Background: 5-Hydroxytryptamine (5HT) modulates N-methyl-d-aspartate (NMDA) depolarization.
Results: 5HT2C co-immunoprecipitates with GluN2A and enhances NMDA motoneuronal depolarization through phosphorylation of SrcTyr-416.
Conclusion: 5HT modulates NMDA through Src phosphorylation in a molecular complex that is localized in the processes of spinal neurons.
Significance: 5HT2C modulation of NMDA excitation is coordinated by a molecular complex.
Keywords: Glutamate Receptors Ionotropic (AMPA, NMDA); Protein Complexes; Serotonin; Signal Transduction; Src; 5HT2C; GluN2A; Multiprotein Complex; Spinal Cord; Tyrosine Phosphorylation
Abstract
N-Methyl-d-aspartate (NMDA)-gated ion channels are known to play a critical role in motoneuron depolarization, but the molecular mechanisms modulating NMDA activation in the spinal cord are not well understood. This study demonstrates that activated 5HT2C receptors enhance NMDA depolarizations recorded electrophysiologically from motoneurons. Pharmacological studies indicate involvement of Src tyrosine kinase mediates 5HT2C facilitation of NMDA. RT-PCR analysis revealed edited forms of 5HT2C were present in mammalian spinal cord, indicating the availability of G-protein-independent isoforms. Spinal cord neurons treated with the 5HT2C agonist MK 212 showed increased SrcTyr-416 phosphorylation in a dose-dependent manner thus verifying that Src is activated after treatment. In addition, 5HT2C antagonists and tyrosine kinase inhibitors blocked 5HT2C-mediated SrcTyr-416 phosphorylation and also enhanced NMDA-induced motoneuron depolarization. Co-immunoprecipitation of synaptosomal fractions showed that GluN2A, 5HT2C receptors, and Src tyrosine kinase form protein associations in synaptosomes. Moreover, immunohistochemical analysis demonstrated GluN2A and 5HT2C receptors co-localize on the processes of spinal neurons. These findings reveal that a distinct multiprotein complex links 5-hydroxytryptamine-activated intracellular signaling events with NMDA-mediated functional activity.
Introduction
NMDA3 receptors, activated by the neurotransmitter glutamate, are critical components of motoneuronal firing patterns of the spinal cord leading to reflexive behavior and locomotion (1–3). NMDA channels are tetraheteromeric and typically contain combinations of two GluN1 subunits plus one or more of GluN2A–D subunits (4–6). Of the GluN2 subunits, the majority of NMDA channels in synapses of the rat spinal cord contain GluN2A and GluN2B (7). Proteonomic approaches indicate NMDA channels (8, 9) are in protein complexes with other membrane receptors, cell adhesion proteins, adaptors, signaling enzymes, and cytoskeletal proteins, but the protein associations of the NMDA channel in the spinal cord have not been well characterized. Functional NMDA channels contain a number of allosteric regulatory and modulatory sites, including those for polyamines, Zn2+, multiple Mg2+-binding sites, a glycine co-agonist site, a PDZ binding domain, a proton site, and a redox site (10). In addition, NMDA subunits have multiple serine-threonine phosphorylation sites that when activated covalently modify receptor structure post-translationally (11).
Serotonin (5-hydroxytryptamine; 5HT) is a major neuromodulator of NMDA-mediated motoneuron output (12–15). Spinal motoneurons express a variety of 5HT receptor subtypes (16–20) that are activated by 5HT released from tracts descending from the raphe nuclei (21) and also by 5HT circulating extrasynaptically (22). Multiple signaling pathways that have been shown to modulate NMDA channel activity (10, 11, 23, 24) are activated by 5HT receptors and include those leading to the production of protein kinase A (PKA), PKC, and Src kinases (25–27). There are 7 classes of 5HT receptors with 1, 2, and 4–7 classes that are generally considered to be G-protein-coupled receptors; the exception, 5HT3, forms an ion channel. NMDA-mediated motoneuronal activity has been shown to be depressed by the activation of 5HT2A and facilitated by 5HT1A and 5HT2B receptor subtypes (12–15). The role of NMDA subunits in 5HT-mediated modulation of NMDA-induced motoneuronal depolarization has not been explored.
