Abstract
OBJECTIVE
Sudomotor symptoms are a common component of diabetic autonomic neuropathy, but the pathology of sudomotor innervation and its relationship with glycemic control have remained obscured.
RESEARCH DESIGN AND METHODS
We enrolled 42 patients (26 males and 16 females aged 56.64 ± 12.67 years) with diabetic neuropathy defined by symmetric distally predominant sensory symptoms, abnormal nerve conduction studies, and reduced intraepidermal nerve fiber density in the leg. Skin biopsies of the distal leg were immunostained with antiprotein gene product 9.5 for nerve fibers and counterstained with Congo red for sweat glands. Sweat gland innervation index (SGII) was quantified with a new computerized area-based morphometric system.
RESULTS
Protein gene product 9.5(+) nerve terminals surrounded secretory coils of the sweat glands in the skin of control subjects. Sudomotor denervation was present in diabetic patients, manifesting as depletion of periglandular nerve fibers with lower SGII compared with 42 age- and sex-matched control subjects (2.54 ± 1.87 vs. 4.68 ± 1.51%, P < 0.001). The SGII was correlated with HbA1c (P = 0.011) and was lower in patients with anhidrosis of the feet compared with those with normal sweating of the feet (0.82 ± 0.69 vs. 3.00 ± 1.81%, P = 0.001). Sudomotor denervation was concordant with cardiac autonomic dysfunction as assessed with reduced heart rate variability (P = 0.003).
CONCLUSIONS
Sudomotor denervation is a significant presentation of diabetic neuropathy, and the SGII was associated with HbA1c. A skin biopsy offers a structural assessment of sudomotor innervation.
Sudomotor failure, an important presentation of diabetic autonomic neuropathy (1), may predispose patients to dry skin and frequent skin wounds, which are risk factors for limb amputation in diabetes owing to poor wound healing (2). Most assessments of autonomic neuropathy depend on functional tests, and there is a lack of direct pathological evidence of peripheral autonomic nerves (3). Previous studies have mainly used nerve biopsies to document the postganglionic neuropathology of nerve degeneration (4). Skin biopsy is a recognized approach to diagnosing small-fiber sensory neuropathy in diabetes by assessing unmyelinated nerve terminals in the skin (5,6). The pathologic hallmarks include epidermal denervation and the presence of degenerated nerve fibers in the dermis (7–9). Since sweat glands are located in the dermis, this skin biopsy approach may have the potential to evaluate autonomic nerve fibers innervating sweat glands (10–13). Recently, we have developed a new morphometry-based method to quantify the pathology of sudomotor innervation on skin biopsies according to the parameter of sweat gland innervation index (SGII) (14). This approach provides an opportunity to investigate the clinical significance of sweat gland innervations in diabetic neuropathy.
Despite there being several large-scale nerve conduction studies on large myelinated nerve parameters (15), the effects of glycemic control on small-fiber parameters, including skin innervation and neuropathic pain, remain elusive. For example, HbA1c was not correlated with intraepidermal nerve fiber (IENF) density or neuropathic pain (8,16). Given that diabetes is a major cause of autonomic neuropathy (1,17), there is a lack of systematic studies exploring the effects of glycemic control on the pathology of sudomotor innervation.
In addition to the sudomotor system, the involvement of the autonomic nervous system in diabetic neuropathy is generally diffuse. Other major manifestations of diabetic autonomic neuropathies include cardiac and gastrointestinal autonomic dysfunctions (18,19). Among these, cardiac autonomic dysfunction, usually assessed by heart rate variability (HRV) (17), is a risk factor of cardiovascular disease and a major cause of morbidity and mortality in diabetes (18,20,21). The relationship of cardiac autonomic dysfunction with sweat gland innervation, however, has remained largely unknown.
In the current study, by applying the newly developed quantitative system of sudomotor innervation we investigated the pathology of sweat gland innervation in diabetes and its clinical significance in relation to 1) glycemic control and 2) functional parameters of autonomic neuropathy.
