Background: P450 2D6 contributes significantly to the metabolic clearance of many drugs.
Results: Binding of prinomastat to P450 2D6 reveals a distinctive active site topology.
Conclusion: P450 2D6 structural flexibility contributes to its catalytic versatility.
Significance: This structure will aid efforts to minimize the impact of genetic variation and drug-drug interactions for new drugs.
Keywords: Cytochrome P450, Drug Metabolism, Enzyme Structure, Protein Structure, Xenobiotics
Abstract
Human cytochrome P450 2D6 contributes to the metabolism of >15% of drugs used in clinical practice. This study determined the structure of P450 2D6 complexed with a substrate and potent inhibitor, prinomastat, to 2.85 Å resolution by x-ray crystallography. Prinomastat binding is well defined by electron density maps with its pyridyl nitrogen bound to the heme iron. The structure of ligand-bound P450 2D6 differs significantly from the ligand-free structure reported for the P450 2D6 Met-374 variant (Protein Data Bank code 2F9Q). Superposition of the structures reveals significant differences for β sheet 1, helices A, F, F′, G″, G, and H as well as the helix B-C loop. The structure of the ligand complex exhibits a closed active site cavity that conforms closely to the shape of prinomastat. The closure of the open cavity seen for the 2F9Q structure reflects a change in the direction and pitch of helix F and introduction of a turn at Gly-218, which is followed by a well defined helix F′ that was not observed in the 2F9Q structure. These differences reflect considerable structural flexibility that is likely to contribute to the catalytic versatility of P450 2D6, and this new structure provides an alternative model for in silico studies of substrate interactions with P450 2D6.
Introduction
Human cytochrome P4503 2D6 mediates the principal route of metabolic clearance for >15% of the 200 most marketed drugs that are primarily cleared by P450-mediated hepatic metabolism (1). P450 2D6 oxidizes a variety of anti-psychotic, anti-depressant, and anti-arrhythmic drugs, which reflects the capacity of the enzyme to oxidize moderately sized, basic substrates (2). Genetic polymorphisms contribute to a wide variation of 2D6 expression and activity, leading to four phenotypes as follows: poor, intermediate, extensive, and ultra-fast drug metabolizers (1, 3). Drugs such as thioridazine, debrisoquine, phenformin, and captopril, which exhibit comparatively narrow therapeutic windows, can be problematic when used by 2D6 poor metabolizers, which represent ∼10% of the Caucasian population (3–5).
A structure for P450 2D6, PDB4 2F9Q, crystallized without a substrate bound in the active site was reported previously (6). This study sought to determine the structure of the enzyme with a substrate or inhibitor bound in the active site. P450s can exhibit significant differences in the active site architecture between ligand-bound and ligand-free states (7–9), and this was observed for P450 2D6 in this study. Moreover, the successful crystallization and determination of structures for P450s 1A2 (10) and 1B1 (11) required the stabilization of the enzymes with a ligand during isolation and crystallization to maintain normal substrate binding as judged by spectral properties of the enzyme complexes. As described here, this was also evident for P450 2D6, and the enzyme was purified and crystallized with prinomastat bound in the active site. The resulting structure differs significantly from the ligand-free structure and provides an alternative model for understanding substrate and inhibitor binding to P450 2D6 as well as demonstrating the pliant active site architecture of the enzyme.
EXPERIMENTAL PROCEDURES
Expression of Modified P450 2D6 in Escherichia coli
To reduce aggregation and increase solubility, the amino acid sequence of the N-terminal trans-membrane helix of P450 2D6 was removed by replacing the first 33 residues with a shorter amino acid sequence, MAKKTSSKGKL. Additionally, a four-histidine expression tag was added to the C terminus to facilitate purification using nickel-nitrilotriacetate-agarose affinity chromatography. These changes were introduced by amplification of the P450 2D6 cDNA with upper primer, 5′-CGGAATTCCATATGGCTAAGAAAACGAGCTCTAAACCACCAGGCCCCCTGCCAC-3′, and lower primer, 5′-CGG AAT TCC GAA GCT TTC AGT GGT GGT GGT GGC GGG GCA CAG CAC AAA GCTC-3′. The PCR employed 30 cycles at 94 °C for 30 s, 60 °C for 30 s, and 68 °C for 120 s using high fidelity Pfu Ultra (Agilent). In all other respects, the expression cassette corresponded to the coding sequence for the major human allele, CYP2D6*1. The expression cassette was inserted between the NdeI and HindIII restriction sites of the pCWori vector (12, 13). As an NdeI site was present in the coding sequence for P450 2D6, two fragments were generated from the PCR product by NdeI/BsmI and BsmI/HindIII digestion, which were combined with the NdeI/HindIII-digested vector backbone in a single ligation reaction to produce the expression vector. The construct was verified by sequencing the expression cassette.
E. coli strain DH5α was transformed with both the CYP2D6 expression plasmid and the pGro7 plasmid for elevated expression of the chaperone proteins GroEL and GroES (Takara Bio Inc., Shiga, Japan). The selected and validated transformant was grown in 500 ml of terrific broth containing ampicillin and chloramphenicol at 37 °C, 220 rpm in a tabletop C24KC refrigerated incubator/shaker (New Brunswick Scientific, Edison, NJ) until an absorbance of ∼0.5 at a wavelength of 600 nm was obtained. The temperature was lowered to 30 °C, and the incubation was continued at 190 rpm. After about 30 min, when the absorbance at 600 nm was ∼0.7–0.8, δ-aminolevulinic acid (5 mm), isopropyl β-d-thiogalactopyranoside (1 mm), and arabinose (4 g/liter) (Sigma) were added to induce the expression of P450 2D6 and of the chaperones GroEL and GroES. Cells were harvested after 24 h.
Purification of P450 2D6
For protein extraction, spheroplasts were prepared as described (14) and suspended in a 500 mm potassium phosphate buffer, pH 7.4, containing 20% glycerol, v/v, 0.2 mm prinomastat (Pfizer Global Research and Development, La Jolla), 10 mm β-mercaptoethanol, 14 mm CHAPS (Anatrace, Maumee, OH), and 1 mm phenylmethylsulfonyl fluoride. P450 2D6 was purified from the protein extract by nickel-nitriloacetate-agarose (Qiagen, Valencia, CA) affinity chromatography. After several washes, the protein was eluted using a 10 mm potassium phosphate buffer, pH 7.4, containing 30 mm histidine, 1 m NaCl, 0.05 mm prinomastat, 14 mm CHAPS, 10 mm β-mercaptoethanol, 1 mm phenylmethylsulfonyl fluoride, and 20% v/v glycerol. The pooled fractions were dialyzed overnight against the same buffer with the NaCl concentration lowered to 150 mm and without histidine before application to a column containing hydroxylapatite-agarose beads (HA Ultrogel, BioSepra Inc) equilibrated with the same buffer. The protein was eluted in 120 mm potassium phosphate, pH 7.4, containing 20% v/v glycerol, 0.05 mm prinomastat, 10 mm β-mercaptoethanol, 14 mm CHAPS, and 1 mm phenylmethylsulfonyl fluoride. The protein solution was concentrated to 0.68 mm for crystallization using an Amicon ultracentrifugal filtration device with a 50K molecular weight exclusion limit (Millipore). P450 concentrations were determined by CO-difference spectroscopy using an extinction coefficient of 0.091 μm−1 cm−1 (15). As prinomastat reduces the formation of the CO complex, concentrations of the purified P450 2D6 prinomastat complex used for crystallization were estimated by the intensity of the Soret absorption band. An extinction coefficient of 0.113 ± 0.006 μm−1 cm−1 was estimated for the complex by titration of the ligand-free enzyme with prinomastat as described below. The extinction coefficient was calculated by dividing the absorbance of the complex observed at saturating concentrations of prinomastat by the concentration of the ligand-free enzyme determined by CO-difference spectroscopy. The mean and standard deviation are reported for seven replicate experiments. Protein purity was assessed by SDS-PAGE followed by staining with Coomassie Brilliant Blue.