We sought to understand whether there is an association between 5HT2C receptors and NMDA-induced motoneuronal excitation. 5HT2C receptors are found on motoneurons (28, 29) and become constitutively active months after spinal transection. 5HT2C activation is implicated in restoring large persistent L-type Ca2+ currents involved in amplification and duration of synaptic input, motoneuronal recovery, and post-injury spasticity (29, 30). In addition, similar to NMDA channels, 5HT2C receptors are in protein complexes with other membrane receptors, cell adhesion proteins, adaptors, signaling enzymes, and cytoskeletal proteins (31, 32).
Here, we provide evidence that 5HT2C receptors enhance NMDA-induced depolarization in spinal motoneurons recorded in situ. The facilitation involves 5HT2C-mediated activation of Src tyrosine kinase. In addition, we explored the role of NMDA subunits in 5HT2C modulation and found that GluN2A subunits, 5HT2C receptors, and Src protein kinases form protein associations in synaptosomes. These findings reveal a multiprotein complex containing a GluN2A subunit and a 5HT2C receptor that links enhancement of spinal motoneuronal NMDA responses to 5HT2C activation of Src tyrosine kinase.
EXPERIMENTAL PROCEDURES
All animal protocols were approved by the University of Miami Institutional Animal Care and Use Committee (IACUC) and are in accordance with National Research Council guidelines for the care and use of laboratory animals.
Electrophysiology
Adult frogs (mixed sex Rana pipiens, ∼2 inches in length from head to tail) were anesthetized by cooling on crushed ice and pithed. Spinal cords with attached dorsal and ventral roots were removed. Cords were hemisected and placed in a bath superfused at a rate of 15–20 ml/min with freshly made Ringer's solution containing the following: NaCl 114 mm, KCl 2.0 mm, CaCl2 1.9 mm, NaHCO3 10 mm, and glucose 5.5 mm; pH was maintained at 7.4 by bubbling with 95% O2, 5% CO2. Magnesium (MgCl2, 1.0 mm) was added to the Ringer's solution in some experiments, as indicated. The IXth ventral root was placed across a 3-mm gap and isolated from the cord with Vaseline; the distal end of the ventral root was placed in a Ringer's bath. Within the gap, sucrose (232 mm) flowed at a rate of 1 ml/s. The isolated dorsal root was placed across silver-silver chloride bipolar electrodes in mineral oil. Membrane potentials of motoneurons in situ were made using DC recordings of the difference in potential between the spinal cord bath and electronically conducted changes at the distal end of the ventral root. Tetrodotoxin (TTX) (0.78 μm) was used to block indirect effects of interneurons and afferents. Temperature was maintained at physiological levels for the frog (18 °C) using a Peltier thermoelectric cooling device. Metabolic activity in the cord is maintained using this preparation (33, 34). The integrity of the preparation was tested by stimulating the dorsal root with a 15.0-V and 1.0-ms rectangular pulse and recording the ventral root potential. Spinal cords with dorsal root-ventral root potentials <5 mV were discarded. Drugs were delivered to the Ringer's superfusate of the hemisected cord using solenoid valves for rapid (<1 s) solution changes. NMDA controls were replicated at least three times and typically varied <1%. When an antagonist was used, NMDA responses were obtained in the absence and presence of MK 212, and responses were compared. Peak amplitude of responses to agonists were measured directly in millivolts and used to compare treatments. Data were expressed as mean ± S.E., and comparisons were made between the NMDA response with and without MK 212 or NMDA in blocker/modulator/condition with and without MK 212. Statistical significance of differences was assessed using Student's t test for correlated means. A significance level of p < 0.05 was accepted as different from control.
Pharmacological Agents
Drugs were obtained from the following suppliers: GMP-PNP, N-(2-aminoethyl)-5-isoquinoline sulfonamide dihydrochloride (H-9), TTX, and staurosporine (Calbiochem); NMDA (Sigma); genistein, 6-chloro-2-(1-piperazinyl)pyrazine hydrochloride (MK 212), [4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo[3,4,-d] pyrimidine (PP2), 8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulfonamido)phenyl-5-oxopentyl]-1,3,8-triazaspiro(4,5)decane-2,4-dionehydrochloride (RS 102221), N-3-pyrinyl-3,5-dihydro-5-methyl-benzo (1,2-b;4,5-b′)dipyrrole-1(2H) (SB 206553), and 1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,2dione (U 73122) (Tocris Cookson).
