Abstract
OBJECTIVE
To extend our previous work on evaluating the use of oligonucleotide arrays to discriminate colonization from infection owing to Staphylococcus aureus in diabetic foot ulcers (DFUs).
RESEARCH DESIGN AND METHODS
Patients admitted to 14 French diabetic foot departments for a DFU were screened for entry into the study. At admission, ulcers were classified based on clinical examination according to the Infectious Diseases Society of America system. Only patients with monomicrobial culture for S. aureus were included. In persons with an uninfected ulcer, a second wound bacterial specimen was obtained 1 month later. Using oligonucleotide arrays, S. aureus resistance and virulence genes were determined, and each isolate was affiliated to a clonal complex (CC).
RESULTS
S. aureus was initially isolated from 75 uninfected and 120 infected ulcers; 35 were methicillin resistant. A total of 44 (59%) strains from uninfected DFUs belonged to CC5/CC8 clones vs. 6 (5%) from infected DFUs (P < 0.001). During follow-up, 57 (76%) of uninfected DFUs healed or had a favorable outcome; the strain in 49 (86%) of them belonged to CC5/CC8. Conversely, 18 (24%) had a poor outcome but not a single strain belonged to CC5/CC8 clone. Moreover, lukDE was significantly associated with a favorable outcome of the wound.
CONCLUSIONS
As suggested by our previous study, the use of DNA arrays appears to be a promising technique that might help distinguishing uninfected from infected wounds, predicting ulcer outcome and then contributing to a more adequate use of antibiotics.
Foot ulcers are common in diabetic patients, with prevalence as high as 25% (1). These ulcers frequently become infected, and spread of infection to soft tissue and bony structures is a major causal factor for lower-limb amputation (2), making early diagnosis and adequate treatment essential. As microorganisms are always present on skin wounds, diagnosis of infection must be based not on microbiological findings but on symptoms and clinical signs, as emphasized by the Infectious Diseases Society of America (IDSA), the International Working Group on the Diabetic Foot (IWGDF), and the French Society for Infectious Pathology (3–5). However, owing to the confounding effect of neuropathy and ischemia on local and systemic inflammatory response, diagnosing foot infection at an early stage in diabetic patients is often difficult. Hence, identification of reliable clinical and/or microbiological criteria for diabetic foot infection would be of great value, allowing differentiation of actually infected ulcers from noninfected (colonized) ulcers.
New technologies, such as DNA microarray and multiplex real-time PCR, offer a unique opportunity to rapidly and reliably detect the presence of genes encoding for various virulence factors (e.g., genes encoding the bicomponent toxin Panton-Valentine leukocidin, lukF-PV and lukS-PV) and antibiotic resistance factors (e.g., SCCmec, the complex mobile genetic elements bringing mecA, the methicillin-resistance gene) (6,7). Using this method, we previously found that the combination of five genes (sea, sei, lukDE, hlgv, and cap8) was highly predictive for differentiating clinically uninfected from infected diabetic foot ulcers (DFUs) (8). Moreover, we showed that the presence of Staphylococcus aureus virulence factors, either at presentation or at follow-up in diabetic patients with clinically uninfected wounds, was predictive of a poor clinical outcome (9).
Since then, technological advances have led to the development of a new generation of miniaturized oligonucleotide array to genotype S. aureus covering a much larger number of genes. The aim of the current study was to validate such a new tool and to assess its potential advantages over the previous technique to type S. aureus isolated from DFUs in several French hospital centers.
RESEARCH DESIGN AND METHODS
Prospective study
We prospectively enrolled a casual sample of outpatients attending 1 of 12 participating French foot clinics (Supplementary Data) between 1 April 2008 and 30 June 2010 for any type of foot ulcer after informed consent was obtained. Patients were included if they had not received any antibiotic agents in the previous week. This study was approved by the local ethics committee (South Mediterranean III) and carried out in accordance with the Declaration of Helsinki as revised in 2000. Every patient was examined by trained physicians to grade infection severity. According to the IDSA/IWGDF criteria (4,5), wounds were considered either uninfected (grade 1) or infected (grade ≥ 2).
