Abstract
OBJECTIVE
To examine the relationship between retinal vascular geometry parameters and development of incident renal dysfunction in young people with type 1 diabetes.
RESEARCH DESIGN AND METHODS
This was a prospective cohort study of 511 adolescents with type 1 diabetes of at least 2 years duration, with normal albumin excretion rate (AER) and no retinopathy at baseline while attending an Australian tertiary-care hospital. AER was quantified using three overnight, timed urine specimen collections and early renal dysfunction was defined as AER >7.5 μg/min. Retinal vascular geometry (including length-to-diameter ratio [LDR] and simple tortuosity [ST]) was quantified from baseline retinal photographs. Generalized estimating equations were used to examine the relationship between incident renal dysfunction and baseline venular LDR and ST, adjusting for age, diabetes duration, glycated hemoglobin (A1C), blood pressure (BP), BMI, and cholesterol.
RESULTS
Diabetes duration at baseline was 4.8 (IQR 3.3–7.5) years. After a median 3.7 (2.3–5.7) years follow-up, 34% of participants developed incident renal dysfunction. In multivariate analysis, higher retinal venular LDR (odds ratio 1.7, 95% CI 1.2–2.4; quartile 4 vs. 1–3) and lower venular ST (1.6, 1.1–2.2; quartile 1 vs. 2–4) predicted incident renal dysfunction.
CONCLUSIONS
Retinal venular geometry independently predicted incident renal dysfunction in young people with type 1 diabetes. These noninvasive retinal measures may help to elucidate early mechanistic pathways for microvascular complications. Retinal venular geometry may be a useful tool to identify individuals at high risk of renal disease early in the course of diabetes.
A diffuse endotheliopathy, affecting all organ systems, contributes to microvascular complications in diabetes. Diabetic nephropathy (DN) begins soon after diabetes diagnosis with a possible genetic predisposition (1). DN is the leading cause of end-stage renal disease (ESRD) in Western countries and accounts for >40% of ESRD in the U.S. In addition, DN is associated with greater risk of severe visual impairment, stroke, and cardiac death. However, early markers that predict subsequent development of ESRD remain elusive. Microalbuminuria (albumin excretion rate [AER] >20 μg/min), which is uncommon in young adolescents with type 1 diabetes early in the course of disease, is a well-established predictor of progression to overt nephropathy. Lower thresholds for early renal dysfunction (AER >7.5 μg/min) have been clearly demonstrated (2–4) as predictors of microalbuminuria. A better understanding of early microvascular changes in diabetes may allow early identification, prevention, and treatment of those at risk for ESRD and its comorbidities. Thus, surrogate measures of risk need to be explored in young people early in the course of diabetes.
There is emerging evidence that retinal vascular changes, such as retinal vascular diameter, are associated with diabetes complications including retinopathy, coronary artery disease, and stroke (5–7), supporting the view that retinal vascular changes may be early markers of systemic endothelial damage (8). In adults with type 1 diabetes, the incidence of gross proteinuria was associated with wider retinal venules, but not arterioles (9).
Retinal vessel diameter represents only a single parameter of the retinal vasculature, which is dynamic and influenced by ambient glycemia particularly early in diabetes (10), whereas longer diabetes duration (>5 years) is associated with wider vascular diameters (11–13). Therefore, parameters such as vessel tortuosity and length-to-diameter ratio (LDR) may provide greater information on the pathobiology of complications early in the course of disease. We recently demonstrated that arteriolar LDR and tortuosity predicted incident diabetic retinopathy in adolescents with type 1 diabetes (14). However, the relationship between these geometric parameters and DN has not been previously studied.
We hypothesized that changes in retinal venular LDR (LDRv) and simple tortuosity (STv) may predict early incident renal dysfunction. In this study we examined whether retinal vascular geometry in young people with type 1 diabetes, free of retinopathy and with normal renal function at baseline assessment, predicted subsequent incident renal dysfunction.
