Abstract
OBJECTIVE
The study objective was to evaluate how the use of a pervasive blood glucose monitoring (BGM) technology relates to glycemic control, report of self-care behavior, and emotional response to BGM of children with type 1 diabetes and their parents.
RESEARCH DESIGN AND METHODS
Forty-eight children aged less than 12 years (mean 8.8 years) with type 1 diabetes were randomly assigned to one of two study groups, a control group (conventional care without technology) or an experimental group (conventional care with technology), and followed for 12 months. Families in the experimental group were given the Automated Diabetes Management System (ADMS), which automatically collects blood glucose (BG) values and sends to parent(s) a 21-day BG trending report via e-mail each night. Measures of glycemic control (HbA1c) were collected at baseline and at quarterly diabetes clinic visits; BGM effect and diabetes self-care behavior measures were obtained at the baseline, 6-month, and 12-month visits.
RESULTS
Children in the experimental group had significantly (P = 0.01) lower HbA1c at 12 months (7.44 ± 0.94, −0.35 from baseline) than controls (8.31 ± 1.24, +0.15 from baseline). Improvement in HbA1c was more profound in families using the ADMS more frequently. In addition, in these families, parents showed a significant improvement in BGM effect (P = 0.03) and children became more meticulous in diabetes self-care (P = 0.04). Children in both experimental and control groups experienced no change in their emotional response to BGM.
CONCLUSIONS
Using the ADMS 1–3 times/week may help children with type 1 diabetes improve glycemic control and gain diabetes self-management skills, as well as improve the BGM effect of parents.
Pervasive computing, also called “ubiquitous computing,” is the notion that applications are no longer restricted to desktop computers but rather embedded into objects used in everyday life and always available. Pervasive technologies are therefore uniquely suited to support children with type 1 diabetes and their families as they cope with the complex, daily routines of diabetes care. For example, pervasive technologies that work together to automatically record and display blood glucose monitoring (BGM) data can be an aid to parents by heightening awareness of blood glucose (BG) trends and sustaining engagement with their child’s diabetes management. Prior research has established the value of parental involvement in the care of children with type 1 diabetes (1,2). A technology that eases the burden of collecting and reflecting on BGM data also stands to improve diabetes self-management in children, which has been correlated with better glycemic control (3). However, there is also potential for these types of monitoring technologies to create a negative attitude toward BGM because of an increased focus on BG values and possible perception by the child of increased surveillance. Negative BGM effect has been associated with poor glycemic control (4). Commercial devices designed to support remote BGM are increasingly being produced; thus, it is important to understand the potential impact of integrating this type of technology into the family context of diabetes management. The goal of this research was to understand how a particular pervasive remote BG monitoring technology influences glycemic control, diabetes self-management, and attitudes toward BGM. In this study, the Automated Diabetes Management System (ADMS) by Diabetech, LP (Dallas, TX; http://healthimo.com/glucomon) was provided to children with established type 1 diabetes and their parents to measure the effect of automated BGM data collection and trend reporting on self-care behaviors, glucose control, and attitudes toward BGM compared with conventional management. Prior research has shown the ADMS to be an effective means of self-monitoring BG, allowing early identification of islet graft dysfunction (5).
RESEARCH DESIGN AND METHODS
Participants
Study participants were children with type 1 diabetes and their parents who were followed at an outpatient diabetes clinic associated with a tertiary care children’s hospital in the Southwestern U.S.. All subjects had type 1 diabetes for at least 1 year. This criterion was intended to minimize the effect of the honeymoon period on HbA1c levels. To minimize the impact of oppositional behaviors on glycemic control, only children with no diagnosed major psychoaffective disorders were eligible to participate.
Patient records were reviewed for the following eligibility criteria: duration of type 1 diabetes >1 year and age <12 years at the time of enrollment. Exclusion criteria were prior involvement with foster care, juvenile justice system, or children’s protective services, or subject expected to live in the same home environment for <1 year; patients with known psychiatric and behavioral disorders other than attention deficit disorder (as defined by a medical diagnosis recorded in the patient chart by the patient’s physician); and HbA1c >12% at enrollment. Written informed consent was obtained from all families before entry.
