Abstract
OBJECTIVE
Biochemical heterogeneity governs functional disparities among lipoproteins. We examined charge-defined VLDL subfractions in metabolic syndrome (MetS) to determine whether their increased electronegativity is associated with increased cytotoxicity and whether high concentrations of highly electronegative subfractions render VLDL harmful to the vascular endothelium.
RESEARCH DESIGN AND METHODS
Plasma VLDL of normal individuals (control subjects) (n = 13) and of those with MetS (n = 13) was resolved into subfractions with increasing negative charge (V1–V5) by anion-exchange chromatography. Human aortic endothelial cells were treated with V1–V5 or unfractionated VLDL.
RESULTS
Compared with the control subjects, individuals with MetS had a significantly higher percentage of V5 VLDL (V5/VLDL%) (34 ± 20 vs. 39 ± 11%, respectively; P < 0.05) and plasma V5 concentration ([V5]) (5.5 ± 4.4 vs. 15.2 ± 8.5 mg/dL, respectively; P < 0.001). Apolipoprotein (apo)B100 levels decreased and apoC levels increased from V1 to V5, indicating that V5 is apoC-rich VLDL. Regression analyses of all 26 individuals showed that [V5] was positively correlated with total cholesterol (P = 0.016), triglyceride (P < 0.000001), and V5/VLDL% (P = 0.002). Fasting plasma glucose, but not waist circumference, exhibited a positive trend (P = 0.058); plasma HDL cholesterol exhibited a weak inverse trend (P = 0.138). V5 (10 μg/mL) induced apoptosis in ~50% of endothelial cells in 24 h. V5 was the most rapidly (<15 min) internalized subfraction and induced the production of reactive oxygen species (ROS) in endothelial cells after 20 min. Unfractionated MetS VLDL, but not control VLDL, also induced ROS production and endothelial cell apoptosis.
CONCLUSIONS
In populations with increased risk of diabetes, the vascular endothelium is constantly exposed to VLDL that contains a high proportion of V5. The potential impact of V5-rich VLDL warrants further investigation.
Patients with metabolic syndrome (MetS) or type 2 diabetes often have increased plasma levels of triglycerides and triglyceride-derived VLDL (1,2). Defined by density, the VLDL class (d = 0.930–1.006 g/mL) contains a heterogeneous group of lipoprotein particles. Normal VLDL particles have been considered nontoxic to vascular cells, but apolipoprotein (apo)CIII-rich VLDL exhibits atherogenicity by enhancing monocyte–endothelial cell adhesion (3,4). Historically, cholesteryl ester-rich VLDL, such as β-VLDL, has been shown to increase endothelial cell permeability to LDL (5). Unlike the LDL class (in which the small, dense particle is considered closely associated with atherosclerosis [6]), large VLDL imparts higher cardiovascular risk than small VLDL (7). However, because the particle size is determined by nuclear magnetic resonance, isolating large VLDL for chemical and functional characterization is technically difficult. Using anion-exchange chromatography, we have separated VLDL into five subfractions, V1–V5, on the basis of surface electrical charge rather than particle size. Apart from increases in the proportion of large VLDL (2), repartition of V1–V5 particles with varying degrees of electronegativity may also contribute to changes in VLDL functionality.
We and others have reported that electronegative LDL particles possess atherogenic properties in cultured vascular cells (8–12). Using anion-exchange chromatography, we previously resolved plasma LDL into five charge-defined subfractions, L1–L5 (8,9). L5, the most negatively charged LDL, is the most potent in inducing endothelial cell apoptosis and monocyte–endothelial cell adhesion and in inhibiting endothelial progenitor cell differentiation. In addition, L5 is more abundant in patients with increased cardiac risks (e.g., hypercholesterolemia, type 2 diabetes, smoking) than in the healthy population (8,9,13–15). It is unknown whether metabolic abnormalities also involve a shift of VLDL particles to a more negative surface electrical charge and whether such a shift adds to the overall atherogenicity in patients with increased diabetic risks, including those with MetS. We hypothesized that V5 is more toxic to vascular endothelial cells than are the other subfractions of VLDL and that the proportion of V5 in total VLDL is higher in patients with MetS than in normal healthy individuals.
