Abstract
OBJECTIVE
To examine persistence of C-peptide production by ultrasensitive assay years after onset of type 1 diabetes and factors associated with preserving β-cell function.
RESEARCH DESIGN AND METHODS
Serum C-peptide levels, a marker of insulin production and surviving β-cells, were measured in human subjects (n = 182) by ultrasensitive assay, as was β-cell functioning. Twenty-two times more sensitive than standard assays, this assay’s lower detection limit is 1.5 pmol/L. Disease duration, age at onset, age, sex, and autoantibody titers were analyzed by regression analysis to determine their relationship to C-peptide production. Another group of four patients was serially studied for up to 20 weeks to examine C-peptide levels and functioning.
RESULTS
The ultrasensitive assay detected C-peptide in 10% of individuals 31–40 years after disease onset and with percentages higher at shorter duration. Levels as low as 2.8 ± 1.1 pmol/L responded to hyperglycemia with increased C-peptide production, indicating residual β-cell functioning. Several other analyses showed that β-cells, whose C-peptide production was formerly undetectable, were capable of functioning. Multivariate analysis found disease duration (β = −2.721; P = 0.005) and level of zinc transporter 8 autoantibodies (β = 0.127; P = 0.015) significantly associated with C-peptide production. Unexpectedly, onset at >40 years of age was associated with low C-peptide production, despite short disease duration.
CONCLUSIONS
The ultrasensitive assay revealed that C-peptide production persists for decades after disease onset and remains functionally responsive. These findings suggest that patients with advanced disease, whose β-cell function was thought to have long ceased, may benefit from interventions to preserve β-cell function or to prevent complications.
In type 1 diabetes, significant destruction of β-cells occurs prior to diagnosis. At the time of clinical onset, only ∼10% of normal β-cell mass remains (1). Levels of plasma C-peptide drop to ∼20% of the maximal mean of healthy people (2). A prospective study found that 2 years after diagnosis, insulin levels, after a mixed-meal stimulation, decreased to nearly 30% of baseline (3). Similar findings contribute to therapeutic nihilism that β-cells are nearly destroyed several years after diagnosis, especially with early age of onset. But is this pessimism warranted?
Studies show that patients with advanced disease do show some residual β-cell function, depending on individual variables (4–7). Low or vanished C-peptide levels are indicative of advancing disease after diagnosis, and undetectable C-peptide is usually observed after ∼1 year of disease duration. Yet, even small amounts of residual β-cell function confer fewer complications in most studies (4,5,8,9,10). The strongest evidence to date, however, finds that while higher and sustained levels of C-peptide are most beneficial, even modest levels of β-cell function in some with long-term disease are associated with lower rates of hypoglycemia and lower incidence of retinopathy and nephropathy (9,11).
The findings raise questions about how long insulin production persists, whether β-cell functioning is fully maintained, and what personal or disease factors predict residual β-cell function. Some studies indicate the protective effects of shorter disease duration, higher age at onset, and female sex, but not all studies agree (4,7,12–14). Islet cell autoantibodies GAD (GADA) and islet antigen 2 (IA-2A) have been found to be associated with rapid loss of β-cell function (15), although other studies have found them to be unrelated (2,16). The recently discovered autoantibody zinc transporter 8 (ZnT8A) has only begun to be studied in relation to β-cell function (17–19).
We studied 182 patients using an ultrasensitive assay of C-peptide to assess residual β-cell function. The assay is the most sensitive available, with a detection limit of 1.5 pmol/L. We first compared the ultrasensitive with a commonly used C-peptide assay, which had a detection limit of 33.1 pmol/L, and then determined whether β-cells above the lower detection limit remained functional. Then, we analyzed the persistence of C-peptide persistence over time, functional response to hyperglycemia, and the relationship of C-peptide with factors often associated with residual β-cell function: disease duration, chronological age, age at onset, sex, and levels of autoantibodies GADA, IA-2A, and ZnT8A (20). Having prolonged β-cell function enables patients, once considered to lack C-peptide by standard assay, to become candidates for interventions to preserve or enhance β-cell function or to prevent diabetes complications.