RESEARCH DESIGN AND METHODS
The study subjects consisted of type 2 diabetic polyneuropathy patients referred to National Taiwan University Hospital. The diagnosis of type 2 diabetes was made according to the revised American Diabetes Association recommendations (1,8,22). Diabetic neuropathy was defined according to 1) symmetrical sensory symptoms in the foot with a distribution of graded stocking pattern, 2) abnormal results of nerve conduction studies, and 3) reduced IENF density on skin biopsy from distal leg. All patients had completed blood tests to exclude other etiologies of neuropathy (23). Neurologic examinations followed routine procedures, and physical examinations including blood pressure measurements during posture change and palpation of feet and socks for moisture were performed on each patient. In total, there were 42 patients (26 males and 16 females) aged 56.64 ± 12.67 years (range 25.0–79.0), fulfilling the above diagnostic criteria of diabetic neuropathy. All patients received oral hypoglycemic agents and/or insulin and regularly visited the diabetes clinic once every 1–3 months to adjust the glycemic control according to the examinations of fasting and 2-h postprandial glucose levels and HbA1c. All data of glycemic control 3 years before the skin biopsy were analyzed for patients with a diabetes duration exceeding 3 years. For patients with a diabetes duration <3 years, all glycemic data before the skin biopsy were collected for analysis.
The definition of symptomatic dysautonomia was made according to physical examinations and a previously validated questionnaire (23), including the cardiovascular system (orthostatic hypotension documented with a reduction of >20 mmHg in systolic blood pressure and/or >10 mmHg in diastolic pressure on postural change), gastrointestinal system (persistent diarrhea or constipation with normal endoscopic findings), genitourinary system (impotence and urination difficulties without prostatic hypertrophy), sudomotor system (anhidrosis of the feet with dry skin on a hot day, during exercise, under stress, or after a hot bath), pupillary system (poor dark accommodation and/or photophobia), and secremotor system (documented dry eyes and dry mouth without anti-Sjögren syndrome antibodies). The presence of one of the above symptoms was classified as having symptomatic autonomic neuropathy.
For comparison of sweat gland innervation with the diabetic group, 42 age- and sex-matched subjects (26 males and 16 females, aged 54.76 ± 11.76 years; P = 0.48 for sex and P = 1 for age) were retrieved from our database (14). The study protocol was approved by the ethics committee of National Taiwan University Hospital. Informed consent was obtained from each subject before the procedures. Detailed descriptions of clinical assessments, skin biopsy procedures, nerve conduction studies, and cardiac autonomic function tests are presented in Supplementary Data.
Area-based morphometry of sweat gland innervation
We quantified sweat gland innervation on Congo red–counterstained sections (14). The methods were modified from the well-established protocols for quantification of sweat gland innervation (12,13). This newly established approach is an area-based morphometry and has been validated with stereology. The efficiency of this method has also been examined on a subset of the current diabetic cohort (35 patients), in whom SGIIs were only used for comparison with those of control subjects, and no further analysis (such as correlations with clinical manifestations, etc.) was performed (14). The details of the methods are described in Supplementary Data. Briefly, skin sections were immunostained with protein gene product 9.5 to demonstrate nerve fibers and then counterstained with Congo red to reveal sweat glands. All information on the slides was masked before quantification. The clinical identity was only decoded after the quantification procedures were completed. Sections were observed under a ×20 objective on a Leica DM2500 light microscope (Leica Microsystems, Wetzlar, Germany) equipped with a Leica DFC490 CCD (Leica Microsystems). For each sweat gland and its surrounding nerve fibers, an image of 2,176 × 1,632 pixels (with a resolution of 72 pixels per inch) was captured, and one pixel was equivalent to 353 μm at this magnification.
The acquired image was then opened with Photoshop CS3 (Adobe Systems, San Jose, CA) to quantitate sweat gland innervation. The areas of nerve fibers and sweat glands were measured in each section. We designated SGII of the selected sweat gland according to the following formula: SGII = nerve fiber area/sweat gland area × 100%, which represented the area of nerve fibers normalized to the area of a sweat gland. The mean of all SGIIs (three to seven sweat glands from three different sections for each subject) was defined as the SGII of a given subject. This approach had a high intrarater correlation coefficient (r = 0.99, P < 0.001) and interrater correlation coefficient (r = 0.99, P < 0.001). According to the normative data from 107 healthy subjects (55 males and 52 females, aged 49.54 ± 13.74 years [range 20.0–82.0]), SGIIs were related to sex (P < 0.001) but independent of age (P = 0.83), with mean ± SD normative values of 4.33 ± 1.32% (5th percentile value 1.58%) for males and 5.33 ± 1.41% (2.63%) for females.
