Abstract
Humans transformed Western Atlantic coastal marine ecosystems before modern ecological investigations began. Paleoecological, archeological, and historical reconstructions demonstrate incredible losses of large vertebrates and oysters from the entire Atlantic coast. Untold millions of large fishes, sharks, sea turtles, and manatees were removed from the Caribbean in the 17th to 19th centuries. Recent collapses of reef corals and seagrasses are due ultimately to losses of these large consumers as much as to more recent changes in climate, eutrophication, or outbreaks of disease. Overfishing in the 19th century reduced vast beds of oysters in Chesapeake Bay and other estuaries to a few percent of pristine abundances and promoted eutrophication. Mechanized harvesting of bottom fishes like cod set off a series of trophic cascades that eliminated kelp forests and then brought them back again as fishers fished their way down food webs to small invertebrates. Lastly, but most pervasively, mechanized harvesting of the entire continental shelf decimated large, long-lived fishes and destroyed three-dimensional habitats built up by sessile corals, bryozoans, and sponges. The universal pattern of losses demonstrates that no coastal ecosystem is pristine and few wild fisheries are sustainable along the entire Western Atlantic coast. Reconstructions of ecosystems lost only a century or two ago demonstrate attainable goals of establishing large and effective marine reserves if society is willing to pay the costs. Historical reconstructions provide a new scientific framework for manipulative experiments at the ecosystem scale to explore the feasibility and benefits of protection of our living coastal resources.
The persistent myth of the oceans as wilderness blinded ecologists to the massive loss of marine ecological diversity caused by overfishing and human inputs from the land over the past centuries. Until the 1980s, coral reefs, kelp forests, and other coastal habitats were discussed in scientific journals and textbooks as “natural” or “pristine” communities with little or no reference to the pervasive absence of large vertebrates or the widespread effects of pollution. This is because our concept of what is natural today is based on personal experience at the expense of historical perspective. Thus, “natural” means the way things were when we first saw them or exploited them, and “unnatural” means all subsequent change (1, 2). As in Magritte's masterpiece, La Condition Humaine, we see the world through a model of our own creation that organizes and filters understanding (3). In the present context, that filter is the sum total of anthropogenic change that took place in the oceans before we were born.
Not all ecological change is anthropogenic, however. Natural conditions in the oceans fluctuate greatly and sometimes suddenly on time scales that extend for decades to millennia. Thus, the filter of individual experience has two components. Changes caused by humans are the signal and natural variability constitutes the noise that obscures the human footprint (4–6). An important example of the potential magnitude of natural change comes from annually layered sediments of the Santa Barbara Basin (7). Abundances of fish scales of anchovies and sardines preserved in these sediments fluctuate more than an order of magnitude and exhibit nine major collapses and subsequent recoveries over 1700 years. These data and shorter records of fish catches suggest population cycles of 50 to 70 years associated with alteration of warm and cold physical regimes (4, 8). These cycles exceed the longest instrumental temperature records for the region and greatly complicate management of fisheries. How can one determine a sustainable catch against a background of such extreme natural variation?
Conventional ecological data are clearly inadequate to measure the ecological impacts of fishing or any other long-term human disturbance (4, 5, 9). Most observational records are much too short, too poorly replicated, and too uncontrolled to encompass even a single cycle of natural environmental variation. For example, detailed ecological observations of reef corals began only in the 1930s. There are a few “before and after” comparisons of community composition between surveys conducted up to a century ago and the present (10). However, the longest quantitative time series comprises only a few small intertidal quadrats on one small island over 30 years (11), and the longest comparable subtidal records encompass less than 20 years (12). In both cases, the interval studied is much less than the generation times of most common coral species and the intervals between some kinds of major disturbances in coral reef environments (13). Several kelp forests and rocky intertidal communities have been surveyed for about 25 years over scales of several hectares, so that the data approximate or exceed generation times of most important species, but not the periodicity of major climatic cycles (5, 6). Ecological data for oyster reefs, seagrass meadows, level bottoms, and virtually all other marine communities have similar limitations (14–18).
Paleoecological, archeological, and historical data are the only means for extending ecological records back long enough to document the characteristic variability of marine ecosystems and the magnitude of earlier anthropogenic change. Here I review the transformation of five Western Atlantic coastal ecosystems over the past few centuries as a result of human exploitation and pollution. My goals are to demonstrate the extraordinary magnitude of ecological changes that have been largely forgotten and to show how awareness of these changes can benefit efforts for conservation and restoration of coastal ecosystems. My focus is on benthic communities because extreme overfishing of pelagic species such as Atlantic whales, tuna, salmon, and herring is well known (19, 20). Transformations of benthos are subtler and known only to a few specialists. I also focus on ecological extinction because the magnitude of ecological changes is not generally understood (1, 2, 5, 9), and documentation of actual extinctions of marine species is just beginning (21). More importantly, too great a focus on species detracts attention from the transformation and loss of habitats and collapse of natural ecosystems that drive the processes of extinction.
Caribbean Coral Reefs
Coral reefs are the largest durable biological constructions on earth. Reefs determine the physical structure of coastlines and adjacent ecosystems, including seagrass beds and lagoons. Coral reefs are the most taxonomically diverse marine ecosystems and provide complex habitat for myriad sessile and mobile organisms (22, 23). Recent discoveries of numerous sibling species suggest diversity is even greater than already described (24).
Species composition of Caribbean coral communities was stable for at least 125 thousand years, until the collapse in the 1980s (25–29). Different environments were dominated by distinct species assemblages of the corals Acropora, Montastrea, Diploria, and a few other genera, and the composition of these assemblages was similar over tens of kilometers of coastline for tens of thousands of years. Within each habitat, community membership was more predictable than expected by random sampling of the habitat-specific species pool. Thus, there was a clear baseline of coral community composition that serves for comparison with today.
Western Atlantic reef corals suffered catastrophic mortality in the 1980s (30–34). Live coral abundance declined to 1–2% cover from values of 50% or more. Dominant framework species of Acropora and Montastrea were severely affected. Besides overall reduction in coral abundance there was a shift in life histories of surviving species (13, 31–33). Western Atlantic Acropora and Montastrea are long-lived and reproduce by mass spawning of gametes that are fertilized and develop in the water column. These taxa are being replaced by smaller, shorter-lived Agaricia and Porites with internal fertilization and direct development, presumably because of selection for shorter life cycles in a regime of increased human disturbance.
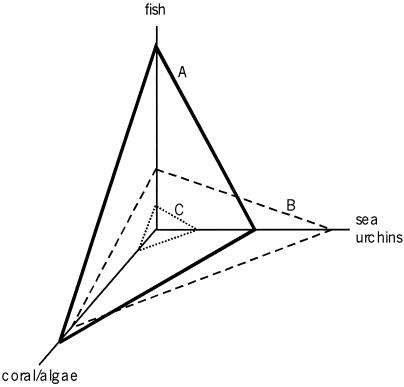
The principal cause of coral mortality was overgrowth by macroalgae that exploded in abundance after an unidentified pathogen caused mass mortality of the enormously abundant grazing sea urchin Diadema antillarum in 1983–1984 (33, 35, 36). Increasing frequency of coral disease and bleaching were also major factors (30, 37, 38). A likely explanation for the formerly great abundance of Diadema is overfishing of major fish predators on Diadema and of large herbivorous fishes that had competed with Diadema for algal food (refs. 33, 36, 39–41; Fig. 1).