RESEARCH DESIGN AND METHODS
Patients with type 1 diabetes were recruited over a 10-year period by the Massachusetts General Hospital with informed consent. The study received full institutional approval. We studied serum samples from 182 separate participants for whom we had a complete set of clinical characteristics, listed in Table 1, but without any foreknowledge of C-peptide values. When two or more samples were available, the most recent sample was analyzed in order to include patients with the most advanced disease. Serum had been collected and frozen at −80°C until analysis. All blood samples tested for C-peptide assay were <5 years old. None of the patients whose samples were older than 5 years or for whom we did not have the complete set of clinical characteristics, and who thus could not be included, died or withdrew from our clinical sample. Subjects were asked to appear in the research clinic fasting. The most recent single serum sample was collected and analyzed from each of the 182 subjects (Figs. 2 and 3 and Supplementary Figs. 1 and 3). Their glycemic levels were also evaluated. In addition to the 182 patients under study, we separately evaluated four long-term patients, shown in Fig. 1 and Supplementary Fig. 2, who provided serum samples weekly for up to 20 consecutive weeks. At one of the visits, we tested each patient for fasting and nonfasting–stimulated C-peptide. In some of the analyses, we did test patients for nonfasting C-peptide levels (Supplementary Fig. 2).
Table 1.
Characteristics of study subjects
Figure 2.
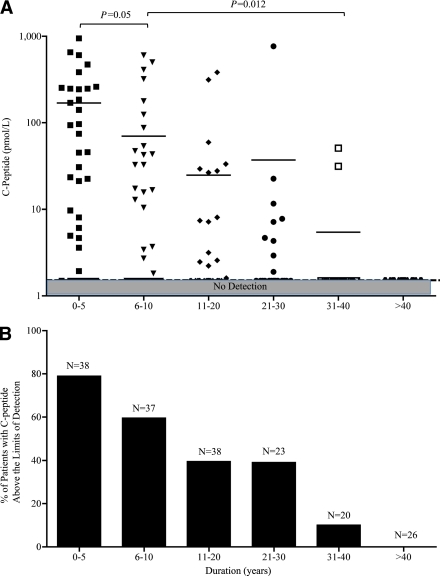
The relationships between C-peptide production and disease duration in subjects with type 1 diabetes (n = 182) by ultrasensitive assay. A: Stratified by six intervals of disease duration, in years, subjects with shorter disease duration tended to produce higher levels of C-peptide by the ultrasensitive assay for values up to 230 pmol/L or with the Mercodia regular assay for values >230 pmol/L, which persisted decades after disease onset. The shaded area across the lower portion of the panel displays the limit of detection of the ultrasensitive assay 1.5 pmol/L. B: Percentage of patients with detectable C-peptide above detection limits. (A high-quality color representation of this figure is available in the online issue.)
Figure 3.
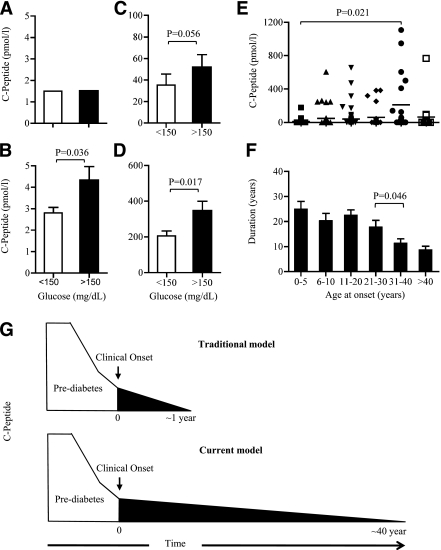
Investigating functionality of insulin-secreting β-cells based on blood glucose (A–D) or age at onset and lifetime duration of the C-peptide response (E and F) defines the time line of persistent C-peptide section (G). Hyperglycemic (n = 52) or normoglycemic (n = 52) samples were examined in the same subjects (n = 52), who gave samples on consecutive weeks and stratified into four ranges of C-peptide levels: 0–5 pmol/L (nonresponsive), 0–5 pmol/L (responsive), 5–100 pmol/L, and >100 pmol/L (A–D). Significantly higher C-peptide levels were produced in hyperglycemic samples, except around the lower levels of assay detection (range 0–5 pmol/L), where β-cells from some samples did not respond to hyperglycemia. Subjects divided into six groups based on age at onset produced varying levels of C-peptide, with the highest levels produced at ages 31–40 years and a sudden drop in the group >40 years old and studied for remaining C-peptide secretion (E and F). C-peptide production during and after type 1 diabetes diagnosis. Reduction of C-peptide levels starts sometime during the prediabetes stage and keeps going down with the progress of the disease. With the new ultrasensitive C-peptide assays, remaining β-cell function extends for years after loss of pancreas function and in some cases extends to 40 years after diagnosis (G).