Statistical analysis
Numerical variables are expressed as means ± SD and were compared using a t test if the data followed a Gaussian distribution. For variables not following a Gaussian distribution, data are expressed as median (range) and were analyzed with the nonparametric Mann-Whitney U test. A regression analysis was performed using the statistical software Stata (StataCorp, College Station, TX) and GraphPad Prism (GraphPad Software, San Diego, CA) to evaluate correlations between numerical variables. Categorical variables were analyzed with Fisher exact test. Receiver operating characteristic (ROC) curve analysis was performed with MedCalc (Mariakerke, Belgium) to determine the performance of SGII in the diagnosis of autonomic neuropathy and classifying patients with anhidrotic symptoms. Results were considered significant at P < 0.05.
RESULTS
Clinical data of patients
In the 42 diabetic patients, the duration of diabetes was 9.85 ± 5.97 years (range 1–23). Mean fasting and 2-h postprandial glucose levels and HbA1c for these patients were 163.80 ± 64.11 mg/dL, 244.10 ± 104.50 mg/dL, and 8.49 ± 2.47%, respectively. All patients had sensory symptoms with a stocking distribution and reduced IENF density in the distal leg (0.82 ± 0.18 fibers/mm) according to normative values of IENF density from our laboratory (Supplementary Data) (8). Regarding the autonomic dysfunction, 20 patients (47.6%) were classified as having symptomatic autonomic neuropathy, including 2 patients (4.76%) with cardiovascular dysautonomia, 13 (30.95%) with gastrointestinal dysautonomia, 5 (11.90%) with genitourinary dysautonomia, and 9 (21.43%) with anhidrosis of feet.
Pathologic demonstration of sweat gland denervation in diabetes
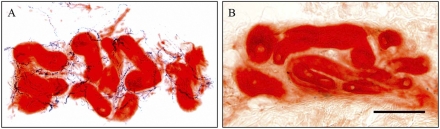
Nerve fibers exhibited linear and dense immunoreactivities (Fig. 1A) in the sweat glands of control subjects. In diabetic patients, there was significant reduction of nerve fibers innervating sweat glands and only a small portion of the entire sweat glands was surrounded by nerve fibers (Fig. 1B). A significant proportion of immunoreactive nerve fibers showed degeneration, i.e., became fragmented (6–8).
Figure 1.
Sweat gland innervation in control and diabetic subjects. A: In the skin of control subjects, distinct sweat glands (red) and characteristic periglandular innervation (dark blue) are noticeable. Sudomotor nerve fibers exhibit linear and dense immunoreactivities and form a reticulated pattern. B: In skin of diabetic subjects, only sparse and fragmented immunoreactive profiles (dark blue) surround sweat glands (red), reflecting nerve degeneration. Bar = 100 μm. (A high-quality digital representation of this figure is available in the online issue.)
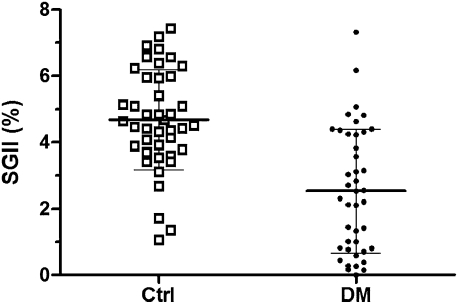
We quantitatively compared the above findings. The SGII in the diabetic group was significantly lower than that in age-and sex-matched control subjects (2.54 ± 1.87 vs. 4.68 ± 1.51%, P < 0.001 (Fig. 2).
Figure 2.
