Abstract
The angiopoietins/Tie-2 system is essential for the maintenance of vascular integrity and angiogenesis. The functional role of angiopoietin-2 (Ang-2) in the regulation of angiogenesis is dependent on other growth factors such as VEGF and a given physiopathological conditions. This study investigates the potential role of Ang-2 in myocardial angiogenesis and fibrosis formation in the diabetic db/db mouse. Diabetic db/db mice received intramyocardial administration of either adenovirus Ang-2 (Ad-CMV-Ang-2) or Ad-β-gal. The levels of Tie-2, VEGF, caspase-3, Wnt7b, fibroblast-specific protein-1 (FSP-1), and adhesion molecules (ICAM-1 and VCAM-1) expression were measured. Apoptosis, capillary density, and cardiac fibrosis were also analyzed in the db/db mouse hearts. Overexpression of Ang-2 suppressed Tie-2 and VEGF expression in db/db mouse hearts together with significant upregulation of Wnt7b expression. Overexpression of Ang-2 also sensitizes ICAM-1 and VCAM-1 expression in db/db mouse hearts. Immunohistochemical analysis revealed that overexpression of Ang-2 resulted in a gradual apoptosis as well as interstitial fibrosis formation, these leading to a significant loss of capillary density. Data from these studies were confirmed in cultured mouse heart microvascular endothelial cells (MHMEC) exposed to excessive Ang-2. Exposure of MHMEC to Ang-2 resulted in increased caspase-3 activity and endothelial apoptosis. Knockdown of Ang-2 attenuated high glucose-induced endothelial cell apoptosis. Further, counterbalance of Ang-2 by overexpression of Ang-1 reversed loss of capillary density and fibrosis formation in db/db mouse hearts. Our data demonstrate that Ang-2 increases endothelial apoptosis, sensitizes myocardial microvascular inflammation, and promotes cardiac fibrosis and thus contributes to loss of capillary density in diabetic diseases.
Keywords: type 2 diabetes, vascular endothelial growth factor, myocardial capillary density, apoptosis
microvascular rarefaction (loss of capillary), a major cause of end-stage organ failure in diabetes, results in a decreased coronary blood flow reserve rendering the myocardium vulnerable to ischemia and exacerbation of heart failure. Severe microvascular rarefaction has been detected in the myocardium of diabetic patients and animal models of diabetes (28, 29). The progressive microvasculature rarefaction is related to the duration of diabetes (22, 37). The reduction in capillary density leads to cardiac dysfunction following myocardial ischemia (37), and its preservation improves recovery of left ventricular function in diabetes (22). These studies strongly suggest that insufficient angiogenesis and microvascular rarefaction may represent one of the most critical mechanisms involved in the pathogenesis and progression of diabetic cardiac dysfunction.
Angiogenesis is thought to depend on a perfectly coordinated balance among a variety of angiogenic factors and is mainly regulated by the interplay between VEGF and angiopoietins (13, 14). Angiopoietin-2 (Ang-2) is a ligand of Tie-2 receptor, and its role in the regulation of angiogenesis is dependent on the VEGF. In the presence of VEGF, Ang-2 promotes endothelial proliferation and migration and induces vessel sprouting. However, in the absence of VEGF, Ang-2 increases endothelial apoptosis and results in vessel regression (20). Under physiological conditions, systemic delivery of Ang-2 has been shown to increase angiogenesis in the lung and the heart without a concomitant upregulation of VEGF (3). However, overexpression of Ang-2 in tumors results in disorganized vasculature with endothelial cells apoptosis and tumor vessel regression (27). These data implicate that the functional role of Ang-2 in the regulation of angiogenesis or vessel regression is also dependent on a given physiopathological conditions. An increased Ang-2 is a powerful predictor of adverse outcomes in diabetic cardiovascular diseases. Ang-2 expression, but not Ang-1, is abnormally elevated in patients with diabetes and congestive heart failure, and increased Ang-2 is strongly associated with cardiovascular dysfunction (8, 9, 16–18). Previously, we (4, 5) demonstrated that Ang-2 expression was increased whereas VEGF expression and angiogenesis were reduced in diabetic db/db mice subjected to myocardial ischemia. So far, no study has done to investigate the direct effects of Ang-2 on the diabetes-associated cardiovascular complications. Whether overexpression of Ang-2 mimic diabetic ischemic conditions results in loss of VEGF expression and capillary density remains unexplored. The present study uses the diabetic db/db mouse to test the concept that excess of Ang-2 impairs angiogenesis and promotes myocardial fibrosis by suppressing Tie-2 and VEGF signaling, which thereby enhances endothelial cell apoptosis as well as proinflammatory and profibrotic responses. Furthermore, whether overexpression of Ang-1 counterbalances Ang-2 and reverses these abnormalities of diabetes.
MATERIALS AND METHODS
In vivo intramyocardial injection of adenovirus-Ang-2, adenovirus-Ang-1, or adenovirus-β-gal.
C57BL/6J (wild type), db/+, and diabetic db/db male mice (12 wk of age) were purchased from Jackson Laboratory (Bar Harbor, ME). Before injection, db/db mice were anesthetized with ketamine (100 mg/kg) plus xylazine (15 mg/kg), intubated, and artificially ventilated with room air. A left thoracotomy was performed, and the left anterior descending coronary artery was exposed. Adenovirus (Ad)-Ang-2, Ad-Ang-1, or Ad-β-gal (1 × 109 platelet-forming units) was mixed with 100 μl of saline. A 30-G needle bent at a right angle was inserted into the left anterior descending coronary artery zone, its tip was turned into the border zone, and a maximum volume of 25 μl of the Ad-mixture was injected at four equidistant sites (4, 5). Mice were killed 48 h or 14 days after intramyocardial viral transfection for the analyses described below.
Analysis of Ang-1, Ang-2, Tie-2, VEGF, Wnt7b, VCAM-1, and ICAM-1 expression.