RESEARCH DESIGN AND METHODS
We conducted a prospective cohort study in 951 young people aged 12–20 years with type 1 diabetes of minimum 2 years duration at a tertiary hospital in Australia between 1990 and 2007 as previously described (14). All were free of retinopathy at baseline and had a minimum of two diabetes complication assessments. The number of people with baseline photographs suitable for grading was 736 (77%). No baseline urine samples were available for 70 (10%) who were excluded from the study. A1C in those without urine samples at baseline was higher (8.9 vs. 8.5%; P = 0.04) but no other baseline differences were observed. Thus, 666 participants qualified for the study. Of these, 511 had normal AER at baseline (AER ≤7.5 μg/min) and 155 had elevated AER. Predictive models were based on the 511 with normal AER. Baseline characteristics are described for all 666 participants (Table 1). Participants were prospectively followed using standardized interviews, examinations, and laboratory investigations at each visit as previously described (15). The study was approved by The Children’s Hospital at Westmead Ethics Committee, and written informed consent was obtained from all participants.
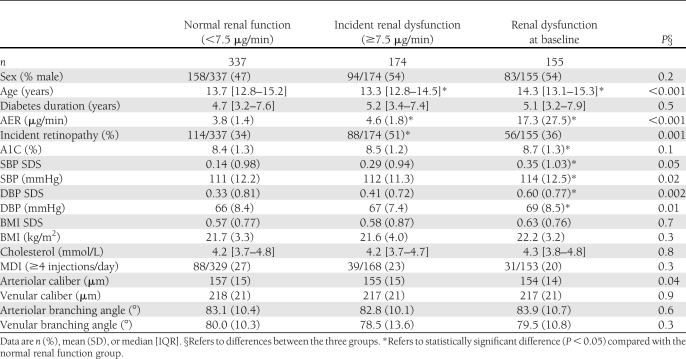
Table 1.
Baseline characteristics by renal function status (AER >7.5 μg/min) through the study period
Definition of early renal dysfunction
Incident renal dysfunction was defined as an AER >7.5 μg/min from three overnight, timed urine collections. This cutoff is above the 95th percentile of the normal adolescent population and predictive of microalbuminuria (3,4). Urinary albumin was measured using a polyclonal radioimmunoassay (Pharmacia RIA, Uppsala, Sweden) from 1997 to March 2000, changed to nephelemetry (IMMAGE analyzer; Beckman Coulter, Sydney, Australia) until 2003, and then changed to Immulite analyzer (Siemans, Los Angeles, CA) from 2004 onwards. A cross-validation analysis found no significant differences or systematic bias between methods in the estimation of urinary albumin concentration. The slope of the fitted regression between the values obtained from each of the assays did not differ significantly from unity, nor did an intercept term differ significantly from zero.
Retinal photography
Mydriatic seven-field stereoscopic fundal photography was performed using a Topcon Fundus Camera (TRC50-VT; Tokyo-Optical, Tokyo, Japan) using film and, subsequently, digital photography (16). Changeover from film to digital photography took place in September 2004. Camera settings, including angle of retinal photography, remained unchanged. All film images prior to September 2004 were individually scanned in a high-resolution scanner (3071 × 2048 pixels) in raw format to avoid pixel and color compression (CanoScan FS2710; Canon, Tokyo, Japan). Each image was selected and deemed appropriate for retinal vascular geometry grading by a single grader (M.B.S.). Digitized images were used to assess baseline retinal vascular geometry. Retinopathy was assessed by a single ophthalmologist masked to participants’ clinical characteristics, according to the modified Airlie House classification (12). Incident retinopathy was defined as at least one microaneurysm/hemorrhage in either eye (Early Treatment of Diabetic Retinopathy Study [ETDRS] level 21, minimal nonproliferative diabetic retinopathy or greater).
Retinal vascular geometry
Retinal vascular geometry was analyzed from baseline retinal photographs. All slides were individually assessed for grading suitability. Right-eye, digitized retinal photographs of each patient were analyzed by a single grader (M.B.S.), masked to participants’ characteristics, using a semiautomated, computer-assisted image program (Singapore I Vessel Assessment or SIVA) as previously described (14,15). The grader ensured that the vessel type selected by the program was correct. The software then combined the individual measurements into summary indices:
LDR was calculated as the length from the midpoint of the first branch to the midpoint of the second branch, divided by the diameter of the parent vessel at the first branch.
ST was calculated as the ratio between the actual path length of the vessel segment (measured by tracking) and the straight-line length of the same segment.
Retinal vessel caliber measurements, represented by the central retinal arteriolar equivalent (CRAE) and the central retinal venular equivalent (CRVE), were calculated as previously described (17).
Branching angle in degrees (°) represented the angle between two daughter vessels (18).