Figure 1.
Model of the consequences for reef corals of the increase in the sea urchin Diadema antillarum caused by overfishing of large predatory and herbivorous fishes and the subsequent mass mortality of Diadema caused by disease. Reproduced with permission from ref. 41 (Copyright 1994, The Royal Society). Plane A, pristine condition, with high ratio of corals to macroalgae because of intense grazing of macroalgae by fishes. Plane B, abundant Diadema grazed macroalgae formerly consumed by herbivorous fishes so the ratio of corals to macroalgae remained high despite intensive fishing. Plane C, mass mortality of Diadema caused by infectious disease allowed macroalgae to proliferate and overgrow corals.
Overfishing allowed Diadema to increase in abundance and compensate for loss of herbivorous fishes that ate macroalgae before overfishing began. Then, when Diadema died out there were no other large grazers remaining to consume the algae. A key question is when overfishing began (9). Jamaican and other Caribbean reefs were so severely overfished in the 19th century that northern salt cod were imported en masse to stave off human starvation (42, 43). This early overfishing distorted ecological perspective to the point that reef fishes are described in the best modern textbook as small “aquarium species” rarely greater than 20–30 cm long (44). Most species of reef fishes are indeed small like other animals (45), but this says nothing about size–frequency distributions of communities of reef fishes before overfishing (and ecological investigations) began. Indeed, several of the earliest European explorers of the Caribbean (46, 47) carefully described large-scale native and early colonial fisheries of sharks, groupers, and other large fishes that have rarely been seen by most ecologists. Remarkably, the same modern textbook does not mention these species.
The stage for the collapse of Caribbean reef corals was set by the loss of large fishes sometime in the 19th century (9). The first modern study of Caribbean coral reefs in the 1950s (48) described coral communities like those in the Pleistocene when humans were absent from the Americas (25, 49). Coral communities did not change noticeably until the epidemic mortality of Diadema antillarum in the 1980s because ecological redundancy of herbivores obscured the potential effects of the loss of large herbivorous fishes for well over a century (9, 33, 50). Macroalgae were not able to overgrow corals until the last major herbivore was lost from the system. Lapointe suggested that nutrient enrichment might have tipped the competitive balance of macroalgae over corals (51), but this seems unlikely (40, 52, 53).
In contrast to macroalgal overgrowth, outbreaks of coral disease are not understood (54). Climatic variability, humans as agents of dispersal of pathogens, habitat degradation, and pollutants have all been invoked as factors that favor increase of pathogens (55). However, there is no clear model or mechanism for how these factors could affect some species and not others, or consideration of the profound historical changes that previously affected reef ecosystems. Outbreaks of disease may be increasing because of the reduction of other species that once kept specific pathogens in check. In contrast, increasing frequency of severe episodes of coral bleaching is strongly correlated with high sea surface temperatures, and may truly reflect changes in global climate (56).
Caribbean Seagrass Meadows
Tropical American seagrasses are less diverse than corals, but seagrass meadows cover much greater areas than coral reefs (18, 57). Seagrasses enhance sediment stability, decrease wave energy, and increase water clarity as well as providing forage, habitat, and nurseries for diverse and abundant invertebrates and fishes (57, 58). The most common Caribbean species are turtle grass (Thalassia testudinum), manatee grass (Syringodium filiforme), and shoal grass (Halodule wrightii) (57). Seagrasses do not fossilize as readily as corals. However, well-preserved fossil assemblages of bivalve mollusks that inhabit the rhizome mat of seagrasses (59) suggest the persistence of seagrass communities throughout the Pleistocene. Seagrass beds were also persistent features on nautical charts.
Seagrasses along the Florida coast experienced mass mortality in the 1980s because of a wasting disease (60, 61). Mortality was positively density dependent and correlated with high temperatures and salinities, sulfide toxicity, self-shading, hypoxia, and infection by the slime mold Labyrinthula sp. Ecologists search for causes of seagrass mortality in terms of recent changes in hydrography and pollution (55, 61). However, all of the above factors except salinity and temperature have changed greatly because of massive exploitation centuries ago of sea turtles and manatees that gave the seagrasses their popular names.
Green turtles (Chelonia mydas) were extraordinarily abundant when Columbus arrived in the Caribbean (9, 62). Estimates of adult populations have been calculated, based on the assumption that population size was regulated by food limitation and by extrapolating from early hunting data from the Cayman Islands. Population sizes based on the carrying capacity of turtle grass range from 16 to 586 million 50-kg adults (62), whereas estimates based on early hunting data range from 33 to 39 million large nesting adults (9). Even the smallest estimate for green turtles exceeds the highest recorded wildebeest abundances in the Serengeti (63)!
What were the effects on seagrass beds of such enormous numbers of turtles? Blades of turtlegrass grow upward from the base and can reach 30 cm or more in length (57). Older, more distal portions are commonly heavily overgrown by microorganisms, fungi, algae, and invertebrates, and are broken off and transported en masse during storms (64). Green turtles crop turtlegrass 2–4 cm above the base, and individuals commonly return repeatedly to the same plots that are maintained by continuously cropping grazed areas to feed on more nutritious new shoots of the turtlegrass (64). When density of turtles is comparatively high, individual grazing plots may merge so that the entire turtlegrass bed is closely cropped (65). Such close cropping matches Dampier's (46) description of turtlegrass blades as “six Inches long” (15 cm) when turtles were abundant, in comparison with much greater lengths typically observed today (57). Grazing by green turtles also reduces 20-fold the flux of detritus and nitrogen to seagrass sediments and alters their microbial ecology (64, 66–68). This happens because turtles (i) consume more of the blades than fishes and invertebrates, (ii) metabolize cellulose of cell walls by microbial fermentation in their hindguts, and (iii) disperse feces and urine over large areas well away from seagrass beds. In contrast, fishes and invertebrates feeding on turtlegrass cannot metabolize the cellulose and do not migrate over such large areas (68).
Now consider the potential significance of the ecological demise of green turtles for turtlegrass in Florida Bay. Green turtles were formerly very abundant in South Florida (69), and all of the factors identified in seagrass die-offs except changes in temperature and salinity would have been profoundly altered by abundant green turtles. Concentration of sulfides in sediments increases with accumulation of organic material that may also cause anoxia within sediments and hypoxia of overlying waters (70), but green turtles greatly decrease accumulation of organic matter in sediments (68). Self-shading is due to the density and foliage height of the leaves, which also are greatly reduced by green turtles. Finally, infection by slime molds is positively correlated with density of turtlegrass (61) and probably depends on the amount of time senescing leaf tissues are exposed to the environment. Scientific descriptions of the sites of infections are vague, but leaf segments free of lesions caused by slime molds for use in experiments were always obtained from mid-to-basal sections of leaves (71), which are the youngest portions (57). In addition, infections illustrated in photographs occur along the distal portion of the blade (http://www.floridamarine.org/). Thus infection begins on those older portions of leaves that were typically grazed away when turtles were abundant. Elimination of green turtles is implicated on four counts as the ultimate factor in die-offs of turtlegrass; a hypothesis that could be tested by manipulative experiments of abundance of green turtles in turtlegrass beds on an appropriately massive scale.