Forty-eight hours after either Ad-Ang-2 or Ad-β-gal injection, the hearts were harvested and homogenized in lysis buffer. Fifty micrograms of total protein were separated using SDS-gel electrophoresis. The membranes were immunoblotted with Ang-2, Ang-1, Tie-2, VEGF, Wnt7b, ICAM-1, and VCAM-1 antibodies (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were washed and incubated with a secondary antibody coupled to horseradish peroxidase, and densitometric analysis was carried out using image acquisition and analysis software (TINA 2.0).
Analysis of myocardial capillary density.
Fourteen days after Ad-Ang-2, Ad-Ang-1, or Ad-β-gal treatment, the hearts were harvested and immediately flash frozen in super friendly freeze'it (Fisher). Five-micrometer sections were cut and incubated with fluorescent-labeled Griffonia Bandeiraea Simplicifolia Isolectin B4 (1:200; IB4; Molecular Probes, Invitrogen). The number of capillaries (IB4-positive endothelial cells) was counted and expressed as capillary density per field (10×) of myocardium (5, 32).
Colocalization of cleaved caspase-3 with Ang-2.
Sections of myocardium were incubated with Ang-2 and cleaved caspase-3 (1:200; Cell Signaling, Danvers, MA) antibodies overnight, followed by incubation with streptavidin-Alexa Fluor 488 for Ang-2, and Fluorolink Cy3 for cleaved caspase-3. Colocalization of cleaved caspase-3 with Ang-2 was visualized with a fluorescence microscope (Nikon TE 2000). Sections were counterstained with DAPI.
Myocardial cell apoptosis.
Sections of myocardial tissue were labeled with transferase deoxyuridine nick end labeling (TUNEL) following the manufacturer's instructions (Promega, Madison, WI). Sections were counterstained with DAPI. Apoptosis was indexed by counting TUNEL-positive cells per 100 nuclei.
Myocardial Ang-2, Wnt7b, fibroblast-specific protein-1, ICAM-1, and VCAM-1 expression.
Heart tissue sections were incubated with Ang-2, Wnt7b, fibroblast-specific protein-1 (FSP-1), ICAM-1, and VCAM-1 (1:200) antibodies overnight, followed by incubation with FITC-labeled goat anti-mouse IgG antibodies for Ang-2, ICAM-1, and VCAM-1, and Fluorolink Cy3-labeled goat anti-mouse IgG antibodies for Wnt7b and FSP-1 (1:200). Colocalization of either Ang-2 with Wnt7b or ICAM-1 and VCAM-1 with FSP-1 was visualized with a fluorescence microscope (Nikon TE 2000). Sections were counterstained with DAPI.
Cardiac hypertrophy and interstitial fibrosis.
Fourteen days after Ad-Ang-2, Ad-Ang-1, or Ad-β-gal treatment, the heart was removed and weighed and the weight was divided by the total body weight of each mouse, resulting in a ratio representative of cardiac hypertrophy. To determine cardiac fibrosis, sections were stained with Masson's trichrome (Sigma, St. Louis, MO). Myocardial interstitial fibrosis was quantified by measuring the Masson's trichrome-stained (blue) area using NIH Image analysis software as previously described (4).
Mouse heart microvascular endothelial cells.
Mouse heart microvascular endothelial cells (MHMEC) were isolated from C57BL/6J mouse hearts and cultured as previously described (6, 7). Primary cultures of MHMEC, between passages 4 and 10, were used in all experiments.
Endothelial cell apoptosis and caspase-3 activity.
MHMEC were exposed to either high glucose (HG; 30 mmol/l) or normal glucose (NG; 5 mmol/l) for 72 h. To induce apoptosis, MHMEC were exposed to serum-free medium for 48 h under HG or NG conditions. Endothelial cell apoptosis was measured by counting TUNEL-positive cells per 100 endothelial cells following the manufacturer's instructions (Promega). Caspase-3 activity was measured using the caspase-3 kit (Sigma).
Ang-2 short interfering RNA transfection.
MHMEC (∼80% confluent) were treated with Ang-2 short interfering (si)RNA (mouse; Santa Cruz Biotechnology) for 24 h to inhibit Ang-2 expression according to the manufacturer's instructions. Knockdown of Ang-2 was confirmed by Western blot analysis of Ang-2 protein expression.
Statistical analysis.
The results are expressed as the means ± SD. Differences between groups were compared using either the unpaired Student's t-test or by multiple ANOVA. Significance was set at P < 0.05.
All procedures conformed to the Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals and were approved by the Vanderbilt University Institutional Animal Care and Use Committee.
RESULTS
Overexpression of Ang-2 inhibits Tie-2 and VEGF expression in the db/db mouse hearts.
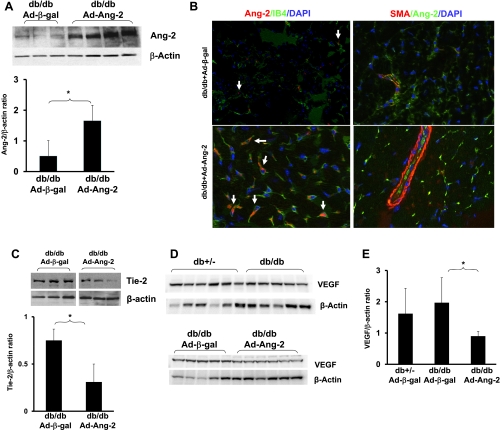
As shown in Fig. 1A, 48 h following intramyocardial administration of Ad-Ang-2 resulted in a significant increase in Ang-2 expression in db/db mouse hearts compared with hearts receiving Ad-β-gal. Immunofluorescence studies confirmed that the increased Ang-2 localized within IB4-positive endothelial cells but not with smooth muscle cell actin in Ad-Ang-2-treated db/db mouse hearts (Fig. 1B). This was accompanied by a significant decrease in Tie-2 expression (Fig. 1C). The basal levels of VEGF expression were not significantly different in the Ad-β-gal-treated db/db and nondiabetic db/+ mouse hearts. Overexpression of Ang-2 resulted in a significant reduction of VEGF expression in db/db mouse hearts (Fig. 1, D and E). Myocardial Ang-1 expression also remained unchanged (data not shown).