Study design
After wound debridement, samples for bacterial culture were obtained by swabbing the wound base, needle aspiration, or tissue biopsy and immediately sent to the bacteriology department. Only patients with monomicrobial culture for S. aureus were included in the study. Patients with uninfected ulcers did not undergo antibiotic treatment and were monitored closely for 30 days to definitively assess the wound status (infected vs. uninfected). If the wound was considered to be worsening before the follow-up visit, patients were instructed to return to the outpatient facility for early review and a further sample was taken for bacterial culture. At the follow-up visit, if the wound was healing but not completely reepithelialized, a sample for bacterial culture was obtained and the outcome was considered favorable. For completely reepithelialized wounds, no microbiological specimens were sampled and the wound was considered healed.
Microbiological study
Genus, species, and antibiotic susceptibilities were determined using the Vitek 2 card (BioMérieux, Marcy-l’Etoile, France) and interpreted according to the recommendations of the French Society for Microbiology (10). Susceptibility to methicillin was screened by agar diffusion using cefoxitin disks (BioRad, Marnes-La-Coquette, France) (10).
Oligonucleotide DNA arrays and genotyping
Each S. aureus strain collected during the study was analyzed at the INSERM laboratory in Nîmes, France. All the experiments were performed by a PhD student blind to the DFU grade. The Alere StaphyType DNA microarray was used according to protocols and procedures previously described (6,7). The test was able to screen numerous markers simultaneously in 5 h. The DNA microarray covers 334 target sequences including the main virulence and resistance genes. Primer and probe sequences have previously been published (6,7). DNA was extracted from each S. aureus strain, and after amplification and hybridization, markers were identified. This technology determines the clonal complex (CC) of strains. A CC may be defined as a cluster of strains (clones) that are close enough together to be claimed to share a common origin. Thus, this group of bacteria is genetically identical to a single ancestral clone. This affiliation of isolates was assessed by an automated comparison of hybridization profiles to a collection of reference strains previously characterized (6,7,11).
In order to determine the CCs of the colonizing strains, we first analyzed the 11 S. aureus strains from clinically uninfected ulcers that we isolated in our previous study (8). More information regarding oligonucleotide DNA arrays and genotyping can be found in Supplementary Data.
Statistical analysis
The presence of each gene in S. aureus strains was compared as to ulcer grades and outcome of ulcers using Fisher exact test. The most predictive genes for a favorable outcome or healing were expressed by sensitivity, specificity, and positive and negative predictive values; area under the receiver operating characteristic (ROC) curve was calculated by the nonparametric Hanley method. To assess the usefulness of combining several virulence markers, we used logistic regression with a backward procedure to select the most relevant markers; only markers for which area under the ROC curve was >0.80 were initially entered as explanatory variables in the regression analysis. An ROC curve was then generated for the combination of relevant genes derived from the regression model, and its area was compared with that of every single virulence marker by a nonparametric method adapted to paired data (12). Statistical analysis was performed using the S-Plus 2000 software package (Insightful, Seattle, WA), and results were considered significant for P < 0.05.
