Abstract
OBJECTIVE
Chronic infections could be contributing to the socioeconomic gradient in chronic diseases. Although chronic infections have been associated with increased levels of inflammatory cytokines and cardiovascular disease, there is limited evidence on how infections affect risk of diabetes.
RESEARCH DESIGN AND METHODS
We examined the association between serological evidence of chronic viral and bacterial infections and incident diabetes in a prospective cohort of Latino elderly. We analyzed data on 782 individuals aged >60 years and diabetes-free in 1998–1999, whose blood was tested for antibodies to herpes simplex virus 1, varicella virus, cytomegalovirus, Helicobacter pylori, and Toxoplasma gondii and who were followed until June 2008. We used Cox proportional hazards regression to estimate the relative incidence rate of diabetes by serostatus, with adjustment for age, sex, education, cardiovascular disease, smoking, and cholesterol levels.
RESULTS
Individuals seropositive for herpes simplex virus 1, varicella virus, cytomegalovirus, and T. gondii did not show an increased rate of diabetes, whereas those who were seropositive for H. pylori at enrollment were 2.7 times more likely at any given time to develop diabetes than seronegative individuals (hazard ratio 2.69 [95% CI 1.10–6.60]). Controlling for insulin resistance, C-reactive protein and interleukin-6 did not attenuate the effect of H. pylori infection.
CONCLUSIONS
We demonstrated for the first time that H. pylori infection leads to an increased rate of incident diabetes in a prospective cohort study. Our findings implicate a potential role for antibiotic and gastrointestinal treatment in preventing diabetes.
Cardiovascular diseases and diabetes disproportionately affect people of low socioeconomic status and minority race in the U.S. (1). Behavioral risk factors, such as poor diet, smoking, and physical inactivity, are well-known contributors to the disparity, but only partially explain the gap in health states (2,3). Other contributing factors may include chronic infections that are more prevalent among minorities and individuals of low-socioeconomic status (4). In fact, studies suggest that pathogen burden, including infectious agents, such as cytomegalovirus, herpes simplex virus (HSV), Chlamydia pneumoniae, and Helicobacter pylori, may have an impact on cardiovascular conditions and metabolic syndrome (5–7) potentially mediated by elevations in inflammatory markers such as C-reactive protein (CRP) and interleukin (IL)-6 (5). Inflammation and activated innate immunity have also been implicated in the pathogenesis of diabetes through insulin resistance (8). For example, elevated levels of inflammatory cytokines may lead to phosphorylation of serine residues on the insulin receptor substrate, which prevents its interaction with insulin receptors, inhibiting insulin action (8). Lipopolysaccharides from pathogens in the gut, such as H. pylori, have also been linked to the activation of Toll-like receptors, resulting in energy harvesting, fat accumulation and stimulation of the innate immune system, and consequent insulin resistance (9). However, epidemiological studies investigating the impact of pathogen burden on diabetes have been limited. A previous study found increased risk of diabetes in people with a high burden of periodontal bacteria (10). On the other hand, cross-sectional studies examining other more systemic pathogens and insulin resistance or prevalent diabetes have produced equivocal findings (11–13), with higher titers of antibodies to HSV2 and C. pneumoniae showing decreased insulin sensitivity in one study (11) and infection with C. pneumoniae, H. pylori, cytomegalovirus, HSV, and/or hepatitis A showing no association with insulin resistance or prevalent diabetes in other studies (12,13). These earlier studies were limited by cross-sectional analyses, and there are no prospective studies, of which we are aware, to elucidate whether there is a potential causal relationship between different types of pathogens or pathogen burden and incident diabetes. In addition, there has been a lack of epidemiological studies examining the potential pathways by which pathogens may affect diabetes, such as inflammation. In this study, we aim to examine the effects of specific pathogens and pathogen burden, as measured by seropositivity to five pathogens—HSV1, varicella virus (VZV), cytomegalovirus (CMV), Toxoplasma gondii, and H. pylori—on incident diabetes over 10 years and whether these relationships are mediated by inflammation and insulin resistance. We selected HSV1, CMV, and H. pylori because they have previously been investigated with regard to pathogen burden and diabetes or cardiovascular disease. Among other pathogens for which data were available, we also included VZV, since it is a herpes virus closely related to HSV1 and CMV, as well as T. gondii, since it has been associated with other chronic conditions, including neuropathy and cognitive function.
