Abstract
OBJECTIVE
To evaluate the efficacy and safety of taspoglutide monotherapy in drug-naive patients with type 2 diabetes inadequately controlled.
RESEARCH DESIGN AND METHODS
In this 24-week double-blind, placebo-controlled, multicenter trial, 373 patients with type 2 diabetes naive to antihyperglycemic medication were randomized to weekly subcutaneous taspoglutide 10 or 20 mg or placebo.
RESULTS
HbA1c reductions from baseline were greater with taspoglutide 10 and 20 mg than placebo (least squares mean [SE] changes: –1.01% [0.07], –1.18% [0.06], and –0.09% [0.07], respectively; both P < 0.0001 vs. placebo). Decreases in bodyweight were greater with taspoglutide 10 mg (–1.45 kg [0.32]) and with 20 mg (–2.25 kg [0.30]) than placebo (–1.23 kg [0.31]; P = 0.61 and P = 0.02 for taspoglutide 10 and 20 mg vs. placebo, respectively). Gastrointestinal adverse events and injection site reactions were more common with taspoglutide than placebo.
CONCLUSIONS
In drug-naive patients, once-weekly taspoglutide improved glycemic control, reduced body weight, and was generally well tolerated.
Taspoglutide is a human glucagon-like peptide 1 analog with a pharmacokinetic profile suitable for once-weekly subcutaneous administration (1). In a phase 2 clinical trial, once-weekly taspoglutide added to metformin lowered HbA1c by up to 1%, reduced body weight, and was generally well tolerated (2). The efficacy and safety of taspoglutide monotherapy as a first-line agent was investigated in a phase 3 trial in patients with early type 2 diabetes who were inadequately controlled with diet and exercise and naive to antihyperglycemic therapy.
RESEARCH DESIGN AND METHODS
This 24-week, randomized, double-blind, placebo-controlled study (clinical trial reg. no. NCT00744926) was conducted at 53 centers internationally and in accordance with the principles of the Declaration of Helsinki. An institutional review/ethics board at each center approved the protocol, and written informed consent was obtained from all patients.
Eligible patients were adults (aged ≥18 and ≤80 years) with type 2 diabetes naive to antihyperglycemic therapy and uncontrolled with diet and exercise (HbA1c 6.5−10%; BMI 25−45 kg/m2).
Patients were excluded if they had significant complications associated with type 2 diabetes, symptomatic gastrointestinal diseases, history of bariatric surgery, pancreatic disease, cardiac disease within the past 6 months, history of unstable hypertension, treatment with chronic corticosteroids within the past month, and treatment with weight-lowering agents within the past 12 weeks.
Patients were randomly assigned (1:1:1) to subcutaneous taspoglutide 10 mg weekly, taspoglutide 20 mg weekly (after 10 mg weekly for the initial 4 weeks), or placebo. Patients were stratified by baseline HbA1c (<8.0 or ≥8.0%). If glycemic control deteriorated, additional antihyperglycemic rescue medication was prescribed and the patient could continue in the study.
The primary efficacy end point was absolute change from baseline in HbA1c after 24 weeks. Secondary efficacy end points included HbA1c response rates (≤6.5 and ≤7%) and changes in fasting plasma glucose (FPG), fructosamine, body weight, fasting proinsulin, fasting proinsulin-to-insulin ratio, and homeostasis model assessment of β-cell function (HOMA-B). Exploratory end points are listed in Supplementary Table 1.
Safety assessments included adverse events (AEs), vital signs, physical examinations, clinical laboratory tests, electrocardiograms, and hypoglycemia.
Statistical analysis
Approximately 130 patients per treatment arm were needed to detect a difference from placebo in HbA1c of 0.6% between groups with a power of 90%, assuming an SD of change from baseline of 1.2. Primary efficacy analyses were conducted using intention-to-treat population. The primary end point was analyzed using ANCOVA with treatment, region, and baseline value of the end point as the covariate. Missing data were imputed using the last observation carried forward method. HbA1c was tested in each of the two active arms versus placebo using the two-sided 95% CI for treatment difference.
