volumes in IDDM due to defect in connective tissue? Diabetes 1988;37:829–831 [DOI] [PubMed] [Google Scholar]
- 32.Bottini P, Scionti L, Santeusanio F, Casucci G, Tantucci C. Impairment of the respiratory system in diabetic autonomic neuropathy. Diabetes Nutr Metab 2000;13:165–172 [PubMed] [Google Scholar]
- 33.Page MM, Watkins PJ. Cardiorespiratory arrest and diabetic autonomic neuropathy. Lancet 1978;1:14–16 [DOI] [PubMed] [Google Scholar]
- 34.Kabitz HJ, Sonntag F, Walker D, et al. Diabetic polyneuropathy is associated with respiratory muscle impairment in type 2 diabetes. Diabetologia 2008;51:191–197 [DOI] [PubMed] [Google Scholar]
- 35.Parekh B. The mechanism of dead-in-bed syndrome and other sudden unexplained nocturnal deaths. Curr Diabetes Rev 2009;5:210–215 [DOI] [PubMed] [Google Scholar]
- 36.Tantucci C, Scionti L, Bottini P, et al. Influence of autonomic neuropathy of different severities on the hypercapnic drive to breathing in diabetic patients. Chest 1997;112:145–153 [DOI] [PubMed] [Google Scholar]
- 37.Huizinga M, Elasy T, Villegas R, Signorello L, Blot W, Cavanaugh K. Validation of diabetes self-report and characteristics of undiagnosed diabetes in the Southern Community Cohort Study (Abstract). Diabetes 2009;58(Suppl. 1):A279 [Google Scholar]
- 38.Mühlhauser I, Sawicki PT, Blank M, Overmann H, Richter B, Berger M. Reliability of causes of death in persons with type I diabetes. Diabetologia 2002;45:1490–1497 [DOI] [PubMed] [Google Scholar]
- 39.Pambianco G, Costacou T, Orchard TJ. The prediction of major outcomes of type 1 diabetes: a 12-year prospective evaluation of three separate definitions of the metabolic syndrome and their components and estimated glucose disposal rate. Diabetes Care 2007;30:1248–1254 [DOI] [PubMed] [Google Scholar]
- 40.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–2538 [DOI] [PubMed] [Google Scholar]