Abstract
In heart failure (HF), arrhythmogenic Ca2+ release and chronic Ca2+ depletion of the sarcoplasmic reticulum (SR) arise due to altered function of the ryanodine receptor (RyR) SR Ca2+-release channel. Dantrolene, a therapeutic agent used to treat malignant hyperthermia associated with mutations of the skeletal muscle type 1 RyR (RyR1), has recently been suggested to have effects on the cardiac type 2 RyR (RyR2). In this investigation, we tested the hypothesis that dantrolene exerts antiarrhythmic and inotropic effects on HF ventricular myocytes by examining multiple aspects of intracellular Ca2+ handling. In normal rabbit myocytes, dantrolene (1 μM) had no effect on SR Ca2+ load, postrest decay of SR Ca2+ content, the threshold for spontaneous Ca2+ wave initiation (i.e., the SR Ca2+ content at which spontaneous waves initiate) and Ca2+ spark frequency. In cardiomyocytes from failing rabbit hearts, SR Ca2+ load and the wave initiation threshold were decreased compared with normal myocytes, Ca2+ spark frequency was increased, and the postrest decay was potentiated. Using a novel approach of measuring cytosolic and intra-SR Ca2+ concentration (using the low-affinity Ca2+ indicator fluo-5N entrapped within the SR), we showed that treatment of HF cardiomyocytes with dantrolene rescued postrest decay and increased the wave initiation threshold. Additionally, dantrolene decreased Ca2+ spark frequency while increasing the SR Ca2+ content in HF myocytes. These data suggest that dantrolene exerts antiarrhythmic effects and preserves inotropy in HF cardiomyocytes by decreasing the incidence of diastolic Ca2+ sparks, increasing the intra-SR Ca2+ threshold at which spontaneous Ca2+ waves occur, and decreasing the loss of Ca2+ from the SR. Furthermore, the observation that dantrolene reduces arrhythmogenicity while at the same time preserves inotropy suggests that dantrolene is a potentially useful drug in the treatment of arrhythmia associated with HF.
Keywords: arrhythmogenesis, calcium waves, excitation-contraction coupling, ryanodine receptor
the ryanodine receptor (RyR) is an intracellular Ca2+ release channel located on the sarcoplasmic reticulum (SR)/endoplasmic reticulum Ca2+ store (42). There are three known mammalian isoforms of the RyR (RyR1, RyR2, and RyR3), each with a distinct pattern of cellular and tissue expression and functional properties. RyR1 is the predominant isoform found in skeletal muscle (18), whereas RyR2 is the most abundant in cardiac tissues (2). RyRs respond to increased cytosolic Ca2+ concentration ([Ca2+]i), leading to the release of Ca2+ from intracellular stores in a process termed Ca2+-induced Ca2+ release (CICR). The RyR channels have been well characterized at both the cellular and single channel levels, providing detailed insight into their biophysical and physiological properties (16, 19). Numerous mutations of the RyR channel have been implicated in disease states, emphasizing its central role in regulating cellular Ca2+ homeostasis (25, 27, 49).
The drug dantrolene is a skeletal muscle relaxant (15) that acts by stabilizing uncontrolled SR Ca2+ release during excitation-contraction (E-C) coupling. E-C coupling describes the sequence of events where action potential (AP) depolarization of the cell membrane results in an increase in [Ca2+]i, which activates Ca2+-sensitive contractile proteins in muscle cells. Dantrolene is a well-established treatment for malignant hyperthermia, a skeletal muscle condition associated with mutations of RyR1 (17), where it has been used clinically at concentrations of ∼10 μM (27, 48). Treatment with the drug suppresses the life-threatening symptoms of malignant hyperthermia (27) that are triggered by volatile anesthetics in susceptible patients. Dantrolene has been shown to bind to amino acids 590–609 of RyR1, which presumably stabilizes the channel protein, thus providing evidence for a direct action of dantrolene on RyRs (32). This stabilizing effect inhibits aberrant activation of the channel and prevents excessive Ca2+ release from intracellular stores. Furthermore, dantrolene binds to the corresponding sequence (amino acids 601–620) of RyR2 (33, 47) and has recently been proposed to have effects on cardiac RyR2 (24, 50); however, no effect of the drug on the properties of native RyR2 channels has been observed in single channel analysis (12). Recent evidence has suggested that dantrolene may ameliorate abnormal RyR2-mediated Ca2+ release associated with heart failure (HF) (24, 31). In HF (7), it has been hypothesized that interdomain interactions between the NH2-terminal and central domains of the RyR2 channel become destabilized or unzipped [possibly through posttranslational modifications such as phosphorylation (1, 30) or oxidation (44)], thus contributing to channel dysfunction and altering cellular Ca2+-handling properties. Ca2+ leak from the RyR during diastole increases, along with a higher propensity for RyR-mediated activation of potentially arrhythmogenic spontaneous Ca2+ waves (13, 40). Additionally, Ca2+ leak through RyR2 contributes to the net loss of Ca2+ from the cell as this Ca2+ is subsequently extruded via Na+/Ca2+ exchanger (NCX) (37). The loss of Ca2+ from the cell decreases SR Ca2+ content and subsequently weakens myocyte contraction as less Ca2+ is available for beat-to-beat activation of the contractile proteins (26, 35). Recent investigations have suggested that dantrolene may correct the abnormal RyR2-mediated Ca2+ release associated with HF by stabilizing the interdomain interactions of RyR2 and preventing diastolic SR Ca2+ release (23, 24, 31).