RESEARCH DESIGN AND METHODS
Plasma samples were isolated from asymptomatic individuals who did (MetS subjects) or did not (control subjects) meet the criteria for MetS according to the National Cholesterol Education Program–Adult Treatment Panel III guidelines (16). All participants gave informed consent for the use of their plasma; the study was conducted according to the principles in the Declaration of Helsinki. Total VLDL and LDL (d = 1.019–1.063 g/mL) were isolated by sequential ultracentrifugation (8). VLDL and LDL samples were resolved into V1–V5 and L1–L5 subfractions by anion-exchange chromatography. In brief, the lipoprotein samples were injected through a UnoQ12 column (BioRad, Hercules, CA) that had been equilibrated with buffer A (0.02 mol/L Tris-HCl, pH 8.0, containing 1 mmol/L EDTA). Subfractions were eluted by use of a multistep gradient of buffer B (1 mol/L NaCl in buffer A). Samples equilibrated with buffer A were eluted by using a linear gradient program at a flow rate of 2 mL/min. Effluent was monitored at 280 nm and protected from ex vivo oxidation with 5 mmol/L EDTA; protein concentrations were determined by the Lowry method (8). Paired Student t tests were used to compare the percentage of V5 VLDL (V5/VLDL%) and plasma V5 concentrations ([V5]) between the control and MetS groups. Linear regression analyses with a 95% CI were used to examine the correlation between demographic/blood parameters and [V5] in the combined control and MetS cohorts (n = 26). Data are expressed as means ± SD. The normality of the data was verified with the Kolgomorov-Smirnov test. A P value of <0.05 was considered significant.
To evaluate whether the VLDL subfractions vary in protein content, V1–V5 were analyzed by electrophoresis in 0.7% agarose (50 mmol/L sodium barbital, pH 8.4). For SDS-PAGE, the subfractions were delipidated, solubilized, and separated on 4–20% SDS gels (Invitrogen, Carlsbad, CA) at room temperature as previously described (9). To identify the endothelial cell–damaging VLDL subfractions and to determine whether MetS VLDL is more toxic than control VLDL, we exposed cultured human aortic endothelial cells to 10–50 μg/mL of V1–V5 and unfractionated VLDL from control and MetS subjects. For comparison, cells were treated with L1–L5 in some settings. The effects of V1–V5 and L1–L5 on endothelial cell apoptosis were assessed 24 h after treatment by examining nuclear morphology with Hoechst dye 33342 and membrane integrity with calcein acetoxymethyl ester and propidium iodide (Invitrogen) with the use of a Zeiss inverted microscope (8). We have previously shown that the results derived from this approach are compatible with those obtained from DNA laddering and cytoplasmic histone-associated DNA fragmentation assays (8,15). To compare the rate of particle internalization of V5 and V1–V4, we labeled the particles with dialkyindocarbocyanine iodide (DiI) and examined their endocytosis under a fluorescence microscope (15). We evaluated the effects of V1–V5 on oxidative stress by measuring the generation of reactive oxygen species (ROS) after 20 min of exposure to the VLDL subfractions with the use of a total ROS detection kit (Enzo, New York, NY).
RESULTS
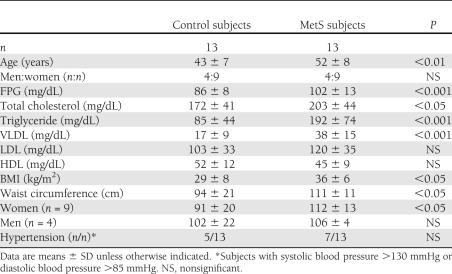
Table 1, with the demographic and lipid profiles, shows that the control and MetS groups differed significantly in age, fasting plasma glucose (FPG), total cholesterol, triglycerides, VLDL cholesterol, BMI, and waist circumference. Anion-exchange chromatography of VLDL samples showed that the distribution of the five subfractions, V1–V5, was shifted toward the most negatively charged subfractions (i.e., V5) more in the MetS group than in the control group (Fig. 1A). In group analyses, V5/VLDL% was significantly increased in the MetS vs. control group (39 ± 11 vs. 34 ± 20%, respectively; P < 0.05) (Fig. 1B). Moreover, the plasma concentration of V5 was 2.8-fold higher in the MetS group than in the control group (15.2 ± 8.5 vs. 5.5 ± 4.4 mg/dL, respectively; P < 0.001) (Fig. 1C). For each unit amount of total protein in the MetS samples, the relative content of apoB100 progressively decreased in the direction of V1 to V5, whereas that of apoE and apoC progressively increased (Fig. 1D). In vitro studies showed that even at low concentrations (10 μg/mL), V5 induced significant apoptosis (50%; P < 0.001 vs. PBS) in cultured human aortic endothelial cells after 24 h (Fig. 1E). The apoptotic effect decreased from V4 to V1, with the latter having a negligible effect (Fig. 1E). We resolved LDL samples from control and MetS subjects into L1–L5 subfractions. As expected, MetS L5 (50 μg/mL) induced significant apoptosis (30%) after 24 h (Fig. 1E). However, VLDL and LDL from MetS subjects differed in that V5 was more cytotoxic than L5 at lower (10 μg/mL) and higher (50 μg/mL) concentrations and that V3 and V4 were also cytotoxic (although to a lesser degree than V5) compared with the relatively benign L1–L4.