Drugs were dissolved in Ringer's solution shortly before use to minimize chemical degradation. Dimethyl sulfoxide (DMSO) (0.25%, Sigma) was used to dissolve the following: staurosporine, genistein, MK 212, PP2, and RS 102221.
Preparation of RNA and Reverse Transcriptase-PCR
For this and other studies using mammalian spinal cord, adult male Sprague-Dawley rats (250–350 g) were anesthetized under 3% halothane, 70% N2O, and a balance of O2 to achieve deep sedation. Rat cervical and thoracic spinal cords were removed. Tissue samples were snap-frozen in liquid nitrogen and stored at −80 °C until the time of assay. Primers and probes were designed on the basis of published sequences (35) and were checked for specificity via a BLAST (NCBI) search. Sense and antisense primer sequences flanking the 5HT2C receptor editing region were designed using Primer3 software, where sense and antisense sequences were 5′-CCT GTC TCT GCT TGC AAT TCT-3′ and 5′-GCG AAT TGA ACC GGC TATG-3′, respectively (Sigma). 6-Carboxyfluorescein (FAM)-labeled TaqMan® MGB probes were custom-synthesized (Applied Biosystems) for four 5HT2C receptor mRNA isoforms as follows: the nonedited (INI), FAM-TAG CAA TAC GTA ATC CTA TTG AMGBNFQ; fully edited (VGV, 6FAM-TAG CAG TGC GTG GTC CTG TTG AMGBNFQ; and partially edited (VNV), 6FAM-TAG CAG TGC GTA ATC CTG TTG AMGBNFQ, and (VSV), 6FAM-TAG CAG TGC GTA GTC CTG TTG AMGBNFQ. The sense and antisense primers flanking the editing region were used in quantitative RT-PCR assays with each TaqMan® MGB probe to assess the different 5HT2C receptor mRNA edited isoform levels. Total RNA was extracted from the tissue via homogenization in a guanidinium thiocyanate lysis buffer, extracted with phenol, and precipitated in isopropyl alcohol. The RNA was pelleted by centrifugation and resuspended in buffer containing MgCl2 and ribonuclease-free deoxyribonuclease (DNase). Incubation for 15 min at 37 °C degraded any contaminating DNA. The RNA was precipitated and resuspended. Two micrograms of total RNA from each sample were reverse-transcribed into complementary DNA by using Superscript III reverse transcriptase (Invitrogen), according to the protocol provided by the manufacturer, with oligo(dT) to prime the first-strand synthesis. One-tenth of the complementary DNA product was used in a PCR with TaqMan Universal PCR master mix (Applied Biosystems) and the above-mentioned primers. Real time PCR was performed on an Applied Biosystems 7300 machine and threshold cycles (Ct) were determined. Quantitative PCR for each sample was performed in triplicate, and data analysis utilized the ΔΔCt method and actin average (36). Between the group differences in immunoblots were analyzed using one-way analysis of variance, followed by Tukey post hoc comparison, and significance level of p < 0.05 was accepted as different.
Spinal Neuronal Culture
Spinal neuronal cultures were prepared by dissociation of 16–17-day Sprague-Dawley rat embryonic spinal cords. The tissue was disrupted into a cell suspension by gentle trituration, and the cells were grown on poly-l-lysine-coated tissue culture dishes in N5 medium that contained 5% serum fraction that supports the long term survival of neurons as described (37). For pharmacological experiments, cultures were treated with vehicle (serum + DMSO), MK 212, MK 212 + genistein, or MK 212 + RS 102221. Cells were lysed and immunoblotted as described below. Sixty independent cultures from five rats (∼12 embryo/rat) were used for co-immunoprecipitation analysis (n = 5 for each group).