In this investigation, we tested the hypothesis that dantrolene exerts potentially antiarrhythmic and positive inotropic effects in HF by examining multiple aspects of intracellular Ca2+ handling in ventricular myocytes, specifically those mediated by the RyR. We monitored the intra-SR Ca2+ concentration ([Ca2+]SR) to gain direct insights into SR Ca2+ handling in normal and HF myocytes before and after dantrolene treatment. Using a novel approach of measuring [Ca2+]i and [Ca2+]SR directly, we found that dantrolene exerts antiarrhythmic effects in HF cardiomyocytes by decreasing the occurrence of spontaneous SR Ca2+-release events while at the same time preserving inotropy. This makes dantrolene a potentially useful drug in the treatment of HF, particularly through its antiarrhythmic effects.
METHODS
Solutions and chemicals.
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Fluorescent Ca2+ indicator dyes were purchased from Molecular Probes/Invitrogen (Carlsbad, CA). Dantrolene stock solutions were made at a concentration of 10 mM in DMSO and used at a working concentration of 1 μM. Dantrolene was applied 5 min before the beginning of recording and was present throughout the experiment. Tyrode solution contained (in mM) 130 NaCl, 4 KCl, 7 CaCl2, 1 MgCl2, 10 d-glucose, and 10 HEPES; pH 7.4 with NaOH. Basal Ca2+ spark rates and the propensity for spontaneous Ca2+ waves are extremely low in intact rabbit ventricular myocytes. Therefore, all experiments were performed in elevated extracellular Ca2+ concentration.
Myocyte isolation.
Ventricular myocytes were isolated from New Zealand White rabbits (25 animals, 2.5 kg, Myrtle's Rabbitry, Thompsons Station, TN). Rabbits were anesthetized with pentobarbital sodium (50 mg/kg), and hearts were excised and mounted on a Langendorff apparatus. Hearts were retrogradely perfused with nominally Ca2+-free Tyrode solution for 5 min followed by minimal essential medium Eagle (MEM) solution containing 20 μM Ca2+ and 45 μg/ml Liberase Blendzyme TH (Roche Applied Science, Indianapolis, IN) for 20 min at 37°C. The left ventricular free wall was removed from the heart and digested for an additional 5 min in the enzyme solution at 37°C. Digested tissue was then minced, filtered, and washed in MEM solution containing 50 μM Ca2+ and 10 mg/ml BSA. Isolated cells were kept in MEM solution with 50 μM Ca2+ at room temperature (22–24°C) until indicator dye loading and subsequent experimentation. In addition, left ventricular myocytes were isolated from rabbits with nonischemic HF (n = 5) induced by combined pressure and volume overload and characterized by the combination of gradual HF development (over 4–9 mo, monitored by serial echocardiography), depressed systolic function, and arrhythmogenesis in an animal that has human-like Ca2+ handling and elecrophysiological properties (36). This model has been extensively studied and is well characterized at structural, biochemical, molecular, Ca2+-handling, and electrophysiological levels (11, 13, 36–38, 40, 51). All protocols were approved by the Institutional Animal Care and Use Committee.
Ca2+ concentration measurements.
To directly monitor [Ca2+]SR, the SR was loaded with the low-affinity Ca2+ indicator fluo-5N by incubation of ventricular myocytes with 10 μM of membrane-permeable fluo-5N AM together with 0.25% Pluronic F-127 in nominally Ca2+-free Tyrode solution for 2.5 h followed by a 30-min wash, all at 37°C. For measurements of [Ca2+]i, myocytes were incubated at room temperature with either 5 μM indo-1 AM or 20 μM fluo-4 AM for 20 min followed by a 20-min wash.
Global [Ca2+]i was monitored in single intact myocytes using the ratiometric dye indo-1, with dye excitation at 360 ± 20 nm and emission collected simultaneously (sampling frequency of 100 Hz) at 410 ± 20 nm (F410) and 485 ± 25 nm (F485). F410 and F485 signals were background subtracted, and changes in [Ca2+]i are expressed as follows: R = F410/F485, where R is the ratio of the fluorescence signals F410 and F485. The AP-induced Ca2+ transient amplitude was defined as follows: ΔR = Rpeak − Rdiast, where Rpeak is the peak R signal and Rdiast is the minimum R signal before the Ca2+ transient. The caffeine-induced Ca2+ transient amplitude was defined as ΔR = Rpeak − Rrest, where Rrest refers to the steady-state R signal preceding the caffeine-induced Ca2+ transient.
Confocal microscopy (Nikon A1R, Nikon, Melville, NY) was used to image [Ca2+]SR (fluo-5N) and Ca2+ sparks (fluo-4), with both dyes excited at 488 nm and emission collected at >500 nm. [Ca2+]SR measurements were acquired using high-speed frame scan (two-dimensional) imaging (60 fps, A1R resonant scanner), and the fluorescence signal was integrated over the entire area of the cell. Ca2+ spark measurements were acquired from intact myocytes during rest from 1-Hz stimulation in line-scan mode at 2 ms/line with a pixel size of 0.12 μm. All fluorescence signals were background subtracted. Changes in [Ca2+]i are expressed as ΔF/F0, where ΔF is the change in fluorescence [measured fluorescence (F) − F0] and F0 is resting baseline fluo-4 fluorescence. Changes in SR Ca2+ are expressed as follows: [Ca2+]SR = (F − Fmin)/(Fmax − Fmin). Fmin is the intra-SR fluorescence in the absence of Ca2+, which was obtained after the complete emptying of SR Ca2+ with 10 mM caffeine. Fmax is the diastolic fluorescence in the presence of 1 μM isoproterenol during 1-Hz pacing. In the presence of isoproterenol, [Ca2+]SR increases to levels where intra-SR fluo-5N is nearly saturated with Ca2+, and this therefore approximates maximal fluo-5N fluorescence (39).