Table 1.
Demographic and lipid parameters of control and MetS subjects
Figure 1.
VLDL subfractions in control (Ctrl) and metabolic syndrome (MetS) subjects. A: Distribution of V1–V5 (labeled as 1–5) in VLDL of control and MetS subjects resolved by anion-exchange chromatography (representative of 13 subjects for each group). mAU, absorbance at 280 nm. B: V5/VLDL% in control and MetS subjects. C: [V5] in control and MetS subjects. D: SDS-PAGE gel of V1–V5 from VLDL of MetS subjects showing relative amounts of apoB100, apoE, and apoC. E: Nuclear staining of human aortic endothelial cells exposed to 10 μg/mL V1–V5 and 50 μg/mL L1–L5 viewed under epifluorescence microscopy. Condensed or fragmented nuclei indicate cells undergoing apoptosis. Images are representative of six experiments with V1–V5 and L1–L5.The percentage of cells undergoing apoptosis was evaluated in six samples. *P < 0.05, **P < 0.01, ***P < 0.001 vs. PBS; †P < 0.05 between V5 and L5. (A high-quality color representation of this figure is available in the online issue.)
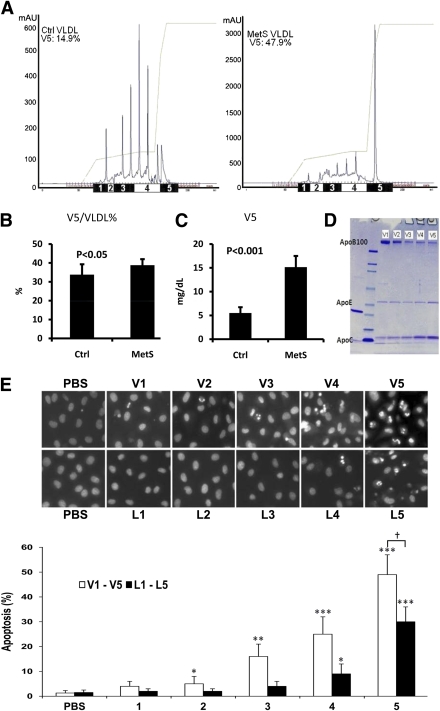
To examine the relationship between [V5] and the components of MetS criteria (i.e., FPG, waist circumference, HDL cholesterol, and triglyceride), total cholesterol, and V5/VLDL, we performed linear regression analysis of the combined groups (MetS and control; n = 26). A positive, though nonsignificant, trend was observed between FPG and [V5] (Fig. 2). We did not see a forward, inverse relationship between [V5] and either enlarged waist circumference or reduced HDL cholesterol levels, although both are components of the MetS criteria (Fig. 2). As expected, [V5] elevation was closely correlated with increases in both V5/VLDL% (R = 0.584; P = 0.002) and plasma triglyceride concentration (R = 0.82; P < 0.000001) (Fig. 2). Probably attributable to the contribution of the triglyceride component in total cholesterol, [V5] elevation was also correlated with increased total cholesterol levels (R = 0.469; P = 0.016) (Fig. 2).
Figure 2.
Linear regression analyses of correlations between [V5] and the components of MetS criteria (i.e., FPG, waist circumference [WC], HDL cholesterol, and triglyceride [TG]), total cholesterol (TC), and V5/VLDL.