Figure 1.
Sensitivity of standard (Cobas/Roche) versus ultrasensitive (Mercodia) C-peptide assays in a cohort of individuals with type 1 diabetes for whom weekly samples were taken for up to 20 weeks (n = 4 subjects, n = 54 blood samples). Each blood sample was tested by both assays. The shaded areas represent the areas below the detection limits of the assay. A: Standard assay failed to detect C-peptide in any of the selected serum sample. The shaded area across the lower portion of the panel displays the limit of detection of the Cobas assay (33.1 pmol/L), a limit defined as a maintained CV of 1.9%. B: Ultrasensitive assay detected C-peptide in the majority of the same samples. The dashed horizontal line across the entire lower portion of the panel displays the limit of detection (1.5 pmol/L). C and D: Samples taken from four patients studied for functional responses to glycemic level. Samples depicting hyperglycemia (glucose >150 mg/dL) and normoglycemia (glucose ≤150 mg/dL) revealed that C-peptide level was elevated with mild hyperglycemia, as opposed to normoglycemia, by ultrasensitive assay (D). The standard assay is not sensitive enough to reveal this functional response (C). (A high-quality color representation of this figure is available in the online issue.)
C-peptide samples for 182 subjects were commercially tested by Mercodia equipment (Uppsala, Sweden) using the regular (cat. no. 10-1136-01) or ultrasensitive (cat. no 10-1141-01) C-peptide ELISA kits. Both assays were calibrated against the International Reference Reagent for C-peptide, C-peptide 84/510 (a World Health Organization standard), and listed with the U.S. Food and Drug Administration as Class I in vitro diagnostic devices. Mercodia’s regular C-peptide assay has a lower detection limit of 15 pmol/L with inter- and intra-assay coefficients of variation (CVs) at 4.8 and 4.8%, respectively, at 304 pmol/L (cat. no. 10-1136-01; Mercodia) (Supplementary Table 1). The ultrasensitive assay’s lower detection limit is 1.5 pmol/L, with inter- and intra-assay CVs at 5.5 and 3.8% at 37 pmol/L (cat. no. 10-1141-01; Mercodia). For the four serially followed subjects who appeared in the clinic fasting, C-peptide levels were first tested at the Mayo Clinic, which uses the standard C-peptide assay (cat. no. 03184-897-190, Cobas; Roche Diagnostics) (Fig. 1 and Supplementary Table 1); then, these samples were restudied in the Mercodia ultrasensitive assay. The Cobas assay’s range of detection is 330–1470 pmol/L for the 5–95% range (Supplementary Fig. 1) and can maintain a 1.9% CV to 31 pmol/L. The percentages of CVs are determined by SD/mean × 100%. For the rest of the samples in this study, C-peptide levels were first tested in the Mercodia ultrasensitive assay and, only if sample values were above the range of this assay, then the few samples outside this range were rerun in the Mercodia regular assay that then captured all sample ranges. It should also be mentioned that all samples studied in either of the two Mercodia assays were commercially run blinded by the Mercodia laboratories, since their assay performance data with automated plate washes and other clinically standardized innovations exceeded our abilities with the same tight CVs. One-way ANOVA was used to calculate intra- and interassay variations. Autoantibodies GADA, IA–2A, and ZnT8A were commercially measured by Protein A radio binding assays at the Diabetes Research Institute of Forschergruppe Diabetes (Munich, Germany) as previously described (17).