The demise of green turtles is better documented (9, 69) than that of manatees (Trichechus manatus), which feed on manatee grass and other submerged vegetation (46, 72) and can metabolize cellulose as green turtles do (68). One- to two-ton manatees were sufficiently abundant along the low-lying and swampy coasts of Central America and northern South America to merit extensive and detailed descriptions of their natural history and how they were commonly hunted (46). Moreover, the much better documented and more recent demise of the dugong (Dugong dugong) in Australia suggests populations of these enormous relatives of the manatee of about 1 million along the Australian coast only a century ago (73). Dugongs plow through seagrass beds in Australia, reducing shoot density and biomass of by up to 90% (74). We will likely never know the equivalent ecological consequences of manatee grazing in pristine seagrass environments. However, once again Dampier (46) gives us a clue when he describes manatee grass as “7 or 8 Inches long” (20 cm) compared with lengths commonly exceeding 20 inches (50 cm) today (57).
Chesapeake Bay
Chesapeake Bay is the largest and historically most productive estuary in North America. During the 20th century, once very extensive meadows of seagrasses, oyster beds, clams, blue crabs, and fish declined precipitously, while abundance and production of phytoplankton, eutrophication, and episodes of hypoxia and anoxia correspondingly increased (75). Overfishing and increasing runoff of freshwater, nutrients, and sediment from the land seem the obvious culprits, but physical conditions are extremely variable (76) and hypoxia was first reported in the 1930s when modern ecological research was only just beginning (77, 78). Thus it is impossible to determine the extent of human influence solely on the basis of modern observations.
The stratigraphic record of sedimentation, pollen, seeds, diatoms, and geochemistry in sediment cores was used to reconstruct the ecological history of the northern half of the watershed over the past 2,000 years (75, 78–80). Environmental and biological fluctuations since European settlement exceed all earlier changes severalfold. Sedimentation rate and concentrations of organic carbon, sulfur, and ragweed pollen increased suddenly at the end of the 18th century. Diversity of diatom species, the ratio of benthic to planktonic diatoms, and the occurrence of seeds of benthic macrophytes gradually declined. Altogether, the results from the cores demonstrate an ecological shift in the upper Chesapeake Bay from predominantly benthic to predominantly planktonic primary production that was well under way by the early 19th century.
These results were corroborated by more recent observations of increasing phytoplankton biomass and decreasing submerged aquatic vegetation over the past 50–75 years (14, 81). Decline of the eelgrass Zostera marina was due primarily to wasting disease caused by the slime mold Labyrinthula sp., the same genus of pathogen affecting turtlegrass in Florida Bay (14, 82). Earliest reports of declines in eelgrass date from the 1890s, but mortality affecting >90% of eelgrass populations along the entire East Coast of North America occurred in the 1930s (14, 82).
Increase in phytoplankton was compounded by massive overfishing and physical destruction of oyster beds in the 19th century (15, 16, 77) in addition to increased loading of nutrients, especially nitrogen. Like seagrasses, oysters stabilize the substratum and provide complex habitat for hundreds of other species (16). Large oyster beds were a major hazard to navigation in bays and estuaries from New England to west Florida until the mid-19th century, when large-scale mechanized harvesting began. Both the spatial extent of oyster beds and body size of individual oysters diminished greatly by the mid-19th century (15, 16). Numerous shell middens at least one quarter of a million cubic meters in volume attest to long history of aboriginal exploitation, but these great harvests were apparently sustainable. Shells in middens commonly exceed 30 cm, which agrees with colonial reports that oysters had to be cut in two to be eaten (16).
The filtration power of so many suspension-feeding animals must have been truly enormous (83). Calculations suggest that oysters before the 1870s filtered the equivalent of all of the water in Chesapeake Bay in less than 1 week, compared with 46 weeks for depleted modern stocks (84), a 50-fold difference! Subsequent model calculations suggest that this intense filtration would have reduced phytoplankton and zooplankton to a small fraction of present abundance regardless of increases in nutrients (85). These calculations are supported by striking reductions in abundance of phytoplankton after population explosions of introduced clams in lakes and estuaries (16).
Overfishing of oysters, decreased sediment stability, reduced benthic oxygen production because of loss of seagrasses, and increased nutrients from runoff acted synergistically to increase phytoplankton production at the expense of benthic resources and habitat. Increased eutrophication, frequency and scope of hypoxia, outbreaks of toxic microbes, and explosions of sea nettles and other noxious gelatinous zooplankton that feed on zooplankton and the larvae of invertebrates and fish are the result. Today Chesapeake Bay is a bacterially dominated ecosystem with a totally different trophic structure from a century ago (86). Similarly intense eutrophication occurs in other estuaries like Pamlico Sound (87), as well as along the continental shelf near the outflow of the Mississippi River (88). Oxygen deficiency is no longer restricted to bays and estuaries, but has spread to the open coastal ocean.
Kelps and Codfish in the Gulf of Maine
Kelp forests characterize large areas of warm temperate to subpolar coastal waters worldwide (89). Kelps provide complex habitat for a great diversity of fishes and invertebrates, including many commercially important species (89). Atlantic cod and other predatory ground fish were extremely abundant in kelp forests all along the coast of New England and eastern Canada until this century, but have now been fished to exhaustion (90). Loss of predatory fishes set off a series of complex ecological transformations that are still going on (90–92).
Large and abundant cod were fished from the Gulf of Maine for 5,000 years before the 19th century with no evidence of decline (92, 93). Cod remains constitute 80–90% of the bone mass in middens in Maine dating from 500 to 2,500 years ago. Vertebrae in middens suggest that cod commonly reached 1½ to 2 m in length, a size in accord with early European illustrations of drying cod the size of fishermen (19, 94). Large cod remained abundant until the 1920s, when mechanized trawling replaced traditional hook-and-line fishing. Cod abundance and size declined precipitously thereafter. Cod were virtually eliminated from coastal habitats in the 1980s and the average size of the few fishes caught was less than 30 to 40 cm. Today cod are so rare throughout the region that no cod were observed during hundreds of hours of underwater observations by diving and video cameras in the 1990s (90). Remaining fishes include small sculpins, skates, and dogfish, whereas cod has become ecologically extinct.
Elimination of cod and other large predatory ground fish resulted eventually in great increases in lobsters, crabs, sea urchins, and other invertebrate grazers and predators during the latter half of the 20th century (90–92). Lobsters had been fished down in size and abundance before mechanized fishing of cod, but subsequently increased in abundance with the elimination of coastal predators other than humans. Newly abundant sea urchins consumed all of the kelp, which was replaced by structurally “barren” substrata covered by encrusting coralline algae. Fishes and invertebrates dependent on kelps as habitat were also necessarily reduced. Subsequent “fishing down the food web” (95) of sea urchins beginning in 1987 resulted in rapid return of kelp forests, but without large populations of ground fishes. Humans are now the dominant predators in the Gulf of Maine coastal ecosystem. Hunting and fishing caused similar changes in kelp forests in Alaska and Southern California (5, 96, 97).