Fig. 1.
A: Western blot analysis demonstrating that intramyocardial administration of adenovirus (Ad)-angiopoietin-2 (Ang-2) resulted in a significant increase in Ang-2 expression in the db/db mouse hearts (n = 3–4 mice; *P < 0.05). B: Ang-2 expression localized in endothelial cells (EC) but not in smooth muscle cells (SMC) in heart tissue. Left: Ang-2 was immunostained with anti-Ang-2 (red). EC were stained with Isolectin B4 (IB4; green). Merged image revealed that Ang-2 localized to EC. Right: Ang-2 was stained with anti-Ang-2 (green). SMC were stained with smooth muscle actin (SMA; red), and nuclei were counterstained with DAPI (blue). Merged images showed that Ang-2 did not localize to SMC in the vessel wall. Fluorescence immunohistochemistry analysis was conducted on heart tissue in triplicate studies. C: Western blot and densitometric analysis showing treatment with Ad-Ang-2 in db/db mice led to a significant decrease in Tie-2 expression compared with Ad-β-gal-treated db/db mice (n = 3 mice; *P < 0.05). Representative images were from same loading Western blot. D and E: basal level of VEGF expression was similar in the Ad-β-gal-treated db/db mice compared with db+/− mice. Overexpression of Ang-2 in db/db mice resulted in a significant reduction of VEGF expression compared with Ad-β-gal-treated db/db mice (n = 5–6 mice; *P < 0.05).
Overexpression of Ang-2 leads to loss of capillary and increase in endothelial apoptosis via activation of caspase-3 in the db/db mouse hearts.
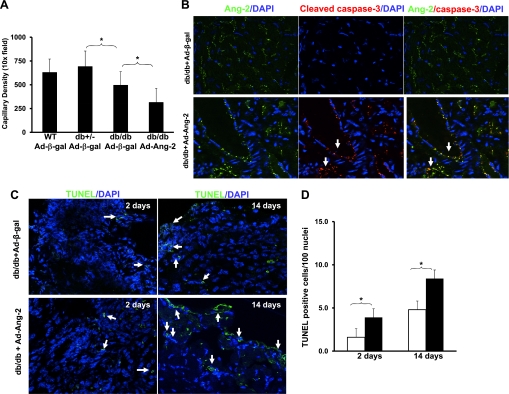
Assessment of capillary density at 14 days revealed that overexpression of Ang-2 in db/db mouse hearts led to a significant loss of capillary density compared with db/db mice treated with Ad-β-gal (Fig. 2A). To investigate whether loss of capillary in the overexpressed Ang-2 db/db mouse hearts was due to endothelial cell apoptosis, colocalization of Ang-2 and cleaved caspase-3 in Ad-Ang-2- and Ad-β-gal-treated mouse hearts was examined. As shown in Fig. 2B, overexpressed Ang-2 was colocalized with cleaved caspase-3 in the vascular wall of Ad-Ang-2-treated mouse hearts. Surprisingly, cleaved caspase-3 expression was not detected in Ad-β-gal-treated db/db mouse hearts (Fig. 2B). Quantitative analysis of TUNEL staining at days 2 and 14 following Ad-Ang-2 treatment showed a gradual and statistically significant time-dependent increase in apoptosis in db/db mouse hearts compared with db/db mice treated with Ad-β-gal (Fig. 2, C and D).
Fig. 2.
A: Quantitative analysis by IB4-stained EC showing a significant decrease in myocardial capillary density in Ad-β-gal-treated db/db mice and Ad-Ang-2-treated db/db mice, compared with db/+ or Ad-β-gal-treated db/db mice, respectively (n = 5–8 for each group; *P < 0.05). WT, wild type. B: representative images of myocardium cleaved caspase-3 staining. Left: Ang-2 protein was imunostained with anti-Ang-2 (green), and nuclei were stained with DAPI (blue). Middle: caspase-3 was immunostained with cleaved caspase-3 (red). Right: merged image revealed that Ang-2 was localized with cleaved caspase-3 in the Ad-Ang-2-treated db/db mouse hearts but not in Ad-β-gal-treated hearts. Areas of colocalization appear yellow. Immunofluorescence microscopy was conducted on heart tissue in triplicate. C and D: representative images and quantitative analysis of apoptotic cells by transferase deoxyuridine nick end labeling (TUNEL) staining in db/db mice hearts at day 2 and 14. Apoptosis was indexed by counting TUNEL-positive cells per 100 nuclei in the affected area. Apoptotic cells were significantly increased at day 2 and 14 after Ad-Ang-2 treatment (solid box) compared with Ad-β-gal-treated db/db mice (open box), respectively (n = 5 mice in each group; *P < 0.05).
Overexpression of Ang-2 increases Wnt7b expression in the db/db mouse hearts.
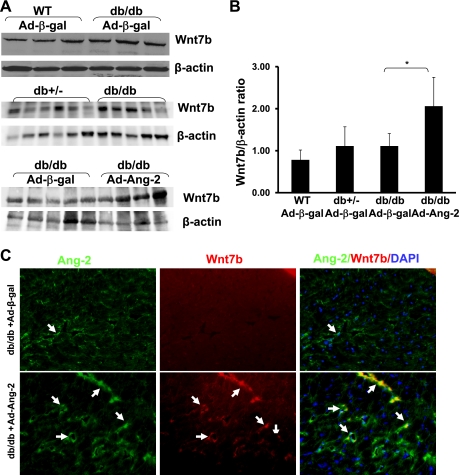
Recent studies (21, 23) implicate Wnt7b activation in Ang-2-mediated programmed cell death and vessel regression in the retina. To investigate the association between Ang-2 and Wnt7b activation in db/db mouse hearts, Wnt7b expression and its colocalization with Ang-2 were examined. Western blot analysis revealed that basal levels of Wnt7b expression were not significantly different in the Ad-β-gal-treated db/db, db/+, and wild-type mouse hearts (Fig. 3, A and B). Overexpression of Ang-2 in db/db mouse hearts led to a significant increase in Wnt7b expression compared with Ad-β-gal-treated db/db mouse hearts (Fig. 3, A and B). Immunohistochemical analysis further revealed that overexpressed Ang-2 was colocalized with Wnt7b (Fig. 3C).