Characterization of Ligand Binding by Visible Absorption Spectroscopy
Binding constants were estimated by monitoring the concentration-dependent effects of ligands on the visible absorption spectrum of the modified P450 2D6. For comparison, full-length P450 2D6 was expressed in E. coli, and membranes containing P450 2D6 were isolated (16). Stock solutions of thioridazine hydrochloride and quinidine sulfate were dissolved in water. Prinomastat was dissolved in DMSO, and the final concentration of DMSO in titrations was <1%. Difference spectra were calculated by subtracting the absorbance spectrum determined in the absence of the ligand from spectra obtained with ligand added to the sample and reference cuvettes in equal amounts. Alternatively, difference spectra were determined directly by addition of equal volumes of the ligand to the sample cuvette and of the solvent to the reference cuvette, where both cuvettes contained the solution of the protein. Peak to trough differences for the Soret absorption band were calculated and plotted against the concentration of the ligand. Dissociation constants, Kd, and maximum absorbance changes, ΔAmax, were estimated by nonlinear regression fitting of the results with a one-site ligand binding model. Kd and ΔAmax are reported as means ± S.D. determined from at least three experiments. Alternatively, when the protein concentration was near or exceeded the apparent Kd value, the quadratic form of the binding equation was employed with the assumption that the maximal change in absorbance reflects a 1:1 stoichiometry, and where P is the concentration of P450 2D6, and L is the total concentration of the ligand as shown in Equation 1.
Protein Crystallization
The modified P450 2D6 prinomastat complex was crystallized by hanging drop vapor diffusion. The drop contained 1 μl of the concentrated protein solution (620 μm P450), 0.25 μl of 70 mm HEGA-10 detergent (Anatrace, Maumee, OH), 0.625 μl of the protein buffer containing 0.01 mm prinomastat without CHAPS, and 0.625 μl of precipitant solution containing 20% w/v PEG-3350 and 0.1 m sodium cacodylate, pH 7.0, and the drop was set to equilibrate at 296 K against 0.5 ml of reservoir solution composed of the same precipitant solution containing 6% v/v glycerol.
Structure Determination
A dataset collected from a single crystal of P450 2D6 complexed with prinomastat was used for structure determination. The crystal was flash-frozen in liquid nitrogen, and the data were collected at 100 K using the Stanford Synchrotron Radiation Lightsource beamline 7-1. MOSFLM and SCALA (17) were used to index, integrate, and scale the data. The structure was solved by molecular replacement using PHASER (18). The model of the 2D6 prinomastat complex (PDB code 3QM4) was built using COOT (19) and refined using CNS (20) to 2.85 Å in the C2 space group with two molecules in the asymmetric unit. Noncrystallographic symmetry restraints were used initially. At later stages of refinement, these restraints were removed for divergent regions. The final structural model of P450 2D6 prinomastat complex encompasses residues 33–497 of the protein for chain A. There was insufficient density to model the N-terminal residues preceding Leu-33 of chains A and B and residues 230–237 between helices F′ and G″ of chain B. Prinomastat was modeled in the active site pocket above the heme prosthetic group in each chain. A nickel ion, identified by x-ray fluorescence, was bound on the surface of chain B. Additionally, seven water molecules were added in the final stages of refinement. Data processing and model refinement statistics are listed in Table 1.
TABLE 1.
Data collection and refinement statistics
1 Values for the highest resolution shell, 3.00 to 2.85 Å, are shown in parentheses.
2 Ramachandran plot indicates 89.2% of residues in most favored regions, 98.7% in allowed regions, and 1.3% in disfavored regions.
3 The full sequence corresponds to residues 23–497 when numbered according to the native protein. There was insufficient density to model residues 23–32 for chains A and B and residues 230–237 for chain B.
4 A nickel ion is coordinated with His-258(B) on the protein surface in the interface with a symmetry related copy of chain B.
RESULTS
Protein Design
P450 2D6 was modified for expression in E. coli and to facilitate purification and crystallization. These changes entailed removal of the N-terminal transmembrane leader sequence to improve solubility and to reduce aggregation. This was accomplished by replacing the codons for amino acid residues 2–33 of a cDNA encoding the major CYP2D6 allele (UNP: P10635-1) with selected codons encoding a short hydrophilic sequence AKKTSSKGKL as previously employed for the crystallization of other mammalian microsomal P450s (21–24). Additionally, four codons for histidine were inserted upstream of the translation stop site as an expression tag for affinity chromatography. The resulting construct is similar in design to the one used by Rowland et al. (6) to determine the structure of P450 2D6 in the absence of a bound ligand (PDB code 2F9Q). The coding region of the latter construct differed by using a cDNA for a rare allelic variant of P450 2D6 (25, 26) exhibiting a V374M substitution and by the introduction of two additional mutations, L230D and L231R, that increased the solubility of the protein (6).
Effects of Substrates and Inhibitors on the Ligand Binding Properties of Modified P450 2D6
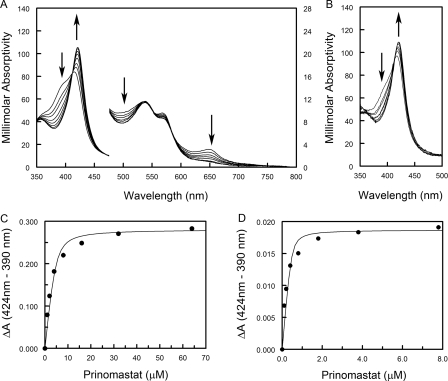
For our studies, initial protein extracts were prepared by lysis of spheroplasts in buffers containing a substrate or inhibitor of the enzyme as well as the detergent CHAPS. The detergent was necessary to maintain solubility of the protein during purification. When prepared in this manner, P450 2D6 exhibited substrate-dependent differences in its absorption spectrum indicative of substrate or inhibitor binding. As shown in Fig. 1A, when the modified P450 2D6 was isolated in the presence of 50 μm quinidine (green spectrum), the shape of the Soret band exhibits a shoulder indicative of increase in the proportion of the high spin state relative to P450 2D6 prepared in the absence of quinidine (black spectrum). A larger increase in the proportion of high spin P450 is evident when the enzyme is isolated in the presence of 50 μm thioridazine (Fig. 1A, blue spectrum). The thioridazine complex exhibits a broad bifurcated peak indicative of roughly equal amounts of high and low spin heme iron. Ligand-dependent increases in the proportion of high spin heme iron are generally termed type 1 spectral changes and are thought to reflect perturbations of water binding to the iron. In contrast, the prinomastat complex displays a spectrum for a predominantly low spin P450 indicative of the coordination of the prinomastat pyridyl nitrogen to the heme iron (Fig. 1A, red spectrum). This is generally described as a type 2 spectral change.