Benthic Communities on Continental Shelves
Direct and indirect effects of dredging and trawling on subtidal benthic communities have been reviewed extensively elsewhere (21, 98, 99). Most studies are from the North Sea and around the British Isles or from New Zealand and Australia, but similar effects are known from the Atlantic coasts of North America (99). Mechanized bottom fishing reduces abundance of echinoderms, mollusks, and worms by 10–90% each time the bottom is fished (98, 99), and the formerly abundant and long-lived skate Raja laevis has been trawled to ecological extinction (100). Most areas are dredged many times per year, thereby flattening the bottom (98, 99). Large sponges, bryozoans, corals, worms, or bivalves that provide important habitat for commercially important fishes and numerous smaller invertebrates are virtually eliminated (21, 98, 99). Large species that form these habitats grow so slowly that they cannot recover for decades to centuries.
Except for Northern Europe, intensive trawling and dredging on continental shelves began more recently than the overfishing described previously for other habitats (21, 98, 99). Nevertheless, few of the habitats affected were studied before mechanized fishing began, so that the quantitative effects of mechanized bottom fishing remain poorly documented in all but a few cases. The key point is that bottom fishing is already so intensive and pervasive that it is now effectively impossible to find “control” systems to help identify effects of fishing damage on natural communities. The only alternative to waiting decades or centuries for their recovery will be examination of changes in taxa from old museum collections and paleoecological analyses of Holocene shelf communities. Modern benthic communities already have been transformed beyond recognition on virtually the entire continental shelf of eastern North America.
Emerging Patterns
Five general patterns emerge from this brief review of Western Atlantic coastal ecosystems. The first three are well known from comparable effects of humans on terrestrial ecosystems. The last two patterns are less important in terrestrial environments because of differences in trophic levels harvested in the sea and on the land, and the insignificance of farming and absence of domesticated species in the oceans.
Vulnerability of Large Vertebrates.
Large, long-lived vertebrates such as manatees, sea turtles, large fishes, and sharks were the first to disappear from coastal ecosystems in response to human activities because of their life history characteristics and large body size that attracted the most attention. Low fecundity, late maturation, and long generation times greatly reduce speed of recovery after harvesting or disease for all these organisms. Age of first reproduction for female manatees is about 6–10 years, after which they bear single offspring with a gestation period of about 1 year (72). Female sea turtles do not reach reproductive maturity for 7–30 years, after which they produce 1 to 7 clutches of ≈100–200 eggs every 1 to 3 years (101, 102). Moreover, these estimates of age of first reproduction are probably too young, and the true ages may range from as much as 40–60 years for some species (103).
Age (as opposed to size) of first reproduction for female groupers is poorly known but is only 6–7 years for the jewfish, which is the largest species (104), and numbers of eggs spawned are in the millions (104, 105). However, groupers reproduce in spawning aggregations that previously numbered in the tens to hundreds of thousands and occurred only at specific places and times of the year (105–107). As for sea turtles nesting on beaches, dense spawning aggregations make groupers easy to fish just at the time when they have the greatest potential to contribute to future generations (108).
Approximately 70% of living sharks and rays bear live young, and hammerheads exhibit placental viviparity (109, 110). Ages of maturation typically range from 6 to 18 years, but lemon sharks take 24 years. Gestation periods are long (6 to 22 months) and clutch sizes small (2 to 135). Thus, it is hardly surprising that sharks exhibit sudden collapse and slow recovery after relatively few years of intensive fishing (111, 112).
Collapse of Sessile Ecosystem Engineers.
“Ecosystem engineers” are species that modify, maintain, or create habitats, thereby modulating availability of resources to other species (113). Reef-building corals, seagrasses, oysters, and kelps are among the most important ecosystem engineers in marine coastal environments. Their massive physical presence and three-dimensional complexity help stabilize the physical environment and provide habitat to thousands of generally smaller associated species (6, 22, 23, 50, 57, 58, 89, 91, 114). Once-vast populations of ecosystem engineers have now collapsed along the Western Atlantic coast from the southern Caribbean to the Gulf of Maine. The reasons range from complex shifts in competitive abilities of corals, seagrasses, and kelps after the removal of keystone consumer species or outbreaks of disease (refs. 18, 33, 61, 90–92; Fig. 1) to direct physical destruction of oyster beds and sponge–bryozoan gardens by mechanical dredging and trawling (15, 16, 98, 99, 114, 115).
Once, great coral reefs, seagrass meadows, and oyster reefs were products of growth of dominant framework species and accumulation of sediments and skeletal debris. Dead skeletons remain partially intact for various periods after the death of corals and oysters unless removed by mechanized harvesting, whereas sea grasses and kelps do not produce such durable remains, so that three-dimensionality rapidly disappears (90). Loss of habitat structure decreases growth and larval recruitment and increases mortality of engineering species (12, 31, 115). Diversity and abundance of associated species also drops precipitously (18, 116).
Time Lags Between Effects of Overfishing and Collapse of Ecosystem Engineers.
Lengthy time lags between initial harvesting and many of the resulting ecological consequences are pervasive in tropical forests (117). Similarly in the coastal ocean, time lags of decades to centuries occurred between initial harvesting or destruction of large vertebrates and subsequent collapse of ecosystem engineers such as reef corals, seagrasses, or kelps (9, 33, 61, 90). Similar lags are apparent between increased fluxes of nutrients and sediments into coastal environments and collapse of reef corals (118), submerged macrophytes (14, 76), or oysters (15, 16, 85). Of course, oysters were intensively harvested by mining down the habitat, so their abundance declined much more rapidly than unfished corals and seagrasses.
One likely explanation for time lags is ecological redundancy, whereby other species take over the ecological role of species removed by harvesting. This is presumably what happened after overfishing on coral reefs (ref. 33; Fig. 1) and extirpation of green turtles in the Caribbean (9). Ecological redundancy should increase with taxonomic diversity, which may explain why time lags in the destruction of ecosystem engineers appear to decrease northward from corals and seagrasses in the Caribbean to kelps in the Gulf of Maine. Another important factor is widespread occurrence of threshold effects on human altered ecosystems (33, 119–122). These may involve simple thresholds in physiological tolerance to decreasing light or increasing sediments and nutrients, or more subtle density-dependent consequences of reduced abundance on fertilization, recruitment, or the ability to filter large volumes of water that reduces abundance of phytoplankton. Such negative feedbacks are exacerbated by the fact that both over-harvesting and increased nutrients and primary production work synergistically to reduce abundance of sessile ecosystem engineers (9, 76, 85, 114).
Fishing Down Food Webs.
Top carnivores were never an important part of the human diet on land (123) but are the preferred large prey in the sea except for green turtles and sirenians. Smaller and smaller fishes, sea urchins, lobsters, and shrimps are replacing large fishes, turtles, and sharks as the remnant fisheries in all of the coastal ecosystems discussed herein (9, 33, 90, 95, 124). Free-living animals larger than 1 kg are increasingly rare and nearly absent on the reefs of Jamaica and many other sites throughout the Caribbean (33, 125). The process is reversible, but only by regulation of fishing.