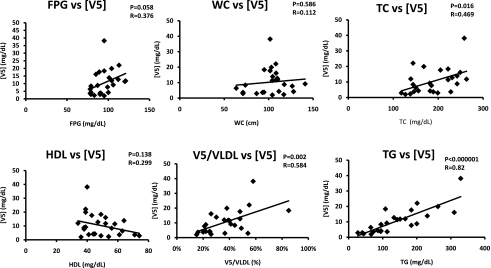
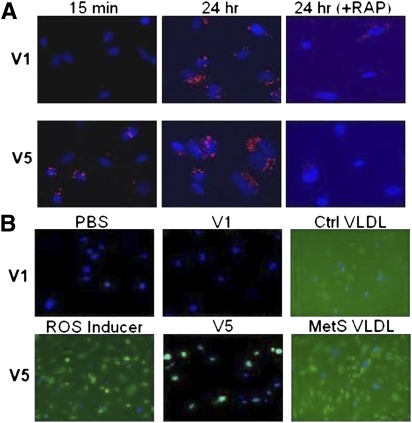
Endothelial cells internalize VLDL via the VLDL receptor; binding of ligands to the VLDL receptor can be diminished by receptor-associated protein (RAP), which acts as a molecular chaperone (17). Fluorescently visible DiI-V5, but not DiI-V1, was internalized rapidly (within 15 min); internalization of both was complete by 24 h and was sensitive to RAP blockage (Fig. 3A). V5, but not V1, elicited oxidative stress in endothelial cells as indicated by the production of ROS within 20 min of exposure (Fig. 3B). Moreover, unfractionated VLDL from MetS but not control subjects induced ROS production in 20 min (Fig. 3B) and endothelial cell apoptosis in 24 h (Fig. 4). Furthermore, MetS V5, but not V1, induced substantial apoptosis in endothelial cells (Fig. 4).
Figure 3.
Internalization of VLDL particles and ROS induced by VLDL subfractions V1 and V5 in human aortic endothelial cells. A: Internalization of DiI-labeled (red) V1 and V5 at 15 min and 24 h (hr) after incubation with endothelial cells with or without pretreatment with RAP. B: Detection of ROS production (green) after 20 min of exposure to PBS, V1, control (Ctrl) VLDL, ROS inducer (pyocyanin), V5, or MetS VLDL. (A high-quality digital representation of this figure is available in the online issue.)
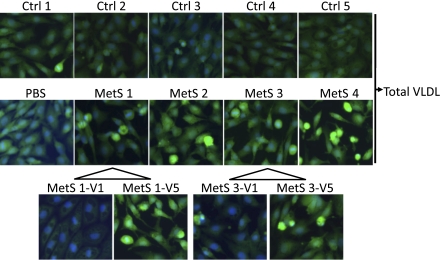
Figure 4.
Differences in proapoptotic potency between control (Ctrl) and MetS VLDL. Nuclear staining of human aortic endothelial cells exposed to 20 μg/mL VLDL from five control (Ctrl1–Ctrl5) subjects, PBS, or 20 μg/mL VLDL from four MetS (MetS1–MetS4) subjects viewed under epifluorescence microscopy. Effects of V1 and V5 from MetS1 and MetS3 were also examined. Normal nuclei appear blue with DAPI staining. Condensed or fragmented nuclei (bright green) indicate cells undergoing apoptosis. (A high-quality digital representation of this figure is available in the online issue.)
CONCLUSIONS
We report novel findings that charge-defined heterogeneity results in functional disparities among VLDL particles. The findings show that highly negatively charged VLDL subfractions, such as V5, are particularly cytotoxic. In the current study, both the V5/VLDL% and [V5] increased as plasma triglyceride levels increased, which occurs often in MetS. Our study suggests that VLDL rich in V5 content is directly harmful to the vascular endothelium and adds to the overall lipoprotein-associated atherogenicity in patients with cardiometabolic disorders, which may lead to the development of diabetes.
Although MetS has been considered a constellation of interrelated metabolic risk factors seemingly related to the development of diabetes and cardiovascular disease, the inclusion criteria have continued to change, and thus the usefulness of MetS for diagnostic and therapeutic guidance has recently been challenged (18,19). The new terms that imply abnormal cardiac metabolism, such as cardiometabolic derangement and cardiometabolic disorders, are also incompletely defined. In this study, we used the National Cholesterol Education Program–Adult Treatment Panel III guidelines to categorize study participants into control (non-MetS) and MetS groups; in the combined cohort, we also examined the effects of individual risk factors that may suggest a degree of cardiometabolic derangement on V5 abundance. Of note, three of the five major MetS criteria—waist circumference, decreased HDL cholesterol, and hypertension—did not correlate with [V5] elevation. A promising correlative trend was observed between FPG and increased [V5] that may reach statistical significance in a larger cohort with an expanded FPG spectrum and readjusted study power. High triglyceride levels were the only MetS criterion that correlated significantly with increased [V5]. The increased [V5] value was a result of multiplication between the increased V5/VLDL% and increased VLDL cholesterol levels.