Procedure
In total, there were 120 children followed in the practice who met the inclusion criteria, 12 of whom were excluded on the basis of the defined criteria. Patients were recruited when they came into the clinic for routine visits or by phone until the maximum number of participants was met with a total of 64 families invited to participate in the study. Subjects were randomly assigned to one of two study groups: the control group (conventional care without ADMS) or the experimental group (conventional care with ADMS). During the 12-month study period, all subjects from both study groups were seen five times beginning with an initial baseline screening appointment, during which various demographic, glycemic control, and psychosocial data were collected. Families were seen quarterly for routine diabetes clinic visits, during which relevant information was gathered. Neither group was asked to monitor BG any more or less frequently than other patients with type 1 diabetes in the practice. Two registered BG meters and test strips (OneTouch Ultra by LifeScan, Inc., Milpitas, CA) were given to families in both the control and experimental groups. Subjects agreed to use only these devices for BGM for the duration of the study.
Both groups were seen by the same health care team and provided the same level of care without additional contact. All participants were told that their BGM information was for their use only and would not be monitored by the diabetes team from a distance. Families attending this clinic are routinely asked to forward BGM information between clinical visits if there is concern about glucose control or if need for changes in diabetes management arises. This practice did not change within the experimental or control group. The health care team did not explicitly encourage use of the ADMS and treated patients in the experimental group without bias.
Participants enrolled in the experimental group were provided the ADMS equipment and service free of charge for the study duration. As an incentive, subjects in the control group were offered use of the ADMS for 6 months after the completion of their participation in the study. There were no other incentives for participation provided to either group.
Intervention
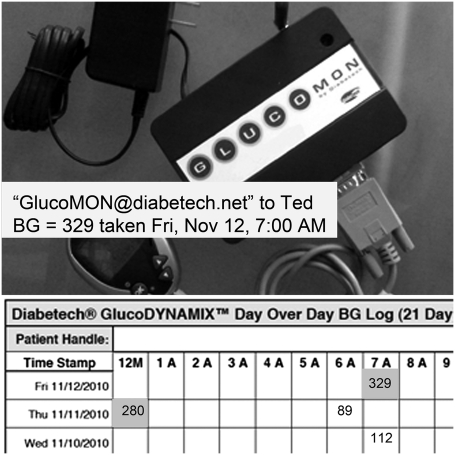
The ADMS is composed of the GlucoMON and GlucoDYNAMIX (Diabetech, LP), which are wireless technologies that work together to provide automated BGM data retrieval, analysis, and reporting. The experimental group subjects had access to two features within the GlucoDYNAMIX, including 1) “real-time alerts,” notification by text message to cell phones or e-mail of the last BG result immediately after the docking of the glucometer to the GlucoMON device, and 2) “trend analysis reports,” a daily e-mail to parents including the system-generated 21-day BG log attached as a PDF document (Fig. 1). The report is color coded and arranged by date (on the y-axis) and time (on the x-axis). Elevated BG values are shown in red, and low BG results are shown in yellow. When two readings occur within the same hour, a blue color is displayed along with the information. Readings in white boxes are “within range.”
Figure 1.
A 21-day trend analysis report, GlucoMON device and real-time alert. BG, blood glucose.
Participants were free to use data from the ADMS as they saw fit for diabetes management purposes. They were advised to dock their meter(s) daily or more often if they wanted, but at a minimum, they should dock weekly. One family had no Internet access, so their trend analysis reports were mailed to their home each week. Reports were not automatically sent to the diabetes care team, and no specific health care provider–initiated action was triggered by ADMS reports. It was left to the family to decide what (if any) action should be taken on the basis of knowledge of self-care gained through glucose pattern management skills training before enrollment.
Measurements
The effectiveness of the intervention was measured in three ways 1) glycemic control, 2) patient and parent effect around BGM, and 3) parent report of child’s self-management behavior.
Measure of glycemic control.