RESULTS
Of 373 randomized patients, 368 received at least one dose of study medication and had at least one follow-up safety measurement, and 354 received at least one dose of study medication and had an evaluable baseline and at least one postbaseline measurement of HbA1c (Supplementary Fig. 1). Treatment groups were well matched at baseline, and mean duration of diabetes was 2.1−2.8 years (Supplementary Table 2).
Least squares mean HbA1c decreased from baseline by −0.09 ± 0.07, −1.01 ± 0.07, and −1.18 ± 0.06% with placebo, taspoglutide 10 mg, and taspoglutide 20 mg, respectively (both P < 0.0001 vs. placebo). Reductions were greater with taspoglutide (10- and 20-mg groups, respectively) in patients with HbA1c ≥8% (–1.69 and –1.72%) vs. <8% (–0.69 and –0.93%) at baseline. A greater proportion of patients in the taspoglutide 10- and 20-mg groups, respectively, achieved HbA1c of ≤6.5% (60 and 66%) and ≤7.0% (80 and 83%) versus placebo (17 and 40%) (Supplementary Fig. 2A and B).
Least squares mean changes from baseline in FPG were –0.08 ± 0.17, –1.55 ± 0.17, and –1.90 ± 0.16 mmol/L with placebo, taspoglutide 10-mg, and 20-mg groups, respectively (both P < 0.001 vs. placebo). Weight loss occurred progressively in all groups and was greater in the taspoglutide 20-mg group versus placebo (–2.25 ± 0.30 kg; P = 0.02).
Significant improvements in HOMA-B were seen with both doses of taspoglutide versus placebo (59 and 65 vs. 2; P = 0.01 and P < 0.01 for taspoglutide 10 mg and 20 mg, respectively). Proinsulin-to-insulin ratios significantly decreased with taspoglutide 10 and 20 mg versus placebo (Supplementary Table 3).
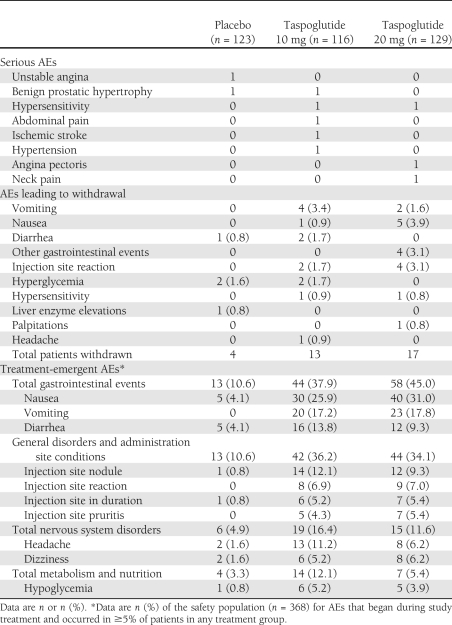
Treatment-emergent AEs were reported in 44.7, 69.0, and 75.2% of patients in the placebo, taspoglutide 10-mg, and taspoglutide 20-mg groups, respectively. Serious AEs were reported in 10 patients (Table 1). Hypersensitivity reactions were reported in two patients receiving taspoglutide 10 mg (one moderate rash on left forearm, which occurred shortly after the first injection of trial medication, and one moderate systemic urticaria, which occurred on study day 163 after the last injection) and in two patients receiving taspoglutide 20 mg (one case of face redness moderate in intensity that started on study day 29 immediately after the patient had received the fifth dose of taspoglutide and one severe edema of the larynx and tongue that occurred shortly after the first dose of taspoglutide 10 mg and resolved 1 h after prednisone administered). No deaths occurred and no cases of pancreatitis were reported.
Table 1.
AEs (safety population)
Gastrointestinal complaints were the most frequently reported AEs (Table 1). Injection site reactions occurred at a higher frequency in patients receiving taspoglutide than placebo. No cases of severe hypoglycemia were reported.
Withdrawal from treatment as a result of AEs occurred in 3.3, 11.2, and 13.2% of the placebo, taspoglutide 10-mg, and taspoglutide 20-mg groups, respectively (Table 1).