For quality control of retinal vascular measures, we performed intragrader reliability analysis on 120 randomly selected images to which the grader was masked. The intragrader correlation coefficients (ICCs) for each parameter were as follows: LDRv 0.80, arteriolar LDR 0.84, STv 0.81, arteriolar ST 0.96, CRAE 0.79, CRVE 0.89, arteriolar branching angle 0.65, and venular branching angle 0.4.
Statistical analysis
The population was classified into three groups: 1) normal renal function throughout the study (n = 337); 2) incident renal dysfunction (n = 174), and 3) renal dysfunction at baseline (n = 155). The latter group was excluded from multivariate analyses exploring predictors of incident nephropathy, leaving 511 participants. Descriptive statistics are presented as mean and SD for normally distributed data or median and IQR for skewed distributions. ANOVA was used to determine differences between renal function groups with Tukey test for post hoc analysis for normally distributed variables. The Kruskal-Wallis test was used to examine between group differences for skewed data, and differences in categorical variables were compared using the χ2 test (Table 1). The effect of diabetes duration on retinal vascular measures was explored by comparing groups with shorter (≤5 years) and longer (>5 years) diabetes duration using independent sample Student t tests (Table 2). Cox proportional hazard regression analysis was used to study threshold effects for LDR and ST using quartiles as predictors of time to development of early renal dysfunction and retinopathy.
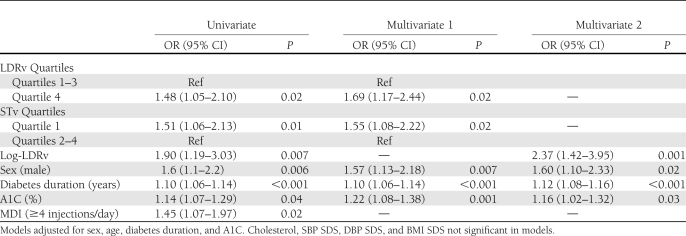
Table 2.
LDRv and STv predict incident renal dysfunction. Predictive GEE univariate and multivariate models
Longitudinal analysis was performed using generalized estimating equations (GEEs) with presence of renal dysfunction at the respective visit as the outcome variable. Predictive models were fitted to explore the association between retinal vascular geometry and development of renal dysfunction (AER >7.5 μg/min) for those with normal renal function at baseline (n = 511). The use of GEE allowed all visits to be included in the analysis and accounted for correlations between repeated observations for a given patient. Age, sex, diabetes duration, A1C, systolic blood pressure (SBP) SDS, diastolic blood pressure (DBP) SDS, BMI SDS, total cholesterol, and insulin therapy intensity (as a binary variable multiple daily injections (MDIs) greater than or equal to four insulin injections per day versus less than four) were included in the models as explanatory variables. Baseline arteriolar and venular ST and LDR were log transformed for analysis as continuous variables and categorized into quartiles to examine for threshold effects. Relevant interaction terms were included in the models. Results of significant variables in GEE models are reported as odds ratio (OR) and 95% CIs, and results of Cox regression are reported as hazard ratio (HR) and 95% CI. Statistical procedures were performed using PASW version 18.0 (2009 SPSS Inc., Chicago, IL).
RESULTS
At baseline, median age was 13.5 (IQR 12.8–14.9) years and diabetes duration 4.8 (3.3–7.5) years. Baseline characteristics stratified by renal function status are shown in Table 1. Those with renal dysfunction at baseline were older and had higher AER, A1C, SBP, and DBP than the other groups (Table 1).
Overall baseline CRAE was smaller in those who had renal dysfunction at baseline, whereas CRVE was not significantly different. In the incident renal dysfunction group, arteriolar branching angle was significantly greater among those with longer diabetes duration, whereas venular branching angles did not differ (Table 1).
Of the 511 participants with normal AER at baseline, median follow-up was 3.7 (IQR 2.3–5.7) years, with 3.0 (2.0–4.0) visits per participant and 1,898 clinical assessments. Incident renal dysfunction was evident in 34%; those who developed incident renal dysfunction had higher mean baseline AER (P < 0.001) and were generally younger (P = 0.02) than those who had normal AER throughout follow-up. No significant differences were observed with respect to sex distribution, diabetes duration, BP SDS, BMI SDS, insulin therapy intensity, or glycemic control (A1C) between these groups (Table 1). A greater proportion of those who developed incident renal dysfunction also developed incident retinopathy (P < 0.001). There was no significant correlation between LDRv and STv (Pearson correlation 0.04, P = 0.4).