Immunoblot Analysis
Tissue sections were homogenized in a Dounce homogenizer with extraction/lysis buffer (w/v) (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Triton X-100, 1 mm EDTA, 1 mm EGTA, 2.5 mm pyrophosphate, 1 mm β-glycerophosphate) containing protease and phosphatase inhibitor mixtures (Sigma) and then centrifuged at 15,300 × g for 2 min. Lysates were mixed with 2× Laemmli loading buffer. Equal amounts of protein were resolved on 10–20% gradient Tris-HCl Criterion pre-casted gels (Bio-Rad), to separate proteins with a wide range of molecular weights, transferred to polyvinylidene fluoride (PVDF) membranes, and placed in blocking buffer (0.1% Tween 20, 0.4% I-block in PBS) for 1 h (38). Membranes were then incubated with primary antibodies followed by the appropriate HRP-conjugated secondary antibody. Visualization of the signal was enhanced by chemiluminescence using a Phototope-HRP detection kit (Cell Signaling). Independent samples from rat were used for immunoblot analysis (n = 8 for each group), and data were replicated three times per independent sample. Data are expressed as mean ± S.E. Quantification of bands corresponding to changes in protein levels was made using scanned densitometric analysis and National Institutes of Health Image Program 1.62f. The between group differences in immunoblots were analyzed using one-way analysis of variance, followed by Tukey post hoc comparison. Cultured spinal neurons lysates were normalized to total Src, and a significance level of p < 0.05 was accepted as different from control.
Isolation of Synaptosomes from Rat Spinal Cord
Synaptosomal membranes were isolated from adult rat spinal cords using sucrose density gradient ultracentrifugation at 4 °C. Spinal cord tissue was homogenized in a Dounce homogenizer in a solution containing 0.32 m sucrose, 1 mm NaHCO3, 1 mm MgCl2, 0.5 mm CaCl2 with protease inhibitors (10 mm DTT, 1 mm PMSF, 5 μg/ml leupeptin, 1 μg/ml pepstatin A). The homogenate was centrifuged at 800 × g for 10 min at 4 °C. The supernatant (S1) was saved, and the pellet washed and centrifuged at 800 × g for 10 min at 4 °C. The supernatant (S2) was saved. S1 and S2 were then combined and centrifuged at 13,800 × g for 10 min at 4 °C. The pellet (P1) was suspended with 0.32 m sucrose (1 mm NaHC3)-containing protease inhibitors as described above, and the samples were placed on a discontinuous gradient of 3 ml of 1.2 m sucrose, 3 ml of 1 m sucrose, and 3 ml of 0.85 m sucrose, respectively. The sucrose gradient was centrifuged at 82,500 × g for 2 h at 4 °C, and synaptosomal fractions were extracted from the interface between the 1.2 and 1.0 m sucrose solutions. Isolated synaptosomal proteins were resuspended and solubilized in an equal volume of detergent extraction buffer (2% Triton X-100, pH 7.4), followed by centrifugation at 100,000 × g for 2 min, to remove insoluble material. Synaptosomes were verified by the presence of the presynaptic protein, synaptophysin, and the postsynaptic protein PSD 95. The nonsynaptic peripheral membrane protein annexin V and the nuclear membrane protein lamin IB were used as negative controls. Six independent samples from rat were used for synaptosomal isolation and immunoblot analysis (n = 6 for each group), and data were replicated three times per independent sample.
Co-immunoprecipitation
Synaptosomal fractions from rat (cervical) spinal cords (2 mm2) were isolated as described above. Seventy microliters of TrueblotTM anti-mouse or anti-rabbit IgG immunoprecipitation beads (Ebioscience) were added to 200 μg of sample, and the mixture was rotated at 4 °C for 2 h in a microcentrifuge tube before preclearing. The beads were pelleted by centrifugation at 15,300 × g for 30 s. The supernatant was recovered and mixed with appropriate primary antibody and incubated at 4 °C overnight. Seventy microliters of anti-mouse or anti-rabbit IgG beads was added and incubated for 2 h and then centrifuged at 15,300 × g for 30 s. The pelleted beads were washed six times in extraction/lysis buffer (described above), resuspended in 2× Laemmli loading buffer, and heated at 95 °C for 3 min. Immunoprecipitates were separated on 10–20% Tris-HCl Criterion pre-casted gels and analyzed by immunoblotting using the appropriate antibodies. Preimmune serum lacking the primary antibody was run as a control. Six independent samples from rat were used for co-immunoprecipitation and immunoblot analysis (n = 6 for each group), and data were replicated three times per independent sample.