Farming of the sea, or aquaculture, is a possible alternative to fishing, but one that carries its own set of potentially harmful consequences to coastal ecosystems, including eutrophication, pollution, and the spread of disease (126, 127). Cultured species include a wide diversity of algae, oysters, shrimps, and various fishes from mullets to salmon. Most of the problems of aquaculture of algae and herbivorous animals could be alleviated if goals were broadened to include ecosystem conservation and management, rather than only to produce food. For example, benthic algae could be farmed to remove excess nitrogen from the water column, and oysters and other suspension-feeding bivalves could be farmed to reduce algal blooms induced by eutrophication.
Rise of Microbes.
Fishing down marine food webs and increasing pollution from the land are resulting in increasing abundance and widespread dominance of ecosystem processes by microbes. Eutrophication is most apparent in bays and estuaries like Chesapeake Bay (86), but it has extended onto the continental shelf (88). Outbreaks of previously rare or unreported toxic microbes and diseases are another example of the increasing importance of microbial disruption of coastal ecosystems (30, 35, 54, 55).
General Model of Coastal Ecosystem Collapse.
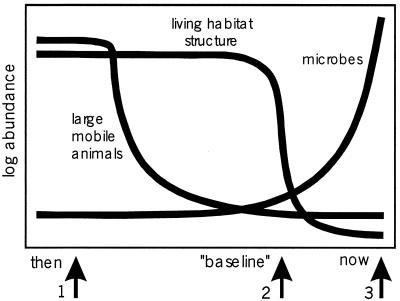
I summarized much of the above in the simple qualitative model in Fig. 2 showing the demise of large animals and ecosystem engineers and the rise of microbes since European colonization of the Americas. The model is based on Western Atlantic case studies reviewed in this paper, but I predict the same general pattern will obtain for the entire global coastal ocean. The y-axes are logarithmic to capture the orders of magnitude changes in these variables. The time axis is deliberately general because onset of major changes depends more on timing of the onset of intensive harvesting or development of new fishing technologies than chronological age.
Figure 2.
Model of the collapse of Western Atlantic coastal ecosystems caused by overfishing. Arrows indicate the three major ecological transitions discussed in the text.
Early ecological extinction of large mobile animals defines the first major transition in the history of coastal marine ecosystems. Extirpation of large vertebrates preceded ecological investigations so that their absence has been uncritically accepted as the natural “baseline” condition. Their precipitous decline reflects greater economic desirability, ease of capture, and limited capacity for increase that is well documented for sea turtles, manatees, large fishes such as cod and groupers, and sharks. The second major transformation reflects sudden collapse of sessile ecosystem engineers (reef corals, seagrasses, and kelps) caused by indirect effects of overfishing large vertebrates. Ecological dominance of microbes at the expense of macroorganisms (86) and increasing frequency of invasions of exotic species (128, 129) define the third major transition that is increasingly upon us (54, 55, 86, 88).
Why History Matters
Oceans are not wilderness and no Western Atlantic coastal habitat is pristine. The same is almost certainly true of coastal oceans worldwide, but this assertion needs rigorous documentation. Neotropical forests are greatly threatened by human activities and may disappear entirely within this century (117). The facts about tropical forests are widely known and much discussed by governments, international agencies, and the general public. By comparison, Neotropical coral reefs are already effectively “deforested” throughout their entire range, but this fact received almost no comparable attention until the 1990s (33, 130, 131). Moreover, human activities leading to the destruction of coral and oyster reefs, seagrass beds, or kelp forests began early in the 19th century or earlier, long before comprehensive scientific study began. In general, we are more aware of the mass extinction of large vertebrates at the end of the Pleistocene (123) than what happened in coastal seas only a century ago!
As in geology, the present is not always the key to the past, or to the future (132). Understanding what was natural is important not just for historical curiosity, but for rational management and conservation of coastal oceans in the future. I conclude with three basic points that emerge from comparisons of present conditions with historical baselines.
(1) No wild Atlantic coastal fishery is sustainable at anything close to present levels of exploitation. Coastal marine ecosystems already have been changed beyond recognition because of direct and indirect effects of overfishing. Most fishing is unsustainable because (i) inexorable growth of the human population drives increasing demand, (ii) development of mechanized fishing technologies severely damages the environment, (iii) cheap and rapid transportation makes even the most distant populations vulnerable to exploitation, and (iv) management has consistently failed to conserve depleted stocks (9, 15, 16, 33, 43, 77, 90, 98, 99). Evidence for ecological transformation and loss of fisheries resources on Western Atlantic coral reefs, seagrass beds, bays, estuaries, and the continental shelves is scientifically sound, and the burden of proof belongs on those who would still fish rather than the other way around (133). Monitoring is a basic tool for management, but no more monitoring is required to know what we have lost. Scientific efforts should be redirected toward evaluating options for restoration of resources rather than perpetuating the myth of sustainable fisheries. It is hard to imagine how increasingly sophisticated and frequent environmental monitoring and micromanagement could do a fraction of the good of simply stopping fishing. There is no rational scientific basis to continue fishing of wild stocks along the Atlantic coast of North America or in the Caribbean for the foreseeable future.
(2) Paleoecological, archeological, and historical reconstructions of coastal marine ecosystems provide the best evidence for predicting ecological consequences of establishing very large-scale marine reserves and other forms of rigorous protection of fisheries. Formerly pristine conditions of seagrass beds and oyster reefs of Chesapeake Bay (14–16), or of Caribbean coral reefs and seagrass beds and the hordes of large animals that lived upon them (9), seem fantastic and unbelievable today. Scientists, as well as the general public, set goals and expectations for marine reserves that are too low because they cannot imagine how coastal ecosystems used to be only a century ago (1, 2). These great changes, and frequently nonlinear transformations among alternative ecosystem states (31, 33, 119–122), make it almost impossible to predict the outcomes of complete protection from fishing and terrestrial inputs based on recent observations alone. Fortunately, historical records tell us what is possible. Because few of the large apex predators and herbivores are extinct, we could restore coastal resources for ecosystem services and managed harvest.
(3) Knowing the former abundance of large animals and ecosystem engineers makes it possible to design experiments to estimate per capita interaction strengths of ecologically extinct species (134, 135). The importance of such studies as a complement to results of human exclusion experiments (136) cannot be overestimated. Even among dedicated advocates, discussions of potential benefits of marine reserves rarely mention swordfish, sharks, sea turtles, or manatees (137), because almost no scientists have ever seen these animals in abundance or contemplated their restoration (1, 2, 9). Large mammals are considered in management plans for the Pacific Northwest because effects of protected sea otters, gray whales, and walrus on benthic communities are well known (96, 138). Most of the time, however, scientific debate revolves around species far down the original food webs, and former top predators and grazers are forgotten or ignored.
But to ignore these large animals is to give up most of what is attainable before we start. Very-large-scale experiments (enclosures of hundreds of hectares) with surviving large green turtles could be carried out in Florida Bay, for example, to determine how their presence affects mass wasting of seagrasses and losses of associated species (60, 61). Even such enormous experiments would probably cost less than increasingly sophisticated monitoring we are doing in so many places for want of a better idea (139). The same would be true for extensive reseeding followed by total protection of oyster beds in entire sections of bays and estuaries, or in entire embayments for cod. It is time scientists began an aggressive series of experiments involving large keystone species on the largest possible spatial and temporal scales. The alternative is absolute microbial domination of coastal ecosystems in 20 to 30 years. Is that the future of evolution in the oceans?