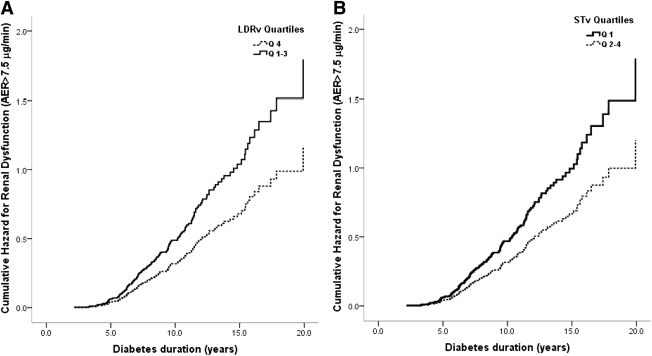
In Cox proportional hazard regression analysis (n = 511), LDRv in the highest quartile (HR 2.0 [95% CI 1.3–2.9]), STv in the lowest quartile (1.5 [1.1–2.1]), and higher DBP SDS and A1C were associated with a higher cumulative risk of early renal dysfunction (Fig. 1). There were no significant differences in risk between quartiles 1–3 for LDRv and quartiles 2–4 for STv.
Figure 1.
A: Cumulative hazard curves for risk of early renal dysfunction by LDRv quartiles: quartile 4 vs. quartiles 1–3 (HR 2.0 [95% CI 1.3–2.9]) adjusted for sex, A1C, and DBP SDS. B: Cumulative hazard curves for risk of early renal dysfunction by STv: quartile 1 vs. quartiles 2–4 (1.5 [1.1–2.1]) adjusted for sex, A1C, and DBP SDS.
In univariate GEE analysis (n = 511), log-LDRv, as a continuous variable, predicted incident renal dysfunction (OR 1.9), whereas log-STv as a continuous variable, was not statistically significant (OR 1.00 [95% CI 0.98–1.01]; P = 0.4). When analyzing for a threshold effect, those in the highest LDRv quartile (OR 1.5) and lowest STv quartile (OR 1.5) were at greater risk of incident renal dysfunction. Other significant risk factors for incident renal dysfunction included longer diabetes duration, higher A1C, and male sex (Table 2).
In multivariate GEE analysis, log-LDRv predicted incident renal dysfunction (OR 2.4) after adjusting for age, sex, diabetes duration, A1C, DBP SDS, BMI, and total cholesterol. When analyzing for a threshold effect, those in the highest LDRv quartile (OR 1.7) and lowest STv quartile (OR 1.6) were at greater risk of incident renal dysfunction (Table 2).
CONCLUSIONS
In this longitudinal study of young people with type 1 diabetes, normal AER, and no retinopathy at baseline, greater retinal venular LDR and lower venular tortuosity predicted incident renal dysfunction independent of established risk factors including diabetes duration, glycemic control, BP, and total cholesterol. We also confirmed our recent findings that lower arteriolar LDR and greater arteriolar tortuosity were associated with incident retinopathy (data not shown) (14).
We recently proposed that, in the diabetic milieu, neuroretinal hypoxia leads to a compensatory increase in afferent blood flow through increased vessel density (earlier arteriolar branching resulting in lower arteriolar LDR) and a corresponding increase in arteriolar tortuosity (14). Although this described the afferent changes in blood flow, it is important to understand the adaptations at the efferent (venular) end and their significance.
Whereas arteriolar changes may best represent localized organ tissue changes (14,15), venular changes may better reflect systemic compensation and maladaptation. Determinants of resistance to blood flow within a vascular network include the individual blood vessel size (caliber and length), the organization of the vascular network (series and parallel), and the flow characteristics (laminar versus turbulent). Although the microcirculation provides most of the systemic vascular resistance, it has low Reynolds numbers and therefore turbulence at this level is unlikely to occur. In this setting, hydrostatic and oncotic pressures, viscosity, and wall shear stress would be most influential. Hyperglycemia causes significant hemodynamic and rheological changes (19), including increased retinal blood flow (20) and blood viscosity (19). Furthermore, changes to particulate components of blood include greater platelet aggregation (19) and increased leukocyte numbers with higher integrin-mediated adhesion properties promoting leukostasis (11). We propose that decreased venular network complexity together with early vascular tone dysregulation and non-Newtonian influences contribute to increased capillary hydrostatic pressure, greater wall shear stress, and vessel damage. Thus, individuals with a simplified venular network may be at greater risk of microvascular complications such as renal dysfunction.