RESULTS
Clinical and bacteriological data
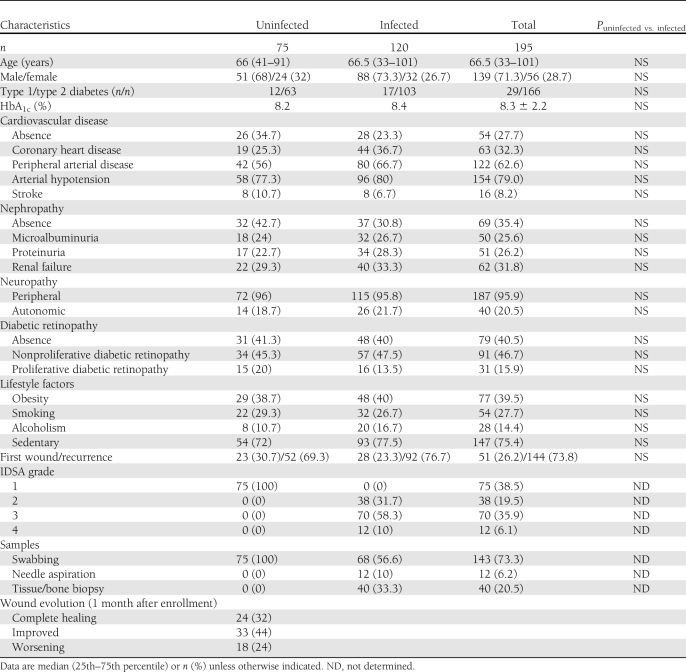
During the study period, 195 patients were recruited in whom S. aureus was the sole organism isolated from the bacterial culture of their wound. Seventy-five wounds (38.5%) were classified as uninfected (grade 1) and 120 as infected (grades 2–4). As shown in Table 1, both groups were well matched for age, sex, type, and complications of diabetes. At the follow-up visit, 24 of initially uninfected DFUs were healed (32%) and 33 had a favorable outcome (44%), whereas 18 (24%) had worsened rapidly (≤ 30 days). No association was found between the uninfected ulcers that worsened and clinical (peripheral arterial disease, neuropathy) or biochemical (HbA1c) parameters. In each case, an S. aureus strain was isolated from the second bacterial sampling carried out in those uninfected ulcers that were not completely healed at the follow-up visit, regardless of whether the outcome was favorable. Overall, there were 246 isolates of S. aureus (195 at baseline and 51 at the follow-up), of which 40 (16.3%) were methicillin resistant (35 [17.9%] at baseline and 5 [9.8%] during the follow-up period).
Table 1.
Demographic and clinical characteristics of study patients
CC distribution
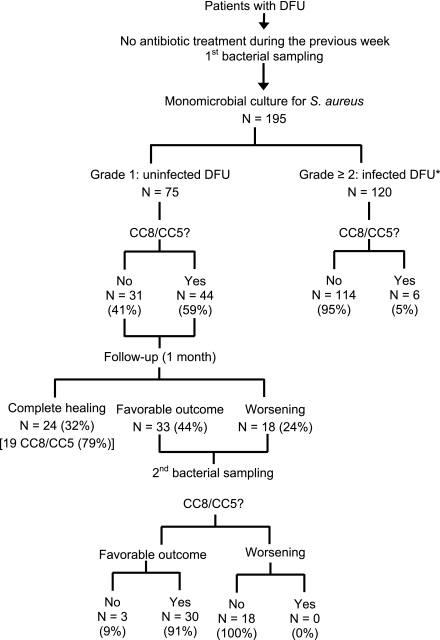
CC distribution of isolates allowed comparison between the strains, examination of the biodiversity of the isolates, and determination of their origin and clonality. Using the new technology, the CCs of the 11 colonizing S. aureus strains isolated in our previous pilot study (8) were shown to belong only to two clones, CC8–methicillin-sensitive S. aureus (MSSA) and CC5-MSSA. Thus, we hypothesized that these two CCs corresponded to colonizing CCs and other CCs were considered infecting CCs.
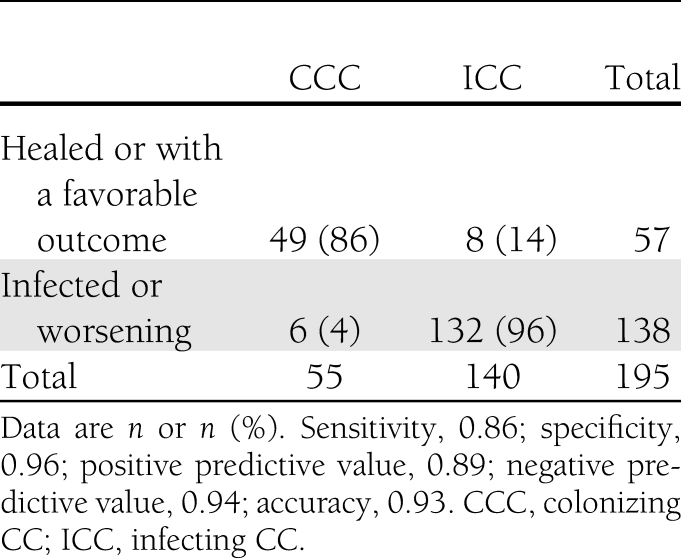
All the strains from patients included in the current study were analyzed. The results are shown in Fig. 1 and in Supplemental Data. On admission, 44 strains (59%) from clinically uninfected wounds belonged to colonizing CCs compared with only 6 (5%) from infected DFUs (P < 0.001). According to the outcome, the prevalence rate of colonizing CCs increased up to 86% if strains isolated from DFUs whose outcome were favorable were also taken into account (Fig. 1 and Table 2). On the other hand, no strains isolated from DFUs with a worsening outcome belonged to colonizing CCs and there was no change of CCs between admission and the follow-up visit. Infecting CCs were recovered from 96% of S. aureus isolated from infected ulcers or with a worsening outcome. Among these infecting CCs, CC45-MSSA was the most commonly identified in 21 strains. If we used the colonizing CCs to differentiate uninfected from infected ulcers, sensitivity was 0.59 and specificity 0.95 and positive and negative predictive values were 0.88 and 0.79, respectively. If colonizing CCs were considered for differentiating DFUs according to the outcome, diagnostic performances were substantially improved (Table 2). Finally, the methicillin-resistant S. aureus (MRSA) strains mainly belonged to CC8-MRSA (Lyon clone) (n = 25 strains), the main clone present in French hospitals.