FIGURE 1.
Effects of ligands on the visible absorption spectra of P450 2D6. A, spectra of P450 2D6 isolated in the presence of 50 μm thioridazine (blue), quinidine (green), or prinomastat (red) or in the absence of an exogenous ligand (black) are shown. For comparison, spectral absorbance is expressed as millimolar absorptivity for each complex, and the four spectra were normalized to each other at 477 nm to reduce the effects of experimental variation. The scale for the apparent millimolar absorptivity is shown on the left for wavelengths below 477 nm and on the right for longer wavelengths. The thioridazine and quinidine complexes show an increase in the high spin state of the enzyme as judged by the increased absorbance at 390 and 650 nm as well as decreases in the absorbance at 418 and 568 nm. In contrast, prinomast produces a low spin enzyme as judged by the loss of the 650 nm band and shift of the Soret band to 421 nm. B, concentration-dependent effects of thioridazine on the spectrum of the 10 μm P450 2D6 prepared in the absence of an exogenous ligand are displayed. Thioridazine in water was added to the sample and reference cuvettes in small increments to final concentrations of 10, 21, 31, 40, 50, 60, 70, 80, 99, 149, 198, and 247 μm. The spectrum (blue) of P450 2D6 isolated in the presence of 50 μm thioridazine exhibits a larger proportion of high spin enzyme than evident for P450 2D6 prepared in the absence of an exogenous ligand at saturating concentrations. C, concentration-dependent effects of prinomastat on 10 μm P450 2D6 prepared without exogenous ligands are depicted. The final concentrations of prinomast were 2, 4, 8, 16, 32, 64, and 127 μm. The maximum concentration of DMSO was 0.66% v/v. The arrows indicate the direction of change in response to addition of each ligand in B and C. Prinomastat converts P450 2D6 to a low spin form similar to that isolated in the presence of prinomastat. Estimates of the Kd and ΔAmax for multiple determinations are reported in the text.
Thioridazine is a 2D6 substrate that was reported to exhibit Ki values of 0.75 μm (27) and 1.4 ± 0.8 μm for competitive inhibition of P450 2D6-catalyzed reactions (28). When thioridazine was added to membranes isolated from E. coli-expressing full-length P450 2D6, a type 1 spectral change was observed by difference spectroscopy with an apparent Kd of 1.4 ± 0.53 μm, which is concordant with the reported Ki values. In contrast, when the enzyme isolated in the absence of an added ligand was titrated with thioridazine (Fig. 1B), the maximal change in the absorbance was less than that observed for the enzyme isolated in the presence of thioridazine, and the apparent Kd of 39.6 ± 1.8 μm was much higher than exhibited by the full-length membrane-bound P450 2D6. The difference in the absorption exhibited by thioridazine complex in the presence of 50 μm ligand and the ligand-free enzyme is 67.8 mm−1 cm−1, which is greater than the estimated ΔAmax of 41.5 ± 3.2 mm−1 cm−1 from the binding curve observed, which is not reached, in turn, until much higher concentrations of thioridazine are added.
Although the extent of the type 1 spectral change and the binding affinity were diminished, the yields of the modified P450 2D6 in the absence of an exogenous ligand were similar to those for the ligand complexes, and there was no evidence of a significant conversion of the ligand-free preparation to an inactive form exhibiting a Soret absorption maximum 420 nm for the ferrous enzyme. Although the apparent increase in Kd values could reflect the binding of unknown competitor to the protein, removal of ligands following isolation resulted in conversion of the modified P450 2D6 to a low affinity form.
Similarly, titration with quinidine of modified P450 2D6 isolated in the absence of an exogenous ligand exhibited a diminished type 1 spectral change, ΔAmax of 8.5 ± 3.0 mm−1 cm−1, when compared with the difference of 15.5 mm−1 cm−1 observed for the spectrum of the complex and the spectrum of the ligand-free enzyme (Fig. 1A). Moreover, the apparent Kd of 42.1 ± 1.4 μm is also much greater than reported for type 1 spectral changes exhibited by the full-length P450 2D6 expressed in E. coli by Hanna et al. (29), who estimated a Kd of 0.7 μm, for the full-length enzyme expressed and purified from E. coli and for the full-length enzyme expressed in yeast microsomes, which exhibited an estimated Kd of 0.1 μm (26).
Hanna et al. (29) reported previously that quinidine did not produce a type 1 spectral change with a similarly truncated P450 2D6 construct. Although the type 1 spectral change was not observed by Hanna et al. (29), they found that the purified, truncated enzyme could be reconstituted with reductase and phospholipids and that the active reconstituted enzyme was inhibited by quinidine at a concentration of 1 μm. Hanna et al. (29) speculated that the absence of the spectral change for the truncated enzyme might reflect a more open conformation for the truncated enzyme that might diminish the effects of the quinidine on the binding of water to the heme iron without indicating whether or not quinidine occupied the active site. Increased conformational dynamics might also contribute to the reduced extent of the type 1 spectral change and to the diminished binding affinity observed in our studies. This explanation is consistent with recent molecular dynamics simulations for P450 2C9 indicating that the structure of N-terminally truncated 2C9 exhibits a larger range of conformational dynamics in solution and for the opening and closing of solvent access channels during simulations than observed for a model of the full-length P450 enzyme embedded in a phospholipid bilayer. Extensive interactions of the distal outer surface of the catalytic domain with the membrane bilayer appear to limit the conformational dynamics of the protein in these simulations. Additionally, a bound substrate reduced the conformational dynamics seen in the simulations (30, 31). Our observation that ligand complexes exhibited expected spectral changes when isolated in the presence of the ligand suggested that isolation of modified P450 2D6 in this manner would be more suitable for crystallization of the complexes. Additionally, it allowed us to monitor the effects of different isolation protocols and conditions on the stability of the complex.