Fig. 3.
A: basal level of Wnt7b expression was similar in the Ad-β-gal-treated db/db, db/+, and WT mouse hearts (top); Wnt7b expression in the Ad-Ang-2-treated hearts was increased compared with that of Ad-β-gal-treated db/db mice (bottom). B: Western blot densitometric analysis showing a significant increase in Wnt7b expression in the Ad-Ang-2-treated hearts compared with that of Ad-β-gal-treated db/db mice (n = 3–5 mice per group, *P < 0.05). C: immunofluorescence images showing colocalization of Ang-2 with Wnt7b in db/db mouse hearts. Ang-2 was stained with mouse Ang-2 antibody (green, ×40). Wnt7b was stained with Wnt7b antibody (red, ×40) and nuclei were stained with DAPI (blue, ×40). Merged images showed that Ang-2 expression colocalized to Wnt7b (yellow) in Ad-Ang-2-treated db/db mice but not in Ad-β-gal-treated db/db mouse hearts.
Overexpression of Ang-2 potentiates cardiac fibrosis in the db/db mouse hearts.
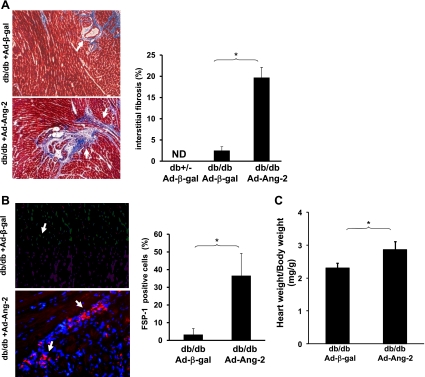
Myocardial interstitial fibrosis was examined by measuring FSP-1 expression and fibrosis formation in the db/db mouse hearts. Overexpression of Ang-2 resulted in a significant increase in myocardial interstitial fibrosis as assessed by an increase in connective tissue compared with the Ad-β-gal-treated mouse hearts (Fig. 4A). The number of FSP-1-positive cells in the myocardial interstitium was also significantly increased in the Ad-Ang-2-treated db/db mouse hearts compared with the Ad-β-gal-treated mouse hearts (Fig. 4B). Further, overexpression of Ang-2 resulted in significant myocardial hypertrophy as assessed by the heart to body weight ratio (Fig. 4C).
Fig. 4.
A: representative images of Masson's trichrome staining and quantitative analysis of myocardial interstitial fibrosis of heart sections. Fibrotic areas are shown in blue (left). Myocardial interstitial fibrosis was significantly increased in db/db mice treated with Ad-Ang-2 compared with control db/db mice treated with Ad-β-gal at 14 days (n = 8 in each group; *P < 0.05). ND, not detected. B: representative images of fibroblast-specific protein-1 (FSP-1) staining and quantitative analysis of myocardial FSP-1 expression of heart sections. FSP-1-positive cells are expressed as a percentage of the total number of nuclei in the injected area. Quantitative analysis demonstrating that the number of FSP-1-positive cells per field was significantly increased in the Ad-Ang-2-treated hearts compared with Ad-β-gal-treated db/db mice (n = 3 mice in each group; *P < 0.05). C: heart:body weight ratio in db/db and Ad-Ang-2-treated db/db mouse hearts at 14 days after myocardial injection. A significant increase in cardiac hypertrophy was observed in db/db mice after 14 days of Ad-Ang-2 treatment compared with those treated with Ad-β-gal (n = 8 in each group; *P < 0.05).
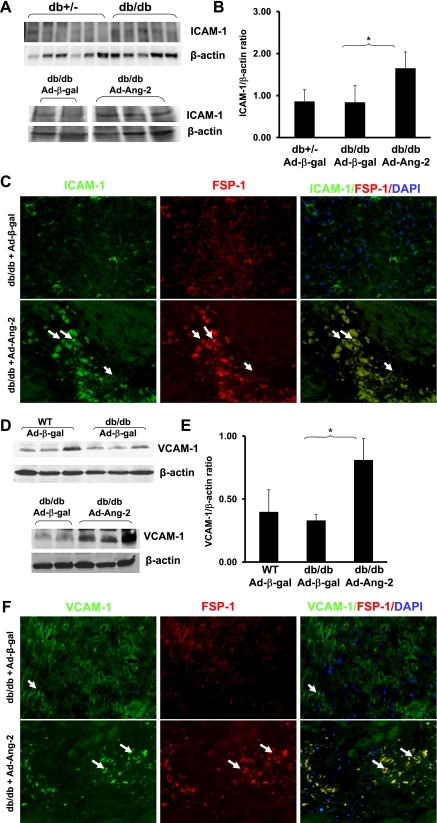
Overexpression of Ang-2 sensitizes VCAM-1 and ICAM-1 expression in the db/db mouse hearts.
Ang-2 has been shown to promote adhesion by sensitizing endothelial cells toward TNF-α and modulating TNF-α-induced expression of endothelial cell adhesion molecules (12). To determine whether excessive Ang-2 augments endothelial cell adhesion molecules and cardiac fibrosis, myocardial ICAM-1 and VCAM-1 expression and their colocalization with FSP-1 were examined. Western blot analysis revealed that basal levels of ICAM-1 expression were not significantly different in the db/db mouse compared with control db/+ mice (Fig. 5, A and B). Overexpression of Ang-2 in db/db mouse hearts significantly enhanced ICAM-1 expression compared with Ad-β-gal-treated db/db mouse hearts (Fig. 5, A and B). Intriguingly, increased ICAM-1 was colocalized with FSP-1 in the Ad-Ang-2-treated mice (Fig. 5C). Similarly, VCAM-1 expression was also enhanced in the Ad-Ang-2-treated db/db mouse hearts compared with the Ad-β-gal-treated mice (Fig. 5D and E). The increased VCAM-1 positive cell was colocalized with FSP-1 in the Ad-Ang-2-treated db/db mouse hearts (Fig. 5F).
