Abstract
OBJECTIVE
Our aim was to analyze the performance of two scores developed for predicting diabetes in nontransplant populations for identifying kidney transplant recipients with a higher new-onset diabetes mellitus after transplantation (NODAT) risk beyond the first year after transplantation.
RESEARCH DESIGN AND METHODS
We analyzed 191 kidney transplants, which had at least 1-year follow-up posttransplant. First-year posttransplant variables were collected to estimate the San Antonio Diabetes Prediction Model (SADPM) and Framingham Offspring Study–Diabetes Mellitus (FOS-DM) algorithm.
RESULTS
Areas under the receiver operating characteristic curve of FOS-DM and SADPM scores to predict NODAT were 0.756 and 0.807 (P < 0.001), respectively. FOS-DM and SADPM scores over 75 percentile (hazard ratio 5.074 and 8.179, respectively, P < 0.001) were associated with NODAT.
CONCLUSIONS
Both scores can be used to identify kidney recipients at higher risk for NODAT beyond the first year. SADPM score detects some 25% of kidney transplant patients with an eightfold risk for NODAT.
New-onset diabetes mellitus after transplantation (NODAT) occurs in a substantial percentage of renal transplant recipients and is associated with reduced patient and graft survival (1–4). Lifestyle and pharmacological interventions can reduce the rate of progression to type 2 diabetes in people with impaired glucose tolerance (5). So, preventive NODAT measures will be more effective when applied to well-identified high-risk-for-diabetes kidney transplant recipients. In the general population, more than 40 diabetes prediction models have been reported (6). The aim of our report was to analyze the performance of two scores developed in nontransplant population for identifying kidney transplant recipients with a higher NODAT risk using variables obtained at first year posttransplantation.
RESEARCH DESIGN AND METHODS
We retrospectively analyzed 191 kidney transplants performed in our center, which had at least 1-year posttransplant follow-up and without previous diabetes diagnosis. NODAT was defined according to the consensus guidelines (7). Relevant information was extracted from the prospectively maintained database of renal transplant patients of our center. The study was conducted according to the Declaration of Helsinki and was approved by the ethics committee of our hospital. San Antonio Diabetes Prediction Model (SADPM) and Framingham Offspring Study–Diabetes Mellitus (FOS-DM) algorithm were calculated from 1-year variables according to the equations reported previously (8,9).
Continuous variables were analyzed using Student t test. Calibration was estimated by the Hosmer-Lemeshow goodness-of-fit test. NODAT discrimination ability was measured by positive and negative predictive values and area under receiver operating characteristic curve (AUC-ROC) statistic. NODAT incidence was calculated with the Kaplan-Meier estimate. Cox model was used to calculate the hazard ratio (HR) for NODAT. Statistical analyses were performed with SPSS, version 15.0 (SPSS, Chicago, IL).
RESULTS
Some 41 patients (13.7%) developed NODAT after the first year. Mean values of both FOS-DM (15.8 ± 6.0 vs. 9.5 ± 6.7, P < 0.001) and SADPM (0.39 ± 0.18 vs. 0.19 ± 0.15, P < 0.001) scores were significantly higher in those patients who developed NODAT.
The AUC-ROC of FOS-DM score to predict NODAT was 0.756 (95% CI 0.664–0.848, P < 0.001). The goodness-of-fit test demonstrates that the FOS-DM risk was well calibrated (P = 0.641). Similarly, the AUC-ROC curve of SADPM score to predict NODAT was 0.807 (95% CI 0.728–0.885, P < 0.001). The Hosmer-Lemeshow test demonstrates that the SADPM score was well calibrated (P = 0.765). The positive and negative predictive values of a FOS-DM score over the 75 percentile were 24.5% and 92.5%. The positive and negative predictive values of a SADPM score over 75 percentile were 31.2% and 93.7%.
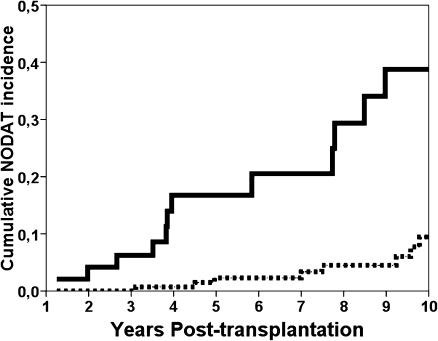
The cumulative incidence of NODAT at 6 years was significantly higher for patients over the 75 percentile of both FOS-DM (10.7% vs. 3.7%, P < 0.001) and SADPM (11.4% vs. 2.1%, P < 0.001) scores (Fig. 1). After other variables were adjusted, FOS-DM over the 75 percentile remained significantly associated with a further development of NODAT (HR 5.074 [95% CI 2.156–11.943], P < 0.001). In a similar way, an SADPM score over the 75 percentile was related with NODAT (HR 8.179 [95% CI 3.528–18.957], P < 0.001). In a model adjusting each score with each other, SADPM score over the 75 percentile remained significantly related to NODAT (HR 4.826 [95% CI 1.716–13.575], P = 0.003) but not the FOS-DM score (HR 2.109 [95% CI 0.739–6.020], P = 0.163).