Immunocytochemistry
Spinal neuronal cultures were prepared as described above. Media were removed, and cultures were fixed in 4% paraformaldehyde for 20 min and then washed twice with phosphate-buffered saline (PBS, pH 7.4). Cultures were then treated with 95% ethanol (−20 °C) for 1 min and washed twice with PBS. Cultures were incubated for 1 h at 4 °C with primary antibodies. Primary antibody binding was detected with Alexa Fluor secondary antibody conjugates (1:2000, Molecular Probes). Controls lacking the primary antibody were run in parallel. Cultures were coverslipped with Vectashield mounting medium (Vector Laboratories) for confocal analysis (Zeiss, LSM 510, scanning confocal microscope).
Perfusion Fixation
Animals were anesthetized with an intramuscular injection of ketamine (87 mg/kg) and xylazine (13 mg/kg). Complete anesthetization was determined by the lack of a stereotypical retraction of the hind paw in response to a nociceptive stimulus. Animals then received an intracardial injection of heparin (0.1 ml) and were perfused transcardially with physiological saline, followed by 300 ml of 4% paraformaldehyde in phosphate-buffered saline (PBS). The spinal cords were removed, placed in 4% paraformaldehyde at 4 °C for 48 h, and then transferred to 20% sucrose in 0.1 m PBS until sectioned.
Immunohistochemistry
Animals were perfused with 4% paraformaldehyde solution as described above, and cervical and thoracic spinal cords were processed for cryostat sectioning (Leica SM 2000R sliding microtome). Sections (50 μm) were stored in free-floating cryostat media (30% ethylene glycol, 30% sucrose, 0.1 m PBS, pH 7.4) at 20 °C and then rinsed with 0.1 m PBS, pH 7.4. Tissue sections were blocked by treatment with 5% goat serum (Vector Laboratories Inc., Burlingame, CA) and 0.4% Triton X-100 (Sigma). Sections were incubated for 48 h at 4 °C with the primary antibodies. Primary antibody binding was detected with Alexa Fluor secondary antibody conjugates (1:200, Molecular Probes, Eugene, OR). Controls lacking the primary antibody were run in parallel. Sections were coverslipped with Vectashield mounting medium (Vector Laboratories Inc., Burlingame, CA) for confocal analysis (Zeiss, LSM 510, scanning confocal microscope).
Antibodies
Antibodies used (with dilutions and sources in parentheses) were as follows: Rabbit polyclonal anti-5HT2B (1:1000, AbCam), anti-5HT2C (1:1000, AbCam), anti-Src527 (1:1000, Cell Signaling), anti-Src527p (1:1000, Cell Signaling), anti-Src416p (1:1000, Cell Signaling), anti-Srctotal (1:1000, Cell Signaling), mouse monoclonal anti-annexin V (1:1000, AbCam), anti-lamin IB (1:1000, AbCam), anti-GLUN1 (1:250, Pharmingen), anti-GLUN2A (1:250, Pharmingen), anti-GLUN2B (1:250, BD Transduction Laboratories), anti-PSD 95 (1:250, Pharmingen), anti-Src416 (1:1000, Cell Signaling), and anti-synaptophysin (1:250, Pharmingen).
RESULTS
5HT2C Activation Enhances NMDA-induced Motoneuron Depolarization
In the presence or absence of Mg2+, the 5HT2C agonist MK 212 (1–100 μm) had no effect on motoneuronal membrane potential. In contrast, MK 212 (1–100 μm) dose-dependently enhanced NMDA-induced depolarization of motoneurons (100 μm, 10 s) in frog spinal cord preparations bathed in nominally Mg2+-free medium (+TTX) (Fig. 1A). At the highest dose of MK 212 tested (100 μm), NMDA responses were 170 ± 29% of control NMDA responses ((NMDA (100 μm) in MK 212 (100 μm)/control NMDA response), n = 4, p < 0.05). In the presence of Mg2+ (1 mm) (+TTX) in the Ringer's bath and using the same doses of NMDA, MK 212 elicits a similar dose-dependent response (Fig. 1B; in 100 μm MK 212, % control NMDA response in Mg2+, 172 ± 15, n = 3, p < 0.05), illustrating that 5HT2C receptor-mediated potentiation of NMDA-evoked motoneuronal depolarization does not involve Mg2+. In experiments with nominally Mg2+-free medium (+TTX) and a single dose of MK 212 (30 μm) in the Ringer's, 100 μm NMDA-induced depolarization was increased by 118 ± 6%, n = 5, p < 0.05).