Sweat gland innervation in diabetes. Diabetic (DM) patients had a reduced SGII compared with age- and sex-matched control (Ctrl) subjects (2.54 ± 1.87 vs. 4.68 ± 1.51%, respectively; P < 0.001).
Sweat gland innervation and glycemic control
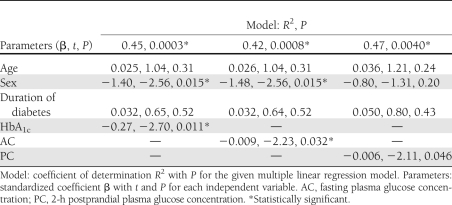
This study further investigated the effects of glycemic control on SGIIs by exploring the correlations between the SGII and glycemic parameters: HbA1c, the fasting plasma glucose concentration, and the 2-h postprandial plasma glucose concentration. Among these parameters, HbA1c (r = 0.42, P = 0.006), fasting plasma glucose concentration (r = 0.32, P = 0.042), and 2-h postprandial plasma glucose concentration (r = 0.46, P = 0.012) were all linearly correlated with the SGII. The above observations were further refined through a multiple linear regression analysis. In these models, the SGII was set as the dependent variable, with age, sex, diabetes duration, and one glycemic parameter as independent variables (Table 1). HbA1c and the fasting plasma glucose concentration were consistently correlated with the SGII. HbA1c was the diabetic parameter most significantly associated with the SGII (P = 0.011) compared with fasting and 2-h postprandial plasma glucose concentrations, suggesting that better glycemic control was associated with a higher SGII.
Table 1.
Correlation of SGII and glycemic parameters by multivariate analysis
SGII in the spectrum of diabetic autonomic neuropathies
We explored clinical significance of SGII in diabetic neuropathy: 1) the relationship with autonomic symptoms and 2) the spectrum of autonomic neuropathies. This study first examined the relationship of the SGII with clinical presentations. The SGII was lower in patients with symptomatic autonomic neuropathy than in those without autonomic symptoms (1.72 ± 1.54 vs. 3.28 ± 1.86%, respectively; P = 0.005) (Supplementary Table 1). We further refined the relationship of SGII with each individual autonomic symptom. Diabetic patients with anhidrosis of the feet had lower SGII than those with normal sweating of the feet (0.82 ± 0.69 vs. 3.00 ± 1.81%; P = 0.001). In contrast, SGII was not associated with autonomic symptoms in the cardiovascular (P = 0.18), gastrointestinal (P = 0.14), or genitourinary (P = 0.55) systems, indicating the specific association of SGII with sudomotor failure of the feet.
Impaired HRV was considered a clinical indicator of cardiac autonomic dysfunctions. In the current study, 51.7% had reduced HRV. In the patients with reduced HRV, 78.6% also had a reduced SGII. The results of the reduced SGII and cardiac autonomic dysfunction were concordant (P = 0.003 on Fisher exact test), and patients with reduced HRV also had a lower SGII than those with a normal HRV (1.21 ± 1.68 vs. 3.32 ± 1.47%, respectively; P = 0.001).
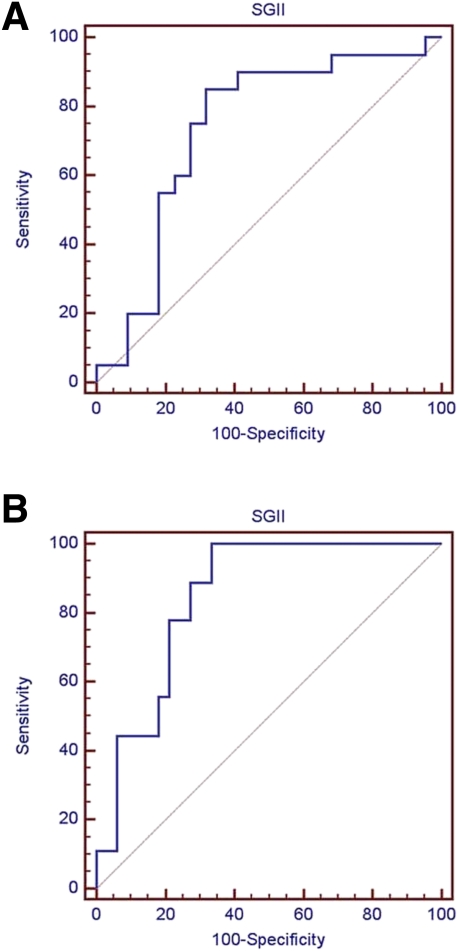
We used ROC curve analysis to evaluate the performance of SGII in diagnosing autonomic neuropathy (Fig. 3A). Area under the curve (AUC) was 0.736 (95% CI 0.578–0.860). The ROC curve analysis was then used to determine the utility of SGII in distinguishing diabetic patients with anhidrotic symptoms from those without such symptoms. AUC from this analysis was 0.845 (0.700–0.938), indicating good classification accuracy (Fig. 3B). The sympathetic skin response at the sole (SSR) has been a surrogate marker of sudomotor function tests. We further compared SGII and SSR in classifying patients with anhidrotic symptoms. With a cutoff of 1.01% in SGII, the diagnostic performance of SGII was higher than SSR in terms of sensitivity (77.8 vs. 50.0%) and specificity (78.8 vs. 74.1%).