For all Ca2+ concentration measurements, cells were placed on laminin-coated coverslips. APs and global Ca2+ transients were elicited by electrical field stimulation using a pair of platinum electrodes (voltage set at ∼50% above the threshold for contraction). Experiments were conducted at room temperature (22–24°C).
Data analysis and statistics.
Ca2+ spark frequency [quantified as the number of sparks per second per 100 μm of scanned distance: (sparks·s−1·(100 μm)−1)], amplitude, spatial width (full width at half-maximum and full width), duration (full duration at half-maximum and full duration), time to peak, maximum steepness of the upstroke, and decay kinetics were calculated using the SparkMaster algorithm (34). Statistical comparisons were made using Student's t-test for paired or unpaired data, the nonparametric Mann-Whitney U-test for two samples, and the Wilcoxon signed-rank test for paired observations, with statistical significance set at P < 0.05. Data are presented as individual observations or as means ± SE of n measurements, where n is the number of cells.
RESULTS
Effects of dantrolene on basal [Ca2+]i, Ca2+ release, and SR content in normal and HF ventricular myocytes.
Resting [Ca2+]i was determined with the ratiometric Ca2+ indicator indo-1. In ventricular myocytes from failing hearts (referred to as HF myocytes), resting [Ca2+]i was significantly higher (normal myocytes: R = 1.37 ± 0.02, n = 21; HF myocytes: R = 1.65 ± 0.03, n = 13, P < 0.001); however, dantrolene had no effect on resting [Ca2+]i, in either normal myocytes (R = 1.35 ± 0.02, n = 16) or HF myocytes (R = 1.64 ± 0.03, n = 12).
Cytosolic Ca2+ transients elicited by field stimulation (1 Hz) were significantly reduced in amplitude (ΔR) in HF myocytes compared with normal cells (P < 0.002; Fig. 1); however, dantrolene treatment had no significant effect on the transient amplitude (Fig. 1C,a). Furthermore, dantrolene had no effect on the kinetics of the Ca2+ transient [normalized averaged Ca2+ transients at an expanded timescale in the presence and absence of dantrolene are shown in Fig. 1, A (normal myocytes) and B (HF myocytes), right].
Fig. 1.
Effects of dantrolene on Ca2+ release and sarcoplasmic reticulum (SR) content in normal and heart failure (HF) ventricular myocytes. A and B, left: representative traces of normal (A) or HF (B) myocytes field stimulated at 1 Hz under control (CTL) conditions or after dantrolene (DAN) treatment and subsequent caffeine (10 mM) application. [Ca2+]i, cytosolic Ca2+ concentration; R, ratio of fluorescence at 410 to 485 nm. Right, averaged and amplitude-normalized Ca2+ transients on an expanded timescale under CTL conditions (black traces) and in the presence of DAN (gray traces). Here and in the following figures, the placement of the solid horizontal bar for caffeine application indicates the point at which solutions were changed and includes a slight delay due to the time it takes for the solution to reach the myocyte. C: summary data of Ca2+ transient amplitudes (a), SR Ca2+ load (b), and fractional release (c). ΔR, change in R; ΔRTwitch/ΔRCaffeine; change in the R signal during twitch/during caffeine treatment. Normal: n = 23; HF: n = 13. *P < 0.005.
To determine the effect of dantrolene on SR Ca2+ load in both normal and HF myocytes, SR Ca2+ was assessed by the application of 10 mM caffeine to empty the SR. The amplitude of the caffeine-induced cytosolic Ca2+ transient (ΔR) served as a quantitative measure of SR Ca2+ content. In normal myocytes (Fig. 1A), dantrolene had no significant effect on SR Ca2+ load (Fig. 1C,b). However, in HF myocytes (Fig. 1B), dantrolene significantly increased the SR Ca2+ load (Fig. 1C,b). Overall, in HF cardiomyocytes, the SR Ca2+ load was significantly diminished compared with normal cells (P < 0.002). In both normal and HF myocytes, the effect of dantrolene on the Ca2+ transient amplitude and SR Ca2+ load at 0.5-Hz pacing was qualitatively the same as observed during 1-Hz pacing (data not shown).
Fractional release refers to the nonlinear relationship between SR Ca2+ release and SR Ca2+ load and serves as a quantitative measure for the efficiency of a given Ca2+ trigger (L-type Ca2+ current) to elicit CICR from the SR (3, 41). Fractional release was quantified as the AP-induced Ca2+ transient amplitude (ΔR) divided by the amplitude of the caffeine-induced Ca2+ transient. HF myocytes had significantly higher fractional release compared with normal myocytes (P < 0.05; Fig. 1C,c), indicative of a compensatory mechanism to partially sustain SR Ca2+ release and contractile strength in HF despite the decrease in SR Ca2+ content (13, 20, 40). Dantrolene had no effect on fractional release in normal myocytes. In HF myocytes, dantrolene caused a decrease in fractional release to levels near that observed in normal cells while not compromising inotropy (i.e., no significant change in the Ca2+ transient amplitude).