Fig. 5.
A: basal level of ICAM-1 expression was similar in the Ad-β-gal-treated db/db mice compared with db/+ mice (n = 5–6 mice per group; top). ICAM-1 expression in the Ad-Ang-2-treated hearts was increased compared with that of Ad-β-gal-treated db/db mice (bottom). Representative images were from same loading Western blot. B: treatment with Ad-Ang-2 resulted in a significant increase in ICAM-1 expression in db/db mice compared with db/db control mice (n = 3–4; *P < 0.05). C: immunofluorescence microscopy showing colocalization of ICAM-1 and FSP-1 in db/db mice. ICAM-1 (green, ×40), FSP-1 (red, ×40), and nuclei were stained by DAPI (blue, ×40). Merged images showed that ICAM-1-positive cells colocalized with FSP-1 (yellow) in the Ad-Ang-2-treated db/db mice, but not the Ad-β-gal-treated db/db mice D and E: basal level of VCAM-1 expression was similar in the Ad-β-gal-treated db/db mice compared with WT mice. VCAM-1 expression in the Ad-Ang-2-treated hearts was increased compared with that of Ad-β-gal-treated db/db mice (bottom). Overexpression of Ang-2 significantly enhanced VCAM-1 expression in db/db mice (n = 3–5 mice; *P < 0.05). F: Immunofluorescence microscopy showing colocalization of VCAM-1 (green, 40×) and FSP-1 in db/db mice. FSP-1 was stained with FSP-1 antibody (red), and nuclei were stained by DAPI (blue). Merged images showed that VCAM-1 and FSP-1(yellow) colocalized in the Ad-Ang-2-treated db/db mice but not the Ad-β-gal-treated db/db mice.
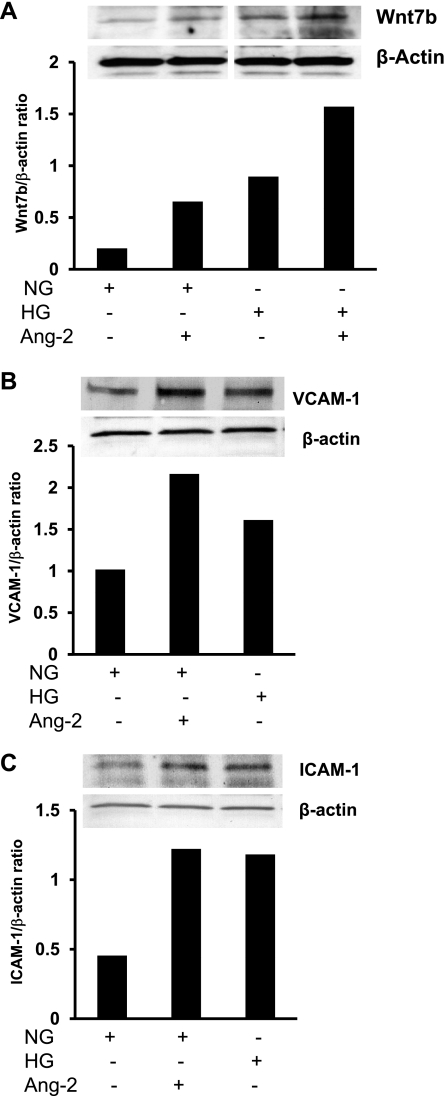
Excess of Ang-2 increases Wnt7b expression, adhesion molecule expression, and endothelial cell apoptosis in MHMEC.
To confirm the histological findings and functional effect of Ang-2 observed in the Ad-Ang-2-treated db/db mouse hearts, parallel studies were performed in cultured MHMEC grown in NG (5 mmol/l) and HG (30 mmol/l) conditions. Exposure of endothelial cells to Ang-2 (250 ng/ml) for 24 h increases Wnt7b expression under NG conditions. Exposure of endothelial cells to HG alone for 72 h also upregulates Wnt7b expression; the HG-induced Wnt7b expression was further enhanced in the presence of Ang-2 (Fig. 6A). Exposure of MHMEC to Ang-2 (250 ng/ml) for 24 h or HG for 72 h also led to a twofold increase in VACM-1 expression (Fig. 6B) and a 50% increase in ICAM-1 expression (Fig. 6C).
Fig. 6.
A: representative Western blot analysis showing that treatment of mouse heart microvascular EC (MHMEC) with Ang-2 led to increased Wnt7b protein expression under normal glucose (NG) and high glucose (HG) conditions. Representative images were from same loading Western blot. These results are representative of 3 different experiments. B: Western blot analysis showing that treatment with Ang-2 led to a dramatic increase in VCAM-1 protein expression. These results are representative of three different experiments. C: representative Western analysis showing that treatment with Ang-2 resulted in an increase in ICAM-1 protein expression. These results represent 3 different experiments.