RESEARCH DESIGN AND METHODS
Study population
We derived the data for our analyses from the Sacramento Area Latino Study on Aging (SALSA), a large representative ongoing prospective cohort study of community-dwelling Latinos in California. Participants were 60–101 years of age at baseline in 1998–1999. Trained bilingual interviewers conducted in-home interviews covering sociodemographics, lifestyle factors, and medical diagnoses. A fasting blood sample was drawn on the day of the interview. A previous publication describes further details of the survey and cohort design (14). At baseline recruitment, 1,789 people were enrolled in the study. We excluded 523 participants without measures of antibody titers to HSV1, VZV, CMV, T. gondii, or H. pylori at baseline and an additional 69 without measures of other covariates of interest at baseline. There were more males and older individuals in the excluded group compared with those not excluded (71.8 vs. 68.8 years, P < 0.0001; 47 vs. 39% male, P = 0.0009), whereas incident rates of diabetes were not significantly different (3.09 vs. 2.95 per 100 person-years, P = 0.77). To determine factors related to incident diabetes, we further excluded 411 participants who already had diabetes at baseline and 4 individuals without follow-up after baseline. Therefore, our study subsample comprised 782 individuals who were diabetes-free at baseline. The SALSA cohort was approved by the institutional review boards at the University of Michigan and the University of California, San Francisco and Davis.
Outcome ascertainment
Follow-up data on diabetes were available through June 2008, with a total of 144 incident diabetes events. We defined incident diabetes as meeting any of the following criteria in semiannual follow-up interviews or annual laboratory examinations: 1) self-report of a physician’s diagnosis of diabetes, 2) fasting blood glucose level of ≥126 mg/dL, 3) usage of diabetes medication (insulin or oral hypoglycemic agent), or 4) diabetes listed as a cause of death anywhere on a death certificate. Age at incident diabetes was computed by adding enrollment age and years of follow-up from recruitment to earliest evidence of diabetes according to the above criteria. Individuals without any evidence of diabetes were censored at the time of the last visit or death because of causes other than diabetes.
Covariates
Socioeconomic status was determined by the years of formal education received. Income was not used because many of these individuals were retired; therefore, income was unlikely to provide a sensitive measure of lifetime socioeconomic status. Place of birth was defined as having been born in the U.S. or Mexico or another Latin American country. BMI was measured at baseline and computed by weight in kilograms divided by height in meters squared. Waist and hip measurements were taken at baseline in inches, and waist-to-hip ratio was determined by dividing the waist measurements by the hip measurements. Smokers were categorized into never-smokers, former smokers, and current smokers. Presence of vascular disease was determined by a self-report of a physician’s diagnosis of heart attack, chest pain due to heart disease, congestive heart failure, intermittent claudication, stroke, irregular heart rhythm, deep vein thrombosis, or coronary/heart catheterization. Systolic and diastolic blood pressures were measured at baseline and in annual follow-up visits in sitting position. Medications for diabetes were determined by a medicine cabinet inventory by a trained interviewer at the baseline and annual interviews.