Baseline characteristics (Table 1) demonstrated overall narrower arteriolar calibers in those with renal dysfunction. However, longer diabetes duration was associated with wider vessel calibers (both arteriolar and venular). This may describe the natural history of microvascular changes in diabetes: 1) early impairment of microvascular autoregulation with inappropriate vasoconstriction, 2) subsequent compensatory dilation, 3) loss of smooth muscle cells and pericytes (or podocytes in the kidney), and 4) loss of wall structural integrity and, finally, irreversible dilation. Early hyperglycemia results in a downregulation of large-conductance, calcium-activated potassium channels necessary for vascular smooth muscle cell relaxation, leading to early vasoconstriction and neuroretinal hypoxia (21). Diabetic rat models demonstrated a similar dysregulation in the renal microvasculature early in diabetes with severe insulin deficiency. It has been speculated that tissue hypoxia beyond a certain threshold may override the vasoconstrictive effect of diabetes (12). Finally, large population-based studies associated wider retinal venules with proteinuria in adults with type 1 diabetes of longer duration (13).
Changes in vessel caliber are most influential in the acute regulation of blood flow. According to the Poiseuille equation, vessel resistance is inversely proportional to the vessel radius to the fourth power (r4). Thus, even small changes in the diameter of arterioles and venules can lead to significant changes in blood flow and capillary pressures. Capillary hypertension has been associated with both retinopathy (12) and nephropathy (22). Classical renal micropuncture studies demonstrated that glomerular hypertension ensued in diabetes models even in the setting of normal BP (23). Diabetes led to an impairment of glomerular circulatory autoregulation, with greater vasodilatation of the afferent arteriole than the efferent arteriole, which resulted in greater intraglomerular capillary pressure (23). Ultimately, this exposes the glomeruli to increased intracapillary pressure, increased shear stress, and mechanical stretch. Mechanical stretch has been observed to favor an accumulation of extracellular matrix (24) and a decrease in podocyte number through effects on cell proliferation, apoptosis, and cell adhesion to the basement membrane (22). In this setting, even minor increments in systemic BP would exacerbate glomerular hypertension in a self-perpetuating cycle of ongoing hypertensive injury with impaired microvascular autoregulation. The efficacy of ACE inhibitors in retarding the progression of DN may in part be due to their vasodilatory effect on the efferent glomerular arteriole, thus decreasing glomerular capillary pressure (25).
Although we observed higher BP in those with baseline renal dysfunction (Table 1), BP measured at each visit did not predict renal dysfunction in GEE longitudinal analysis (Table 2). A longer follow-up period may be required to examine this relationship with adequate statistical power.
In our study, A1C was an independent predictor of early renal dysfunction in keeping with previous reports (26). Loss of capillary autoregulation, higher glomerular capillary pressure, and decrease in podocytes are related to the severity and chronicity of hyperglycemia (22).
This study of normotensive young people with no intercurrent medications provides an insight into the preclinical renal dysfunction associated with diabetes and may assist in identifying those at greatest risk where early intervention may yield greatest benefit. The lack of a statistically significant association between BP and incident renal dysfunction in our multivariate models contrasts with previous findings regarding retinopathy (27). It is noteworthy that a significantly greater proportion of those who developed incident renal dysfunction also developed incident retinopathy. Thus, changes in BP may only become apparent later in the course of renal disease where capillary bed autoregulation may no longer be able to compensate for metabolic demands. This also suggests that retinal hypoxic demands may have a greater influence in BP autoregulation than changes in renal microvasculature. This is consistent with our previous observations between BP and retinopathy in young people with type 1 diabetes (27).
Our findings suggest that a simplified venular network (i.e., greater LDRv and lower STv) results in a greater risk of early renal dysfunction. As previously described, lower LDR can result from shorter axial length due to earlier branching points and/or wider vessel calibers (14).
Our current software does not report vessel length; however, our results suggest venular axial length was increased in view of the wider venules observed with longer diabetes duration. These early venular differences may represent greater tissue metabolic demands, mechanical stress, and an increased propensity for early nephropathy. Our measures of tortuosity and LDR had good reproducibility; however, branching angles are particularly vulnerable to parallax error as reflected in the relatively low intraobserver correlation. Not unexpectedly, therefore, there were no significant associations in GEE models between branching angles and renal dysfunction.