Figure 1.
Flow of patients through the study and results of CC8-MSSA/CC5-MSSA obtained of grade 1–4 inclusion and during follow-up of uninfected ulcers. *Grades according to the IDSA/ IWGDF classification system (4,5): grade 2, n = 38; grade 3, n = 70; grade 4, n = 12.
Table 2.
Distribution of colonizing and infecting clonal complexes of S. aureus according to the outcome of DFUs
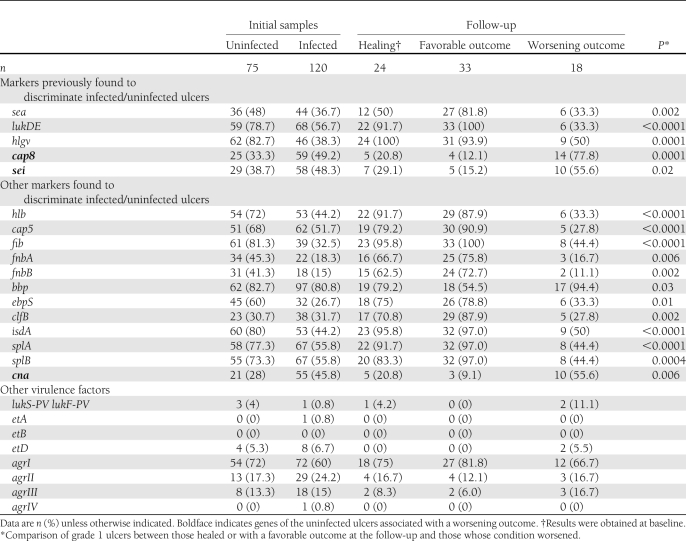
Virulence profile
The univariate analysis found that among the five genes (sea, sei, lukDE, hlgv, and cap8) that we previously identified as predictive for differentiating infected from noninfected DFUs (8), only two (lukDE and hlgv) actually distinguished infected from uninfected DFUs (P < 0.05) (Table 3). However, three of these five genes (cap8, lukDE, and hlgv) differentiated S. aureus isolated from uninfected DFUs with favorable outcome and S. aureus isolated both from infected and uninfected DFUs with a poor outcome (P < 0.05). Moreover, the five genes were significantly associated with the wound outcome: cap8 and sei with a poor outcome as opposed to sea, lukDE, and hlgv, which were associated with DFUs with favorable outcomes (Table 3). In the univariate analysis, 72 additional genes were found to be significantly associated with the outcome of uninfected ulcers (P < 0.05) (data not shown). From the logistic model analysis, lukDE was the most predictive gene for the favorable outcome of uninfected DFU: sensitivity was 0.965 (SD 0.025) and specificity 0.667 (0.063), and positive and negative predictive values were 0.902 and 0.857, respectively. Using this model, only two uninfected ulcers were misclassified as they healed despite the absence of lukDE; interestingly, one ulcer presented a colonizing CC8 strain and the other a strain positive for Panton-Valentine leukocidin (PVL). Conversely, S. aureus from six ulcers with a poor outcome were positive for lukDE gene; however, these strains belonged to infecting CCs.