Laboratory analysis
Measurement of IL-6, CRP, HDL, LDL, glucose, and insulin levels in serum taken from fasting blood samples took place at University of Michigan. IL-6 levels were determined using the Quantiglo Chemiluminsecent Immunoassay (QTA00B and Q6000B, respectively; R&D Systems, Minneapolis, MN). CRP levels were assayed with the CRP Ultra Wide Range Reagent Kit latex-enhanced immunoassay (Equal Diagnostics, Exton, PA). Morning fasting blood samples were used to measure LDL and HDL cholesterol levels. HDL cholesterol was measured directly using the HDL Direct reagent (number 3034569; Roche Diagnostics, Indianapolis, IN). LDL cholesterol levels were determined by LDL Direct Liquid Select (number 7120; Equal Diagnostics). Glucose was measured by the Cobas Mira Chemistry Analyzer (Roche Diagnostics). Insulin levels were measured using a double-antibody radioimmunoassay using a 125I-human insulin tracer (Linco Research, Charles, MO), a guinea pig anti-porcine insulin first antibody (Michigan Diabetes Research and Training Center, 68.5% cross-reaction to human proinsulin), and a goat anti–guinea pig gammaglobulin-polyethylene glycol second antibody (Antibodies Inc., Davis, CA) and standardized against the Human Insulin International Reference Preparation (National Institute for Biological Standards and Control). Homeostatic model assessment for insulin resistance (HOMA-IR) was computed by taking the product of the fasting glucose (milligrams per deciliter) and insulin values and dividing by 405. Frozen (−70°C) serum samples were sent to the Stanley Laboratory of Developmental Neurovirology at the Johns Hopkins University to measure antibody titers to HSV1, VZV, CMV, T. gondii, and H. pylori. A solid-phase enzyme-linked immunosorbent assay was used for detecting type-specific IgG antibody responses to the infectious agents measured by optical density units, as previously described (15).
Statistical analysis
The distributions of baseline covariates were compared across diabetes status by using χ2 tests for categorical variables and Wilcoxon rank-sum test for continuous variables. Participants were considered seropositive for a specific infection if the measured antibody titer levels were ≥1 optical density unit. To determine sensitivity of results to the optical density threshold, we also performed the analysis defining seropositivity as having optical density units ≥1.1. We performed Cox proportional hazards models to quantify the relative hazard of diabetes between pathogen profiles among individuals without diabetes at baseline using age at diabetes diagnosis as the time scale. Two proportional hazards models were constructed: 1) sex- and education-adjusted analysis and 2) multivariate analysis with sex, education, and covariates associated with incident diabetes rate in model 1 at significance levels of P < 0.20. The multivariate models included sex, education, and smoking status as potential confounders because they are established risk factors for both infection and diabetes. Additional variables considered for the multivariate model included vascular disease, BMI, waist-to-hip ratio, diastolic and systolic blood pressure, and HDL and LDL cholesterol, because obesity, hypertension, and lipid profiles were implicated in cardiometabolic disorders and, if related to diabetes, could improve the precision of the model. Because the study population comprised elderly individuals, we further considered the potential for selection bias by performing a competing risk model for all non–diabetes-related deaths. We confirmed that proportional hazards assumption was met by testing the interaction between time and the covariates included in the multivariate model.