The strengths of this study include the longitudinal design, a large patient cohort with multiple visits per individual, and standardized, quantitative evaluation of retinal vascular measures by a single grader masked to participants’ clinical status. Our young cohort without comorbidities avoids confounders present in older groups with established complications and medical therapy. The use of GEEs allowed inclusion of every available patient visit in the analysis and accounts for uneven follow-up and missing data. The reproducibility of LDR and ST measures (ICCs ranging from 0.80 to 0.96) was comparable with retinal vascular caliber measurements in the Atherosclerosis Risk on Community study (ICCs ranging from 0.79 to 0.83) (28). Repeated longitudinal LDR and ST measures would strengthen this study. This cohort from a tertiary referral center may be biased toward closer monitoring, tighter metabolic control, and, thus, underrepresentation of early renal dysfunction; conversely, this underscores the robust nature of the measures studied.
Limitations of our measurements include the use of the central retinal field only and the lack of reporting absolute values for vessel length. Therefore, we cannot generalize our findings to the whole microvascular bed involving peripheral retinal areas that need further study both structurally and functionally. The spherical nature of the retinal surface cannot be accurately assessed in two-dimensional photographs and has an inherent degree of parallax error when evaluating branching angles. Nevertheless, our results demonstrate the potential utility of novel quantitative measurements of retinal vascular geometry from this central field, which is practical and reproducible, in people with diabetes.
In summary, retinal vascular geometry parameters, specifically retinal venular LDR and tortuosity, are independent predictors of incident renal dysfunction in young people with type 1 diabetes. These noninvasive retinal measures may further our understanding of early mechanistic pathways for microvascular complications. Functional studies examining blood flow would complement these findings. Future studies replicating these findings in other populations are needed. Our study highlights the role of venular LDR and tortuosity as tools for risk stratification of individuals at high risk for microvascular complications, and in monitoring of disease progression and therapeutic benefits.
Acknowledgments
This study was supported by the National Health and Medical Research Council (475605) and the Juvenile Diabetes Research Foundation (5-2008-274).
No potential conflicts of interest relevant to this article were reported.
P.Z.B.-A. wrote the manuscript, analyzed data, and digitized photos for grading. M.B.S. graded photographs and reviewed the manuscript. M.E.C. advised on statistical analysis, contributed to the introduction, and reviewed the manuscript. A.J.J. reviewed the manuscript and the conclusions, and contributed to discussion. J.C. contributed to data collection and retrieval. N.C. critically reviewed the manuscript and contributed to the research design and methods. T.Y.W. contributed to the study design and research design and methods and reviewed the manuscript. K.C.D. contributed to the study design, introduction, data analysis, and discussion; reviewed the manuscript; and reviewed and contributed to the conclusions. As the corresponding author and guarantor of this article, K.C.D. takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Preliminary data from this study was presented at the Asia Pacific Pediatric Endocrine Society (APPES) biennial meeting, Xi'an, China, 17–20 November 2010. Parts of this study were presented in abstract form at the 37th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes, Miami, Florida, 19–22 October 2011.
References
- 1.Schultz CJ, Neil HA, Dalton RN, Dunger DB; Oxforn Regional Prospective Study Group Risk of nephropathy can be detected before the onset of microalbuminuria during the early years after diagnosis of type 1 diabetes. Diabetes Care 2000;23:1811–1815 [DOI] [PubMed] [Google Scholar]
- 2.Stone ML, Craig ME, Chan AK, Lee JW, Verge CF, Donaghue KC. Natural history and risk factors for microalbuminuria in adolescents with type 1 diabetes: a longitudinal study. Diabetes Care 2006;29:2072–2077 [DOI] [PubMed] [Google Scholar]
- 3.Chase HP, Marshall G, Garg SK, Harris S, Osberg I. Borderline increases in albumin excretion rate and the relation to glycemic control in subjects with type I diabetes. Clin Chem 1991;37:2048–2052 [PubMed] [Google Scholar]