Table 3.
Main virulence profiles of S. aureus isolated from uninfected (grade 1) ulcer at initial sampling and follow-up
Genes encoding PVL and exfoliatin A, B, and D toxins were found in 4 (2%), 1 (0.5%), 0 (0%), and 12 (6%) of the isolates, respectively. No differences were found between infected and uninfected ulcers.
CONCLUSIONS
Over the last few years, various DNA array technologies have been developed, but most systems are very expensive, time-consuming, technically demanding, and difficult to adapt to the needs of clinical screening, restricting their use to research laboratories (6,7). In contrast, compared with the first generation of miniaturized oligonucleotide array, the new system we used is more convenient and informative. Each DNA microarray carries a set of 334 different probes (vs. 50 for the first generation), and the use of strip-integrated arrays is time-saving (5 h vs. 1 day for the first generation), easy to perform and to interpret, and allows a large number of samples to be analyzed (96-well strip) (6,7) at low cost (∼60 USD, which is three times less expensive than the first generation). Moreover, the new arrays allow for assessment of whether the strains are identical by determining the CCs.
The main result of our multicenter study is that the microarray technology revealed differences between uninfected and infected ulcers and, if the results are confirmed, the use of this technique may enable clinicians to identify infection and to predict the outcome of apparently clinically uninfected wounds. The most interesting contribution of this new microarray is its ability to determine the clonal CCs of the strains. As a result, a lot of CCs were isolated from DFUs (Supplementary Data), demonstrating that a large panel of S. aureus is involved in this pathology. The results of this study also clearly suggest that the colonizing S. aureus strains belonged to 2 CCs: CC8-MSSA and CC5-MSSA. The distribution of these CCs was significantly different in uninfected and infected DFUs (58 vs. 5%, respectively; P < 0.001) and predicted a favorable outcome of the uninfected wounds, as 86% of strains isolated from uninfected ulcers that healed or had a favorable outcome belonged to these CCs. We previously showed that the association of five virulence markers could distinguish uninfected and infected DFUs (8). The current study shows that a single one of the five markers (lukDE) may suffice with a high sensitivity (96.5%). The association of CCs and the lukDE gene is a powerful tool for predicting the wound outcome. For example, none of the six ulcers from which lukDE-positive S. aureus was isolated and that had a poor outcome belonged to a colonizing CC. However, the results of the current study vary from those of our previous study: only two (lukDE and hlgv) of the five previously identified virulence genes actually allowed the distinction between infected and uninfected DFUs. Moreover, it is worth noting that cap8 gene was a marker of colonization in our pilot study but a marker of worsening evolution in the current study. Some explanations may be put forward to account for those discrepancies: the current study is multicentric, and the detection tools are different (PCR versus arrays with different targets for the same gene). Moreover, the time between the two studies coincided with a quite marked decrease in the prevalence of MRSA in France (involving a change in clonal strains). Interestingly, this study showed that all of the uninfected strains that worsened had the same genotype (CC and content of 334 genes), indicating that the wounds were actually infected and not only colonized. These results highlight the potential usefulness of this technique in patients in whom the clinical diagnosis of infection is made difficult by, for example, peripheral arterial disease, neuropathy, or impaired leukocyte functions (13). Additionally, the DNA microarray might provide important help for clinicians and might allow for adequate management of clinically uninfected ulcers carrying S. aureus, according to its genotype profile. Results of the current study raise the question about antibiotic treatment in apparently uninfected DFUs and challenge the dogma about abstention of antibiotic therapy in clinically uninfected ulcers (14,15).
Furthermore, this new generation of microarray rapidly gives information about in vitro susceptibility of S. aureus, especially about the presence of mecA gene, an important therapeutic concern. Worth noting is that that the prevalence of MRSA was low in the current study (18%), confirming the decreasing trends in MRSA prevalence in France (16). While 14 patients with uninfected ulcers had an MRSA strain at baseline, only five cases of MRSA were recovered during the follow-up, suggesting that debridement of the wound alone may be sufficient for eradicating MRSA. The favorable outcome associated with uninfected DFU with S. aureus belonging to the “infecting” CC1 poses a problem. However, this clonal complex does not have to be included in colonizing CC, as three DFUs were actually infected by CC1 S. aureus.