HbA1c was collected using an aqueous finger stick 3-μL blood sample collection kit including a prepaid mailer to the study’s centralized laboratory (DTI Laboratories, Inc., Thomasville, GA). The HbA1c method used for this study used a multimethod sample screening via high-performance liquid chromatography-ion exchange for detecting possible interference followed by high-performance liquid chromatography-bioassay analysis to determine the percent HbA1c. HbA1c was collected using this method at enrollment and 3-month intervals for every participant for the duration of the study.
Measure of patient and parent effect around BGM.
Both parent and child completed the Blood Glucose Monitoring Communication (BGMC) questionnaire at the baseline, 6-month, and 12-month visits to assess the emotional response to BGM when the ADMS is added to routine diabetes care. The BGMC questionnaire is a validated (4) eight-question survey that gauges emotional response to BGM. Children were asked to reflect on how it felt when their BG was out of range. Likewise, questions aimed at parents asked them to report the level of concern that they had for their child in such situations. The total score of the survey was used to evaluate BGM effect where a minimum score of 8 reflected a more positive emotional response and higher scores reflected up to a maximum of 24 points, which are indicative of negative feelings toward BGM.
Measure of diabetes self-management.
All participants completed a Diabetes Self-Management Profile (DSMP) at the baseline, 6-month, and 12-month visits. The DSMP is a semistructured interview that is a validated measure of diabetes self-management (3). Parents were interviewed in this study, but prior work has shown little difference between child and parent responses (3). The questions are grouped into subscales related to each area of self-care and have a range of possible points as follows: exercise (0–12), management of hypoglycemia (0–11), diet (0–17), BG checking (0–33), and insulin administration and adjustment (0–16). Higher scores on the DSMP indicate more rigorous diabetes self-management along the five subscales. High DSMP scores have been associated with improved glycemic control (3).
Statistical analysis
Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (PASW Statistics 18 for Windows; SPSS Inc., Chicago, IL). The analyses included unpaired t tests, ANOVA, two-way ANOVA, Pearson bivariate correlations, χ2 and Fisher exact tests. P values <0.05 were considered significant. Values are reported as mean ± SD unless otherwise noted. Change in HbA1c, BGMC, and DSMP were analyzed according to study group (experimental vs. control) and ADMS use. To evaluate the impact of prestudy glycemic control, these groups were further segmented during analysis by baseline HbA1c: subjects who began the study with 1) HbA1c >8% and 2) HbA1c ≤8%.
RESULTS
Participant characteristics
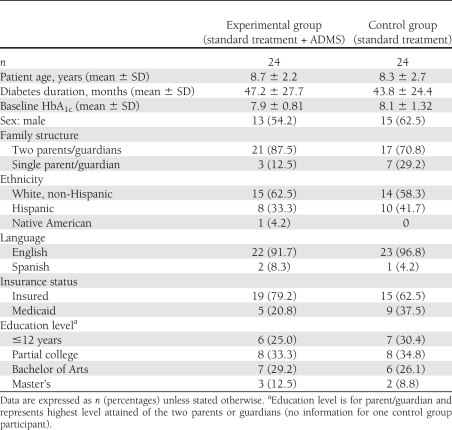
Fifty-four eligible families (84% of the 64 families invited) volunteered to participate and were randomly assigned to one of two study groups: 27 families in the control group (conventional care without ADMS) and 27 families in the experimental group (conventional care with ADMS). Three families from each group dropped out of the study, leaving a total of 48 families: 24 in the experimental group and 24 in the control arm. Five of the 6 families dropped out because of relocation. One family exited the experimental group after 3 months because the patient’s mother found the color-coded log generated by the ADMS to be confusing. Retrospective analysis revealed no statistically significant difference between the experimental and control groups in the demographic and clinical characteristics presented in Table 1.
Table 1.
Participant characteristics
ADMS use
ADMS use rates were defined using system-recorded docking events collected for each subject in the experimental group throughout the study. A docking event is created when a glucometer is connected to the GlucoMON, and all BG readings in the memory are downloaded to the system. Each date and time-stamped event is associated with an individual subject through the GlucoMON identification number.