Furthermore, HOMA-IR, IL-6, and CRP were investigated as potential mediators, because they are known to be involved in inflammatory or endocrine processes that contribute to metabolic disorders. To qualify as mediators, the markers of inflammation or insulin resistance had to satisfy the Baron and Kenny (16) criteria of mediation as follows: 1) pathogen seropositivity is associated with the marker, 2) the marker is associated with diabetes rate, and 3) controlling for the marker significantly alters the association between pathogen seropositivity and incident rate. All statistical analyses were conducted in SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
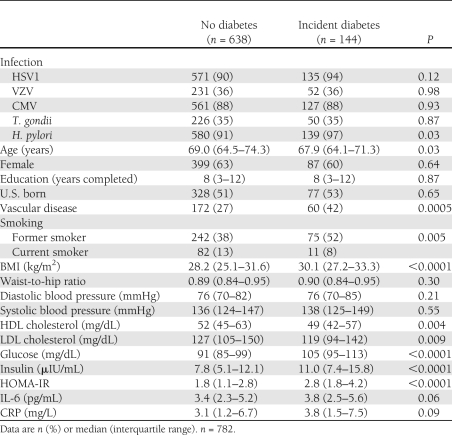
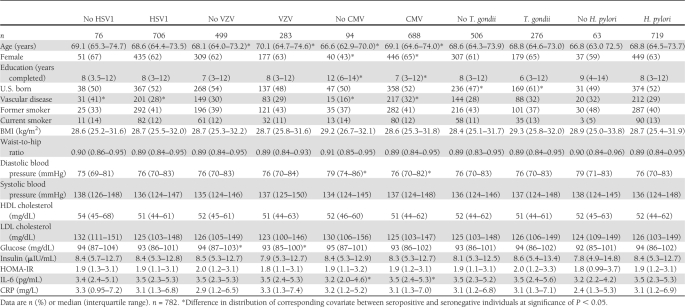
Among 782 diabetes-free participants in the SALSA cohort with information on baseline covariates, 144 individuals developed diabetes and 92 individuals died because of causes other than diabetes during 4,886 person-years of follow-up (incidence rate of diabetes of 2.95 per 100 person-years and incidence rate of death of 1.88 per 100 person-years). The median age of the study population was 68.7 years, and 38% were male. There was no difference by age, sex, and education between individuals who had developed diabetes and individuals who had not (Table 1). Individuals who developed diabetes were more likely to have a history of vascular disease, have smoked in the past, have lower HDL and LDL cholesterol levels, and have higher glucose, insulin, and HOMA-IR levels at baseline. Of 144 individuals who developed diabetes, 64 were found to be taking diabetes medication during follow-up. Seropositivity to HSV1, VZV, CMV, and T. gondii was not significantly different among individuals with and without diabetes, whereas seropositivity to H. pylori was significantly greater among individuals who developed diabetes compared with nondiabetic individuals (Table 1). The incidence rates of diabetes among HSV1-, VZV-, CMV-, T. gondii–, and H. pylori–infected individuals were 3.06, 2.92, 2.98, 2.97, and 3.10 per 100 person-years, respectively, whereas rates among the seronegative individuals were 1.87, 2.97, 2.73, 2.93, and 1.22 cases per 100 person-years, respectively. Distribution of covariates by infection status is summarized in Table 2.
Table 1.
Summary of characteristics by diabetes status in the SALSA cohort
Table 2.
Summary of characteristics by infection status in the SALSA cohort
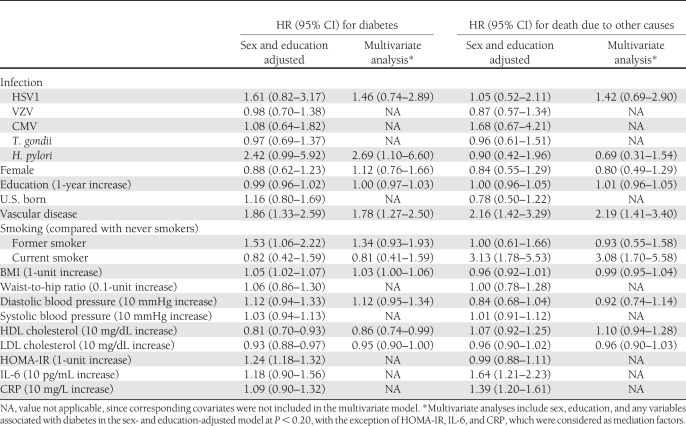
In sex- and education-adjusted model, those seropositive to HSV1 or H. pylori were more likely to develop diabetes at any given time than those who were seronegative (hazard ratio [HR] for HSV1 1.61 [95% CI 0.82–3.17]; HR for H. pylori 2.42 [0.99–5.92]), but the effects did not reach statistical significance. The estimate for H. pylori became significant in a multivariate model (2.69 [1.10–6.60]), additionally adjusting for HSV1, vascular disease, smoking, BMI, diastolic blood pressure, and HDL and LDL cholesterol levels (Table 3). Among individuals seropositive for H. pylori, 11% reported taking medications for acid suppression, peptic disorders, or antacids at baseline, and 0.8% were taking antibiotics used to treat H. pylori infection (i.e., penicillin, macrolides, and tetracylines). Adjusting for these medications did not affect the association between H. pylori and incident diabetes rate (multivariate HR for H. pylori 2.76 [1.12–6.81]). Furthermore, the results did not change in the sensitivity analysis when we assigned a higher cutoff to determine seropositivity to the infections (multivariate HR for H. pylori 3.14 [1.28–7.67]). Tests for interaction between covariates in the multivariate model and time were not significant when each interaction term was tested separately, signifying that the proportional hazards assumption was upheld. Because H. pylori was the only pathogen to reach statistical significance with incident diabetes rate in adjusted proportional hazards models, we did not examine pathogen burden as an independent predictor of incident diabetes rate.
Table 3.