Acknowledgments
Much of this paper was the basis for a proposal to the National Center for Ecological Analysis and Synthesis (NCEAS) to reconstruct the human footprint on coastal marine ecosystems. I thank the members of the resulting NCEAS Marine Records Working Group for excellent discussion and criticism. Suggestions by Michael Graham, Michael Kirby, Nancy Knowlton, Hunter Lenihan, Pete Peterson, and Enric Sala greatly improved the manuscript. To all I am very grateful.
Footnotes
This paper was presented at the National Academy of Sciences colloquium, “The Future of Evolution,” held March 16–20, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Pauly D. Trends Ecol Evol. 1995;10:430. doi: 10.1016/s0169-5347(00)89171-5. [DOI] [PubMed] [Google Scholar]
- 2.Sheppard C. Mar Poll Bull. 1995;30:766–767. [Google Scholar]
- 3.Schama S. Landscape and Memory. New York: Knopf; 1995. [Google Scholar]
- 4.McCall A D. Calif Coop Fish Invest. 1996;37:100–110. [Google Scholar]
- 5.Dayton P K, Tegner M J, Edwards P B, Riser K L. Ecol Appl. 1998;8:309–322. [Google Scholar]
- 6.Dayton P K, Tegner M J, Edwards P B, Riser K L. Ecol Monogr. 1999;69:219–250. [Google Scholar]
- 7.Baumgartner T R, Soutar A, Frerreira-Bartrina V. Calif Coop Fish Invest. 1992;33:24–40. [Google Scholar]
- 8.Anderson P J, Piatt J F. Mar Ecol Prog Ser. 1999;189:117–123. [Google Scholar]
- 9.Jackson J B C. Coral Reefs. 1997;16:S23–S32. [Google Scholar]
- 10.Davis G E. Bull Mar Sci. 1982;32:608–623. [Google Scholar]
- 11.Connell J H, Hughes T P, Wallace C C. Ecol Monogr. 1997;67:461–488. [Google Scholar]
- 12.Hughes T P, Tanner J E. Ecology. 2000;81:2250–2263. [Google Scholar]
- 13.Jackson J B C. BioScience. 1991;41:475–482. [Google Scholar]
- 14.Orth R J, Moore K A. Estuaries. 1984;7:531–540. [Google Scholar]
- 15.Rothschild B J, Ault J S, Goulletquer P, Heral M. Mar Ecol Prog Ser. 1994;111:29–39. [Google Scholar]
- 16.Kennedy V S. J Shellfish Res. 1996;15:177–183. [Google Scholar]
- 17.Hall M O, Durako M J, Fourqurean J W, Zieman J C. Estuaries. 1999;22:445–459. [Google Scholar]
- 18.Fourqurean J W, Robblee M B. Estuaries. 1999;22:345–357. [Google Scholar]
- 19.Cushing D H. The Provident Sea. Cambridge, U.K.: Cambridge Univ. Press; 1988. [Google Scholar]
- 20.Botsford L W, Castilla J C, Peterson C H. Science. 1997;277:509–515. [Google Scholar]
- 21.Carlton J T, Geller J B, Reaka-Kudla M L, Norse E A. Annu Rev Ecol Syst. 1999;30:515–538. [Google Scholar]
- 22.Paulay G. In: Life and Death of Coral Reefs. Birkeland C, editor. New York: Chapman & Hall; 1997. pp. 298–353. [Google Scholar]
- 23.Reaka-Kudla M L. In: Biodiversity II. Reaka-Kudla M L, Wilson D E, Wilson E O, editors. Washington, DC: Joseph Henry Press; 1997. pp. 83–108. [Google Scholar]
- 24.Knowlton N, Jackson J B C. Trends Ecol Evol. 1994;9:7–9. doi: 10.1016/0169-5347(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 25.Jackson J B C. Am Zool. 1992;32:719–731. [Google Scholar]
- 26.Pandolfi J M, Jackson J B C. In: Proceedings of the Eighth International Coral Reef Symposium. Lessios H A, Macintyre I G, editors. Vol. 1. Balboa, Panama: Smithsonian Tropical Research Institute; 1997. pp. 397–404. [Google Scholar]
- 27.Pandolfi J M, Jackson J B C. Ecol Monogr. 2001;71:49–67. [Google Scholar]
- 28.Aronson R B, Precht W F, Macintyre I G. Coral Reefs. 1998;17:223–230. [Google Scholar]
- 29.Greenstein B J, Curran H A, Pandolfi J M. Coral Reefs. 1998;17:249–261. [Google Scholar]
- 30.Gladfelter W B. Bull Mar Sci. 1982;32:639–643. [Google Scholar]
- 31.Knowlton N, Lang J C, Keller B D. Smithson Contr Mar Sci. 1990;31:1–25. [Google Scholar]
- 32.Guzmán H M, Jackson J B C, Weil E. Coral Reefs. 1991;10:1–12. [Google Scholar]
- 33.Hughes T P. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 34.Bak R P M, Nieuwland G. Bull Mar Sci. 1995;56:609–619. [Google Scholar]
- 35.Lessios H A, Robertson D R, Cubit J D. Science. 1984;226:335–337. doi: 10.1126/science.226.4672.335. [DOI] [PubMed] [Google Scholar]
- 36.Lessios H A. Annu Rev Ecol Syst. 1988;19:371–393. [Google Scholar]
- 37.McClanahan T R, Muthiga N A. Environ Conserv. 1998;25:12–130. [Google Scholar]
- 38.Aronson R B, Precht W F. Hydrobiologia. 2000. 1–14. [Google Scholar]
- 39.Hay M E. Ecology. 1984;65:446–454. [Google Scholar]
- 40.Lewis S M. Ecol Monogr. 1986;56:183–200. [Google Scholar]
- 41.Jackson J B C. Proc Roy Soc London Ser B. 1994;344:55–60. [Google Scholar]
- 42.Duerden J E. J. Imp. Agric. Dept. W. Indies. 1901. , 121–141. [Google Scholar]
- 43.Thompson E F. Development and Welfare in the West Indies, Bulletin. 1945. 18 (Bridgetown, Barbados). [Google Scholar]
- 44.Sale P F. In: The Ecology of Fishes on Coral Reefs. Sale P F, editor. San Diego: Academic; 1991. pp. 3–15. [Google Scholar]
- 45.Brown J H. Macroecology. Chicago: Univ. of Chicago Press; 1995. [Google Scholar]
- 46.Dampier W. A New Voyage Around the World. London: James and John Knapton; 1729. ; reprinted (1968) (Dover, New York). [Google Scholar]
- 47.de Oviedo G F. Historia General y Natural de las Indias, Islas y Tierra-Firme del Mar Oceano. 1535–1557. ; reprinted (1959) (Atlas, Madrid). [Google Scholar]
- 48.Goreau T F. Ecology. 1959;40:67–89. [Google Scholar]
- 49.Mesolella K J. Science. 1967;156:638–640. doi: 10.1126/science.156.3775.638. [DOI] [PubMed] [Google Scholar]