Statistical methods
Unpaired t test with Welch correction and Pearson correlation test were used for all 182 subjects. However, the univariate and multivariate regression analysis was limited to 125 patients for whom all variables were collected. We performed regression analysis with Microsoft Excel and t tests with Prism. P values ≤0.05 were considered significant.
RESULTS
Patient characteristics of 182 type 1 diabetic subjects are listed in Table 1. C-peptide values in serum samples from each patient were tested using the Mercodia regular (Supplementary Fig. 1A) and ultrasensitive C-peptide (Supplementary Fig. 1B) ELISA kits. Subjects’ median age was 39 years (range 9–85), disease duration 15 years (0–73), and age at onset 13 years (1–56). Levels of autoantibodies GADA, IA-2A, and ZnT8A were obtained for 125 of 182 subjects.
C-peptide levels and functional capacity in serially studied patients
C-peptide levels for up to 14 weekly serum samples from four patients were first tested with standard (Cobas) versus ultrasensitive (Mercodia) assay. The detection limits of these assays are compared in Supplementary Table 1. C-peptide levels in all serum samples tested by standard assay (n = 54) were below its detection limit at 33.1 pmol/L (Fig. 1A), whereas C-peptide levels by ultrasensitive assay were above detection (1.5 pmol/L) in 34 of 54 samples (63%) (Fig. 1B).
To determine whether the pancreas retained functional activity in this group of four patients, their blood samples, assayed under fasting conditions and stratified by glycemic level (normoglycemia <150 mg/dL vs. hyperglycemia >150 mg/dL), were studied in relation to C-peptide levels. In the ultrasensitive but not the standard assay, samples showing hyperglycemia (n = 12) were associated with higher C-peptide levels than those showing normoglycemia (n = 50) (Fig. 1C and D). Similar findings occurred in the larger sample of 182 patients, as discussed below (Fig. 3A–D). The characteristics of the four patients, all of whom are long-term patients with diabetes, are listed in Supplementary Table 2.
Furthermore, glucose responsiveness was examined in three patients with advanced serially followed up to 20 weeks by measurement of their C-peptide levels under nonfasting conditions. The data show that at three different C-peptide ranges, subjects with extremely low C-peptide (Supplementary Fig. 2, top panel), low C-peptide (Supplementary Fig. 2, middle panel), and moderate C-peptide (Supplementary Fig. 2, bottom panel) levels all demonstrated a linear relationship between glycemic levels and C-peptide (Supplementary Fig. 2). These findings suggest that β-cells remain functional even in subjects with low C-peptide output.
Disease duration and C-peptide levels
To test the relationship between C-peptide and disease duration, additional diabetic subjects (n = 182) from each of whom a single serum sample was obtained were categorized into these 5-year-duration groups: 0–5, 6–10, 11–20, 21–30, 31–40, and >40 years (Fig. 2A). As disease duration increased, C-peptide levels tended to gradually decline, but this was a decline over decades of disease—not a decline over months, as is commonly viewed to be the course of the pancreas. The 0–5 years group exhibited higher C-peptide levels than did the 6–10 years group (P = 0.0498). Similarly, the 6–10 years group showed significantly higher levels than did the 31–40 years group (P = 0.0117). In order of increasing duration, C-peptide levels were above the detection limit at rates of 78.9, 59.5, 39.5, 39.1, 10, and 0% of samples in the six groups (Fig. 2B).
Figure 2A shows that after 31–40 years of type 1 diabetes, two subjects appeared to produce detectable C-peptide. Individual characteristics of these subjects were examined to determine whether there was anything unusual about their clinical course. The first subject was a male who had classic onset of type 1 diabetes in childhood, remained thin all his life, demonstrated a history of ketones early in life, and had a positive family history of both type 1 diabetes (siblings) and other autoimmune diseases. As expected from the sustained C-peptide levels, this subject had excellent HbA1C values and no complications. Patient B also had classic type 1 diabetes, with onset at 10 years of age; was thin; and had hypothyroidism and a very positive family history of autoimmune diseases. He was still positive for both GADA and IA-2A after 35 years of disease and also had no complications. These two patients are dramatic examples to support past population-based studies that any preservation of C-peptide is associated with decreased complications (10).