Summary of associations with incident diabetes and death due to other causes in the SALSA cohort
Inclusion of HOMA-IR, CRP, and IL-6 in the model did not change the effect of H. pylori (HR 2.72 [95% CI 1.11–6.68]). Additionally, CRP and IL-6 were not associated with H. pylori, or with diabetes, whereas HOMA-IR was associated with increased rate of diabetes. Thus, HOMA-IR, CRP, and IL-6 did not meet the three Baron and Kenny criteria for mediation.
The multivariate model also showed that participants with a history of vascular disease (HR 1.78 [95% CI 1.27–2.50]) experienced higher incidence of diabetes in multivariate analyses. BMI was positively associated with diabetes incidence rate, whereas HDL and LDL were inversely associated (Table 3).
We considered the potential for selection bias because of censoring of individuals who died from causes other than diabetes. We found that individuals who were smoking at baseline (HR 3.08 [95% CI 1.70–5.58]) and individuals with vascular disease (2.19 [1.41–3.40]) were more likely to die from causes other than diabetes during follow-up. On the other hand, H. pylori was not associated with deaths unrelated to diabetes (0.69 [0.31–1.54]) (Table 3).
CONCLUSIONS
In this prospective study of Latino elderly over 10 years, we found that individuals seropositive for H. pylori experienced a greater rate of incident diabetes than individuals without the infection. On the other hand, we did not find a significantly increased rate of incident diabetes in participants seropositive for HSV1, VZV, CMV, and T. gondii compared with seronegative participants and therefore did not examine whether pathogen burden influenced the rate of incident diabetes. Our finding on the effect of H. pylori infection is in accord with the results from previous studies by Gasbarrini et al. (17) and So et al. (18), who found a positive association between H. pylori infection and the prevalence of diabetes in cross-sectional studies. A cross-sectional analysis of the baseline data from the SALSA cohort also showed greater prevalence of diabetes in H. pylori–infected individuals (data not shown). Our results stand in contrast to several other cross-sectional studies that found a lack of association between H. pylori and prevalence of diabetes (13,19,20). We overcome methodological limitations of previous cross-sectional studies by examining the impact of H. pylori infection on development of diabetes over a 10-year follow-up period. Thus, we were able to establish the relative timing of seropositivity and development of diabetes, giving more credence to a potential causal relationship. Furthermore, our study controlled for multiple factors, including age, sex, ethnicity, education, and cardiometabolic risk factors by design and analysis, thereby minimizing bias due to confounding.
The mechanism by which H. pylori infection increases the risk of diabetes remains to be elucidated but may involve inflammation or dyspepsia. Infection with H. pylori was found in previous studies to be correlated with elevated levels of CRP (21), IL-6, and tumor necrosis factor-α (22), which are markers of inflammation implicated in insulin resistance and development of diabetes (8). Furthermore, the presence of Gram-negative bacteria, such as H. pylori, in the gut microbiota leads to increased production of lipopolysaccharide, which also activates innate inflammatory processes (9). The inflammation hypothesis, however, was not substantiated in our analysis, since we could not establish that IL-6 and CRP levels were elevated in H. pylori seropositive individuals or in those who developed diabetes. The fact that other pathogens associated with systemic inflammation such as HSV and CMV were not associated with diabetes, as had been found in a previous study on the association between pathogen burden and diabetes (13), casts some doubt on the role the inflammation. An alternative hypothesis is that gastroduodenal conditions resulting from H. pylori infection could delay gastric emptying (23), which has been postulated to cause poor glucose control in insulin-dependent children with diabetes (24). The biological impact of H. pylori–associated disorders on glucose metabolism and insulin sensitivity should be further investigated.
In addition to our finding for H. pylori, we found that HDL was inversely associated with diabetes, whereas BMI, diastolic blood pressure, and vascular disease were positively associated with diabetes, which is consistent with the current understanding of risk factors for diabetes, such as low HDL, obesity, hypertension, and history of CVD (25). Our finding for LDL was unexpected; however, this phenomenon has been observed previously (26), and high LDL is not an established risk factor for diabetes.