Colleagues at Pfizer, Inc. (La Jolla, CA) suggested that prinomastat, an experimental matrix metalloprotease inhibitor, might provide a good stabilizing ligand for our studies and provided us with sufficient quantities of prinomastat for use in purification and crystallization of P450 2D6. The Pfizer scientists had observed that prinomastat is a potent inhibitor of P450 2D6 with an observed Ki = 0.049 μm and that prinomastat produced a type 2 binding spectrum with an apparent Kd of 0.29 μm with purified E. coli-expressed full-length P450 2D6 obtained from the commercial supplier Panvera.5 Our studies with membrane-bound E. coli-expressed full-length P450 2D6 confirmed the type 2 change with an estimated Kd <0.02 μm, the upper 95% confidence value. The binding of prinomastat shifts the Soret maximum to a longer wavelength (422 nm) and decreases the intensities of the absorbance bands at 568 and 650 nm (Fig. 1, A (red) and C). In this case, the spectrum of the enzyme obtained following titration of the modified P450 2D6 (Fig. 1C) is highly similar to the spectrum obtained for the enzyme isolated in the presence of 50 μm prinomastat (Fig. 1A (red spectrum)), indicating that pyridyl nitrogen binds to the heme iron in both cases. Nevertheless, the estimated Kd of 1.96 ± 1.06 μm is higher than the Kd value determined spectroscopically for the full-length enzyme.
In contrast, the affinity of the isolated thioridazine complex is higher for prinomastat than for the enzyme purified in the absence of a ligand even though thioridazine is present to compete for binding. As shown in Fig. 2A, 100-fold dilution of highly concentrated solution of the thioridazine complex in thioridazine-free buffer to a final concentration 4.6 μm does not significantly reduce the high spin component. At this dilution, the concentration of thioridazine is predicted to be a 5.1 μm-based saturation of a single binding site in the complex and a 100-fold dilution of the 50 μm thioridazine in the buffer. An increased absorbance seen at 310 nm is consistent with this estimate for thioridazine. Under this condition, titration of the protein with prinomastat converts the spectrum to that of the prinomastat complex (Fig. 2A). A fit of the quadratic form of the one-site binding equation by nonlinear least squares regression (Fig. 2C), yields an apparent Kd of 0.62 ± 0.06 μm. As the presence of residual thioridazine competes for binding, the actual Kd value for prinomastat will be lower. The Kd was estimated to be 0.19 ± 0.02 μm using DynaFit (32, 33) when competitive binding by 5.1 μm thioridazine was included in the model with a Kd of 1.4 μm, which is the Kd value observed for the full-length membrane enzyme. When the concentrated thioridazine complex is diluted 1000-fold to a final concentration of 0.46 μm, the predicted concentration of thioridazine is diminished to 0.51 μm. At this dilution, a reduction in the proportion of high spin P450 is evident (Fig. 2B), indicative of dissociation of some thioridazine, which is consistent with the Kd value of 1.4 ± 0.53 μm observed for the full-length enzyme. In this condition, titration with prinomastat converts the modified P450 2D6 to the low spin prinomastat complex (Fig. 2B), with an apparent Kd of 0.064 ± 0.025 μm determined from the fit of the binding equation to these results (Fig. 2D). With reduced residual thioridazine, this value approaches more closely to the Kd blue exhibited by membrane-bound full-length P450 2D6.
FIGURE 2.
Concentration-dependent effects of prinomastat on P450 2D6 isolated in the presence of 50 μm thioridazine. The concentrated P450 2D6 thioridazine complex in buffer containing 120 mm potassium phosphate, pH 7.4, 20% v/v glycerol, 10 mm β-mercaptoethanol, 14 mm CHAPS, and 50 μm thioridazine was diluted into buffer containing 120 mm potassium phosphate, pH 7.4, and 20% v/v glycerol. A, prinomastat in DMSO was added in small increments to final concentrations of 1, 2, 4, 8, 16, 32, and 64 μm to 4.6 μm P450 2D6 thioridazine complex (100-fold dilution). The maximum concentration of DMSO was 0.07% v/v. The scale for the apparent millimolar absorptivity is shown on the left for wavelengths below 477 nm and on the right for longer wavelengths. B, prinomastat was added to 0.46 μm of the P450 2D6 thioridazine complex (1000-dilution). Only the Soret region is shown. The proportion of high spin P450 is reduced at this concentration of the protein based on the lower ratio of absorbance at 390 nm relative to 424 nm. The final concentrations of prinomastat were 0.1, 0.2, 0.4, 0.8, 1.8, 3.8, and 7.8 μm. The maximum concentration of DMSO was 0.02% v/v. The arrows indicate the direction of change in response to addition of each ligand in A and B. Difference spectra were calculated for the spectra in A and B, and the differences of absorbance between 424 and 390 nm were plotted versus the final concentration of prinomastat in C and D, respectively. The binding curves were computed by nonlinear least squares regression to estimate values of ΔAmax and Kd using the quadratic form of the binding equation. Estimates of the Kd values for multiple determinations are reported in the text.
Based on these observations, modified P450 2D6 was isolated in the presence of ligands for crystallization to maintain the native ligand binding properties of the enzyme. As the P450 2D6 prinomastat complex crystallized more readily than other complexes, conditions were optimized to produce crystals that diffracted to better than 2.85 Å. The structure of the prinomastat complex was determined for a dataset collected from a single crystal exhibiting the C2 space group with two molecules of the complex comprising the asymmetric unit. Model refinement statistics are shown in Table 1. The resulting structure defines the binding interactions of prinomastat with the enzyme, and when compared with the 1F9Q structure of P450 2D6 isolated and crystallized in the absence of ligand, the prinomastat complex exhibits a distinctly different conformation for the active site cavity and for the tertiary and secondary structure forming the distal portion of the active site.
Prinomastat-P450 2D6 Interactions
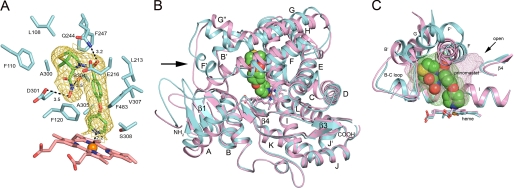
The binding of prinomastat in the active site of P450 2D6 was well defined by a 2|Fo| − |Fc| σA-weighted electron density map calculated for a model that did not include prinomastat (Fig. 3A). The pyridyl nitrogen is located 2.2 Å from the heme iron consistent with the observation that prinomastat shifts the Soret maximum to 422 nm as typically seen for nitrogenous ligands that coordinate to the heme iron. Although prinomastat has two H-bond donor sites and six potential H-bond acceptor sites, excluding the pyridine, only three hydrogen bonding interactions are evident between P450 2D6 and prinomastat. One hydrogen bond is evident between Gln-244 and one of the prinomastat sulfonyl oxygens. Another hydrogen bond is located between the amide hydrogen of the hydroxamic acid moiety and Ser-304, and the third hydrogen bond occurs between the hydroxyl group of the hydroxamic acid moiety and Asp-301. Although many P450 2D6 substrates contain basic nitrogens that are positively charged at neutral pH, as recently reviewed (2), prinomastat is neutral as neither the sulfonamide or the pyridine are likely to be protonated, and the hydroxamic acid moiety is not ionized. With the exception of Glu-216, the remaining contacts between the protein and prinomastat are nonpolar (Fig. 3A).
FIGURE 3.