FIGURE 1.
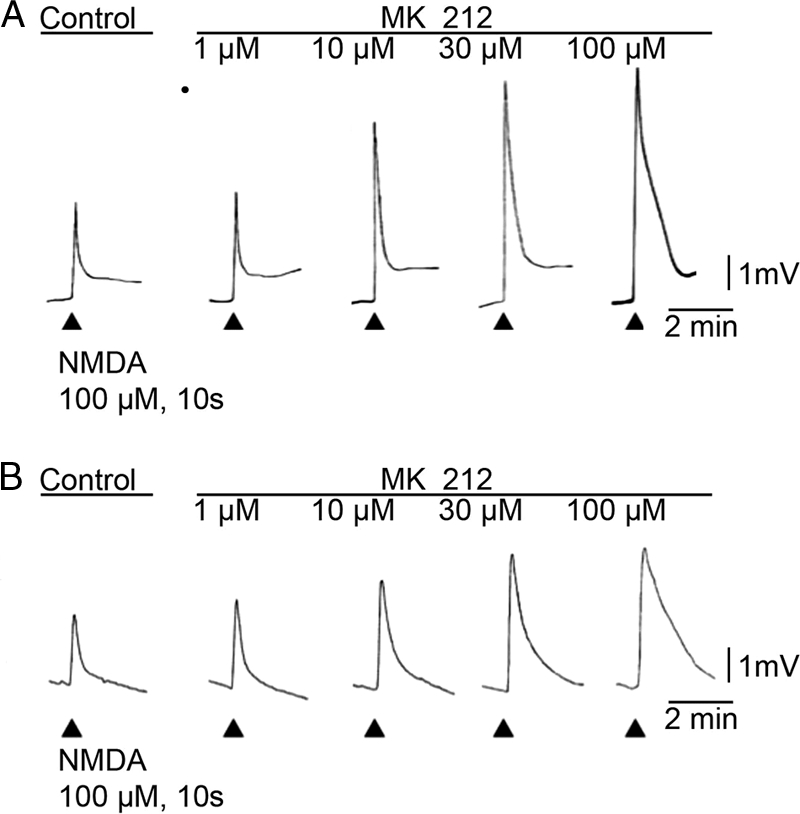
5HT2C agonist MK 212 increases NMDA-induced motorneuronal depolarization in the frog spinal cord. A, 5-HT2C agonist MK 212 enhances NMDA-induced motoneuron depolarizations in a dose-dependent manner. Application of NMDA (100 μm, 10 s) is indicated by the arrowheads below the base line, before and during increasing doses of MK 212 (1–100 μm). The traces show frog motoneuronal membrane potentials conducted along the IX ventral root. In this and other experiments MK 212, at the doses tested (1–100 μm), had no effect on base line motoneuron membrane potentials. Negativity is reflected by upward pen deflection. The Ringer's solution contained TTX (0.783 μm) to block the indirect effects of interneuronal and afferent activation. B, in the presence of 1 mm Mg2+ and TTX (0.783 μm) in the Ringer's bath, MK 212 increases NMDA-evoked motoneuron depolarizations in a dose-dependent manner. Calibration bars for each experiment are located on the right.
To verify the effect of 5HT2C receptor activation on enhancement of NMDA depolarization, the 5HT2B/C-selective receptor antagonist SB 206553 (10 μm) and the 5HT2C selective antagonist RS 102221 (10 μm) were tested in frog spinal cord preparations (+TTX, no Mg2+). Antagonists had no effect on motoneuronal membrane potential. Additionally, in the presence of SB 206553 or RS 102221, MK 212 (30 μm) had no effect on NMDA-induced depolarization of motoneurons (% control NMDA response, 99 ± 4, n = 3, and 98 ± 2, n = 3, respectively), supporting that the enhanced NMDA responses occur through the specific activation of 5HT2C receptors.