CONCLUSIONS
Once-weekly taspoglutide monotherapy improved HbA1c, FPG, and HOMA-B and reduced body weight during a 24-week period in patients with newly diagnosed type 2 diabetes who were naive to antihyperglycemic agents. Notable findings included a mean reduction in HbA1c of nearly 1.2% in patients with a mean baseline of 7.7% treated with taspoglutide 20 mg. In patients with baseline HbA1c of ∼7.0%, patients achieved an HbA1c of 6.1% with 20 mg and 6.3% with 10 mg after 24 weeks of treatment with taspoglutide.
Taspoglutide monotherapy was generally well tolerated; the most frequently reported AEs were nausea and vomiting. Hypersensitivity reactions occurred in four patients; two patients withdrew. In summary, once-weekly taspoglutide given as monotherapy was efficacious and generally well tolerated in patients with type 2 diabetes naive to treatment with antidiabetic agents.
Acknowledgments
This study was sponsored by F. Hoffmann-La Roche. Support for third-party writing assistance for this manuscript, furnished by Susan Sutch, PharmD, CMPP and Linda Wychowski, PhD, CMPP of UBC-Envision Group, was provided by F. Hoffmann-La Roche. I.R. is a consultant for Roche, Novo Nordisk, AstraZeneca, and Bristol-Myers Squibb (money paid to institution of employment) and is a member of the speakers’ bureau for Eli Lilly and Company and Novo Nordisk (money paid to institution of employer). V.F. is a consultant for Roche, Merck, GlaxoSmithKline, Novo Nordisk, sanofi-aventis, and Eli Lilly and Company and has received honoraria from Merck, GlaxoSmithKline, Novo Nordisk, sanofi-aventis, and Daiichi Sankyo. M.K. is an investigator for Roche (money paid to institution of employer) and a consultant for Abbott Laboratories, Gilead Sciences, PPD Development LP, and Takeda. L.D. is an employee of F. Hoffmann-La Roche. M.B. is an employee of Roche. R.B. is a former employee of F. Hoffmann-La Roche and holds Roche stock. No other potential conflicts of interest relevant to this article were reported.
I.R. interpreted data and reviewed and edited the manuscript. V.F. and L.D. contributed to discussion, collected and interpreted data, and reviewed and edited the manuscript. M.K. and J.H. collected and interpreted data and reviewed and edited the manuscript. M.B. provided statistical analyses of data and reviewed and edited the manuscript. R.B. contributed to discussion; researched, collected, and interpreted data; and wrote, reviewed, and edited the manuscript. All authors approved the manuscript for submission. I.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors thank the T-emerge 1 study group, their staff, and clinical trial personnel and patients for participating in the study. A complete list of participating investigators can be found in the Supplementary Data online.
Footnotes
Clinical trial reg. no. NCT00744926, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1942/-/DC1.
R.B. is currently affiliated with Eli Lilly and Company Ltd., Surrey, U.K.
In September 2010, Roche decided to stop dosing patients in the taspoglutide phase 3 trials because higher than expected discontinuation rates of taspoglutide-treated patients were observed, mainly due to gastrointestinal tolerability, and as a result of the implementation of the risk mitigation plan to address serious hypersensitivity reactions. Since this time, Roche has worked on the root cause analysis and on the modified taspoglutide formulations with the input of Ipsen. After further analysis, Roche has now made the decision to stop the development of taspoglutide and to return the product to the originator, Ipsen, which is currently pursuing further investigations.
References
- 1.Retterstøl K. Taspoglutide: a long acting human glucagon-like polypeptide-1 analogue. Expert Opin Investig Drugs 2009;18:1405–1411 [DOI] [PubMed] [Google Scholar]
- 2.Nauck MA, Ratner RE, Kapitza C, Berria R, Boldrin M, Balena R. Treatment with the human once-weekly glucagon-like peptide-1 analog taspoglutide in combination with metformin improves glycemic control and lowers body weight in patients with type 2 diabetes inadequately controlled with metformin alone: a double-blind placebo-controlled study. Diabetes Care 2009;32:1237–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]