A, amino acid side chains (cyan carbons) contacting prinomastat (green carbons) are depicted along with the heme prosthetic group (pink carbons) as stick figures. The gold mesh depicts a 2|Fo| − |Fc| σA-weighted prinomastat omit map contoured at 1σ around the prinomastat molecule. Dashed lines identify hydrogen bonds and the coordination of the pyridyl moiety with the heme iron (orange sphere) with the interatomic distances shown. Amino acid residues are identified by single letter amino acid codes and residue number. Nitrogen, oxygen, sulfur and iron atoms are colored blue, red, yellow, and orange, respectively. B, ribbon diagram showing the superposition of the structure of the prinomastat complex 3QM4 with the substrate-free structure 2F9Q (carbons colored light magenta). Helices are designated by letters and sheets by numbers. The arrow indicates helix F′ seen in the structure of the prinomastat complex. The surrounding region exhibits significant structure differences. C, comparison of the shapes of the active site cavities is depicted by a mesh surface colored green for the prinomastat complex and light magenta for 2F9Q structure. Cavity surfaces were generated by VOIDOO using a 1.4-Å probe. Water molecules were used to terminate the exterior opening of the solvent channel seen for the 2F9Q structure (arrow).
Structural Similarities and Differences between the 3QM4 and 2F9Q Structures of P450 2D6
The structure of the P450 2D6 prinomastat complex defines portions of the structure that were not modeled in the 2F9Q structure, such as the loop between the proline-rich motif and helix A. Additionally, residues 229–239 of the 2F9Q model were modeled as alanine residues because side chains were not defined by electron density maps (6). This region could be modeled completely for chain A of the prinomastat complex, but it could not be modeled for residues 230–237 of chain B.
The most prominent difference between the two structures is the presence of a distinct F′ helix, residues 218–225, in the structure of the prinomastat complex that is almost perpendicular to the axis of helix F, residues 199–215 (see arrow in Fig. 3B). In contrast, helix F′ was not observed in the 2F9Q structure of P450 2D6, where a continuous helix encompasses residues 199–225. This long helix passes over helix I in a direction that passes under the location of helix F′ of the prinomastat complex. As a result of this difference seen for the position of helix F′, there are substantial changes evident for adjacent structural components that include helix A, the loop between the first two strands of sheet β1 (residues 72–77), and helices F, G″, G, and H (Fig. 3B).
Helix F′ is a common feature in structures of family 2 P450s with a turn between helix F and helix F′. Ser-217 and Gly-218 contribute to the flexibility of the polypeptide backbone in this turn because of the small size of the side chain and absence of side chain, respectively. The corresponding amino acids that form this turn are similar for other family 2 P450s. The position of helix F′ in the prinomastat complex is similar to that seen for the structures 2A6 (PDB code 1Z10) and 2C8 (PDB code 2NNJ) as are the positions of helices F and G, the first turn in β-sheet 1, and the N-terminal region before helix A.
An unusual feature of the 2F9Q structure noted by Rowland et al. (6) is a relatively short G helix compared with other P450 family 2 structures. The shortened helix G is preceded by another short helix, which these authors designated G′. As this region is normally part of a longer G helix in other family 2 P450s, we have designated it as helix G″ in Fig. 3B. This unusual feature is conserved for both the 2F9Q and 3QM4 structures. In the prinomastat complex, an almost anti-parallel orientation of the helix F′ and helix G″ is seen. The loop between the two helices corresponds to the portion of the polypeptide chain that forms helix G′ in structures of other family 2 enzymes, such as P450s 2A6 and 2C8. This loop and its side chains are defined in chain A but not in chain B of the 3QM4 structure. This loop does not display a well defined helical structure. A structure-based sequence alignment indicates that the loop appears to have one less amino acid residue relative to the other family 2 P450s.
Active Site Architectural Differences
These differences of tertiary structure are associated with distinctly different shapes for the active site cavities of the prinomastat complex and the 2F9Q structure as depicted by solvent-accessible mesh surfaces shown in Fig. 3C. In the prinomastat complex, helix F and sheet β-4 are positioned to close an open solvent channel evident for 2F9Q structure. The channel passes under helix F and between helix I and sheet β-4. Additionally, the orientations of helices F and G increase the height of the active site cavity above the heme to better accommodate prinomastat with its pyridyl nitrogen oriented for coordination to the heme iron. The cumulative effects of these changes increase the volume of the active site cavity relative to that of the 2F9Q structure, 712 versus 582 Å3 as calculated using VOIDOO (34). As a result, the cavity of the 3QM4 structure is distributed more uniformly above the surface of the heme.
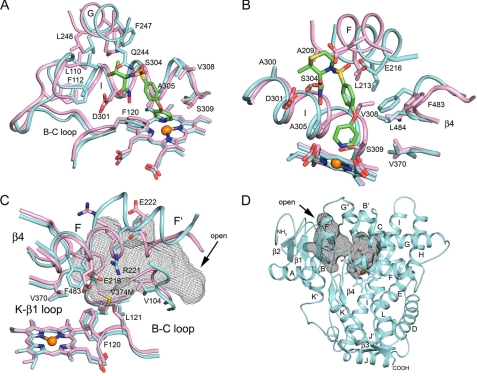
The different cavity shapes and volumes also reflect differences in the position and orientation of amino acid side chains lining the cavities in the two structures. This is illustrated for the sector of the cavity formed by the intersection of the B-C loop and helices G and I in Fig. 4A. The differences are relatively small for residues on helix I with the exception of a distortion in the helix that accommodates the prinomastat pyridyl group bound to the heme iron. This distortion leads to significant changes in the positions of Ser-304, Ala-305, Val-308, and Thr-309 with largest differences, ∼1.9 Å, seen for Ala-305 and Thr-309, which contact the pyridyl group of prinomastat. In contrast, Ala-300 and Asp-301 on helix I and Phe-120 on the adjacent helix B-C loop are not greatly affected. Nevertheless, larger differences of ∼1.5–1.9 Å are seen for Leu-110 and Phe-112 on the helix B-C loop, which moves in to contact prinomastat. The largest differences are evident for residues on helix G, which exhibit >2-Å differences for Cα positions of Gln-244, Phe-247, and Leu-248, and in the case of Gln-244, there is also a significant change in the side chain rotamer to accommodate hydrogen bonding to the sulfonyl oxygen of prinomastat.
FIGURE 4.
A and B show differences for structural features between the two proteins on the right and left sides, respectively, viewed across the heme toward helix I. C shows the view looking across the heme to the side of the cavity opposite helix I with solvent-accessible surface of the antechamber for substrate binding depicted as a black mesh. D shows the ribbon diagram indicating the juxtaposition of the active site cavity above the heme and antechamber for substrate binding. The arrow indicates an open passageway from the surface to the antechamber. The coloring scheme is described in the legend to Fig. 2.