SR Ca2+ content was also measured directly in cells during electrical stimulation (1 Hz) with a novel approach using the low-affinity Ca2+ indicator fluo-5N entrapped within the SR (Fig. 2). In myocytes from failing hearts, diastolic SR Ca2+ levels ([Ca2+]SR) were significantly decreased compared with normal cells (P < 0.001), and treatment with dantrolene significantly increased diastolic [Ca2+]SR in HF myocytes (Fig. 2C,a) while having no effect in normal myocytes, thus confirming the results obtained with indo-1 (Fig. 1). Additionally, fractional release was determined from the fluo-5N measurements as the depletion amplitude (change in [Ca2+]SR during a twitch) divided by diastolic SR Ca2+ level. Fractional release was significantly increased in HF myocytes compared with control myocytes (P < 0.005), and dantrolene again decreased it near to levels seen in normal cells (Fig. 2C,b), replicating the data obtained with indo-1 (Fig. 1). The changes in SR load and fractional release in HF were consistent with previous data from our laboratory (13).
Fig. 2.
DAN increases SR Ca2+ load in HF myocytes as revealed by the direct measurement of SR Ca2+ concentration ([Ca2+]SR). A and B: representative global [Ca2+]SR levels of normal (A) or HF (B) myocytes field stimulated at 1 Hz under CTL conditions or after DAN treatment. F, measured fluorescence; Fmin, intra-SR fluorescence in the absence of Ca2+; Fmax, diastolic fluorescence in the presence of 1 μM isoproterenol during 1-Hz pacing. C: summary data of diastolic SR Ca2+ levels ([Ca2+]SR; a) and fractional release (b). Δ[Ca2+]SR,twitch, change in [Ca2+]SR during twitch; [Ca2+]SR,diast, diastolic [Ca2+]SR. Normal: n = 6; HF: n = 6. *P < 0.02.
Dantrolene decreases the frequency of Ca2+ sparks in HF myocytes.
Ca2+ sparks are elementary SR Ca2+-release events through a cluster of RyRs that summate in time and space to form the global SR Ca2+ transient during E-C coupling (10). However, during diastole, they represent a major pathway for leak of Ca2+ from the SR (21, 40, 51) and pose as a potential substrate for the activation of arrhythmogenic Ca2+ waves (4). Figure 3 shows example confocal line-scan images and corresponding local fluo-4 fluorescence profiles (ΔF/F0) of Ca2+ sparks from intact normal (A) and HF (B) myocytes under control conditions and after dantrolene application. Dantrolene had no effect on Ca2+ spark frequency, amplitude, width, or duration in normal cells (Fig. 3C,a–d). HF myocytes, however, exhibited a significantly increased spark frequency compared with normal cells (P < 0.001; Fig. 3D,a), consistent with previously published reports (13, 40, 51). Treatment with dantrolene significantly decreased spark frequency in HF myocytes to values that were lower than normal myocytes and had no significant effect on the other spark parameters shown (Fig. 3D,b–d). Provided that Ca2+ sparks represent an arrhythmogenic substrate for the activation of Ca2+ waves (4), these results indicate an antiarrhythmogenic effect of dantrolene on HF myocytes. The increased Ca2+ spark frequency in HF myocytes is consistent with the lower SR Ca2+ load observed in these cells (consequence of increased spark-mediated Ca2+ leak), and the inhibition of Ca2+ sparks after dantrolene treatment is further consistent with the significant increase in SR Ca2+ load observed in HF cells in the presence of the drug (Figs. 1C,b and 2C,a).
Fig. 3.
DAN decreases Ca2+ spark frequency in HF myocytes. A and B: examples of line-scan images from intact normal (A) and HF (B) myocytes under CTL conditions or after DAN application (top) and the corresponding fluorescence profiles (ΔF/F0) of Ca2+ sparks recorded from the regions marked by the black boxes (bottom). C and D: summary data for normal (C) or HF myocytes (D) of spark frequency (a), amplitude (b), full width at half-maximum (FWHM; c), and full duration at half-maximum (FDHM; d). Normal: n = 9; HF: n = 6. *P < 0.001.
Dantrolene inhibits postrest decay in HF myocytes.
Postrest decay of SR Ca2+ content is a phenomenon observed in rabbit cardiomyocytes throughout periods of prolonged rest from stimulation. Ca2+ that leaks out of the SR during rest (9, 29) is subsequently extruded from the cell, leading to a net loss of SR and cellular Ca2+. Here, we used the parameter of postrest decay of SR Ca2+ content as a measure of SR Ca2+ leak during rest. To assess the effects of dantrolene on postrest decay in normal and HF conditions, cells were field stimulated at 1 Hz, and SR Ca2+ load was assessed by the application of 10 mM caffeine after 2 s and after 60 s of rest (protocol shown in Fig. 4A). In normal myocytes (Fig. 4B), the postrest decay of SR Ca2+ content was minimal and the caffeine-induced Ca2+ transient amplitude (ΔR) after 60 s of rest amounted to >90% of the amplitude observed immediately (2 s) after the cessation of pacing (Fig. 4D,a). Dantrolene had no effect on the postrest decay in normal myocytes (Fig. 4, B and D,a). In contrast, in HF myocytes, the postrest decay was significantly enhanced compared with control myocytes (P < 0.001; Fig. 4, C and D,b); however, dantrolene treatment effectively restored the postrest decay to levels observed in normal myocytes. These results indicate that dantrolene prevents the leak of Ca2+ from the SR and preserves SR Ca2+ content during rest in HF myocytes.