- 50.Done T J, Ogden J C, Wiebe W J, Rosen B R. In: Functional Roles of Biodiversity: A Global Perspective. Mooney H A, Cushman J H, Medina E, Sala O E, Schulze E-D, editors. London: Wiley; 1996. pp. 393–429. [Google Scholar]
- 51.Lapointe B E. Limnol Oceanogr. 1997;42:1119–1131. [Google Scholar]
- 52.Hughes T P, Szmant A M, Steneck R, Carpenter R, Miller S. Limnol Oceanogr. 1999;44:1583–1586. [Google Scholar]
- 53.Miller M W, Hay M E, Miller S L, Malone D, Sotka E E, Szmant A M. Limnol Oceanogr. 1999;44:1847–1861. [Google Scholar]
- 54.Richardson L L. Trends Ecol Evol. 1998;13:438–443. doi: 10.1016/s0169-5347(98)01460-8. [DOI] [PubMed] [Google Scholar]
- 55.Harvell C D, Kim K, Burkholder J M, Colwell R R, Epstein P R, Grimes D J, Hofman E E, Lipp E K, Osterhaus A D M E, Overstreet R M, et al. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- 56.Aronson R B, Precht W F, Macintyre I G, Murdoch T J T. Nature (London) 2000;405:36. doi: 10.1038/35011132. [DOI] [PubMed] [Google Scholar]
- 57.Zieman J C. The Ecology of the Seagrasses of South Florida: A Community Profile. Office of Biological Services, Washington, DC: U.S. Fish and Wildlife Service; 1982. [Google Scholar]
- 58.Orth R J, Heck K L, Jr, van Montfrans J. Estuaries. 1984;7:339–350. [Google Scholar]
- 59.Jackson J B C. Bull Mar Sci. 1972;23:313–350. [Google Scholar]
- 60.Robblee M B, Barber T R, Carlson P R, Jr, Durako M J, Fourqurean J W, Muehlstein L K, Porter D, Yarbro L A, Zieman R T, Zieman J C. Mar Ecol Prog Ser. 1991;71:297–299. [Google Scholar]
- 61.Zieman J C, Fourqurean J W, Frankovich T A. Estuaries. 1999;22:460–470. [Google Scholar]
- 62.Bjorndal K A, Bolten A B, Chaloupka M Y. Ecol Appl. 2000;10:269–282. [Google Scholar]
- 63.Mduma S A R, Sinclair A R E, Hilborn R. J Anim Ecol. 1999;68:1101–1122. [Google Scholar]
- 64.Ogden J C. In: Handbook of Seagrass Biology: An Ecosystem Perspective. Phillips R C, McRoy C P, editors. New York: Garland; 1980. pp. 173–198. [Google Scholar]
- 65.Williams S L. Mar Biol. 1988;98:447–455. [Google Scholar]
- 66.Ogden J C, Robinson L, Whitlock H, Daganhart H, Cebula R. J Exp Mar Biol Ecol. 1983;66:199–205. [Google Scholar]
- 67.Thayer G W, Engel D W, Bjorndal K A. J Exp Mar Biol Ecol. 1982;62:173–183. [Google Scholar]
- 68.Thayer G W, Bjorndal K A, Ogden J C, Williams S L, Zieman J C. Estuaries. 1984;7:351–376. [Google Scholar]
- 69.King F W. In: Biology and Conservation of Sea Turtles. Bjorndal K, editor. Washington, DC: Smithsonian Inst. Press; 1982. pp. 183–188. [Google Scholar]
- 70.Carlson P R, Yarbro L A, Barber T A. Bull Mar Sci. 1994;54:733–746. [Google Scholar]
- 71.Durako M J, Kuss K M. Bull Mar Sci. 1994;54:727–732. [Google Scholar]
- 72.Reynolds J E, III, Odell D K. Manatees and Dugongs. New York: Facts On File; 1991. [Google Scholar]
- 73.Lanyon J M, Limpus C J, Marsh H. In: Biology of Seagrasses: A Treatise on the Biology of Seagrasses with Special Reference to the Australian Region. Larkum A W D, McComb A J, Shepherd S A, editors. Amsterdam: Elsevier; 1989. pp. 610–634. [Google Scholar]
- 74.Preen A. Mar Ecol Prog Ser. 1995;124:201–213. [Google Scholar]
- 75.Brush G S. In: The Changing Global Environment. Roberts N, editor. Oxford: Blackwell; 1994. pp. 398–416. [Google Scholar]
- 76.Cronin T, Willard D, Karlsen A, Ishman S, Verardo S, McGeehin J, Kerhin R, Holmes C, Colman S, Zimmerman A. Geology. 2000;28:3–6. [Google Scholar]
- 77.Wharton J. The Bounty of the Chesapeake. Virginia Anniversary Celebration Corp.; 1957. , Jamestown 390th Anniversary Historical Booklet 13. [Google Scholar]
- 78.Cooper S R, Brush G S. Science. 1991;254:992–996. doi: 10.1126/science.254.5034.992. [DOI] [PubMed] [Google Scholar]
- 79.Cooper S R, Brush G S. Estuaries. 1993;16:617–626. [Google Scholar]
- 80.Cooper S R. Ecol Appl. 1995;5:703–723. [Google Scholar]
- 81.Harding L W, Jr, Perry E S. Mar Ecol Prog Ser. 1997;157:39–52. [Google Scholar]
- 82.Muehlstein L K. Dis Aquat Organ. 1989;7:211–221. [Google Scholar]
- 83.Officer C B, Smayda T J, Mann R. Mar Ecol Prog Ser. 1982;9:203–210. [Google Scholar]
- 84.Newell R I E. In: Understanding the Estuary: Advances in Chesapeake Bay Research. Lynch M P, Krome E C, editors. CBP/TRS 24/88: Chesapeake Research Consortium 129; 1988. [Google Scholar]
- 85.Ulanowicz R E, Tuttle J H. Estuaries. 1992;15:298–306. [Google Scholar]
- 86.Jonas R B. Am Zool. 1997;37:612–620. [Google Scholar]
- 87.Paerl H W, Pinckney J L, Fear J M, Peierls B L. Mar Ecol Prog Ser. 1998;166:17–25. [Google Scholar]
- 88.Turner R E, Rabalais N N. Nature (London) 1994;368:619–621. [Google Scholar]
- 89.Mann K H. Ecology of Coastal Waters: A Systems Approach. Berkeley: Univ. of California Press; 1982. [Google Scholar]
- 90.Steneck R S. Proceedings of the Gulf of Maine Ecosystem Dynamics Scientific Symposium and Workshop. 1997. , RARGOM Report 91-1, eds. Wallace, G. T. & Braasch, E. F. (Regional Association for Research on the Gulf of Maine, Hanover, NH), pp. 151–165. [Google Scholar]
- 91.Elner R W, Vadas R L. Am Nat. 1990;136:108–125. [Google Scholar]
- 92.Witman J D, Sebens K P. Oecologia. 1992;90:305–315. doi: 10.1007/BF00317686. [DOI] [PubMed] [Google Scholar]
- 93.Bourque B J. Diversity and Complexity in Prehistoric Maritime Societies: A Gulf of Maine Perspective. New York: Plenum; 1995. [Google Scholar]
- 94.de la Morandière C. Histoire de la Pêche Française de la Morue dans l'Amérique Septentrionale. 1–3. Paris: Maisonneuve and Larose; 1962–1966. [Google Scholar]
