Abstract
The less mucoid strain of Brucella canis or M- strain is used for the serologic diagnosis of canine brucellosis. While this strain is avirulent in dogs, we report the case of clinical brucellosis that developed in a laboratory worker a few days after handling live M- cells for antigen production.
Keywords: brucella canis, M- strain, human infection, clinical picture, immune response
Brucella canis is the causative agent of canine brucellosis, which causes contagious abortion, orchiepididymitis, and uveitis. Transmission to human requires close contact with infected animals or bacterial cultures. Symptomatic human infections are rare, probably because of the low virulence of B. canis; 31 human cases have been reported (1).
In contrast to other Brucella species, which are pathogenic for humans (B. abortus, B. melitensis, B. suis) and yield smooth colonies, B. canis colonies are naturally rough. Therefore, serologic tests that use suspensions of smooth brucellae are not useful in diagnosing B. canis infections (2). Since suspensions of wild-type B. canis tend to aggregate even in the absence of specific antibodies, a less mucoid variant termed M-, which does not produce autoagglutination is used for serologic diagnosis (3). The M- strain has reduced virulence in dogs; even high doses of this strain do not induce the typical signs of brucellosis in dogs (4). The pathogenic potential of the M- strain in humans remains unknown, and to the best of our knowledge, human infection by this strain has not been reported. We report a clinical and immunologic study of a human infection by the B. canis M- strain that shows that this strain can produce human disease similar to that produced by wild-type B. canis.
Case Report
A 35-year-old male laboratory worker was referred to a physician with recurrent fever, headache, arthralgia, weakness, and constipation, which had begun 1 month before. The patient worked in a laboratory that produced antigens for diagnostic use. Three weeks before symptoms began, he had been handling a dense culture of live B. canis M- and was using no personal protection; the procedures were not performed in a biological safety cabinet. Moreover, the patient had attempted resuspension by repeated pipetting with his mouth. The clinical examination disclosed cervical adenomegaly, and laboratory tests indicated a mild increase of hepatic enzymes (Aspartate aminotransferase 46 μ/L and alanine aminotransferase 65 μ/L) and neutropenia. The patient reported not having close contact with dogs or other animals. Taking into account the unprotected exposure to B. canis M-, brucellosis was suspected, and blood samples were drawn for culture and serologic studies. Two weeks later blood cultures indicated a Brucella species that was later typed as B. canis. Conventional tests for antibodies to smooth brucellae (agglutination, complement fixation) yielded negative results. In contrast, slide agglutination for B. canis was strongly positive with undiluted serum and was also positive at 1:10 dilution. Serologic tests for hepatotropic viruses and Toxoplasma gondii were negative.
After diagnosis, a course with oral doxycycline, 100 mg twice a day for 42 days, plus parenteral gentamicin, 180 mg once a day for 10 days, was started. The patient clinically recovered, but on the last day gentamicin was administered, symptoms of the left VIII cranial nerve occurred, which resolved with flunarizine and vitamin B12 administration.
Blood cultures performed 2 weeks after antimicrobial therapy ended were negative for B. canis. During follow-up, the patient remained asymptomatic, his cervical adenitis resolved, and serum levels of hepatic enzymes returned to normal. On his last visit, 4 years after infection, the patient was asymptomatic. While he continues to handle B. canis M-, cultures are now performed under strict biological safety measures (biological safety cabinet, personal protection including goggles, gloves and mask, and autoclaving of contaminated material).
Immunologic Studies
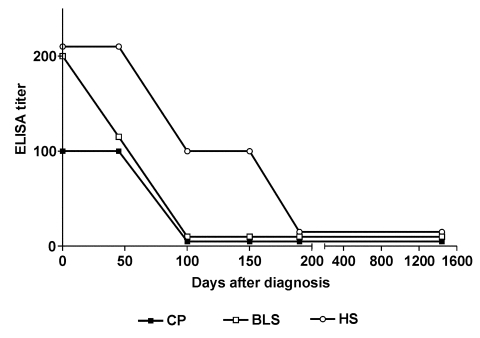
To assess the humoral immune response of the patient to Brucella antigens, the slide agglutination test with B. canis M- and 3 enzyme-linked immunosorbent assays (ELISA) were used. ELISA used a hot-saline extract of B. canis M- (HS, mainly composed of rough lipopolysaccharide [LPS] and outer membrane proteins), a preparation of cytoplasmic proteins of B. abortus depleted of LPS (CP [cytoplasmic proteins]) or recombinant Brucella lumazine synthase (RBLS), which were obtained as described previously (5–7). As shown in the Figure, antibodies to the three antigens were detected at the time of diagnosis, but antibodies to proteinaceous antigens (CP and RBLS) were negative earlier than those against HS. The slide agglutination test that used B. canis M- and undiluted serum was strongly positive at diagnosis and 48 days later (beginning and end of therapy, respectively), weakly positive at 103 and 150 days, and negative at 190 days and 4 years after diagnosis. Positive samples were assayed in serial dilutions starting at 1:10; only the initial sample was positive at 1:10 dilution (negative at 1:20).