Fig. 4.
DAN inhibits postrest decay in HF myocytes. A: protocol used to determine the rest decay of SR Ca2+ content. B and C: representative caffeine-induced transients recorded after 2 s (left) and 60 s (right) of rest after 1-Hz pacing from normal (B) or HF (C) myocytes under CTL conditions or after DAN treatment. D: summary graphs with data presented as a percentage of the original content remaining after 60 s of rest, where t0 is the SR Ca2+ content determined 2 s after the cessation of pacing from normal (a) or HF (b) myocytes. Normal: n = 7; HF: n = 5. *P < 0.001.
Additionally, the rest decay of [Ca2+]SR was measured directly using fluo-5N. To quantify the postrest decay of [Ca2+]SR with this approach, diastolic fluo-5N fluorescence during 1-Hz pacing was compared with remaining fluorescence after 60 s of rest (paired measurements). In normal myocytes (Fig. 5A), the postrest decay of [Ca2+]SR was small (Fig. 5C,a) and there was no significant change when control and dantrolene-treated cells were compared. In contrast, HF myocytes (Fig. 5B) exhibited decreased diastolic [Ca2+]SR levels and the postrest decay of SR Ca2+ content was accelerated. Dantrolene significantly slowed the postrest decay in HF myocytes (Fig. 5C,b). These data further support the notion that in HF myocytes, dantrolene is capable of maintaining SR Ca2+ content by reducing SR Ca2+ leak during rest.
Fig. 5.
Direct measurement of [Ca2+]SR showing that DAN inhibits the postrest decay in HF myocytes. A and B: representative global [Ca2+]SR levels during and after 60 s of rest after 1-Hz field stimulation of normal (A) and HF (B) myocytes under CTL conditions or after DAN treatment. C: summary graphs of normal (a) or HF (b) myocytes. Values are the average percentage of SR Ca2+ remaining after 60 s of rest, where t0 is the diastolic SR Ca2+ level with 1-Hz pacing. Paired experiments were used. Normal: n = 6; HF: n = 4. *P < 0.001.
Dantrolene increases the Ca2+ wave threshold in HF myocytes.
Ca2+ waves are proarrhythmic Ca2+-release events that often occur under SR Ca2+ overload conditions when spontaneous release of Ca2+ from the SR is activated after a distinct threshold level of [Ca2+]SR is reached (14, 28, 43, 46, 52, 53). In the following experiments, we tested for potential effects of dantrolene on the [Ca2+]SR wave threshold in normal and failing myocytes. Spontaneous Ca2+ waves were induced in normal and HF myocytes by incrementally (0.1-Hz steps) increasing pacing frequency in the presence of 7 mM extracellular Ca2+ to facilitate loading of the SR. A period of pacing at a given frequency was followed by 10 s of rest, during which the occurrence of spontaneous Ca2+ waves was monitored. As shown in the protocol shown in Fig. 6A, we determined with this approach, for each individual cell tested, the highest stimulation frequency that failed to result in Ca2+ waves during the subsequent rest period and the lowest frequency at which a Ca2+ wave could be elicited. In separate pacing trains, SR Ca2+ content was measured at these respective frequencies using an application of 10 mM caffeine. In the example shown in Fig. 6A,a, the myocyte was stimulated at 0.6 Hz, pacing was stopped, and no wave was observed in the ensuing rest period. Pacing was resumed at 0.7 Hz and then stopped, and a spontaneous Ca2+ wave was observed during the rest period (Fig. 6A,b). Subsequently, SR Ca2+ loads were assessed by 10 mM caffeine application at these two pacing frequencies (Fig. 6, A,c and d). Stimulation at 0.6 Hz resulted in a SR Ca2+ load that was just below the threshold for wave initiation since no wave was observed during the rest period (Fig. 6A,a; marked by the minus signs in Fig. 6B), whereas at 0.7-Hz pacing, SR Ca2+ content exceeded the Ca2+ wave threshold and a wave was observed (Fig. 6A,b; marked by the plus signs in Fig. 6B). Using this protocol, as shown in Fig. 6A, the loads essentially bracketed the actual wave threshold (i.e., the exact SR Ca2+ level where spontaneous waves occur). The experimental protocol was repeated in the absence and presence of dantrolene for both normal and HF myocytes. Our results show that HF myocytes had a significantly (P < 0.05) decreased wave threshold compared with normal cells (Fig. 6B, open bars in a vs. b). Dantrolene caused a significant increase of the wave threshold in HF myocytes (Fig. 6B,b) whereas in normal cells dantrolene had no effect (Fig. 6B,a). This increase was sufficient to result in a significant decrease in propensity for spontaneous Ca2+ waves. Consistent with a lack of effect of dantrolene on other parameters measured in normal cells (Figs. 1–5), the drug did not alter the wave threshold in normal myocytes.
Fig. 6.
DAN increases the Ca2+ wave threshold in HF myocytes. A: protocol used to determine the Ca2+ wave threshold (see text for details). ●, Spontaneous Ca2+ wave. B: summary data of caffeine-induced Ca2+ transient amplitudes ([Ca]i,Caffeine) just below (−) and above (+) the Ca2+ wave threshold. Normal: n = 5; HF: n = 3. *P < 0.05.