Although experimental group participants were encouraged at enrollment to dock weekly, no encouragement to use the ADMS was given during the study. Three distinct groups of use emerged: 1) docked <1 time each week (0.5 ± 0.2 events/week, n = 13); 2) docked 1–2 times each week (1.4 ± 0.3 events/week, n = 8); and 3) docked >2 times each week (3.1 ± 0.2, n = 3). Multiple ANOVA tests showed no difference in glycemic control, BGMC, or DSMP between groups 2 and 3, so they were combined into a single group defined as docking 1–3 times/week. Therefore, in addition to comparisons between the control group and the experimental group as a whole, the ADMS use groups A (docking <1 time/week), B (docking 1–3 times/week, and C (control group) are used to categorize the study outcomes. There was a steady use of the device in group A throughout the study. Participants in group B used the device more sporadically and never achieved the same level of use on a weekly basis as those in group A. In group B, there was one family who docked only one time and two families who stopped docking completely after 8 months. Other findings around docking patterns are not reported in this article because of space constraints, but they will be reported in a future publication.
Of note, there were no statistically significant differences in baseline demographic or clinical characteristics among ADMS use groups A, B, and C, including age, ethnicity, educational level, insurance status, language, duration of diabetes, baseline HbA1c values, and baseline parental BGMC, child BGMC, or DSMP scores. In addition, there were no statistically significant differences in the demographic characteristics between experimental groups A and B. Both groups had a similar number of parents with a college education or more (5) and similar family structure; group A had two one-parent families versus one found in group B. All families had computer access except one family in group B, which may have contributed to the initial low rate of docking (1–3 times/week) and complete decrease in use during the last 4 months of the study.
Glycemic control
An independent-samples t test was conducted to compare glycemic control between the experimental group and the control group. The results showed that the mean 12-month HbA1c value of the experimental group (7.44 ± 0.94) was significantly lower than of the control group (8.31 ± 1.24), confirming that glycemic control improved in the experimental group when compared with the control group (P = 0.01).
To examine the impact of prestudy glycemic control, the experimental and control groups were divided according to baseline HbA1c: Exp<8 or Ctrl<8 indicate groups that met the American Diabetes Association’s target values, and Exp≥8 or Ctrl≥8 indicate those who were over target (1). A two-way ANOVA found that participants in the Exp≥8 group had a lower mean HbA1c at 12 months (7.68 ± 1.04) compared with counterparts in the Ctrl≥8 group (9.55 ± 1.20), revealing significantly improved glycemic control in the experimental group (P = 0.04). There was no significant difference in HbA1c at 12 months in the Exp<8 group (7.29 ± 0.87) and the Ctrl<8 group (7.69 ± 0.72).
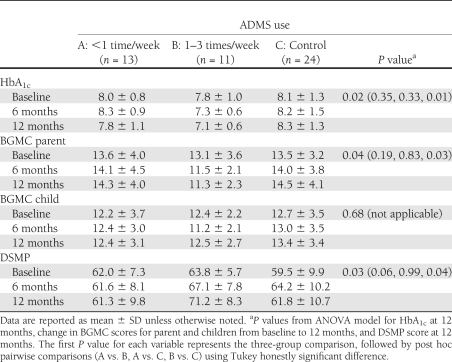
To evaluate the relationship between docking event frequency and changes in HbA1c, an ANOVA was conducted to contrast the change in glycemic control experienced by the three ADMS use groups. As shown in Table 2, there was a significant relationship between docking frequency and glycemic control (P = 0.02). ANOVA tests revealed that subjects using the ADMS 1–3 times/week had significantly lower HbA1c at 12 months when compared with the control group (P = 0.01). The change in HbA1c from baseline to 12 months for subjects using the ADMS 1–3 times/week was also significantly greater than in the control group (P = 0.04). The change in HbA1c for subjects docking <1 time/week did not differ significantly from the control group (P = 0.33) or the group docking 1–3 times/week (P = 0.35).
Table 2.