The cellular immune response against CP from Brucella in peripheral blood mononuclear cells (PBMCS) was also evaluated. In vitro proliferation and cytokine gene expression were investigated as previously described (8). For blastogenesis assays, PBMCS were cultured with CP (10 μg/mL), RBLS (5 μg/mL), or phytohemagglutinin (10 μg/mL). Results were expressed as stimulation index (counts per minute of stimulated cultures divided by counts per minute of unstimulated cultures). Stimulation indices (SI) >2 were considered positive. For reverse transcription-polymerase chain reaction (RT-PCR), RNA was extracted from PBMCS cultured in the presence of CP, RBLS, or phytohemagglutinin for 24 hours. Results were expressed as fold increase over the messenger ribonucleic acid levels of cells cultured in the absence of antigen; increases >2 were considered specific.
CP and RBLS induced T-cell proliferation (SI >2) in PBMCS obtained from the patient before antimicrobial therapy (Table). PBMCS from a healthy person, which were run in parallel, showed no response to CP and RBLS (SI <2) (not shown). In addition, CP and RBLS induced a significant (p<0.001, nonparametric Mann-Withney u test) upregulation of interferon-gamma (IFN-γ), interleukin (IL)-2, and IL-10 transcripts only in PBMCS from the patient. No IL-4 induction was observed with PBMCS from the patient or the healthy control (not shown). The cellular immune response declined with antimicrobial treatment (Table), but CP-specific IFN-γ remained increased 55 days after therapy ended. In the last sample (obtained 250 days after therapy ended), all the parameters of the cellular immune response were normal (not shown).
Table. Cellular immune response in vitro to Brucella cytoplasmic proteinsa.
| Antigen | Lymphocyte proliferation (SI) | IL-2b (fold increase) | IFN-γb (fold increase) | IL-10b (fold increase) | |
|---|---|---|---|---|---|
| Before therapy | BLS | 4 | 8 | 3 | 3 |
| CP | 3 | 10 | 7 | 4.5 | |
| End of therapy | BLS | 1 | 1 | 2 | 1 |
| CP | 2.5 | 6 | 4 | 2 | |
| 55 days after end of therapy | BLS | 1 | 1 | 1 | 1 |
| CP | 1 | 1 | 3 | 1 |
aBLS, Brucella lumazine synthase; CP, cytoplasmic proteins; SI, stimulation indices; IL, interleukin; IFN, interferon. bBy reverse transcription-polymerase chain reaction.
Conclusions
The main finding of our study is that the M- strain of B. canis can produce human disease, which was unexpected in view of the reported avirulent phenotype of this strain in dogs. To the best of our knowledge, this case is the first of human B. canis M- infection ever reported. The M- strain has been widely used for the diagnosis of canine brucellosis because it is less prone to autoagglutination than its wild-type counterpart (called m+). Based on the low virulence of the M- strain in dogs (4), the production protocol of the laboratory where this case occurred did not include bacterial inactivation or personnel protection during initial handling of cultures, which led to a prolonged exposure to a high number of viable bacteria. As the patient was the only person involved in the production of this strain, his co-workers were not tested for B. canis M-. Similar illness in the production plant was not reported. Because Brucella spp. is not usually transmitted from patients to healthy persons, the patient’s family members were not tested for B. canis M- infection.
The clinical manifestations in our patient were similar to those reported for human, wild-type B. canis infections (e.g., fever, headache, anorexia, asthenia, and adenitis). Previous studies in dogs experimentally infected with the M- strain showed that this strain does not revert to the m(+) phenotype in vivo (4). Our case may be analogous to cases of human illness by attenuated strains of Brucella species used for animal vaccination, mainly B. melitensis Rev-1 (9) and B. abortus S19 (10). Altogether, these human infections indicate that attenuation for animals does not necessarily mean immunity for humans and that biological safety measures must be followed in each case.
To assess the humoral immune response to the infection with the M- strain, antibodies against outer membrane antigens (HS) and to internal antigens (CP and Brucella lumazine synthase) were measured. Overall, low titers of antibodies were found by all tests, which is similar to those found for M- infections in dogs (4). Low antibody titers also could be related to early administration of antimicrobial therapy, as has been shown in patients infected with smooth brucellae (11). Antibodies to both external and internal antigens declined after antimicrobial therapy was begun and were undetectable 6 months after diagnosis (Figure). This decline, with longer persistence of antibodies to external antigens, is in agreement with our previous findings in human infections by smooth Brucella species (12).
Figure.
Serological follow-up of a human infection by Brucella canis M-. CP, cytoplasmic proteins; BLS, Brucella lumazine synthase; HS, B. canis hot-saline extract. The enzyme-linked immunosorbent assay titer was calculated as the inverse of the last serum dilution that yielded an optical density higher than the cut-off of the assay.