- 95.Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F., Jr Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- 96.Simenstad C A, Estes J A, Kenyon K W. Science. 1978;200:403–411. doi: 10.1126/science.200.4340.403. [DOI] [PubMed] [Google Scholar]
- 97.Estes J A, Duggins D O, Rathburn G B. Conserv Biol. 1989;3:252–264. [Google Scholar]
- 98.Dayton P K, Thrush S F, Agardy M T, Hofman R J. Aquat Conserv Mar Freshwater Ecosyst. 1995;5:205–232. [Google Scholar]
- 99.Watling L, Norse E A. Conserv Biol. 1998;12:1180–1197. [Google Scholar]
- 100.Casey J M, Myers R A. Science. 1998;281:690–692. doi: 10.1126/science.281.5377.690. [DOI] [PubMed] [Google Scholar]
- 101.Committee on Sea Turtle Conservation, National Research Council. Decline of the Sea Turtles: Causes and Prevention. Washington, DC: Natl. Acad. Press; 1990. [Google Scholar]
- 102.Von Buskirk J. Copeia. 1994;1994:66–81. [Google Scholar]
- 103.Bjorndal K A, Zug G R. In: Biology and Conservation of Sea Turtles. 2nd. Ed. Bjorndal K A, editor. Washington, DC: Smithsonian Inst. Press; 1995. pp. 599–600. [Google Scholar]
- 104.Bullock L H, Murphy M D, Godcharles M F, Mitchell M E. Fish Bull. 1992;90:243–249. [Google Scholar]
- 105.Carter J, Marrow G J, Pryor V. Proc Gulf Caribb Fish Inst. 1990;43:65–111. [Google Scholar]
- 106.Sadovy Y. Proc Gulf Caribb Fish Inst. 1990;43:43–64. [Google Scholar]
- 107.Smith C L. Trans Am Fish Soc. 1972;101:257–261. [Google Scholar]
- 108.Johannes R E. Words of the Lagoon. Berkeley: Univ. of California Press; 1976. [Google Scholar]
- 109.Branstetter S. In: Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries. Pratt H L Jr, Gruber S H, Taniuchi T, editors. Washington, DC: U.S. Dept. of Commerce; 1990. , NOAA Technical Report NMFS 90, pp. 17–28. [Google Scholar]
- 110.Pratt H L, Jr, Casey J G. In: Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries. Pratt H L Jr, Gruber S H, Taniuchi T, editors. Washington, DC: U.S. Dept. of Commerce; 1990. , NOAA Technical Report NMFS 90, pp. 97–109. [Google Scholar]
- 111.Compagno L J V. In: Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries. Pratt H L Jr, Gruber S H, Taniuchi T, editors. Washington, DC: U.S. Dept. of Commerce; 1990. , NOAA Technical Report NMFS 90, pp. 391–414. [Google Scholar]
- 112.Anderson E D. In: Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries. Pratt H L Jr, Gruber S H, Taniuchi T, editors. Washington, DC: U.S. Dept. of Commerce; 1990. , NOAA Technical Report NMFS 90, pp. 473–484. [Google Scholar]
- 113.Jones C G, Lawton J H, Shachak M. Oikos. 1994;69:373–386. [Google Scholar]
- 114.Lenihan H S, Peterson C H. Ecol Appl. 1998;8:128–140. [Google Scholar]
- 115.Lenihan H S. Ecol Monogr. 1999;69:251–275. [Google Scholar]
- 116.Lenihan, H. S., Peterson, C. H., Byers, J. E., Grabowski, J. H., Thayer, G. W. & Colby, D. R. (2001) Ecol. Appl., in press.
- 117.Terborgh J. Requiem for Nature. Washington, DC: Island Press; 1999. [Google Scholar]
- 118.Lewis J B. Coral Reefs. 1984;3:117–122. [Google Scholar]
- 119.Sutherland J P. Am Nat. 1974;108:859–873. [Google Scholar]
- 120.Peterson C H. Am Nat. 1984;124:127–133. [Google Scholar]
- 121.Knowlton N. Am Zool. 1992;32:674–682. [Google Scholar]
- 122.Paine R T, Tegner M J, Johnson E A. Ecosystems. 1998;1:535–545. [Google Scholar]
- 123.Diamond J. Guns, Germs and Steel: The Fates of Human Societies. New York: Norton; 1997. [Google Scholar]
- 124.Steneck R S. Trends Ecol Evol. 1998;13:429–430. doi: 10.1016/s0169-5347(98)01494-3. [DOI] [PubMed] [Google Scholar]
- 125.Munro J L. ICLARM Study Rev. 1983;7:1–276. [Google Scholar]
- 126.Svennevig N, Reinertsen H, New M, editors. Sustainable Aquaculture: Food for the Future? Rotterdam, The Netherlands: Balkema; 1999. [Google Scholar]
- 127.Naylor R L, Goldburg R J, Primavera J H, Kaufsky N, Beveredge M C M, Clay J, Folke C, Lubchenco J, Mooney H, Troell M. Nature (London) 2000;405:1017–1024. doi: 10.1038/35016500. [DOI] [PubMed] [Google Scholar]
- 128.Carlton J T. Conserv Biol. 1989;3:265–273. [Google Scholar]
- 129.Meinesz A. Killer Algae. Chicago: Univ. of Chicago Press; 1999. [Google Scholar]
- 130.Wilkinson C R. In: Proceedings of the Seventh International Coral Reef Symposium. Richmond R, editor. Vol. 1. Mangilao, Guam: Univ. of Guam Press; 1992. pp. 11–21. [Google Scholar]
- 131.Ginsburg R N compiler. Proceedings of the Colloquium on Global Aspects of Coral Reefs: Health, Hazards and History 1993. Univ. of Miami, Miami: Rosenstiel School of Marine and Atmospheric Science; 1994. [Google Scholar]
- 132.Ager D. The New Catastrophism. Cambridge, U.K.: Cambridge Univ. Press; 1993. [Google Scholar]
- 133.Dayton P K. Science. 1998;279:821–822. [Google Scholar]
- 134.Paine R T. Nature (London) 1992;355:73–75. [Google Scholar]
- 135.Wootton J T. Ecol Monogr. 1997;67:45–64. [Google Scholar]
- 136.Castilla J C. Trends Ecol Ecol. 1999;14:280–283. doi: 10.1016/s0169-5347(99)01602-x. [DOI] [PubMed] [Google Scholar]
- 137.Murray S N, Ambrose R F, Bohnsack J A, Botsford L W, Carr M H, Davis G E, Dayton P K, Gotshall D, Gunderson D R, Hixon M A, et al. Fisheries. 1999;24:11–25. [Google Scholar]
- 138.Bowen W D. Mar Ecol Prog Ser. 1997;158:267–274. [Google Scholar]
- 139.Boesch D F. Env Res Sect A. 2000;82:134–142. doi: 10.1006/enrs.1999.4010. [DOI] [PubMed] [Google Scholar]