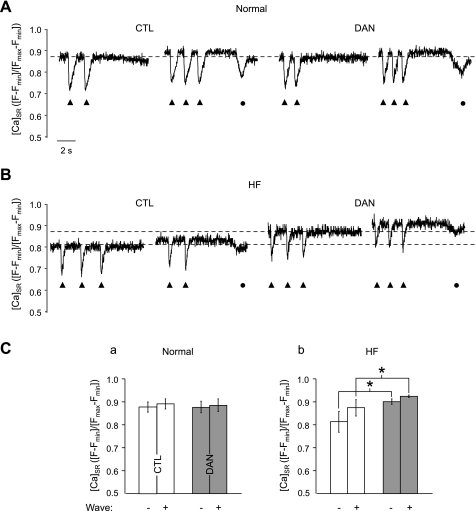
The wave threshold was also directly measured in terms of [Ca2+]SR using fluo-5N entrapped in the SR. Myocytes were field stimulated at increasing frequencies, and the activation of spontaneous Ca2+ waves upon the cessation of pacing was monitored for 10 s. The fluo-5N fluorescence traces shown in Fig. 7 include the last Ca2+ depletion transients triggered by APs (solid triangles). Spontaneous Ca2+ waves during the ensuing rest period are marked by filled circles in Fig. 7. Figure 7A shows that dantrolene had no effect on the [Ca2+]SR levels at which Ca2+ waves occurred in normal cells (summarized in Fig. 7C,a). In HF myocytes (Fig. 7B), however, the wave threshold was decreased compared with normal myocytes (Fig. 7C), but dantrolene significantly increased the wave threshold. Thus, dantrolene exerts antiarrhythmic effects in HF myocytes by increasing the SR Ca2+ wave threshold.
Fig. 7.
Direct measurement of [Ca2+]SR showing that DAN increases the Ca2+ wave threshold in HF myocytes. A and B: representative global [Ca2+]SR levels during field stimulation of normal (A) or HF (B) myocytes under CTL conditions or after DAN treatment. C: summary data of the average [Ca2+]SR just below (−) and above (+) the Ca2+ wave threshold in normal (a) and HF (b) myocytes. ▲, SR Ca2+ depletion transients induced by electrical field stimulation; ●, spontaneous Ca2+ waves. Normal: n = 7; HF: n = 5. *P < 0.001.
DISCUSSION
In HF, profound disturbances in Ca2+ signaling and E–C coupling lead to compromised contractility and an increased propensity of arrhythmia. In HF, the twitch Ca2+ transient amplitude and SR Ca2+ content are typically reduced (8, 35) due to a combination of enhanced diastolic Ca2+ leak, lower sarco(endo)plasmic reticulum Ca2+-ATPase activity, diminished resequestration of Ca2+ into the SR, and enhanced Ca2+ extrusion from the cell via NCX (7, 13, 37, 40). The hypothesis of increased diastolic SR Ca2+ leak in HF was based on the observation of a higher RyR2 open probability measured in single channel lipid bilayer recordings (30), which was later confirmed at the level of intact myocytes by several groups (1, 5, 40, 51). Thus, these studies attested to a central role for RyR2 in the changes in Ca2+ signaling characteristic of HF. The results presented here show that dantrolene, a drug currently used for the treatment of malignant hyperthermia, is able to restore compromised cardiac RyR-mediated Ca2+ signaling in HF myocytes. The most striking effect of dantrolene was its ability to significantly decrease diastolic SR Ca2+-release events in the form of Ca2+ sparks and Ca2+ waves. Through inhibition of the SR Ca2+ depletion and cellular loss of Ca2+ that is typical in HF, dantrolene was able to prevent potentially deleterious spontaneous arrhythmogenic Ca2+ release and preserve cardiac inotropy presumably through the stabilization of RyR2 (23, 24). It has been proposed that during the progression of HF, the RyR2 channel becomes excessively leaky due to defective interdomain interactions (also referred to as unzipping of the domains) (23) and that dantrolene can act on RyR2 by restabilizing these intramolecular interactions (24). This model is consistent with our observation that dantrolene only had effects on Ca2+ signaling in HF myocytes but not in normal cells. Similarly, our data are consistent with the observation that dantrolene had no effect on single RyR2 channel currents in lipid bilayer experiments using SR microsomes isolated from normal rabbit hearts (12), presumably because these channels are from nondiseased animals and have stable interdomain interactions.
HF myocytes had a lower threshold for arrhythmogenic Ca2+ waves, i.e., the [Ca2+]SR at which Ca2+ waves are initiated are lower in HF myocytes compared with cells from normal hearts. This again implicates RyR dysfunction in this model of HF, where NCX upregulation (together with additional changes in Ca2+ signaling and E-C coupling) has been shown to lead to Ca2+-dependent delayed afterdepolarizations and functionally results in an increased propensity for arrhythmogenic Ca2+ waves despite a decrease in SR Ca2+ load (36–38). The effect that HF has on the wave threshold is still a topic of debate. It has been shown that HF myocytes have an unchanged wave threshold (38) and, conversely, that they have a decreased wave threshold (6). The latter is consistent with our results. While dantrolene had no effects on the Ca2+ wave threshold in normal myocytes, it significantly increased the threshold in HF myocytes and therefore exerts a profound antiarrhythmic effect on cellular Ca2+ cycling.