An early and strong cellular Th1-type response to Brucella internal antigens developed in this patient, in agreement with our previous observations in acute human brucellosis (8). The reasons for the decline of this response during follow-up are unknown, but conceivably, bacteria levels were substantially diminished by the early antimicrobial therapy, thus eliminating the internal antigens needed to develop a long-lasting cellular immune response.
In summary, this case shows that, in spite of its reduced virulence in dogs, B. canis M- can produce human disease with a clinical picture similar to that produced by the infection with wild-type strains of B. canis. Therapeutic and immunologic parameters seem to be very similar to those observed in infections by smooth brucellae.
Acknowledgments
We thank Nidia Lucero for bacteriological studies and María M. Wanke for performing B. canis agglutination tests.
This work was supported with grant BID1201/OC-AR PICT99 05-06324 from Agencia Nacional de Promoción Científica y Tecnológica, with a Carrillo-Oñativia Grant from Ministerio de Salud, Argentina, and with a grant from Fundación Antorchas.
Biography
Dr. Wallach is an infectology practitioner at the National Hospital of Infectious Diseases F. J. Muñiz in Buenos Aires. His interests are clinical and immunologic aspects of brucellosis and other infectious diseases.
Footnotes
Suggested citation for this article: Wallach JC, Giambartolomei GH, Baldi PC, Fossati CA. Human infection with M- strain of Brucella canis. Emerg Infect Dis [serial online] 2004 Jan [date cited]. Available from: URL: http://www.cdc.gov/ncidod/EID/vol10no01/02-0622.htm
References
- 1.Madkour MM. Brucellosis: overview. In: Madkour MM, editor. Brucellosis. 2nd edition. Berlin: Springer Verlag; 2001. p. 165–78. [Google Scholar]
- 2.Polt SS, Dismukes WE, Flint A, Schaefer J. Human brucellosis caused by Brucella canis: clinical features and immune response. Ann Intern Med. 1982;97:717–9. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael LE, Joubert JC. A rapid slide agglutination test for the serodiagnosis of Brucella canis infection that employs a variant (M-) organism as antigen. Cornell Vet. 1987;77:3–12. [PubMed] [Google Scholar]
- 4.Carmichael LE, Zoha SJ, Flores-Castro R. Biological properties and dog response to a variant (M-) strain of Brucella canis. Dev Biol Stand. 1984;56:649–56. [PubMed] [Google Scholar]
- 5.Mateu-De-Antonio EM, Martín M, Soler M. Use of indirect enzyme-linked immunosorbent assay with hot saline solution extracts of a variant (M-) strain of Brucella canis for diagnosis of brucellosis in dogs. Am J Vet Res. 1993;54:1043–6. [PubMed] [Google Scholar]
- 6.Goldbaum FA, Rubbi CP, Wallach J, Miguel SE, Baldi PC, Fossati CA. Differentiation between active and inactive human brucellosis by measuring antiprotein humoral immune responses. J Clin Microbiol. 1992;30:604–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldbaum FA, Velikovsky CA, Baldi PC, Mortl S, Bacher A, Fossati CA. The 18 kda cytoplasmic protein of Brucella spp., an antigen useful for diagnosis, is a lumazine synthase. J Med Microbiol. 1999;48:833–9. [DOI] [PubMed] [Google Scholar]
- 8.Giambartolomei GH, Delpino MV, Cahanovich ME, Wallach JC, Baldi PC, Velikovsky CA, et al. Diminished production of T helper 1 cytokines correlates with T-cell unresponsiveness to Brucella cytoplasmic proteins in chronic human brucellosis. J Infect Dis. 2002;186:252–9. 10.1086/341449 [DOI] [PubMed] [Google Scholar]
- 9.Olle-Goig JE, Canela-Soler J. An outbreak of Brucella melitensis infection by airborne transmission among laboratory workers. Am J Public Health. 1987;77:335–8. 10.2105/AJPH.77.3.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young EJ. Clinical manifestations of human brucellosis. In: Young EJ, Corbel MJ, editors. Brucellosis: clinical and laboratory aspects. Boca Raton (FL): CRC Press; 1989. p. 97–126. [Google Scholar]
- 11.Baldi PC, Giambartolomei GH, Wallach JC, Velikovsky CA, Fossati CA. Limited diagnostic usefulness of antibodies to cytoplasmic proteins of Brucella in early-treated human brucellosis. Scand J Infect Dis. 2001;33:200–5. 10.1080/00365540151060842 [DOI] [PubMed] [Google Scholar]
- 12.Baldi PC, Miguel SE, Fossati CA, Wallach JC. Serological follow-up of human brucellosis by measuring IgG antibodies directed to LPS and cytoplasmic proteins of Brucella. Clin Infect Dis. 1996;22:446–55. [DOI] [PubMed] [Google Scholar]