Similarly, when the sector of the cavity formed by the intersection of β-sheet 4 with helices F and I is examined (Fig. 4B), the largest changes in amino acid side-chain positions occur in the upper portion of the cavity with smaller changes observed for Val-370 near the base of the cavity and the heme surface. Residues Ala-209, Leu-213, and Glu-216 on helix F are shifted to expand the cavity to accommodate prinomastat with the position of the carboxylate moiety of Glu-216 displaced by more than 4 Å. In contrast, the side chain of Phe-483 on the turn in the adjacent sheet β-4 swings into the cavity by 4 Å to form favorable nonbonded interactions with prinomastat. The convergence of the amino acid side chains on helix F and sheet β-4 with those on helix I close the open solvent channel evident in the 2F9Q structure.
The largest differences for side-chain positions in the active site reflect the different locations of the helix F′ segment of the polypeptide chain in the two structures (Fig. 4C). A turn following Glu-216 in the prinomastat complex terminates helix F and orients the helix F′ region so that its helical axis points toward the N-terminal side of the helix B-C loop (Fig. 3B). The polar surface of the helix F′ region, which is on the exterior surface of the 2F9Q structure (Fig. 4C), is rotated so that Glu-222 and Arg-221 are directed into the interior of the protein in the prinomastat complex, where a substrate access channel passes below helix F′ and above the surface of the heme and the loop between helix K and β-sheet 1. The outer surface of the entrance channel is formed by β-sheet 1, helix A, and the loop between the proline-rich region at the N terminus of the model and helix A (Fig. 4D). This substrate access channel is not evident for the 2F9Q structure where the extended and combined helices F and F′ pass through this region. Glu-222 is likely to remain hydrated in the substrate access channel of the 3QM4 structure. The access channel is closed off from the active site by the close proximity of Glu-216 on helix F with the guanidinium group of Arg-221 on helix F′, which face into the active site cavity (Fig. 4C). These amino acid side chains together with those of Val-104, Phe-120, and Leu-121 on the helix B-C loop and Phe-483 on the turn in sheet β-4 constrict the connection between the entrance channel and the prinomastat binding cavity, creating a separate solvent-accessible antechamber (Fig. 4, C and D). Additionally, the interaction between Arg-221 and Glu-216 provides a favorable electrostatic interaction between the oppositely charged side chains in the interior of the protein, but the overall electrostatic potential remains rather negative because of the presence of Asp-301 and Glu-222. The constriction of the channel better accommodates the size and shape of prinomastat and increases van der Waal's contacts between prinomastat and the enzyme. Nevertheless, the overall flexibility, implied by comparison of the 3QM4 and 2F9Q structures, suggests that the constriction can open to form a passage to the outside for substrates and products. As shown by the comparisons in Fig. 4, these differences in the tertiary and secondary structure of the prinomastat complex lead to significant shifts in the positions of the contact residues relative to the positions seen in the 2F9Q structure.
DISCUSSION
This study describes the first structure deposited in the PDB of human P450 2D6 complexed with a ligand. The structure was determined with prinomastat bound in the active site. Prinomastat is a matrix metalloprotease inhibitor that is primarily metabolized to a pyridine N-oxide metabolite, and this metabolic pathway is thought to be mediated by P450 2D6 (35). The x-ray diffraction data indicate that prinomastat binds to ferric P450 2D6 with the pyridyl moiety bound to the heme iron consistent with the observation of a red shift in the wavelength maximum of the Soret absorption band observed for the complex. In many cases, coordination of nitrogenous groups on substrates and inhibitors with the heme iron substantially contributes to the stability of the complex when compared with closely related analogs that do not ligate to the heme iron. Although this has often been considered a dead end inhibitor complex, recent studies have emphasized that these heme ligands are oxygenated at significant rates (36). Dissociation of the pyridyl group from the iron followed by oxygenation of the pyridyl group by the reactive intermediate formed by the enzyme would be consistent with the observed formation of the pyridyl N-oxide metabolite.
Our objective in determining the structure of the prinomastat complex was to determine likely differences relative to the structure of the ligand-free protein that occur when prinomastat binds in the active site of P450 2D6. Rowlands et al. (6) described the active site cavity of the ligand-free 2F9Q structure as having the shape of a right foot with the heel positioned above the heme, the arch passing over Phe-120 toward Asp-301, and the calf passing out of the cavity under helix F (Fig. 3C). Extending this analogy, the shape could also be described as a right boot, as the cavity is open and forms a cavity for substrate binding. These authors also discussed docking of the substrate debrisoquine, 3,4-dihydro-1H-isoquinoline-2-carboximidamide (Mr = 175), in the active site and indicated that some degree of protein rearrangement was required for binding, as illustrated by a model obtained from a molecular dynamics simulation. This in silico model allowed debrisoquine to be positioned with its protonated 2-carboximidamide moiety forming a favorable charge-charge interaction with Asp-301 and the 3,4-dihydro-1H-isoquinoline ring positioned for oxygenation by the heme-bound reactive intermediate. This binding orientation was favored by changes in the position of Phe-120 and Phe-483 to open the heel and arch areas of the cavity. Based on the 2F9Q structure and docking studies of Rowland et al. (6), it seemed likely that additional changes would be required to accommodate the larger prinomastat molecule, Mr = 426. As shown in Fig. 3C, a shift in the position of helices F and G is required to accommodate the size of prinomastat as well as coordination of the pyridyl moiety to the heme iron, which requires the plane of pyridyl ring to adopt an almost perpendicular orientation relative to the plane of the heme with the pyridyl nitrogen positioned above the heme iron. This closes the solvent channel formed by the top of the boot and expands the heel and arch regions of the cavity. The resulting structure represents a tertiary structure of the enzyme that can accommodate large substrates and inhibitors in a closed cavity above the heme. Thus, the structure of the prinomastat complex, 3QM4, provides an alternative model for computational examination of ligand interactions with P450 2D6 that complements the existing 2F9Q structural model.
It is unlikely that one or more of the three amino acid differences between the modified proteins used to determine the 3QM4 and 2F9Q structures contributes directly to the observed differences between the structures. The V374M mutation in the allelic variant used for the 2F9Q structure lies outside the active site cavity at the base of the substrate access channel observed in the structure for the prinomastat complex. Although this mutation has been shown to alter catalytic properties for some substrates (25, 26), the larger size of the methionine side chain (Fig. 4C) appears to be easily accommodated in the substrate access channel observed in the structure of the P450 2D6 prinomastat complex. The two additional mutations, L230D and L231R, in the construct used for determination of the 2F9Q structure reside in a loop between helices F′ and G″ that typically correspond to helix G′. Although effects of these differences on protein folding and stability cannot be easily inferred from these considerations, it would appear that these mutations could be accommodated easily in the 3QM4 structure.