Figure 3.
ROC curve analysis of SGII. The graphs show the ROC curves of SGII for diagnosing autonomic neuropathy (A) and for classifying diabetic patients with anhidrotic symptoms (B). A: The AUC was 0.736 (95% CI 0.578–0.860) for autonomic neuropathy. B: The AUC was 0.845 (0.700–0.938) for anhidrotic symptoms. (A high-quality color representation of this figure is available in the online issue.)
CONCLUSIONS
Glycemic control and sweat gland innervation
This is the first study to document the correlation of HbA1c with the SGII, suggesting the potential influence of diabetes control on sweat gland innervation in type 2 diabetes. A previous large-scale study indicated that tight control of diabetes was beneficial to nerve conduction parameters in type 1 diabetes (15). Nerve conduction studies, however, only assess large fibers. Sudomotor neuropathy has traditionally been evaluated using the sympathetic skin response or functional tests in diabetes (2,24,25). Unclear is whether the pathology of small fibers is influenced by glycemic control. According to our analyses with a multiple linear regression, SGIIs were linearly correlated with parameters of glycemic control, and HbA1c was the most significant factor associated with SGII. Because dry skin is a risk factor for skin ulceration and limb amputation, this relationship may indicate a previously underrecognized issue in the care of diabetic patients to prevent injuries to the skin. A prospective study is necessary to establish this relationship, and the current study provides a foundation to examine this issue.
In the current study, there was no correlation between SGII and diabetes duration, although some previous studies showed association between diabetes duration and sudomotor impairment by functional tests (26,27). In previous studies with attempts to quantify the sweat gland or pilomotor innervations on skin biopsies from diabetic patients, there was no correlation between the parameters of innervation and diabetes duration (12,28). Some possible explanations for these findings are as follows: 1) the coexistence of nerve degeneration and regeneration, 2) the simultaneous changes in the volume of sweat glands, and 3) the size of the study cohort. Further prospective study is necessary to elucidate this relationship.
Sudomotor denervation as a parameter of autonomic neuropathy
This study documents sudomotor denervation in type 2 diabetes by demonstrating reduced periglandular nerve fibers in skin biopsies as structural evidence. In addition to assessing IENF density in diabetes (8,9), the current report shows that sweat gland innervation on the same skin biopsy section illustrates another dimension of diabetic neuropathy and expands applications of skin biopsies.
Autonomic symptoms are diverse, encompassing from the cardiovascular system to the sudomotor system (1,3). Previously, most studies have depended on functional tests to diagnose autonomic neuropathy (1,3,24). Except for limited functional sudomotor tests such as the quantitative sudomotor axon reflex test and the dynamic sweat test (24,25,29), the diagnosis of autonomic neuropathy was previously made on the assumption that dysautonomic signs such as reduced sweating are due to postganglionic nerve degeneration. This report provides structural evidence of sweat gland denervation in diabetes.