Dantrolene has also been shown to have beneficial effects in a transgenic mouse model of catecholaminergic polymorphic ventricular tachycardia (CPVT), an inherited arrhythmogenic disease associated with RyR2 mutations where destabilized or unzipped RyR2s cause disturbances of cardiac rhythm and sudden cardiac death. It has been shown that in CPVT, the affinity of calmodulin binding to RyR2 is reduced upon posttranslational modification by PKA phosphorylation (49). This seems to destabilize the channel and be a critical cause of the spontaneous local Ca2+-release events characterizing CPVT, which can be prevented by treatment with dantrolene (49). Furthermore, in a human CPVT mouse model, dantrolene prevented CPVT, presumably by inhibiting Ca2+ leak through RyR2, providing an in vivo antiarrhythmic effect of dantrolene (25).
In our study, dantrolene corrected two key adverse effects of Ca2+ handling in HF: it decreased SR Ca2+ leak (Ca2+ sparks), thus increasing SR Ca2+ load, and it reduced the propensity of proarrhythmic Ca2+-release events (Ca2+ sparks and Ca2+ waves) despite the increase in load. This is the first study to directly measure SR Ca2+ before and after dantrolene treatment in both normal and HF myocytes. In light of the observation that ∼50% of HF patients die from arrhythmogenesis (22), the finding that dantrolene reduces arrhythmogenicity without compromising inotropy makes dantrolene a potentially useful drug for the treatment of HF via its antiarrhythmic effects. Mechanistically, dantrolene can preserve inotropy while at the same time act as an antiarrhythmic agent, given that we have shown that it inhibits diastolic Ca2+ release but not systolic release in HF cells. Our data show that the fractional release in HF myocytes is decreased by dantrolene due to a significant increase in the SR Ca2+ content while not affecting transient amplitude, with the net effect being no change in inotropy. We showed that dantrolene inhibits RyR-mediated leak in the form of Ca2+ sparks, thus preventing the formation of an arrhythmogenic substrate in HF myocytes (4). It was interesting that in our rabbit HF model, we did not see significant inotropic effects during pacing-induced Ca2+ transients, an effect that was observed in a study in canine ventricular myocytes (24). However, in support of our data, a similar result as presented here with dantrolene (decreased diastolic release without compromising inotropy) was shown with tetracaine inhibition of RyR activity in rat ventricular myocytes challenged with isoproterenol (45). Furthermore, as dantrolene produces a strong inhibition of diastolic Ca2+-release events, this raises the possibility that dantrolene could be used in conjunction with positive inotropic agents that normally cause adverse effects due to the arrhythmogenesis associated with positive inotropy, increased SR Ca2+ content, and arrhythmogenic diastolic Ca2+ release. Therefore, in the failing heart, dantrolene can preserve inotropy while at the same time act as an antiarrhythmic agent.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-62231, HL-80101, and HL-101235 and by the Leducq Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.T.M., T.L.D., and L.A.B. conception and design of research; J.T.M. and T.L.D. performed experiments; J.T.M., T.L.D., and L.A.B. analyzed data; J.T.M., T.L.D., and L.A.B. interpreted results of experiments; J.T.M., T.L.D., and L.A.B. prepared figures; J.T.M., T.L.D., and L.A.B. drafted manuscript; J.T.M., T.L.D., and L.A.B. edited and revised manuscript; J.T.M., T.L.D., and L.A.B. approved final version of manuscript.
REFERENCES
- 1. Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97: 1314–1322, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Anderson K, Lai FA, Liu QY, Rousseau E, Erickson HP, Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem 264: 1329–1335, 1989 [PubMed] [Google Scholar]
- 3. Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol Cell Physiol 268: C1313–C1319, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bassani RA, Bers DM. Rate of diastolic Ca release from the sarcoplasmic reticulum of intact rabbit and rat ventricular myocytes. Biophys J 68: 2015–2022, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belevych A, Kubalova Z, Terentyev D, Hamlin RL, Carnes CA, Gyorke S. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J 93: 4083–4092, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, Carnes CA, Gyorke S. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res 90: 493–502, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 21: 380–387, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Bers DM, Despa S, Bossuyt J. Regulation of Ca2+ and Na+ in normal and failing cardiac myocytes. Ann NY Acad Sci 1080: 165–177, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Bers DM, MacLeod KT. Cumulative depletions of extracellular calcium in rabbit ventricular muscle monitored with calcium-selective microelectrodes. Circ Res 58: 769–782, 1986 [DOI] [PubMed] [Google Scholar]
- 10. Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262: 740–744, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation 105: 2543–2548, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Diaz-Sylvester PL, Porta M, Copello JA. Halothane modulation of skeletal muscle ryanodine receptors: dependence on Ca2+, Mg2+, and ATP. Am J Physiol Cell Physiol 294: C1103–C1112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Domeier TL, Blatter LA, Zima AV. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J Physiol 587: 5197–5209, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Domeier TL, Blatter LA, Zima AV. Changes in intra-luminal calcium during spontaneous calcium waves following sensitization of ryanodine receptor channels. Channels (Austin) 4: 87–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellis KO, Castellion AW, Honkomp LJ, Wessels FL, Carpenter JE, Halliday RP. Dantrolene, a direct acting skeletal muscle relaxant. J Pharm Sci 62: 948–951, 1973 [DOI] [PubMed] [Google Scholar]