Functional capacity of β-cells in a large clinical sample
In addition to the two previous analyses of functionality in smaller samples, we sought to use the ultrasensitive assay to determine functional capacity of β-cells in an analysis of our large clinical sample by stratifying patients with and without hyperglycemia. Glycemic levels were divided into hyperglycemic (n = 52) and normoglycemic (n = 52) groups and then compared, with C-peptide levels grouped into four ranges: 0–1.5 pmol/L (nonresponsive), 1.5–5 pmol/L (responsive), 5–100 pmol/L, and >100 pmol/L (responsive). Samples from hyperglycemic subjects had significantly higher C-peptide levels than did those from normoglycemic subjects in each of the C-peptide level ranges, except for the nonresponsive group, which had C-peptide levels below the level of detection of the ultrasensitive assay. As long as C-peptide levels were within the detection range of the ultrasensitive assay, C-peptide levels were responsive to glycemic levels.
Age at onset
C-peptide levels were studied by age at onset, which was categorized into six groups: 0–5 years (N = 28), 6–10 years (N = 37), 11–20 years (N = 57), 21–30 years (N = 25), 31–40 years (N = 19), and >40 years (N = 16) (Fig. 3E and F). C-peptide levels with onset at 31–40 years of age (mean ± SD 210 ± 342 pmol/L) were significantly higher than those with onset at 0–5 years of age (11 ± 34). This is generally consistent with a believed slower disease onset in older patients. Patients with onset at 31–40 years of age had the highest C-peptide levels (210 ± 342.75 pmol/L). The exception to these data and generally accepted concept was that the next age-of-onset group, >40 years, had lower C-peptide levels, 63 ± 90.23 pmol/L, despite short disease duration (Fig. 3E and F). All groups tended to exhibit shorter disease duration as age at onset increased (Fig. 3F). The 31–40 years group produced the highest C-peptide levels, with duration significantly shorter than in the younger groups. Therefore, our data show that C-peptide can be present for decades after disease onset, suggesting that long-term preservation of pancreatic function and C-peptide production is not confined to a short time (∼1 year) after clinical onset but, rather, persists in some cases beyond 30 years (Fig. 3F). A possible exception to this rule that needs to be confirmed in further studies, and in additional subject sets, is whether new-onset type 1 diabetes in subjects older than 40 years of age might be associated with accelerated pancreas loss.
Autoantibodies and sex
GADA was found in the highest fraction of patients (50.4%) (Table 1) but was unrelated in a linear regression analysis of factors affecting C-peptide production either as a univariate or multivariate analysis (Supplementary Table 2). IA-2A and ZnT8A autoantibodies were positively correlated with C-peptide levels (Supplementary Table 2). GADA, IA-2A, and ZnT8A were combined and treated as four separate groups of 3, 2, 1, or 0 positive autoantibodies. No substantial differences were found among the number of positive autoantibodies and C-peptide levels (Supplementary Fig. 3B). Sex was unrelated to C-peptide levels (Supplementary Table 2 and Supplementary Fig. 3A).
Multivariate analysis
By univariate regression analysis, duration, chronological age, IA-2A, and ZnT8A were significantly correlated with C-peptide levels, while age at onset, sex, and GADA were not (Supplementary Table 2). Considering the close correlation between duration and chronological age (r = 0.6757, P < 0.0001) (Supplementary Fig. 3C), all factors except age were chosen as independent variables for multivariate analysis. Duration and ZnT8A emerged as the only factors significantly related to C-peptide levels in multivariate analysis (Supplementary Table 2).