5HT2C Enhancement of NMDA-induced Motoneuron Depolarization Is Independent of a G-protein-coupled Receptor Mechanism
To investigate whether 5HT2C receptor activation and enhancement of NMDA motoneuronal depolarization involves G-protein-coupled receptor intracellular mechanisms, the G-protein-coupled receptor antagonist, GMP-PNP, was tested in frog spinal cord preparations (+TTX, no Mg2+). GMP-PNP (200 μm) had no effect on motoneuronal membrane potential. However, GMP-PNP (200 μm) has previously been shown to block 5HT2B- and 5HT2A-mediated G-protein activation using similar experimental procedures (15).4 Fig. 2 illustrates that GMP-PNP (200 μm) did not block the MK 212 (30 μm)-induced increase of NMDA responses (% control NMDA response, 128 ± 6, n = 4, p < 0.05), suggesting that the 5HT2C receptor effect on NMDA-induced motoneuron depolarization does not involve G-protein signaling.
FIGURE 2.
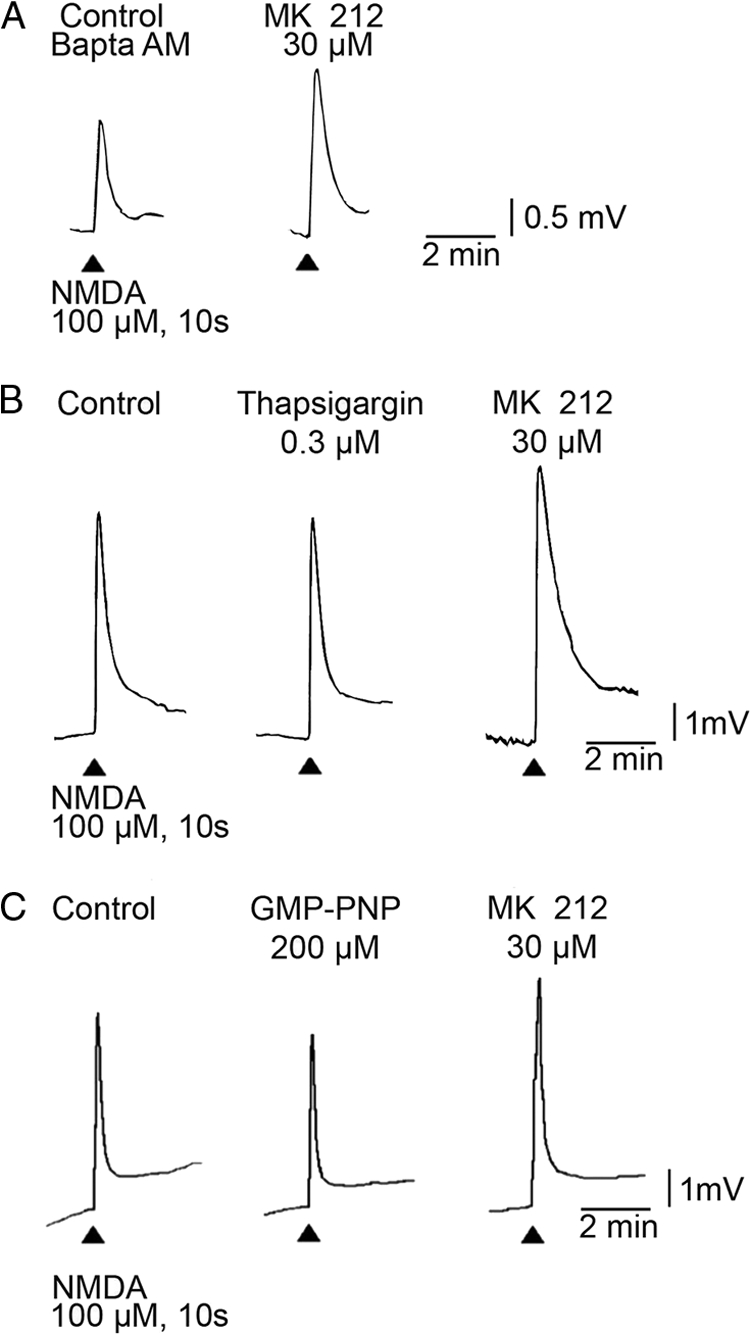
MK 212-mediated increases in NMDA-induced motoneuronal depolarization in the frog spinal cord are not reduced by BAPTA-AM, 0 Ca2+, thapsigargin, or G-protein antagonist GMP-PNP. NMDA (100 μm, 10 s) applications are indicated by the arrowheads. The effect of MK 212 (30 μm) on NMDA motoneuronal depolarization is shown during the presence of BAPTA-AM (A), 0 Ca2+, thapsigargin (B), or G-protein antagonist GMP-PNP (C) in the bath. No changes in base line motoneuron membrane potential were detected when thapsigargin or GMP-PNP was added to the superfusate; a small hyperpolarization was seen in nominally free Ca2+ solutions. TTX (0.783 μm) was present at all times. Calibration bars apply to all responses.