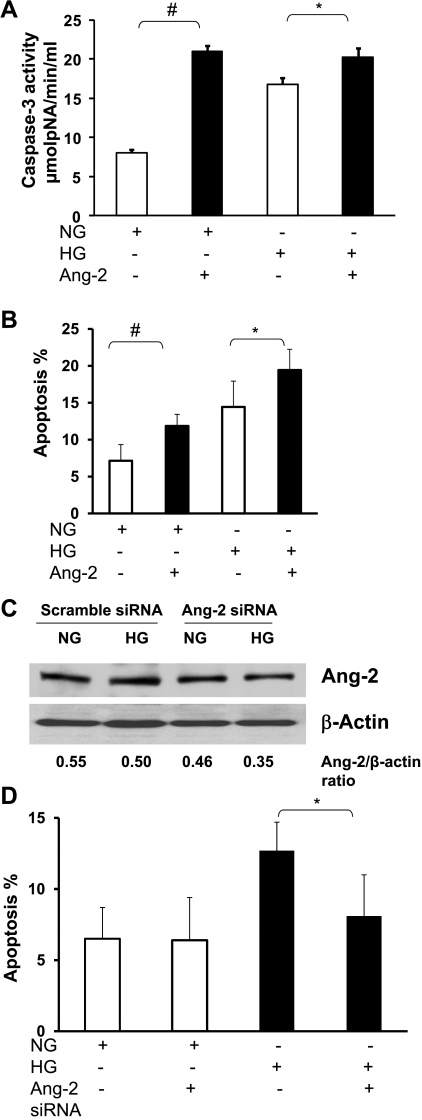
In the presence of exogenous Ang-2 (250 ng/ml) and NG, serum starvation-induced caspase-3 activation and endothelial cell apoptosis were significantly increased compared with NG alone (Fig. 7, A and B). Exposure of MHMEC to HG for 72 h followed by 48 h of serum starvation also resulted in a significant increase in caspase-3 activity and endothelial cell apoptosis, which was further enhanced by exogenous Ang-2 (250 ng/ml; Fig. 7, A and B). To confirm the role of Ang-2 in MHMEC apoptosis, MHMEC were treated with Ang-2 siRNA for 24 h to inhibit Ang-2 expression. Knockdown of Ang-2 was confirmed by Western blot analysis of Ang-2 protein expression as shown in Fig. 7C. Treatment of MHMEC with Ang-2 siRNA significantly blunted HG-induced endothelial cell apoptosis but had little effect on the MHMEC apoptosis under NG conditions (Fig. 7D).
Fig. 7.
A: quantitative analysis demonstrating exogenous Ang-2 significantly enhanced caspase-3 activity under HG and NG conditions in MHMEC (n = 3; *P < 0.05; #P < 0.01). B: quantitative analysis demonstrating that exogenous Ang-2 significantly increased the number of TUNEL-positive cells under HG and NG conditions in MHMEC (n = 3; *P < 0.05; #P < 0.01). C: treatment of MHMEC with Ang-2 siRNA led to a suppression of Ang-2 expression under HG and NG conditions. D: treatment of MHMEC with Ang-2 siRNA significantly attenuated high glucose-induced endothelial apoptosis but had little effect under normal glucose conditions (n = 3; *P < 0.05).
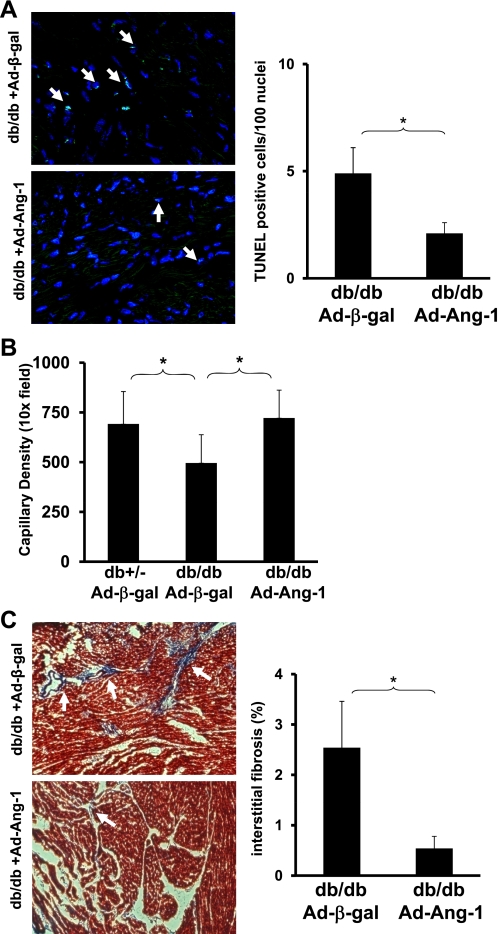
Overexpression of Ang-1 reduces apoptosis and prevents loss of capillary density in db/db mice.
Since Ang-2 is a nature antagonist of Ang-1/Tie-2, we sought to determine whether overexpression of Ang-1 would reverse the progression of myocardial apoptosis and loss of capillary in diabetic mouse hearts. As shown in Fig. 8A, TUNEL-positive cells in the db/db mouse hearts were significantly decreased in Ad-Ang-1-treated db/db mice compared with Ad-β-gal-treated db/db mice. This was accompanied by a significant increase in capillary density (Fig. 8B). Overexpression of Ang-1 also significant reduced myocardial fibrosis formation in db/db mouse (Fig. 8C).
Fig. 8.
A: quantitative analysis of apoptotic cells by TUNEL staining in db/db mice hearts at day 14 after Ad-β-gal and Ad-Ang-1 treatment. TUNEL-positive cells are expressed as a percentage of the total number of nuclei in the affected area (n = 5 in each group; *P < 0.05). B: quantitative analysis by IB4 stained EC showing a significant increase in myocardial capillary density in Ad-Ang-1-treated db/db mice compared with Ad-β-gal-treated db/db mice (n = 5–8 for each group; *P < 0.05). C: myocardial interstitial fibrosis was significantly decreased in db/db mice treated with Ad-Ang-1 compared with control db/db mice treated with Ad-β-gal at 14 days (n = 4 in each group; *P < 0.05).
DISCUSSION
Our present studies demonstrated that overexpression of Ang-2 in diabetic hearts mimicking diabetic ischemic conditions resulted in increased endothelial apoptosis and loss of capillary density. This was accompanied by upregulation of Wnt7b and caspase-3 activation. Intriguingly, overexpression of Ang-2 sensitizes endothelial cell and enhances expression of endothelial adhesion molecules in diabetic db/db mouse hearts. Excessive Ang-2 further promotes cardiac fibrosis formation in db/db mouse hearts. Our data clearly demonstrate that excessive Ang-2 impairs angiogenesis and sensitizes microvascular inflammation in diabetic hearts, and, in addition, plays a critical role in the progression of myocardial fibrosis.