CONCLUSIONS
In summary, results revealed that 1) the ultrasensitive assay, which dramatically improved detection at low C-peptide ranges, found that 10% of patients with disease duration of three to four decades still produced C-peptide; 2) β-cell functioning was intact at C-peptide levels as low as 2.8 ± 1.1 pmol/L; 3) still, longer disease duration was associated with lower levels of C-peptide, according to multivariate analysis; and 4) although some studies have shown that adult-onset diabetes confers a more benign course, subjects in this study with onset at >40 years of age showed a more rapid loss of C-peptide levels despite relatively short disease duration.
With standard assays, many patients are assessed as having lost considerable insulin secretion by the time type 1 diabetes is diagnosed (2,3). Yet even low levels of insulin or C-peptide are consistently linked to lower frequency of late-stage complications (10). Using an ultrasensitive assay, this study found C-peptide detection and functional capacity at levels heretofore thought to be indicative of β-cell destruction. The findings appear to dispute therapeutic nihilism that β-cell functioning cannot be preserved long after diagnosis and suggest a long course of pancreas activity postdiagnosis (Fig. 3G). They also appear to dispute the view that onset in adulthood is less aggressive than in childhood. While onset at 31–40 years of age is generally more benign, these preliminary data suggest that it might be possible that diabetes onset after age 40 years is associated with dramatically lower C-peptide levels, despite short duration. One reason may be that basal C-peptide production is lower in healthy older adults than in younger ones (21,22). Related reasons may be that older adults have less β-cell proliferative and regenerative capacity, shifts in epitopes, and higher levels of proinflammatory cytokines and metabolic control (22). Further studies in the future are needed to confirm these observations in additional subsets of long-term patients with diabetes and to compare various assays (23). In this study, we saw more subjects with C-peptide over a 40 year period but few subjects with C-peptide after 40 years.
Our finding of a strong relationship between longer duration and lower C-peptide is consistent with the findings of previous studies (13). The finding of a strong relationship between higher levels of ZnT8A autoantibodies and higher levels of C-peptide is difficult to interpret. Autoantibodies are generally good predictors for disease onset but are not specific regarding disease outcome (18,20). The recently discovered ZnT8A autoantibody is reported to decline with disease duration, especially 25 years’ duration, but the reasons behind this relationship are highly complex (19). Greater investigation of ZnT8A is clearly needed.
In conclusion, decades after onset, C-peptide detection and functioning β-cells are observed with an ultrasensitive assay. The assay offers a novel approach to identify patients, even with advanced disease, who upon glycemic stimulation may benefit from treatments to retain or enhance β-cell function. Patients with low C-peptide levels or advanced disease should neither be uniformly excluded from clinical trials nor seen as having complete islet cell destruction.
Acknowledgments
This study was supported by the Iacocca Foundation.
No potential conflicts of interest relevant to this article were reported.
L.W. researched and analyzed data and drafted the manuscript. N.L. recruited subjects. D.L.F. designed experiments and reviewed and edited the manuscript. As the corresponding author and guarantor of this article, D.L.F. takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
The authors thank the subjects of the study for their generous participation. The authors also thank M. Davis, PhD, of their team (Massachusetts General Hospital), for her technical and editorial comments and L. Murphy (Massachusetts General Hospital) for her technical comments.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1236/-/DC1.
See accompanying commentary, p. 459.