Our study was limited by a number of factors that would be ameliorated by conducting similar studies in other more diverse populations. First, only a small percentage of the population was seronegative for H. pylori (7%) and HSV1 (10%), which limited the power of the study and increased the width of our confidence intervals. Our study had 16–19% power to detect an HR of 1.25, 40–47% power to detect an HR of 1.50, 77–85% power to detect an HR of 2.00, and 92–96% power to detect an HR of 2.50 given the distribution of HSV1 and H. pylori seropositivity in the study. Despite the low power, it is possible that our finding for H. pylori may have resulted from chance given the small sample of seronegative individuals. In addition, we could not distinguish recent versus historic H. pylori infection. However, a U.S. population-based study of H. pylori has shown that 63% of Hispanics are infected by the age of 60 years (27). Very few participants in the SALSA cohort were taking a proton pump inhibitor or antibiotics used to treat H. pylori at baseline. Thus, treatment for recent infection is unlikely, and most of these elderly individuals have probably been infected before enrollment without overt symptoms. Our finding may not be generalizable to younger individuals considering that they have a shorter history of infection. Future studies should consider investigating a younger population among whom acquisition of H. pylori infection actively occurs. Furthermore, because this was an observational study, there is potential for confounding by factors such as diet and family history of diabetes that may be linked to vulnerability to infection and diabetes. In addition, the inverse association between age and diabetes indicate a potential for survivor bias. In fact, results from the competing risk model show that individuals with vascular disease and who smoked at baseline were more likely to die from nondiabetes causes, indicating that the surviving population was healthier. However, we did not find that individuals with a H. pylori infection were more likely to die from nondiabetes causes. Thus, it is unlikely that survivor bias affected our result for the association between H. pylori and diabetes.
In conclusion, we found that serological evidence of H. pylori infection was associated with an increased rate of incident diabetes in a Latino elderly cohort. Inflammatory cytokines (i.e., CRP and IL-6) did not appear to mediate the effect. Larger prospective studies investigating the impact of H. pylori infection on diabetes and corresponding mediating factors are warranted. If H. pylori plays an etiological role in the development of type 2 diabetes, preventive measures such as increased hygiene and treatments such as antibiotics and proton pump inhibitor combinations for treatment of H. pylori should be explored as targets of intervention in high-risk communities.
Acknowledgments
M.N.H. and A.E.A. were supported by National Institutes of Health (NIH) Grant AG12975 and DK60753. A.E.A. also received funding from the Stanley Medical Research Institute for pathogen laboratory testing and NIH Grant R56DK087864. C.Y.J. was supported by an NIH T32 Training Grant from the National Institute of Allergy and Infectious Diseases.
No potential conflicts of interest relevant to this article were reported.
C.Y.J. and A.E.A. conceived the specific aims of the article. M.N.H. designed and collected data for the SALSA cohort. E.R.C. and E.R.M. prepared the data for analysis. C.Y.J. and C.C. conducted the analysis. C.Y.J. and A.E.A. wrote the initial draft. M.N.H., C.C., E.R.M., and J.W.M. contributed to the interpretation of the results. All authors contributed to the final draft of the manuscript. A.E.A. is guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
See accompanying commentary, p. 463.