Our ROC analysis demonstrated the potential utility of SGII in the diagnosis of autonomic neuropathy. In diabetic patients with anhidrotic symptoms, SGII had a classification accuracy superior to that of a functional sudomotor test, SSR. This suggests that pathological changes in sweat gland innervations closely follow sudomotor symptoms, and SGII could combine with functional sudomotor parameters to provide a more comprehensive assessment of the severity of sudomotor neuropathy.
The current study does not exclude the possible involvement of sweat glands themselves in diabetes; however, it provides opportunities to dissect the pathophysiological basis of anhidrosis, which can lead to new therapies at different levels of nerve fibers and sweat glands. In addition to functional studies (17,30), the addition of this pathologic approach will provide an integrated assessment of sweat gland innervation in autonomic neuropathy of diabetes.
In the current study, there was no association between SGII and autonomic symptoms in cardiovascular, gastrointestinal, or genitourinary systems. This finding suggests the independent involvement of different autonomic systems in diabetes. In our study, symptomatic autonomic neuropathy was defined by the specified dysautonomic symptoms and signs. The manifestations of dysautonomia, however, are usually diffuse and variable (3). This limitation might underestimate the autonomic dysfunctions in our patients, which could be responsible for the absence of the association.
Spectra of diabetic neuropathies
Diabetic autonomic neuropathies encompass a wide range of clinical presentations (1). For example, cardiac autonomic dysfunction is a risk factor for cardiovascular diseases in diabetes (3,17) and is usually diagnosed according to impaired HRV (1,18). We investigated the spectrum of autonomic neuropathies in diabetes by comparing the comorbidity of cardiac autonomic dysfunction and sudomotor denervation. There were parallel changes in abnormal HRV and reduced sweat gland innervation; i.e., diabetic patients with cardiac autonomic dysfunction had lower SGIIs. Taken together, these findings indicate that subclinical involvement of different components of autonomic neuropathy exists in type 2 diabetes and indicates the necessity of performing multimodality examinations for diabetic autonomic neuropathy (1,30).
Acknowledgments
This work was supported by the National Science Council (NSC97-2320-B-002-042-MY3), National Health Research Institute (NHRI-EX99-9736NI), and Translational Medicine Project of National Taiwan University College of Medicine and National Taiwan University Hospital (99C101-201).
No potential conflicts of interest relevant to this article were reported.
K.-R.L. researched data, contributed to discussion, and wrote the manuscript. C.-C.C. researched data and contributed to discussion. P.-C.H. researched data and wrote the manuscript. J.-H.L. and S.-T.H. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. S.-T.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1607/-/DC1.
References
- 1.Tesfaye S, Boulton AJ, Dyck PJ, et al. ; Toronto Diabetic Neuropathy Expert Group Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tentolouris N, Marinou K, Kokotis P, Karanti A, Diakoumopoulou E, Katsilambros N. Sudomotor dysfunction is associated with foot ulceration in diabetes. Diabet Med 2009;26:302–305 [DOI] [PubMed] [Google Scholar]
- 3.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003;26:1553–1579 [DOI] [PubMed] [Google Scholar]
- 4.Vallat JM, Vital A, Magy L, Martin-Negrier ML, Vital C. An update on nerve biopsy. J Neuropathol Exp Neurol 2009;68:833–844 [DOI] [PubMed] [Google Scholar]
- 5.Panoutsopoulou IG, Wendelschafer-Crabb G, Hodges JS, Kennedy WR. Skin blister and skin biopsy to quantify epidermal nerves: a comparative study. Neurology 2009;72:1205–1210 [DOI] [PubMed] [Google Scholar]
- 6.Kennedy WR, Wendelschafer-Crabb G. Innervation of nonglabrous skin. In Clinical Autonomic Disorders. 3rd ed. Low PA, Benarroch EE, Eds. Philadelphia, Lippincott Williams & Wilkins, 2008, p. 264–271 [Google Scholar]
- 7.Reinisch CM, Traxler H, Piringer S, Tangl S, Nader A, Tschachler E. Rarefaction of the peripheral nerve network in diabetic patients is associated with a pronounced reduction of terminal Schwann cells. Diabetes Care 2008;31:1219–1221 [DOI] [PubMed] [Google Scholar]