Ang-2 is produced by endothelial cells, and its expression is limited to sites of vascular remodeling (19, 25, 26, 30). Ang-2 is thought to stimulate angiogenesis by sensitization of endothelial cells to VEGF, a key factor that launches angiogenesis, including endothelial cell migration and proliferation (20). In a recent cross-sectional designed study, it was shown that diabetic patients with macrovascular complications, in particular those with cardiovascular disease, had higher serum levels of Ang-2 than those without macrovascular complications (24). Although plasma Ang-2 levels are increased in patients with diabetes with acute coronary syndrome (9, 16–18), the direct role of Ang-2 on angiogenesis in diabetic hearts has not been reported. Our findings that 1) Tie-2 expression is significantly decreased in db/db mouse hearts overexpressing Ang-2; 2) the increased Ang-2 is colocalized with cleaved caspase-3 and this is accompanied by a significant loss of capillary density in diabetic db/db mouse hearts; 3) exogenous Ang-2 exacerbates HG-induced caspase-3 activation and myocardial endothelial cell apoptosis; and 4) knockdown of Ang-2 significantly attenuates HG-induced endothelial cell apoptosis in vitro suggest that Ang-2 increases endothelial apoptosis via suppression of Tie-2 signaling and activation of caspase-3 pathway. These results implicate a critical role of Ang-2 in the progression of loss of capillary density in diabetes. Our present data demonstrate that overexpression of Ang-2 in diabetic hearts leads to a significant decrease in VEGF expression, accompanied by a more aggressive endothelial apoptosis and loss of capillary, implicating that increased Ang-2 after myocardial ischemia is responsible, at least in part, for the downregulation of VEGF and impairment of angiogenesis seen in diabetic db/db mouse ischemic hearts (4, 5). Given the importance of Ang-2/VEGF in the regulation of angiogenesis and disease progression of diabetes, therefore, balancing Ang-2/VEGF should be considered as a novel therapeutic strategy in treatment of diabetes-associated impairment of angiogenesis.
Although hyperglycemia-induced Ang-2 expression and enhanced endothelial cell apoptosis and loss of capillary have been observed in our previous in vitro and in vivo studies (31, 32), the intracellular molecular mechanisms by which Ang-2 mediates endothelial cell apoptosis and loss of capillary in diabetes are not completely understood. Wnt signaling is required for different aspects of cardiac and vascular development. Studies show that inhibition of Wnt signaling leads to increased angiogenesis and attenuated cardiac hypertrophy (2, 10, 33–35). Inhibition of Wnt signaling by Wnt pathway antagonist FrzA led to a significant decrease in myocardial apoptosis (1). Accumulating evidence demonstrates that the Wnt7b stimulates cell cycle entry of vascular endothelial cells and couples apoptosis and programmed cell death (21). Endothelial cell apoptosis was dependent on Wnt7b-stimulated entry to the cell cycle and transit through the restriction point in mid-G1 phase (21, 23). Ang-2-induced endothelial cell death and vessel regression in the hyaloid of the eye is mediated, in part, by suppression of Tie-2/Akt survival signaling in endothelial cells and upregulation Wnt7b in the macrophage (23). Moreover, treatment with Ang-2 dramatically increased Wnt7b expression together with endothelial apoptosis and vessel regression, whereas treatment with Ang-1 significantly decreased Wnt7b expression (15). Consistent with these studies, our present study reveals that Ang-2 suppresses Tie-2 and VEGF and increases Wnt7b expression as well as endothelial apoptosis and loss of capillary in diabetic hearts. Overexpression of Ang-1 reverses diabetes-associated myocardial apoptosis and loss of capillary. Both of these paradigms implicate the angiopoietins/Tie-2-VEGF-Wnt7b pathway as a potential target for the treatment of diabetic capillary rarefaction. Further studies are needed to investigate the role of VEGF-Wnt7b in diabetes-associated loss of capillary density.
Diabetic cardiomyopathy is characterized by a progressive fibrosis formation, which also plays a key role in loss of capillary density in the disease progression of diabetes (11). Overexpression of Ang-2 in the mouse heart has been shown to lead to myocardial fibrosis (36). Consistent with these findings, our present study demonstrates that excessive Ang-2 increases FSP-1 expression and promotes cardiac fibrosis. In contrast, overexpression of Ang-1 reversed diabetes-induced cardiac fibrosis formation and prevented loss of capillary in diabetic hearts. The findings suggest that Ang-1 operates as a counterbalance to Ang-2 in diabetes. Our present study demonstrates that not only does excessive Ang-2 impair angiogenesis, it also promotes diabetes-associated microvascular inflammation by sensitizing endothelial adhesion molecules. Taken together, our data implicate that Ang-1 and Ang-2, as agonists and antagonists of Tie-2, have opposite effects on the diabetic cardiac fibrosis and angiogenesis. Perturbing equilibrium of angiopoietins/Tie-2 sensitizes diabetic inflammatory response and promotes the diabetic disease progression.
Our present study suggests that an excess of Ang-2 promotes microvascular inflammation and cardiac fibrosis results in loss of capillary in diabetes. Results from the present studies provide the foundation for exploitation of the angiopoietins/Tie-2 system, especially a targeted reduction in Ang-2, to ameliorate or reverse the diabetic abnormal vessel remodeling and microvascular rarefaction that characterizes the diabetic state.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-102042 (to J. X. Chen) HL-077395 and HL096967 (to J. Reese).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.-X.C. conception and design of research; J.-X.C. analyzed data; J.-X.C. interpreted results of experiments; J.-X.C. prepared figures; J.-X.C. drafted manuscript; J.-X.C., J.R., J.L.A., and B.M. edited and revised manuscript; J.-X.C., J.R., J.L.A., and B.M. approved final version of manuscript; H.Z. performed experiments.