References
- 1.Kanjilal S, Gregg EW, Cheng YJ, et al. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971–2002. Arch Intern Med 2006;166:2348–2355 [DOI] [PubMed] [Google Scholar]
- 2.Lindquist CH, Gower BA, Goran MI. Role of dietary factors in ethnic differences in early risk of cardiovascular disease and type 2 diabetes. Am J Clin Nutr 2000;71:725–732 [DOI] [PubMed] [Google Scholar]
- 3.Williams ED, Tapp RJ, Magliano DJ, Shaw JE, Zimmet PZ, Oldenburg BF. Health behaviours, socioeconomic status and diabetes incidence: the Australian Diabetes Obesity and Lifestyle Study (AusDiab). Diabetologia 2010;53:2538–2545 [DOI] [PubMed]
- 4.Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. J Gerontol A Biol Sci Med Sci 2009;64:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georges JL, Rupprecht HJ, Blankenberg S, et al. Impact of pathogen burden in patients with coronary artery disease in relation to systemic inflammation and variation in genes encoding cytokines. Am J Cardiol 2003;92:515–521 [DOI] [PubMed] [Google Scholar]
- 6.Simanek AM, Dowd JB, Aiello AE. Persistent pathogens linking socioeconomic position and cardiovascular disease in the US. Int J Epidemiol 2009;38:775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabipour I, Vahdat K, Jafari SM, Pazoki R, Sanjdideh Z. The association of metabolic syndrome and Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, and herpes simplex virus type 1: the Persian Gulf Healthy Heart Study. Cardiovasc Diabetol 2006;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev 2010;31:817–844 [DOI] [PubMed] [Google Scholar]
- 10.Demmer RT, Jacobs DR, Jr, , Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care 2008;31:1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Real JM, López-Bermejo A, Vendrell J, Ferri MJ, Recasens M, Ricart W. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care 2006;29:1058–1064 [DOI] [PubMed] [Google Scholar]
- 12.Howard BV, Best L, Comuzzie A, et al. C-reactive protein, insulin resistance, and metabolic syndrome in a population with a high burden of subclinical infection: insights from the Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN) study. Diabetes Care 2008;31:2312–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutsey PL, Pankow JS, Bertoni AG, Szklo M, Folsom AR. Serological evidence of infections and type 2 diabetes: the MultiEthnic Study of Atherosclerosis. Diabet Med 2009;26:149–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc 2003;51:169–177 [DOI] [PubMed] [Google Scholar]
- 15.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry 2001;58:1032–1037 [DOI] [PubMed] [Google Scholar]
- 16.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–1182 [DOI] [PubMed] [Google Scholar]
- 17.Gasbarrini A, Ojetti V, Pitocco D, et al. Helicobacter pylori infection in patients affected by insulin-dependent diabetes mellitus. Eur J Gastroenterol Hepatol 1998;10:469–472 [DOI] [PubMed] [Google Scholar]
- 18.So WY, Tong PC, Ko GT, et al. Low plasma adiponectin level, white blood cell count and Helicobacter pylori titre independently predict abnormal pancreatic beta-cell function. Diabetes Res Clin Pract 2009;86:89–95 [DOI] [PubMed] [Google Scholar]
- 19.Xia HH, Talley NJ, Kam EP, Young LJ, Hammer J, Horowitz M. Helicobacter pylori infection is not associated with diabetes mellitus, nor with upper gastrointestinal symptoms in diabetes mellitus. Am J Gastroenterol 2001;96:1039–1046 [DOI] [PubMed] [Google Scholar]
- 20.Ko GT, Chan FK, Chan WB, et al. Helicobacter pylori infection in Chinese subjects with type 2 diabetes. Endocr Res 2001;27:171–177 [DOI] [PubMed] [Google Scholar]
- 21.Diomedi M, Stanzione P, Sallustio F, et al. Cytotoxin-associated gene-A-positive Helicobacter pylori strains infection increases the risk of recurrent atherosclerotic stroke. Helicobacter 2008;13:525–531 [DOI] [PubMed] [Google Scholar]
- 22.Hamed SA, Amine NF, Galal GM, et al. Vascular risks and complications in diabetes mellitus: the role of helicobacter pylori infection. J Stroke Cerebrovasc Dis 2008;17:86–94 [DOI] [PubMed] [Google Scholar]
- 23.Ojetti V, Pellicano R, Fagoonee S, Migneco A, Berrutti M, Gasbarrini A. Helicobacter pylori infection and diabetes. Minerva Med 2010;101:115–119 [PubMed] [Google Scholar]
- 24.Burghen GA, Murrell LR, Whitington GL, Klyce MK, Burstein S. Acid peptic disease in children with type I diabetes mellitus: a complicating relationship. Am J Dis Child 1992;146:718–722 [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saely CH, Eber B, Pfeiffer KP, Drexel H; LIIFE-IN-LIFE Study Group Low serum LDL cholesterol in patients with type 2 diabetes: an analysis on two different patient populations. Int J Cardiol 2010;144:394–398 [DOI] [PubMed] [Google Scholar]
- 27.Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis 2000;181:1359–1363 [DOI] [PubMed] [Google Scholar]