References
- 1.Gianani R, Campbell-Thompson M, Sarkar SA, et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia 2010;53:690–698 [DOI] [PubMed] [Google Scholar]
- 2.Madsbad S, Krarup T, Regeur L, Faber OK, Binder C. Insulin secretory reserve in insulin dependent patients at time of diagnosis and the first 180 days of insulin treatment. Acta Endocrinol (Copenh) 1980;95:359–363 [DOI] [PubMed] [Google Scholar]
- 3.Steele C, Hagopian WA, Gitelman S, et al. Insulin secretion in type 1 diabetes. Diabetes 2004;53:426–433 [DOI] [PubMed] [Google Scholar]
- 4.Eff Ch, Faber O, Deckert T. Persistent insulin secretion, assessed by plasma C-peptide estimation in long-term juvenile diabetics with a low insulin requirement. Diabetologia 1978;15:169–172 [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi K, Watanabe C. Rate of β-cell destruction in type 1 diabetes influences the development of diabetic retinopathy: protective effect of residual β-cell function for more than 10 years. J Clin Endocrinol Metab 2008;93:4759–4766 [DOI] [PubMed] [Google Scholar]
- 6.Liu EH, Digon BJ, Hirshberg B, et al. Pancreatic beta cell function persists in many patients with chronic type 1 diabetes, but is not dramatically improved by prolonged immunosuppression and euglycaemia from a beta cell allograft. Diabetologia 2009;52:1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madsbad S, Faber OK, Binder C, McNair P, Christiansen C, Transbøl I. Prevalence of residual beta-cell function in insulin-dependent diabetics in relation to age at onset and duration of diabetes. Diabetes 1978;27(Suppl 1):262–264 [DOI] [PubMed] [Google Scholar]
- 8.Sjöberg S, Gunnarsson R, Gjötterberg M, Lefvert AK, Persson A, Ostman J. Residual insulin production, glycaemic control and prevalence of microvascular lesions and polyneuropathy in long-term type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1987;30:208–213 [DOI] [PubMed] [Google Scholar]
- 9.Panero F, Novelli G, Zucco C, et al. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care 2009;32:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med 1998;128:517–523 [DOI] [PubMed] [Google Scholar]
- 11.Steffes MW, Sibley S, Jackson M, Thomas W. β-Cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 [DOI] [PubMed] [Google Scholar]
- 12.Sochett EB, Daneman D, Clarson C, Ehrlich RM. Factors affecting and patterns of residual insulin secretion during the first year of type 1 (insulin-dependent) diabetes mellitus in children. Diabetologia 1987;30:453–459 [DOI] [PubMed] [Google Scholar]
- 13.The DCCT Research Group Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual β-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). J Clin Endocrinol Metab 1987;65:30–36 [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, Nakanishi K, Sugimoto T, et al. Maleness as risk factor for slowly progressive IDDM. Diabetes Care 1989;12:7–11 [DOI] [PubMed] [Google Scholar]
- 15.Mortensen HB, Swift PGF, Holl RW, et al. ; Hvidoere Study Group on Childhood Diabetes Multinational study in children and adolescents with newly diagnosed type 1 diabetes: association of age, ketoacidosis, HLA status, and autoantibodies on residual beta-cell function and glycemic control 12 months after diagnosis. Pediatr Diabetes 2010;11:218–226 [DOI] [PubMed] [Google Scholar]
- 16.Jaeger C, Allendörfer J, Hatziagelaki E, et al. Persistent GAD 65 antibodies in longstanding IDDM are not associated with residual beta-cell function, neuropathy or HLA-DR status. Horm Metab Res 1997;29:510–515 [DOI] [PubMed] [Google Scholar]
- 17.Achenbach P, Lampasona V, Landherr U, et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009;52:1881–1888 [DOI] [PubMed] [Google Scholar]
- 18.Brorsson C, Vaziri-Sani F, Bergholdt R, et al. ; Danish Study Group of Childhood Diabetes Correlations between islet autoantibody specificity and the SLC30A8 genotype with HLA-DQB1 and metabolic control in new onset type 1 diabetes. Autoimmunity 2011;44:107–114 [DOI] [PubMed] [Google Scholar]
- 19.Wenzlau JM, Walter M, Gardner TJ, et al. Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J Clin Endocrinol Metab 2010;95:4712–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherry NA, Tsai EB, Herold KC. Natural history of β-cell function in type 1 diabetes. Diabetes 2005;54(Suppl. 2):S32–S39 [DOI] [PubMed] [Google Scholar]
- 21.Pacini G, Beccaro F, Valerio A, Nosadini R, Crepaldi G. Reduced beta-cell secretion and insulin hepatic extraction in healthy elderly subjects. J Am Geriatr Soc 1990;38:1283–1289 [DOI] [PubMed] [Google Scholar]
- 22.Akirav E, Kushner JA, Herold KC. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes 2008;57:2883–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keenan H, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010; 59:2846–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]