Figure 1.
Kaplan-Meier analysis of cumulative NODAT incidence comparing patients with a SADPM score over the 75 percentile (continuous line) with patients under the 75 percentile (dashed line).
CONCLUSIONS
The main finding of our report is that scores which predict type 2 diabetes in the general population can also predict NODAT in kidney transplant recipients. Not only was the mean value of both scores significantly different between patients who will or will not develop NODAT, but patients in the top quartile of both scores also had a significantly higher risk for NODAT.
But, to assess the performance of a prediction model, we must determine discrimination and calibration. On the one hand, discrimination was quantified with the AUC-ROC, which showed that 75% and 80% of patients were adequately classified as at risk for NODAT according to FOS-DM and SADPM scores, respectively. On the other hand, the Hosmer-Lemeshow test showed that both scores were well calibrated to predict NODAT.
Despite the good performance of both scores, their relative low positive predictive value means that they cannot be used to identify the future evolution of an individual patient. By contrast, these scores are adequate to identify a high risk for NODAT kidney transplant population. The high negative predictive value (over 90%) of the top quartile of both scores means that we can consider this 25% of kidney transplant recipients as high risk for NODAT. In this sense, by using these scores, we may identify a specific kidney transplant population with a high NODAT risk that can be used in prospective studies to prevent NODAT.
Chakkera et al. (10) have recently developed a risk score from seven pretransplant variables that predict 1-year NODAT with an AUC-ROC of 0.72 and allow identifying kidney transplant recipients with a first-year higher risk for NODAT. Because of the different NODAT incidence and risk factors after the first year, a different score must be used to predict NODAT at first year posttransplant. A two-step approach to predict NODAT first at pretransplant with the score of Chakkera et al. (10) and second at first year with FOS-DM or SADPM scores could be recommended.
We found that the SADPM score performed better than the FOS-DM score to predict NODAT. SADPM score was developed in a population with an absolute risk for type 2 diabetes greater than in the Framingham population, perhaps more similar to transplant risk (8,9). Although there are no specifically designed scores to predict NODAT at first year posttransplantation, the SADPM score can be used to identify high-risk kidney transplant recipients.
To conclude, FOS-DM and SADPM scores can be used to identify a kidney transplant population with a higher risk for developing NODAT beyond the first year. Both scores are simple and effective tools with good discrimination ability and well calibrated and use variables easy to obtain at first year posttransplantation. The SADPM score detects some 25% of kidney transplant patients with eight times the risk for NODAT, defining a target population for prevention programs.
Acknowledgments
This work was supported by ISCIII (REDINREN 06/16), Fundación Marqués de Valdecilla-IFIMAV. E.R. was supported by grants of Fundación Marqués de Valdecilla-IFIMAV and Sociedad Española de Nefrología.
No potential conflicts of interest relevant to this article were reported.
E.R. participated in research design, research performance, data analysis, and manuscript writing. L.S. participated in research design, research performance, and data analysis. C.P., J.C.R.S.M., M.E.Q., C.T., N.A., and C.G.-A. researched data. M.A. participated in research design, research performance, and manuscript review. As the corresponding author and guarantor of this article, E.R. takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
The authors thank John Hawkins, Santander, for assistance in the preparation of the manuscript.
Footnotes
The contents of this article are the sole responsibility of the authors.
References
- 1.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care 2002;25:583–592 [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 2003;3:178–185 [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Fresnedo G, Escallada R, de Francisco ALM, et al. Posttransplant diabetes is a cardiovascular risk factor in renal transplant patients. Transplant Proc 2003;35:700. [DOI] [PubMed] [Google Scholar]
- 4.Cosio FG, Kudva Y, van der Velde M, et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int 2005;67:2415–2421 [DOI] [PubMed] [Google Scholar]
- 5.Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis [article online], 2007. Available from http://www.bmj.com/content/334/7588/299?view=long&pmid=17237299. Accessed 10 October 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins GS, Mallett S, Omar O, Yu L-M. Developing risk prediction models for type 2 diabetes: a systematic review of methodology and reporting [article online], 2011. Available from http://www.biomedcentral.com/1741-7015/9/103. Accessed 10 October 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson J, Wilkinson A, Dantal J, et al. ; International Expert Panel New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 2003;75(Suppl.):SS3–SS24 [DOI] [PubMed] [Google Scholar]
- 8.Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med 2002;136:575–581 [DOI] [PubMed] [Google Scholar]
- 9.Wilson PWF, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007;167:1068–1074 [DOI] [PubMed] [Google Scholar]
- 10.Chakkera HA, Weil EJ, Swanson CM, et al. Pretransplant risk score for new-onset diabetes after kidney transplantation. Diabetes Care 2011;34:2141–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]