The helix F′–G′ region normally exhibits a reverse amphipathicity that contributes to the formation of a hydrophobic exterior surface that is thought to interact with the membrane bilayer, as reviewed in Ref. 30. In this respect, it is important to note that the polar surface of helix F′ of P450 2D6 is populated by the charged residues Glu-216, Arg-221, and Glu-222 that correspond to polar neutral residues for structures of other family 2 P450s. The charged nature of these side chains could contribute to the stability of the solvent-exposed orientation of this surface in the 2F9Q structure. This surface is oriented toward the interior of the protein in the 3QM4 structure of the prinomastat complex, where Arg-221 forms a salt bridge with Glu-216 that stabilizes the buried location of the two residues in the prinomastat complex. Moreover, Glu-222 resides at the entrance to the putative substrate access channel, where it remains hydrated in the 3QM4 structure.
In contrast to prinomastat, many substrates and inhibitors of P450 2D6 are thought to bind as positively charged species at neutral pH, and it has been proposed that the binding of these positively charged compounds is stabilized by the formation of salt bridges with Glu-216, Asp-301, or possibly Glu-222. Mutagenesis of these residues indicates that the acidic nature of these side chains is important for maintaining efficient metabolism of cationic substrates, as recently reviewed (2). The presence of Asp-301 is not unique to P450 2D6 as a corresponding aspartate residue is found at this location in structures of 1A1, 1B1, 2C5, 2C8, 2C9, and 2E1, which do not contribute substantially to the metabolism of cationic substrates. Charged substrates could encounter a kinetic barrier for binding that might otherwise be stabilized by interactions with the conserved aspartic acid residue. Conversely, acidic residues corresponding to Glu-221 and Glu-216 are not found in these enzymes. Glu-216 is positioned, albeit differently, to support substrate binding in both the 3QM4 and the 2F9Q structures. Glu-222 is located on the outside of the protein in the 2F9Q structure and in a putative substrate-binding entrance channel seen in the 3QM4 structure. However, the presence of Glu-222 in a substrate access channel could play a role in substrate binding by providing “bait” for the initial binding of positively charged substrates. This is consistent with the observation that the E222A mutant exhibits a greatly reduced Vmax without significantly affecting the Km value or enantiomer selectivity exhibited for bufuralol 1′-hydroxylation (37). In conjunction with Glu-216 and Asp-301, Glu-222 likely facilitates substrate entry and proper orientation for metabolism followed by subsequent transit further into the cavity for interaction with Glu-216 and/or Asp-301 during catalysis.
In summary, the 3QM4 structure is the first deposited in the PDB for P450 2D6 complexed with a substrate or inhibitor. As judged by a comparison to ligand-free structure 2F9Q determined by Rowland et al. (6), the binding of prinomastat in the active site is associated with a number of changes in the structure of the enzyme that were anticipated based on the size and shape of the compound and its coordination to the heme iron. This study reiterates the potential usefulness of employing visible spectroscopy to monitor substrate and inhibitor binding continuously during the purification of the enzyme and the selection of detergents for purification and crystallization. The substantial differences exhibited by the 3QM4 and 2F9Q structures provide two complementary models for computational approaches to examine substrate and inhibitor binding to the enzyme. Efforts are under way to obtain structures for additional complexes to better define the contributions of the acidic residues to P450 2D6 substrate binding interactions and to delineate adaptive changes in the active site for the binding of structurally distinct substrates and inhibitors.
Acknowledgments
The technical support of LacThu Tonnu for the preparation and crystallization of P450 2D6 and the help provided by the support staff at Stanford Synchrotron Radiation Lightsource for data collection are greatly appreciated. We also thank Pfizer Global Research and Development for providing prinomastat for the purification and crystallization of the prinomastat P450 2D6 complex and Ben Burke, Caroline Lee, Michael Wester, and Michael Zientek for helpful discussions during the course of this work. The Stanford Synchrotron Radiation Lightsource, Structural Molecular Biology Program, is supported by the United States Department of Energy, Office of Biological and Environmental Research, and by National Institutes of Health, NCRR, Biomedical Technology Program, and NIGMS.
This work was supported, in whole or in part, by National Institutes of Health Grant GM031001 (to E. F. J.). This work was also supported by Pfizer Global Research and Development. Portions of this work were carried out at the Stanford Synchrotron Radiation Lightsource, a national user facility operated by Stanford University on behalf of the United States Department of Energy, Office of Basic Energy Sciences.
The atomic coordinates and structure factors (code 3QM4) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
CYP or P450 is a generic term for a cytochrome P450 enzyme, and individual P450s are identified using a number-letter-number format based on amino acid sequence relatedness.
Michael Zientek (Pfizer, Inc., La Jolla, CA), personal communication.
- PDB
- Protein Data Bank.
REFERENCES
- 1. Zanger U. M., Turpeinen M., Klein K., Schwab M. (2008) Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal. Bioanal. Chem. 392, 1093–1108 [DOI] [PubMed] [Google Scholar]
- 2. Wang B., Yang L. P., Zhang X. Z., Huang S. Q., Bartlam M., Zhou S. F. (2009) New insights into the structural characteristics and functional relevance of the human cytochrome P450 2D6 enzyme. Drug Metab. Rev. 41, 573–643 [DOI] [PubMed] [Google Scholar]
- 3. Ingelman-Sundberg M., Sim S. C. (2010) Pharmacogenetic biomarkers as tools for improved drug therapy; emphasis on the cytochrome P450 system. Biochem. Biophys. Res. Commun. 396, 90–94 [DOI] [PubMed] [Google Scholar]
- 4. Kroemer H. K., Eichelbaum M. (1995) “It's the genes, stupid.” Molecular bases and clinical consequences of genetic cytochrome P450 2D6 polymorphism. Life Sci. 56, 2285–2298 [DOI] [PubMed] [Google Scholar]
- 5. Zhou S. F., Liu J. P., Chowbay B. (2009) Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab. Rev. 41, 89–295 [DOI] [PubMed] [Google Scholar]
- 6. Rowland P., Blaney F. E., Smyth M. G., Jones J. J., Leydon V. R., Oxbrow A. K., Lewis C. J., Tennant M. G., Modi S., Eggleston D. S., Chenery R. J., Bridges A. M. (2006) Crystal structure of human cytochrome P450 2D6. J. Biol. Chem. 281, 7614–7622 [DOI] [PubMed] [Google Scholar]