REFERENCES
- 1. Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D, Duplaa C. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation 108: 2282–2289, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Blankesteijn WM, van de Schans VA, ter Horst P, Smits JF. The Wnt/frizzled/GSK-3 beta pathway: a novel therapeutic target for cardiac hypertrophy. Trends Pharmacol Sci 29: 175–180, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bureau W, Van SP, Jones J, Han RN, Ward NL, Stewart DJ, Dumont DJ. Chronic systemic delivery of angiopoietin-2 reveals a possible independent angiogenic effect. Am J Physiol Heart Circ Physiol 291: H948–H956, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Chen JX, Stinnett A. Ang-1 gene therapy inhibits hypoxia-inducible factor-1alpha (HIF-1alpha)-prolyl-4-hydroxylase-2, stabilizes HIF-1alpha expression, and normalizes immature vasculature in db/db mice. Diabetes 57: 3335–3343, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol 28: 1606–1613, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen JX, Zeng H, Lawrence ML, Blackwell TS, Meyrick B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol 291: H1563–H1572, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chen JX, Zeng H, Tuo QH, Yu H, Meyrick B, Aschner JL. NADPH oxidase modulates myocardial Akt, ERK1/2 activation, and angiogenesis after hypoxia/reoxygenation. Am J Physiol Heart Circ Physiol 292: H1664–H1674, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chong AY, Caine GJ, Freestone B, Blann AD, Lip GY. Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor tie-2 levels in congestive heart failure. J Am Coll Cardiol 43: 423–428, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Chung NA, Makin AJ, Lip GY. Measurement of the soluble angiopoietin receptor tie-2 in patients with coronary artery disease: development and application of an immunoassay. Eur J Clin Invest 33: 529–535, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Dufourcq P, Couffinhal T, Ezan J, Barandon L, Moreau C, Daret D, Duplaa C. FrzA, a secreted frizzled related protein, induced angiogenic response. Circulation 106: 3097–3103, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Fein FS. Diabetic cardiomyopathy. Diabetes Care 13: 1169–1179, 1990 [DOI] [PubMed] [Google Scholar]
- 12. Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12: 235–239, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284: 1994–1998, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 18: 5356–5362, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Lee HJ, Ahn BJ, Shin MW, Jeong JW, Kim JH, Kim KW. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ 16: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Lee KW, Lip GY, Blann AD. Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation 110: 2355–2360, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Lim HS, Blann AD, Chong AY, Freestone B, Lip GY. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care 27: 2918–2924, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Lim HS, Lip GY, Blann AD. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis 180: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Lin TN, Wang CK, Cheung WM, Hsu CY. Induction of angiopoietin and Tie receptor mRNA expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 20: 387–395, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA 99: 11205–11210, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437: 417–421, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Messaoudi S, Milliez P, Samuel JL, Delcayre C. Cardiac aldosterone overexpression prevents harmful effects of diabetes in the mouse heart by preserving capillary density. FASEB J 23: 2176–2185, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Rao S, Lobov IB, Vallance JE, Tsujikawa K, Shiojima I, Akunuru S, Walsh K, Benjamin LE, Lang RA. Obligatory participation of macrophages in an angiopoietin 2-mediated cell death switch. Development 134: 4449–4458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rasul S, Reiter MH, Ilhan A, Lampichler K, Wagner L, Kautzky-Willer A. Circulating angiopoietin-2 and soluble Tie-2 in type 2 diabetes mellitus: a cross-sectional study. Cardiovasc Diabetol 10: 55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ray PS, Estrada-Hernandez T, Sasaki H, Zhu L, Maulik N. Early effects of hypoxia/reoxygenation on VEGF, ang-1, ang-2 and their receptors in the rat myocardium: implications for myocardial angiogenesis. Mol Cell Biochem 213: 145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Ray PS, Sasaki H, Estrada-Hernandez T, Zu L, Maulik N. Effects of hypoxia/reoxygenation on angiogenic factors and their tyrosine kinase receptors in the rat myocardium. Antioxid Redox Signal 3: 89–102, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Reiss Y, Knedla A, Tal AO, Schmidt MH, Jugold M, Kiessling F, Burger AM, Wolburg H, Deutsch U, Plate KH. Switching of vascular phenotypes within a murine breast cancer model induced by angiopoietin-2. J Pathol 217: 571–580, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, Marra C, Nasti R, Marfella R, Cozzolino D, Indolfi C, Cotrufo M, Torella R, Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol 46: 827–834, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Schiekofer S, Galasso G, Sato K, Kraus BJ, Walsh K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler Thromb Vasc Biol 25: 1603–1609, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Shyu KG, Chang CC, Wang BW, Kuan P, Chang H. Increased expression of angiopoietin-2 and Tie2 receptor in a rat model of myocardial ischaemia/reperfusion. Clin Sci (Lond) 105: 287–294, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Tuo QH, Xiong GZ, Zeng H, Yu HD, Sun SW, Ling HY, Zhu BY, Liao DF, Chen JX. Angiopoietin-1 protects myocardial endothelial cell function blunted by angiopoietin-2 and high glucose condition. Acta Pharmacol Sin 32: 45–51, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tuo QH, Zeng H, Stinnett A, Yu H, Aschner JL, Liao DF, Chen JX. Critical role of angiopoietins/Tie-2 in hyperglycemic exacerbation of myocardial infarction and impaired angiogenesis. Am J Physiol Heart Circ Physiol 294: H2547–H2557, 2008 [DOI] [PubMed] [Google Scholar]
- 33. van Gijn ME, Daemen MJ, Smits JF, Blankesteijn WM. The wnt-frizzled cascade in cardiovascular disease. Cardiovasc Res 55: 16–24, 2002 [DOI] [PubMed] [Google Scholar]
- 34. van dS V, Smits JF, Blankesteijn WM. The Wnt/frizzled pathway in cardiovascular development and disease: friend or foe? Eur J Pharmacol 585: 338–345, 2008 [DOI] [PubMed] [Google Scholar]
- 35. van dS V, van den Borne SW, Strzelecka AE, Janssen BJ, van d V, Langen RC, Wynshaw-Boris A, Smits JF, Blankesteijn WM. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension 49: 473–480, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF). Proc Natl Acad Sci USA 99: 8219–8224, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 111: 2073–2085, 2005 [DOI] [PubMed] [Google Scholar]