Similar to L5 (9), V5 is an apoC-rich lipoprotein. In contrast with normal VLDL or LDL, both apoCIII-rich VLDL and apoCIII-rich LDL can enhance monocyte adhesion to endothelial cells by activating β1-integrin through protein kinase C-α (3,4). Our SDS-PAGE results (Fig. 1) indicated the progressive enrichment of all apoC proteins from V1 to V5, and our preliminary proteomic analysis showed an increase of apoCIII (data not shown). Understanding how apoCIII may participate in the bioactivities of V5 will require further investigation, but its low isoelectric point (pH 4.93 [20]) contributes to the electronegativity of V5. Although both V5 and L5 are apoCIII-rich lipoproteins that are toxic to vascular endothelial cells, they may use different signaling mechanisms. Discretely different from the other LDL subfractions, L5 is not recognized by LDL receptor and its entry into endothelial cells is not blocked by the inhibitory chaperone effect of RAP (15). Rather, it signals through lectin-like oxidized LDL receptor-1 and is internalized by lectin-like oxidized LDL receptor-1 (15). In contrast with the internalization of L5, that of V5 was sensitive to the blockade effect of RAP, which also prevented endocytosis of V1–V4. V5 was internalized more rapidly than V1–V4, suggesting a high affinity between V5 components and the binding sites of the VLDL receptor. Once internalized, the lipolysis products of VLDL may either initiate physiologic reactions, such as the activation of peroxisome proliferator–activated receptor-α (21), or participate in pathologic activities, such as the induction of apoptosis (22). In preliminary experiments in which we extracted total lipids from V1–V5 by the Bligh-Dyer method (23), we found that V5 lipids induced more extensive endothelial cell apoptosis than did V1–V4 lipids (data not shown).
Modified LDL–induced oxidative stress in vascular cells has received ample attention and is considered an important event in lipoprotein-associated cell pathology (24). To our knowledge, it has not been reported whether abnormal VLDL also produces similar effects in vascular cells. We present results showing that naturally occurring cytotoxic VLDL, such as V5, can directly induce oxidative stress in endothelial cells. That V1 failed to induce oxidative stress further indicates the importance of minimizing the concentration of V5 in the plasma. Importantly, we found that V5-rich VLDL from MetS subjects induced oxidative stress and apoptosis in endothelial cells, whereas V5-poor VLDL from control subjects did not; this finding may have significant clinical implications.
In summary, we present novel evidence that V5, a highly negatively charged VLDL subfraction, directly damages the endothelium. In patients with cardiometabolic disorders, including MetS, increased levels of V5 render the circulating VLDL highly toxic to the vascular endothelium. Our results strongly suggest that hypertriglyceridemia is an independent cardiac risk factor that acts, in part, via the direct endothelium-damaging effects of V5. Future work will examine the mechanisms of V5 bioactivities and the potential clinical benefits of reducing plasma V5 levels.
Acknowledgments
This study was supported in part by research grant 1-04-RA-13 from the American Diabetes Association and grant HL-63364 from the National Institutes of Health and by the Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH99-TD-B-111-004).
This study was also supported by a research grant from Merck/Schering-Plough Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
J.L., S.-H.C., R.Y.H., H.R.Y., and J.D. contributed to experimental procedures and data organization. M.A.E. performed the statistical analysis. R.A.F.D. helped with the study design and manuscript writing. C.-Y.Y. first isolated V5 and is the coinvestigator of the study. C.-H.C. is the principal investigator and the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented as an audio tour poster at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors thank Rebecca Bartow, PhD, and Nicole Stancel, PhD, of the Texas Heart Institute at St. Luke’s Episcopal Hospital, Houston, Texas, for editorial assistance.