Summary results categorized by ADMS use groups
BGM effect
An independent-samples t test was conducted to determine whether there were differences in BGMC scores of the experimental group compared with the control group. The results show that there were no significant differences in BGMC scores of children. The t test examining the change in parental BGMC scores showed the experimental group had a trend toward improvement in BGMC when compared with parents in the control group (−0.652 ± 2.72 vs. 1.04 ± 3.41; P = 0.07). In addition, the experimental group had a significantly greater number of subjects with a stable or improved parental BGMC scores from baseline to 12 months compared with the control group (78 vs. 46%; P = 0.02). ANOVA showed that prestudy glycemic control had no relationship to the BGMC scores of parents or children.
An ANOVA was conducted to investigate the relationship between docking frequency and change in BGMC scores. As shown in Table 2, there were no significant differences in the BGMC scores of children; however, the change in BGMC scores of parents between baseline and 12 months was significantly different among the three groups (P = 0.04). Post hoc, pairwise comparisons show that parents in the group using the ADMS 1–3 times/week had significantly improved BGM effect than the control group (P = 0.03). There was no significant difference in the BGM effect between those using the ADMS <1 time/week and the control group (P = 0.83).
Diabetes self-management
An independent-samples t test was conducted to determine whether there were differences in DSMP scores of the experimental group compared with the control group. There were no significant differences found between experimental and control at any phase of the study, at baseline, 6 months, or 12 months. ANOVA showed that prestudy glycemic control, as measured by baseline HbA1c, also was not related to DSMP scores.
To investigate possible connections between use rates of the ADMS and the change in diabetes self-care, ANOVA tests were conducted evaluating the relationship of docking frequency with DSMP scores. There was a significant difference in the 12-month DSMP scores of the three groups shown in Table 2 (P = 0.03). Pairwise comparisons, also shown in Table 2, indicate that subjects using the ADMS 1–3 times/week had significantly higher DSMP scores than subjects in the control group (P = 0.04) and a trend for higher scores than those who docked with the ADMS less than once/week (P = 0.06).
The scores for each of the five subscales within the DSMP were also analyzed. No differences were found between ADMS docking groups at baseline; however, by the 12-month interview there were significantly different scores for the BG checking subsection (P = 0.03). Pairwise comparisons indicated that those using the ADMS 1–3 times/week had higher 12-month scores in the BGM subscale (26.8 ± 4.35) than the control group (21.96 ± 5.53; P = 0.03) and the group using the ADMS <1 time/week (21.92 ± 4.44; P = 0.06).
CONCLUSIONS
To be successful, data capture technologies, such as the ADMS, must reduce the burden of collecting and analyzing the data to a point where it does not interfere with ordinary activities of daily living (7). Toward this goal, our study was designed to understand realistic use of the ADMS by simply giving the device to families and studying the ways in which they appropriated the technology. We do not know whether any relationship exists between BGM frequency and ADMS use because the study was conducted in a clinic setting; therefore, these data were incomplete. However, our findings do suggest that frequency of docking had a significant relationship with the measures examined. Specifically, docking 1–3 times/week (an average of 1.9 times/week over the 12 months) was associated with significant improvements in the glycemic control and self-care skills in children, and had a positive effect on the BGM effect of parents.
The significant reduction in HbA1c coupled with the improved and stable BGMC scores of parents and children, respectively, is a particularly important finding from this research. The emotional response to viewing the results of a BG check on a glucometer has an established relationship with glycemic control, that is, lower scores on the BGMC, indicating a more positive BGM effect, have been correlated with lower HbA1c values in prior research (4). Therefore, an important consideration for the design of technology for children with type 1 diabetes is to, at the very least, do no harm to the emotions of the child or parents with interventions aimed at BGM. Although the BGMC scores of children were not significantly changed through the use of the ADMS, they did not get any worse, and we assume that no harm was done. This is an important finding given that this technology is designed to increase the amount of reflection on BG values through real time alerts and daily trending reports; thus, it has the potential to make bad feelings worse.
