Abstract
OBJECTIVE
To identify the characteristics associated with glycemic response to newly initiated insulin therapy.
RESEARCH DESIGN AND METHODS
We identified 1,139 type 2 diabetic patients who initiated insulin therapy between 1 January 2009 and 30 June 2010. Outcomes of interest were the proportion of patients achieving A1C <7% and mean change in A1C within 3–9 months.
RESULTS
Mean A1C at insulin initiation was 8.2 vs. 9.2% among those who did and did not attain A1C <7% (P < 0.001). Within a mean of 5 months, 464 (40.7%) patients attained A1C <7%. In multivariable analyses controlling for insulin regimen, dose, and oral agent use, preinsulin A1C was responsible for nearly all the explained variance in A1C change. Each one percentage point of preinsulin A1C reduced the probability of attaining <7% by 26% (odds ratio 0.74 [95% CI 0.68–0.80]).
CONCLUSIONS
Insulin initiation at lower levels of A1C improves goal attainment and independently increases glycemic response.
Most type 2 diabetic patients require ongoing therapy intensification that eventually includes exogenous insulin administration (1). Despite the theoretical ability of insulin to correct any amount of hyperglycemia, in clinical practice only 30–37% of insulin patients achieved A1C <7% in any given quarter over 7 years of observation (2). Our objective was to examine the characteristics associated with better glycemic response to insulin and achievement of A1C targets.
RESEARCH DESIGN AND METHODS
The data for this observational study were extracted from the pharmacy, laboratory, and electronic medical record systems of Kaiser Permanente Northwest (KPNW). Details of KPNW and its data systems have been recently described (3). For the current observational study, we selected 1,139 KPNW members who met the following inclusion criteria: 1) entered the diabetes registry prior to 1 January 2008; 2) had no insulin dispensed in 2008; 3) had a new dispense of insulin between 1 January 2009 and 30 June 2010; 4) were aged ≥45 years as of the date of dispense; 5) had continuous health plan membership for at least 9 months following the first insulin dispense; 6) had at least one A1C measurement in the year prior to initiating insulin; and 7) had at least one A1C measurement 90–270 days following insulin initiation. The study observation period for each patient began on the date of insulin initiation and ended on the date of the first A1C measured 90–270 days later.
Outcomes of interest were the proportion of patients who achieved A1C <7% within 3–9 months of initiating insulin and mean change (decline) in A1C, calculated by subtracting the value of the first A1C measure taken 90–270 days following insulin initiation from the A1C value preceding insulin initiation. We identified three insulin regimens: long-acting alone (glargine, detemir, or NPH); short-acting alone (regular or rapid insulins); and combinations of long- and short-acting insulins.
RESULTS
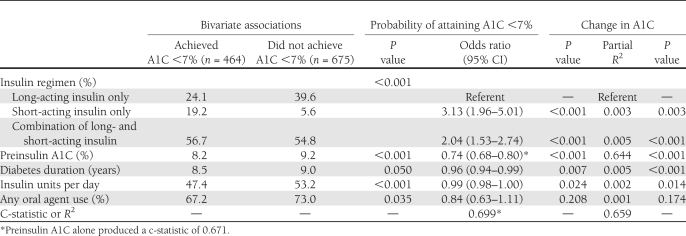
Of 1,139 patients, 464 (40.7%) attained A1C <7% following insulin initiation (Table 1). Patients who attained A1C <7% were older (aged 66.1 vs. 62.6 years; P < 0.001), were less likely to be of a nonwhite race (7.1 vs. 12.2%; P = 0.006), and had slightly shorter duration of diabetes (8.5 vs. 9.0 years; P = 0.050) compared with patients who did not attain A1C <7%. Mean A1C at insulin initiation was 8.2% among those who subsequently attained <7% compared with 9.2% among those who did not (P < 0.001). Mean decline in A1C with insulin was greater among patients who attained the 7% goal (1.9 vs. 1.3%; P < 0.001). Patients who achieved A1C <7% did so with fewer mean units of insulin per day (47.4 vs. 53.2 units/day; P < 0.001) and less use of any oral agent (67.2 vs. 73.0%; P = 0.035). The majority of patients used a combination of insulin types (55.6%), whereas 33.3% used only long-acting and 11.2% used only short-acting insulins.
Table 1.
Bivariate associations and multivariable models of the probability of attaining A1C <7% and change in A1C following insulin initiation
In multivariable analyses, A1C prior to insulin initiation was the dominant factor in goal attainment; each one percentage point of A1C prior to insulin reduced the probability of attaining A1C <7% by 26% (odds ratio 0.74 [95% CI 0.68–0.80]). Although other variables were statistically significant, A1C prior to initiation accounted for 96% of the discriminatory power of the model. Likewise, A1C prior to insulin initiation was responsible for nearly all of the explained variance of change in A1C. Relative to a regimen of long-acting insulin only, short-acting insulin only or combination regimens were significantly associated with goal attainment and glycemic response. Micro- and macrovascular complications and the use of other nondiabetes medications were not statistically significant covariates in either model.