- 7. Wester M. R., Johnson E. F., Marques-Soares C., Dansette P. M., Mansuy D., Stout C. D. (2003) Structure of a substrate complex of mammalian cytochrome P450 2C5 at 2.3 Å resolution. Evidence for multiple substrate binding modes. Biochemistry 42, 6370–6379 [DOI] [PubMed] [Google Scholar]
- 8. Mast N., White M. A., Bjorkhem I., Johnson E. F., Stout C. D., Pikuleva I. A. (2008) Crystal structures of substrate-bound and substrate-free cytochrome P450 46A1; the principal cholesterol hydroxylase in the brain. Proc. Natl. Acad. Sci. U.S.A. 105, 9546–9551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halpert J. R. (2011) Structure and function of cytochromes P450 2B. From mechanism-based inactivators to x-ray crystal structures and back. Drug Metab. Dispos. 39, 1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sansen S., Yano J. K., Reynald R. L., Schoch G. A., Griffin K. J., Stout C. D., Johnson E. F. (2007) Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J. Biol. Chem. 282, 14348–14355 [DOI] [PubMed] [Google Scholar]
- 11. Wang A., Savas U., Stout C. D., Johnson E. F. (2011) Structural characterization of the complex between α-naphthoflavone and human cytochrome P450 1B1. J. Biol. Chem. 286, 5736–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gegner J. A., Dahlquist F. W. (1991) Signal transduction in bacteria: CheW forms a reversible complex with the protein kinase CheA. Proc. Natl. Acad. Sci. U.S.A. 88, 750–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnes H. J. (1996) Maximizing expression of eukaryotic cytochrome P450s in Esherichia coli. Methods Enzymol. 272, 3–14 [DOI] [PubMed] [Google Scholar]
- 14. Wester M. R., Stout C. D., Johnson E. F. (2002) Purification and crystallization of N-terminally truncated forms of microsomal cytochrome P450 2C5. Methods Enzymol. 357, 73–79 [DOI] [PubMed] [Google Scholar]
- 15. Omura T., Sato R. (1964) The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J. Biol. Chem. 239, 2379–2385 [PubMed] [Google Scholar]
- 16. Dehal S. S., Kupfer D. (1997) CYP2D6 catalyzes tamoxifen 4-hydroxylation in human liver. Cancer Res. 57, 3402–3406 [PubMed] [Google Scholar]
- 17. Collaborative Computational Project, Number 4 (1994) The CCP4 suite. Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 18. McCoy A. J., Grosse-Kunstleve R. W., Storoni L. C., Read R. J. (2005) Likelihood-enhanced fast translation functions. Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 [DOI] [PubMed] [Google Scholar]
- 19. Emsley P., Cowtan K. (2004) Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 20. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Crystallography & NMR system. A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 21. Wester M. R., Johnson E. F., Marques-Soares C., Dijols S., Dansette P. M., Mansuy D., Stout C. D. (2003) Structure of mammalian cytochrome P450 2C5 complexed with diclofenac at 2.1 Å resolution. Evidence for an induced fit model of substrate binding. Biochemistry 42, 9335–9345 [DOI] [PubMed] [Google Scholar]
- 22. Schoch G. A., Yano J. K., Wester M. R., Griffin K. J., Stout C. D., Johnson E. F. (2004) Structure of human microsomal cytochrome P450 2C8. Evidence for a peripheral fatty acid-binding site. J. Biol. Chem. 279, 9497–9503 [DOI] [PubMed] [Google Scholar]
- 23. Scott E. E., He Y. A., Wester M. R., White M. A., Chin C. C., Halpert J. R., Johnson E. F., Stout C. D. (2003) An open conformation of mammalian cytochrome P450 2B4 at 1.6-Å resolution. Proc. Natl. Acad. Sci. U.S.A. 100, 13196–13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yano J. K., Hsu M. H., Griffin K. J., Stout C. D., Johnson E. F. (2005) Structures of human microsomal cytochrome P450 2A6 complexed with coumarin and methoxsalen. Nat. Struct. Mol. Biol. 12, 822–823 [DOI] [PubMed] [Google Scholar]
- 25. Crespi C. L., Steimel D. T., Penman B. W., Korzekwa K. R., Fernandez-Salguero P., Buters J. T., Gelboin H. V., Gonzalez F. J., Idle J. R., Daly A. K. (1995) Comparison of substrate metabolism by wild type CYP2D6 protein and a variant containing methionine, not valine, at position 374. Pharmacogenetics 5, 234–243 [DOI] [PubMed] [Google Scholar]
- 26. Ellis S. W., Rowland K., Ackland M. J., Rekka E., Simula A. P., Lennard M. S., Wolf C. R., Tucker G. T. (1996) Influence of amino acid residue 374 of cytochrome P-450 2D6 (CYP2D6) on the regio- and enantio-selective metabolism of metoprolol. Biochem. J. 316, 647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Bahr C., Spina E., Birgersson C., Ericsson O., Göransson M., Henthorn T., Sjöqvist F. (1985) Inhibition of desmethylimipramine 2-hydroxylation by drugs in human liver microsomes. Biochem. Pharmacol. 34, 2501–2505 [DOI] [PubMed] [Google Scholar]
- 28. Shin J. G., Soukhova N., Flockhart D. A. (1999) Effect of antipsychotic drugs on human liver cytochrome P-450 (CYP) isoforms in vitro. Preferential inhibition of CYP2D6. Drug Metab. Dispos. 27, 1078–1084 [PubMed] [Google Scholar]
- 29. Hanna I. H., Kim M. S., Guengerich F. P. (2001) Heterologous expression of cytochrome P450 2D6 mutants, electron transfer, and catalysis of bufuralol hydroxylation. The role of aspartate 301 in structural integrity. Arch. Biochem. Biophys. 393, 255–261 [DOI] [PubMed] [Google Scholar]
- 30. Berka K., Hendrychová T., Anzenbacher P., Otyepka M. (2011) Membrane position of ibuprofen agrees with suggested access path entrance to cytochrome P450 2C9 active site. J. Phys. Chem. A 115, 11248–11255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cojocaru V., Balali-Mood K., Sansom M. S., Wade R. C. (2011) Structure and dynamics of the membrane-bound cytochrome P450 2C9. PLoS Comput. Biol. 7, e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuzmic P., Hill C., Janc J. W. (2004) Practical robust fit of enzyme inhibition data. Methods Enzymol. 383, 366–381 [DOI] [PubMed] [Google Scholar]
- 33. Kuzmic P. (2009) DynaFit. A software package for enzymology. Methods Enzymol. 467, 247–280 [DOI] [PubMed] [Google Scholar]
- 34. Kleywegt G. J., Jones T. A. (1994) Detection, delineation, measurement, and display of cavities in macromolecular structures. Acta Crystallogr. D Biol. Crystallogr. 50, 178–185 [DOI] [PubMed] [Google Scholar]
- 35. Hande K. R., Collier M., Paradiso L., Stuart-Smith J., Dixon M., Clendeninn N., Yeun G., Alberti D., Binger K., Wilding G. (2004) Phase I and pharmacokinetic study of prinomastat, a matrix metalloprotease inhibitor. Clin. Cancer Res. 10, 909–915 [DOI] [PubMed] [Google Scholar]
- 36. Pearson J., Dahal U. P., Rock D., Peng C. C., Schenk J. O., Joswig-Jones C., Jones J. P. (2011) The kinetic mechanism for cytochrome P450 metabolism of type II binding compounds. Evidence supporting direct reduction. Arch. Biochem. Biophys. 511, 69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Masuda K., Tamagake K., Okuda Y., Torigoe F., Tsuzuki D., Isobe T., Hichiya H., Hanioka N., Yamamoto S., Narimatsu S. (2005) Change in enantioselectivity in bufuralol 1"-hydroxylation by the substitution of phenylalanine-120 by alanine in cytochrome P450 2D6. Chirality 17, 37–43 [DOI] [PubMed] [Google Scholar]