Finally, an additional valuable feature of the array technology is its ability to identify PVL genes, since these genes coding for a cytotoxin are claimed to be a major threat in severe tissue necrosis (17–21). However, these strains are rarely isolated from chronic wounds (22,23) and their pathogenicity is low in this setting; accordingly, some PVL+ strains isolated from grade 1 ulcers were healed 1 month later. This suggests that the portal of entry of PVL+ strains is not the chronic wound. The DNA array represents a powerful tool for predicting the evolution of uninfected ulcer, and a clinical study on the cost-effectiveness of this technology on the healing time and amputation rate would be interesting.
One of the main limitations of this study is that it was conducted exclusively on DFUs with S. aureus as the sole pathogen, while the flora of DFUs is often polymicrobial. Nevertheless, if we focus on uninfected ulcers, most of them are monomicrobial, and S. aureus is one of the most frequently isolated microorganisms. S. aureus is also the most common pathogen in infected ulcers, even if the infection is polymicrobial (24). Moreover, to eliminate any influence of another pathogen in the clinical course of the wounds, it was useful to study wounds with only S. aureus strains. Hence, this clinical platform appears suitable for use under routine conditions in a microbiology laboratory. Another limitation is that the specimens for culture were not obtained by the same method in all patients. Finally, despite the fact that the clinical diagnosis of infection was made by experts in the field, the interobserver variability in diagnosing infected (vs. uninfected) DFUs was not tested and is largely unknown.
In conclusion, as our previous study suggested, the miniaturized oligonucleotide arrays are an interesting tool for managing DFUs: they allow an early discrimination between infection and colonization of wounds by S. aureus. Some results of this study challenge the current belief that antibiotic therapy is not needed for clinically uninfected DFUs. One concern is that this may encourage clinicians to use this technology for screening apparently uninfected ulcers, contrary to current guidelines.
Acknowledgments
This study was supported by grants from the French Ministry of Health (Hospital Project of Clinical Research, 2007, A00678-45), the French Society of Diabetes (l'Association de Langue Française pour l'Etude du Diabète et des Maladies Métaboliques Grant 2008), the Languedoc-Roussillon Region (Chercheur d’avenir Grant 2009), and the National Institute of Health and Medical Research (INSERM). This study was also supported by a Pfizer grant. No other potential conflicts of interest relevant to this article were reported.
A.S. and J-L.R. researched data and wrote the manuscript. N.Me. researched data. N.Mo. performed the statistical analyses. N.J., S.S., A.S., and C.C. researched data and reviewed and edited the manuscript. B.C., L.L., and G.L. researched data and contributed to the discussion. J-P.L. was involved in the design of the overall study, designed the analysis plan, supervised the analysis, and wrote the manuscript. As the corresponding author and guarantor of this article, J.-P.L. takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. All the participants of the French Study Group on the Diabetic Foot recruited the patients and reviewed and edited the manuscript.
The authors thank all the participants of the foot clinics for help in recruiting patients. The authors thank Laure Vidal-Navarro, INSERM U1047, for her technical assistance and Anne Blanc-Potard, Julien Chamard, and Céline Groul-Viaud, the research clinical technicians of the study, Nîmes University Hospital, for their help in data processing.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1352/-/DC1.
A complete list of the members of the French Study Group on the Diabetic Foot can be found in the Supplementary Data.