- 16. Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev 82: 893–922, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Fill M, Coronado R, Mickelson JR, Vilven J, Ma JJ, Jacobson BA, Louis CF. Abnormal ryanodine receptor channels in malignant hyperthermia. Biophys J 57: 471–475, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fill M, Ma JJ, Knudson CM, Imagawa T, Campbell KP, Coronado R. Role of the ryanodine receptor of skeletal muscle in excitation-contraction coupling. Ann NY Acad Sci 560: 155–162, 1989 [DOI] [PubMed] [Google Scholar]
- 19. Fill M, Ramos J. Calcium regulation of single cardiac ryanodine receptor channels. J Muscle Res Cell Motil 25: 603–604, 2004 [PubMed] [Google Scholar]
- 20. Guo T, Ai X, Shannon TR, Pogwizd SM, Bers DM. Intra-sarcoplasmic reticulum free [Ca2+] and buffering in arrhythmogenic failing rabbit heart. Circ Res 101: 802–810, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Gyorke S, Carnes C. Dysregulated sarcoplasmic reticulum calcium release: potential pharmacological target in cardiac disease. Pharmacol Ther 119: 340–354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J 115: 869–875, 1988 [DOI] [PubMed] [Google Scholar]
- 23. Kobayashi S, Bannister ML, Gangopadhyay JP, Hamada T, Parness J, Ikemoto N. Dantrolene stabilizes domain interactions within the ryanodine receptor. J Biol Chem 280: 6580–6587, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, Xu X, Uchinoumi H, Okuda S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol 53: 1993–2005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Yamamoto T, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circ J 74: 2579–2584, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Lindner M, Erdmann E, Beuckelmann DJ. Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol 30: 743–749, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Loke J, MacLennan DH. Malignant hyperthermia and central core disease: disorders of Ca2+ release channels. Am J Med 104: 470–486, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Lukyanenko V, Subramanian S, Gyorke I, Wiesner TF, Gyorke S. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricular myocytes. J Physiol 518: 173–186, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacLeod KT, Bers DM. Effects of rest duration and ryanodine on changes of extracellular [Ca] in cardiac muscle from rabbits. Am J Physiol Cell Physiol 253: C398–C407, 1987 [DOI] [PubMed] [Google Scholar]
- 30. Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Ono M, Yano M, Hino A, Suetomi T, Xu X, Susa T, Uchinoumi H, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M. Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca2+ release in heart failure. Cardiovasc Res 87: 609–617, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paul-Pletzer K, Yamamoto T, Bhat MB, Ma J, Ikemoto N, Jimenez LS, Morimoto H, Williams PG, Parness J. Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor. J Biol Chem 277: 34918–34923, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Paul-Pletzer K, Yamamoto T, Ikemoto N, Jimenez LS, Morimoto H, Williams PG, Ma J, Parness J. Probing a putative dantrolene-binding site on the cardiac ryanodine receptor. Biochem J 387: 905–909, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol 293: C1073–C1081, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res 85: 38–46, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation 92: 1034–1048, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res 85: 1009–1019, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual β-adrenergic responsiveness. Circ Res 88: 1159–1167, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res 93: 40–45, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 93: 592–594, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Sheehan KA, Zima AV, Blatter LA. Regional differences in spontaneous Ca2+ spark activity and regulation in cat atrial myocytes. J Physiol 572: 799–809, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith JS, Imagawa T, Ma J, Fill M, Campbell KP, Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol 92: 1–26, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stokke MK, Briston SJ, Jolle GF, Manzoor I, Louch WE, Oyehaug L, Christensen G, Eisner DA, Trafford AW, Sejersted OM, Sjaastad I. Ca2+ wave probability is determined by the balance between SERCA2-dependent Ca2+ reuptake and threshold SR Ca2+ content. Cardiovasc Res 90: 503–512, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 103: 1466–1472, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Venetucci LA, Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Reducing ryanodine receptor open probability as a means to abolish spontaneous Ca2+ release and increase Ca2+ transient amplitude in adult ventricular myocytes. Circ Res 98: 1299–1305, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res 100: 105–111, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Wang R, Zhong X, Meng X, Koop A, Tian X, Jones PP, Fruen BR, Wagenknecht T, Liu Z, Chen SR. Localization of the dantrolene binding sequence near the FKBP binding site in the three-dimensional structure of the ryanodine receptor. J Biol Chem 286: 12202–12212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ward A, Chaffman MO, Sorkin Dantrolene EM. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in malignant hyperthermia, the neuroleptic malignant syndrome and an update of its use in muscle spasticity. Drugs 32: 130–168, 1986 [DOI] [PubMed] [Google Scholar]
- 49. Xu X, Yano M, Uchinoumi H, Hino A, Suetomi T, Ono M, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ikemoto N, Matsuzaki M. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem Biophys Res Commun 394: 660–666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao F, Li P, Chen SR, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J Biol Chem 276: 13810–13816, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Zima AV, Bovo E, Bers DM, Blatter LA. Ca2+ spark-dependent and -independent sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J Physiol 588: 4743–4757, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zima AV, Picht E, Bers DM, Blatter LA. Partial inhibition of sarcoplasmic reticulum Ca release evokes long-lasting Ca release events in ventricular myocytes: role of luminal Ca in termination of ca release. Biophys J 94: 1867–1879, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res 103: e105–e115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]