CONCLUSIONS
With sufficient doses and appropriate lifestyle management, insulin can reduce any level of elevated A1C to the therapeutic goal (4). In our observational study of 1,139 patients in a clinical practice setting, we found that only 41% achieved A1C <7% within a mean of 5 months after initiating insulin. Our findings were remarkably similar to a recent observational study in five European countries (5) and to the Treating To Target in Type 2 Diabetes (4-T) study (6). Thus, even in a rigorous clinical trial setting, A1C goal attainment with insulin therapy is less than optimal.
We examined a wide variety of demographic, clinical, and therapy-related variables in an effort to identify factors that contributed to A1C goal attainment and that were associated with glycemic response to insulin initiation. We found that the level of A1C prior to starting insulin was by far the single most important factor, accounting for 95% of the discriminatory ability to predict the probability of goal attainment and 96% of the explainable variance in A1C change. The recent INSTIGATE (INSulin TItration \x{2013} GAining an understanding of the burden of Type 2 diabetes in Europe) observational study also reported that change in A1C over the first 6 months of new insulin therapy was almost entirely dependent on baseline A1C (5), a relationship also noted in studies of oral antihyperglycemic medications (7,8). This is not surprising; achieving any given A1C goal should be easier for the patient who is closer to the goal when the therapy is initiated. However, although mean A1C among patients who achieved A1C <7% was lower prior to insulin, these patients also had a significantly greater mean change in A1C after starting the therapy, and shorter diabetes duration also was independently associated with A1C and greater A1C reduction. Thus, initiating insulin earlier in the course of oral agent failure seems to improve glycemic goal attainment as well as improve glycemic response.
An important limitation of the current study was that we could not observe insulin titration schedules. We studied glycemic response 3–9 months following insulin initiation, but it is possible that some patients who did not achieve A1C goals in this time frame were being titrated more slowly. If so, the proportion that will ultimately attain A1C goals is likely higher. Another limitation is that we estimated units per day from dispensing records; we could not observe actual units consumed.
In summary, we found that less than one-half of patients newly initiating insulin therapy in a clinical practice setting achieved the recommended A1C goal of <7% within 3–9 months. After considering a large number of demographic and clinical characteristics, it seems that the key to glycemic success with insulin, as with oral agents, is intensifying therapy quickly when current therapies begin to fail.
Acknowledgments
Funding for this research was provided by Merck Research Laboratories. In addition to Merck, G.A.N. has received research funding from Takeda Pharmaceuticals America, GlaxoSmithKline, Novartis Pharmaceuticals, and Tethys Bioscience. G.A.N. and T.M.K. are employees of Kaiser Permanente. J.B.H. was previously employed by and is a shareholder of Merck and currently is employed by Regeneron. T.D.K. and K.G.B. are employees and shareholders of Merck. No other potential conflicts of interest relevant to this article were reported.
G.A.N. contributed to the study conception, design, and interpretation of the results; researched the data; and developed the first draft of the manuscript. T.M.K. contributed to the study conception, design, and interpretation of the results and researched the data. J.B.H. and T.D.K. contributed to the discussion and reviewed and edited the manuscript. K.G.B. contributed to the study conception, design, and interpretation of the results; contributed to the discussion; and reviewed and edited the manuscript. The final draft for submission was approved by all authors. G.A.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
References
- 1.Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 2.Nichols GA, Gandra SR, Chiou CF, Anthony MS, Alexander-Bridges M, Brown JB. Successes and challenges of insulin therapy for type 2 diabetes in a managed-care setting. Curr Med Res Opin 2010;26:9–15 [DOI] [PubMed] [Google Scholar]
- 3.Nichols GA, Vupputuri S, Lau H. Medical care costs associated with progression of diabetic nephropathy. Diabetes Care 2011;34:2374–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Standards of medical care in diabetes: 2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liebl A, Jones S, Benroubi M, et al. Clinical outcomes after insulin initiation in patients with type 2 diabetes: 6-month data from the INSTIGATE observational study in five European countries. Curr Med Res Opin 2011;27:887–895 [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Thorne KI, Farmer AJ, et al. ; 4-T Study Group Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–1730 [DOI] [PubMed] [Google Scholar]
- 7.Nichols GA, Conner C, Brown JB. Initial nonadherence, primary failure and therapeutic success of metformin monotherapy in clinical practice. Curr Med Res Opin 2010;26:2127–2135 [DOI] [PubMed] [Google Scholar]
- 8.Karter AJ, Moffet HH, Liu J, et al. Glycemic response to newly initiated diabetes therapies. Am J Manag Care 2007;13:598–606 [PMC free article] [PubMed] [Google Scholar]