References
- 1.Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004;27:1496–1504 [DOI] [PubMed] [Google Scholar]
- 2.Liao Y, Kwon S, Shaughnessy S, et al. Critical evaluation of adult treatment panel III criteria in identifying insulin resistance with dyslipidemia. Diabetes Care 2004;27:978–983 [DOI] [PubMed] [Google Scholar]
- 3.Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation 2006;113:691–700 [DOI] [PubMed] [Google Scholar]
- 4.Kawakami A, Osaka M, Aikawa M, et al. Toll-like receptor 2 mediates apolipoprotein CIII-induced monocyte activation. Circ Res 2008;103:1402–1409 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Navab M, Hough GP, Berliner JA, et al. Rabbit beta-migrating very low density lipoprotein increases endothelial macromolecular transport without altering electrical resistance. J Clin Invest 1986;78:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai M, Otokozawa S, Asztalos BF, et al. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem 2010;56:967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, Ridker PM. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes 2010;59:1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CH, Jiang T, Yang JH, et al. Low-density lipoprotein in hypercholesterolemic human plasma induces vascular endothelial cell apoptosis by inhibiting fibroblast growth factor 2 transcription. Circulation 2003;107:2102–2108 [DOI] [PubMed] [Google Scholar]
- 9.Yang CY, Raya JL, Chen HH, et al. Isolation, characterization, and functional assessment of oxidatively modified subfractions of circulating low-density lipoproteins. Arterioscler Thromb Vasc Biol 2003;23:1083–1090 [DOI] [PubMed] [Google Scholar]
- 10.Bancells C, Benitez S, Ordonez-Llanos J, et al. Immunochemical analysis of the electronegative LDL subfraction shows that abnormal N-terminal apolipoprotein B conformation is involved in increased binding to proteoglycans. J Biol Chem 2011;286:1125–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bancells C, Villegas S, Blanco FJ, et al. Aggregated electronegative low density lipoprotein in human plasma shows a high tendency toward phospholipolysis and particle fusion. J Biol Chem 2010;285:32425–32435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mello AP, da Silva IT, Abdalla DS, Damasceno NR. Electronegative low-density lipoprotein: origin and impact on health and disease. Atherosclerosis 2011;215:257–265 [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Jiang W, Yang JH, et al. Electronegative LDL impairs vascular endothelial cell integrity in diabetes by disrupting fibroblast growth factor 2 (FGF2) autoregulation. Diabetes 2008;57:158–166 [DOI] [PubMed] [Google Scholar]
- 14.Tang D, Lu J, Walterscheid JP, et al. Electronegative LDL circulating in smokers impairs endothelial progenitor cell differentiation by inhibiting Akt phosphorylation via LOX-1. J Lipid Res 2008;49:33–47 [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Yang JH, Burns AR, et al. Mediation of electronegative low-density lipoprotein signaling by LOX-1: a possible mechanism of endothelial apoptosis. Circ Res 2009;104:619–627 [DOI] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 17.Striekland DK, Ashcom JD, Williams S, et al. Primary structure of alpha 2-macroglobulin receptor-associated protein. Human homologue of a Heymann nephritis antigen. J Biol Chem 1991;266:13364–13369 [PubMed] [Google Scholar]
- 18.Oda E. Metabolic syndrome: its history, mechanisms, and limitations. Acta Diabetol 2011;48:79–88 [DOI] [PubMed] [Google Scholar]
- 19.Reaven GM. The metabolic syndrome: time to get off the merry-go-round? J Intern Med 2011;269:127–136 [DOI] [PubMed] [Google Scholar]
- 20.Catapano AL, Jackson RL, Gilliam EB, Gotto AM, Jr, Smith LC. Quantification of apoC-II and apoC-III of human very low density lipoproteins by analytical isoelectric focusing. J Lipid Res 1978;19:1047–1052 [PubMed] [Google Scholar]
- 21.Ruby MA, Goldenson B, Orasanu G, Johnston TP, Plutzky J, Krauss RM. VLDL hydrolysis by LPL activates PPAR-alpha through generation of unbound fatty acids. J Lipid Res 2010;51:2275–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinbold M, Hufnagel B, Kewitz T, Klumpp S, Krieglstein J. Unsaturated fatty acids liberated from VLDL cause apoptosis in endothelial cells. Mol Nutr Food Res 2008;52:581–588 [DOI] [PubMed] [Google Scholar]
- 23.Marathe GK, Silva AR, de Castro Faria Neto HC, et al. Lysophosphatidylcholine and lyso-PAF display PAF-like activity derived from contaminating phospholipids. J Lipid Res 2001;42:1430–1437 [PubMed] [Google Scholar]
- 24.Park K, Gross M, Lee DH, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care 2009;32:1302–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]