- 4.Couper JJ, Clarke CF, Byrne GC, et al. Progression of borderline increases in albuminuria in adolescents with insulin-dependent diabetes mellitus. Diabet Med 1997;14:766–771 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TT, Wang JJ, Wong TY. Retinal vascular changes in pre-diabetes and prehypertension: new findings and their research and clinical implications. Diabetes Care 2007;30:2708–2715 [DOI] [PubMed] [Google Scholar]
- 6.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010;376:124–136 [DOI] [PubMed] [Google Scholar]
- 7.Cheung N, Rogers SL, Donaghue KC, Jenkins AJ, Tikellis G, Wong TY. Retinal arteriolar dilation predicts retinopathy in adolescents with type 1 diabetes. Diabetes Care 2008;31:1842–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awua-Larbi S, Wong TY, Cotch MF, Durazo-Arvizu R, Jacobs DR, Jr, Klein BE, Klein R, Lima J, Liu K, Kramer H. Retinal arteriolar caliber and urine albumin excretion: the multi-ethnic study of atherosclerosis. Nephrol Dial Transplant 2011;26:3523–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong TY, Coresh J, Klein R, et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol 2004;15:2469–2476 [DOI] [PubMed] [Google Scholar]
- 10.Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci 1996;37:886–897 [PubMed] [Google Scholar]
- 11.Patel JI, Saleh GM, Hykin PG, Gregor ZJ, Cree IA. Concentration of haemodynamic and inflammatory related cytokines in diabetic retinopathy. Eye (Lond) 2008;22:223–228 [DOI] [PubMed] [Google Scholar]
- 12.Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group Ophthalmology 1991;98(5 Suppl.):786–806 [PubMed] [Google Scholar]
- 13.Wong TY, Shankar A, Klein R, Klein BE. Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes 2004;53:179–184 [DOI] [PubMed] [Google Scholar]
- 14.Benitez-Aguirre P, Craig ME, Sasongko MB, et al. Retinal vascular geometry predicts incident retinopathy in young people with type 1 diabetes: a prospective cohort study from adolescence. Diabetes Care 2011;34:1622–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasongko MB, Wang JJ, Donaghue KC, et al. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care 2010;33:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohsin F, Craig ME, Cusumano J, et al. Discordant trends in microvascular complications in adolescents with type 1 diabetes from 1990 to 2002. Diabetes Care 2005;28:1974–1980 [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology 2004;111:1183–1190 [DOI] [PubMed] [Google Scholar]
- 18.Zamir M. Optimality principles in arterial branching. J Theor Biol 1976;62:227–251 [DOI] [PubMed] [Google Scholar]
- 19.Marcovecchio MLMD, Dalton RNPHD, Chiarelli FMD, Dunger DBMD. A1C variability as an independent risk factor for microalbuminuria in young people with type 1 diabetes. Diabetes Care 2011;34:1011–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pemp B, Polska E, Garhofer G, Bayerle-Eder M, Kautzky-Willer A, Schmetterer L. Retinal blood flow in type 1 diabetic patients with no or mild diabetic retinopathy during euglycemic clamp. Diabetes Care 2010;33:2038–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alder VA, Su EN, Yu DY, Cringle SJ, Yu PK. Diabetic retinopathy: early functional changes. Clin Exp Pharmacol Physiol 1997;24:785–788 [DOI] [PubMed] [Google Scholar]
- 22.Giunti S, Barit D, Cooper ME. Mechanisms of diabetic nephropathy: role of hypertension. Hypertension 2006;48:519–526 [DOI] [PubMed] [Google Scholar]
- 23.Hostetter TH, Rennke HG, Brenner BM. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med 1982;72:375–380 [DOI] [PubMed] [Google Scholar]
- 24.Cortes P, Zhao X, Riser BL, Narins RG. Role of glomerular mechanical strain in the pathogenesis of diabetic nephropathy. Kidney Int 1997;51:57–68 [DOI] [PubMed] [Google Scholar]
- 25.Bernadet-Monrozies P, Rostaing L, Kamar N, Durand D. The effect of angiotensin-converting enzyme inhibitors on the progression of chronic renal failure. Presse Med 2002;31:1714–1720 [in French] [PubMed] [Google Scholar]
- 26.Chaturvedi N, Bandinelli S, Mangili R, Penno G, Rottiers RE, Fuller JH. Microalbuminuria in type 1 diabetes: rates, risk factors and glycemic threshold. Kidney Int 2001;60:219–227 [DOI] [PubMed] [Google Scholar]
- 27.Gallego PH, Craig ME, Hing S, Donaghue KC. Role of blood pressure in development of early retinopathy in adolescents with type 1 diabetes: prospective cohort study. BMJ 2008;337:a918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couper DJ, Klein R, Hubbard LD, et al. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. Am J Ophthalmol 2002;133:78–88 [DOI] [PubMed] [Google Scholar]