- 8.Shun CT, Chang YC, Wu HP, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain 2004;127:1593–1605 [DOI] [PubMed] [Google Scholar]
- 9.Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain 2004;127:1606–1615 [DOI] [PubMed] [Google Scholar]
- 10.Hilz MJ, Axelrod FB, Bickel A, et al. Assessing function and pathology in familial dysautonomia: assessment of temperature perception, sweating and cutaneous innervation. Brain 2004;127:2090–2098 [DOI] [PubMed] [Google Scholar]
- 11.Donadio V, Montagna P, Nolano M, et al. Generalised anhidrosis: different lesion sites demonstrated by microneurography and skin biopsy. J Neurol Neurosurg Psychiatry 2005;76:588–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbons CH, Illigens BM, Wang N, Freeman R. Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology 2009;72:1479–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbons CH, Illigens BM, Wang N, Freeman R. Quantification of sudomotor innervation: a comparison of three methods. Muscle Nerve 2010;42:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo KR, Chao CC, Chen YT, et al. Quantitation of sudomotor innervation in skin biopsies of patients with diabetic neuropathy. J Neuropathol Exp Neurol 2011;70:930–938 [DOI] [PubMed] [Google Scholar]
- 15.Charles M, Soedamah-Muthu SS, Tesfaye S, et al. ; EURODIAB Prospective Complications Study Investigators Low peripheral nerve conduction velocities and amplitudes are strongly related to diabetic microvascular complications in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 2010;33:2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spallone V, Morganti R, D’Amato C, et al. Clinical correlates of painful diabetic neuropathy and relationship of neuropathic pain with sensorimotor and autonomic nerve function. Eur J Pain 2011;15:153–160 [DOI] [PubMed] [Google Scholar]
- 17.Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care 2010;33:434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007;115:387–397 [DOI] [PubMed] [Google Scholar]
- 19.Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE. Natural history of diabetic gastroparesis. Diabetes Care 1999;22:503–507 [DOI] [PubMed] [Google Scholar]
- 20.Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH; EURODIAB Prospective Complications Study Group Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008;31:1360–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pop-Busui R, Evans GW, Gerstein HC, et al. ; Action to Control Cardiovascular Risk in Diabetes Study Group Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:1578–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2009;32(Suppl. 1):S62–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang NC, Lee MJ, Chao CC, et al. Clinical presentations and skin denervation in amyloid neuropathy due to transthyretin Ala97Ser. Neurology 2010;75:532–538 [DOI] [PubMed] [Google Scholar]
- 24.Provitera V, Nolano M, Caporaso G, Stancanelli A, Santoro L, Kennedy WR. Evaluation of sudomotor function in diabetes using the dynamic sweat test. Neurology 2010;74:50–56 [DOI] [PubMed] [Google Scholar]
- 25.Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve 2006;34:57–61 [DOI] [PubMed] [Google Scholar]
- 26.Kamenov ZA, Petrova JJ, Christov VG. Diagnosis of diabetic neuropathy using simple somatic and a new autonomic (neuropad) tests in the clinical practice. Exp Clin Endocrinol Diabetes 2010;118:226–233 [DOI] [PubMed] [Google Scholar]
- 27.Levy DM, Reid G, Rowley DA, Abraham RR. Quantitative measures of sympathetic skin response in diabetes: relation to sudomotor and neurological function. J Neurol Neurosurg Psychiatry 1992;55:902–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolano M, Provitera V, Caporaso G, Stancanelli A, Vitale DF, Santoro L. Quantification of pilomotor nerves: a new tool to evaluate autonomic involvement in diabetes. Neurology 2010;75:1089–1097 [DOI] [PubMed] [Google Scholar]
- 29.Illigens BM, Gibbons CH. Sweat testing to evaluate autonomic function. Clin Auton Res 2009;19:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diem P, Laederach-Hofmann K, Navarro X, Mueller B, Kennedy WR, Robertson RP. Diagnosis of diabetic autonomic neuropathy: a multivariate approach. Eur J Clin Invest 2003;33:693–697 [DOI] [PubMed] [Google Scholar]