References
- 1.Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM. Preventive foot care in people with diabetes. Diabetes Care 1998;21:2161–2177 [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 3.Société de Pathologie Infectieuse de Langue Française Recommandations pour la pratique clinique. Prise en charge du pied diabétique infecté. Med Mal Infect 2007;37:26–50 [in French] [DOI] [PubMed] [Google Scholar]
- 4.Lipsky BA, Berendt AR, Deery HG, et al. ; Infectious Diseases Society of America Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2004;39:885–910 [DOI] [PubMed] [Google Scholar]
- 5.Lipsky BA; International consensus group on diagnosing and treating the infected diabetic foot A report from the international consensus on diagnosing and treating the infected diabetic foot. Diabetes Metab Res Rev 2004;20(Suppl. 1):S68–S77 [DOI] [PubMed] [Google Scholar]
- 6.Monecke S, Jatzwauk L, Weber S, Slickers P, Ehricht R. DNA Microarray based genotyping of methicillin-resistant Staphylococcus aureus strains from Eastern Saxony. Clin Microbiol Infect 2008;14:534–545 [DOI] [PubMed] [Google Scholar]
- 7.Monecke S, Slickers P, Ehricht R. Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol Med Microbiol 2008;53:237–251 [DOI] [PubMed] [Google Scholar]
- 8.Sotto A, Lina G, Richard JL, et al. Virulence potential of Staphylococcus aureus strains isolated from diabetic foot ulcers: a new paradigm. Diabetes Care 2008;31:2318–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotto A, Richard JL, Jourdan N, Combescure C, Bouziges N, Lavigne JP; Nîmes University Hospital Working Group on the Diabetic Foot (GP30) Miniaturized oligonucleotide arrays: a new tool for discriminating colonization from infection due to Staphylococcus aureus in diabetic foot ulcers. Diabetes Care 2007;30:2051–2056 [DOI] [PubMed] [Google Scholar]
- 10.Soussy CJ, Carret G, Cavallo JD; Antibiotic Susceptibility Testing Committee of the French Society for Microbiology. Antibiotic susceptibility testing [article online], 2011. French Society for Microbiology. Available from http://www.sfm-microbiology.org Accessed 7 March 2011
- 11.Monecke S, Coombs G, Shore AC, et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE 2011;6:e17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845 [PubMed] [Google Scholar]
- 13.Richard JL, Lavigne JP, Sotto A. Diabetes and foot infection: more than double trouble. Diabetes Metab Res Rev 2012;28(Suppl 1):46–53. [DOI] [PubMed] [Google Scholar]
- 14.Lipsky BA. Evidence-based antibiotic therapy of diabetic foot infections. FEMS Immunol Med Microbiol 1999;26:267–276 [DOI] [PubMed] [Google Scholar]
- 15.Ogunshe AA. Letter: microbiological and clinical mismanagement of non healing diabetic leg ulcers? Int Wound J 2011;8:542–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarlier V, Trystram D, Brun-Buisson C, et al. ; Collégiale de Bactériologie-Virologie-Hygiène des Hôpitaux Universitaires de l’Ile de France Curbing methicillin-resistant Staphylococcus aureus in 38 French hospitals through a 15-year institutional control program. Arch Intern Med 2010;170:552–559 [DOI] [PubMed] [Google Scholar]
- 17.Lina G, Piémont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 1999;29:1128–1132 [DOI] [PubMed] [Google Scholar]
- 18.Jarraud S, Mougel C, Thioulouse J, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun 2002;70:631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenesch F, Etienne J. How to prevent transmission of MRSA in the open community? Euro Surveill 2004;9:5. [PubMed] [Google Scholar]
- 20.Robinson DA, Kearns AM, Holmes A, et al. Re-emergence of early pandemic Staphylococcus aureus as a community-acquired meticillin-resistant clone. Lancet 2005;365:1256–1258 [DOI] [PubMed] [Google Scholar]
- 21.Dufour P, Gillet Y, Bes M, et al. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis 2002;35:819–824 [DOI] [PubMed] [Google Scholar]
- 22.del Giudice P, Blanc V, de Rougemont A, et al. Primary skin abscesses are mainly caused by Panton-Valentine leukocidin-positive Staphylococcus aureus strains. Dermatology 2009;219:299–302 [DOI] [PubMed] [Google Scholar]
- 23.Del Giudice P, Bes M, Hubiche T, et al. Panton-Valentine leukocidin-positive Staphylococcus aureus strains are associated with follicular skin infections. Dermatology 2011;222:167–170 [DOI] [PubMed] [Google Scholar]
- 24.Lipsky BA, Armstrong DG, Citron DM, Tice AD, Morgenstern DE, Abramson MA. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double-blinded, multicentre trial. Lancet 2005;366:1695–1703 [DOI] [PubMed] [Google Scholar]