This study contributes to the larger body of knowledge about how remote data capture technology may be beneficial to children and families coping with type 1 diabetes. Taken together, the results of this study and others that have investigated the use of pervasive technologies to promote BG monitoring (6,8–10) reveal the promise of such devices. The limitations of this study include the relatively small sample size of 48 families. To make stronger claims about the benefit of the ADMS, the study would need to be replicated with a larger set of participants. In addition, approximately half of the families in the experimental group used the ADMS less than one time each week throughout the study and some stopped completely after several months of use, revealing a need for further design work aimed at encouraging the sustained use of the technology. It is difficult to believe that the high level of engagement seen in the group docking 1–3 times/week would have been maintained if the parents or children did not look at the alerts or trending reports. A higher number of docking events translates to more system-generated real-time alerts and more complete 21-day trending reports. Therefore, it may be that improvements found in families docking more frequently were related to an increased reflection on BG data, but there may have been any myriad factors contributing to this relationship. Future work should examine the personal and family contextual factors that present barriers to the use of technologies that ease the burden of collecting and reflecting on BG data.
Acknowledgments
Funding for the study was provided by The Morris L. Lichtenstein, Jr. Medical Research Foundation.
Diabetech, LP provided the technology for the study and was paid for the use of the GlucoMON-ADMS during the study. No other potential conflicts of interest relevant to this article were reported.
T.R.T. and S.W.P. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. B.J.A., M.B.D., and M.L.L. contributed to discussion and reviewed and edited the manuscript. E.M.-G. and P.R. researched data. E.L. and K.L.M. researched data, contributed to discussion, and reviewed and edited the manuscript. T.R.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Driscoll Children’s Hospital, participating clinicians, and families for their commitment to this research endeavor.
References
- 1.Silverstein J, Klingensmith G, Copeland K, et al. ; American Diabetes Association Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 2005;28:186–212 [DOI] [PubMed] [Google Scholar]
- 2.Anderson BJ, Vangsness L, Connell A, Butler D, Goebel-Fabbri A, Laffel LM. Family conflict, adherence, and glycaemic control in youth with short duration Type 1 diabetes. Diabet Med 2002;19:635–642 [DOI] [PubMed] [Google Scholar]
- 3.Harris MA, Wysocki T, Sadler M, et al. Validation of a structured interview for the assessment of diabetes self-management. Diabetes Care 2000;23:1301–1304 [DOI] [PubMed] [Google Scholar]
- 4.Hood KK, Butler DA, Volkening LK, Anderson BJ, Laffel LMB. The Blood Glucose Monitoring Communication questionnaire: an instrument to measure affect specific to blood glucose monitoring. Diabetes Care 2004;27:2610–2615 [DOI] [PubMed] [Google Scholar]
- 5.Takita M, Matsumoto S, Noguchi H, et al. Early identification of islet graft dysfunction using self-monitoring of blood glucose in clinical islet cell transplantation for type 1 diabetes. Diabetes Care 2011;34:1799–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gammon D, Årsand E, Walseth OA, Andersson N, Jenssen M, Taylor T. Parent-child interaction using a mobile and wireless system for blood glucose monitoring. J Med Internet Res 2005;7:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes GR. Documenting and understanding everyday activities through the selective archiving of live experiences. In Proceedings of the 24th annual ACM Conference on Human Factors in Computing Systems Montréal, Québec, Canada, 2006 New York, NY, Association for Computing Machinery, p. 1759–1762 [Google Scholar]
- 8.Carroll AE, DiMeglio LA, Stein S, Marrero DG. Using a cell phone-based glucose monitoring system for adolescent diabetes management. Diabetes Educ 2011;37:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franklin V, Waller A, Pagliari C, Greene S. “Sweet Talk”: text messaging support for intensive insulin therapy for young people with diabetes. Diabetes Technol Ther 2003;5:991–996 [DOI] [PubMed] [Google Scholar]
- 10.Hanauer DA, Wentzell K, Laffel N, Laffel LM. Computerized Automated Reminder Diabetes System (CARDS): e-mail and SMS cell phone text messaging reminders to support diabetes management. Diabetes Technol Ther 2009;11:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]