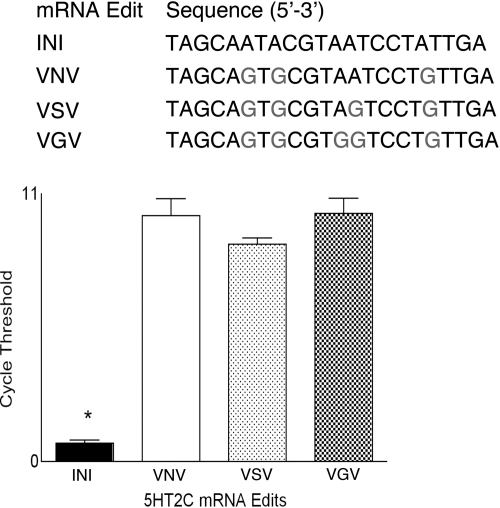
G-protein coupling efficiency of the 5HT2C receptor is directly linked to pre-mRNA editing (39). Rat thoracic spinal cord tissue was utilized to examine the levels of four major isoforms of the 5HT2C receptor pre-mRNA (Fig. 3A). RT-PCR was performed using primers specific for unedited 5HT2C (INI), fully edited 5HT2C (VGV), and two closely related isoforms (VNV and VSV) to examine the relative amount of these 5HT2C mRNA isoforms in the rat spinal cord. Fig. 3B shows that mRNA for VNV, VSV, and VGV 5HT2C isoforms was present in rat spinal cord, whereas significantly less of the unedited INI isoform was detected. The presence of edited versus unedited versions of 5HT2C receptor isoforms in rat spinal cord may explain our findings on the lack of involvement of a G-protein in 5HT2C-mediated enhancement of NMDA responses (40).
FIGURE 3.
5HT2C mRNA isoforms in the rat spinal cord. Top, edited regions of four major isoforms of the 5HT2C receptor pre-mRNA. Bottom, quantification of mRNA for VNV, VSV, and VGV 5HT2C isoforms in rat thoracic spinal cord. Significantly less of the unedited INI isoform was detected (p < 0.05). Cycle thresholds obtained with RT-PCR using primers specific for unedited 5HT2C (INI), fully edited 5HT2C (VGV), and two closely related isoforms (VNV and VSV).
Ca2+ Is Not Involved in the 5HT2C-mediated Enhancement of NMDA Responses on Motoneurons
Several conditions were used to assess the involvement of Ca2+ in 5HT2C-mediated signaling and effect on NMDA responses (Fig. 2). In frog spinal cord preparations (+TTX, no Mg2+), 0 mm Ca2+ Ringer's conditions did not block 30 μm MK 212-evoked enhancement of NMDA-induced responses (control NMDA response, 128 ± 6, n = 4, p < 0.05). The intracellular Ca2+ chelator BAPTA-AM in the 0 mm Ca2+ condition did not reduce NMDA responses in 30 μm MK 212 (119% control NMDA response). Additionally, administration of thapsigargin, which blocks Ca2+ re-uptake and hence the release of Ca2+ by inositol triphosphate, did not change 5HT2C receptor-mediated increases in NMDA-induced responses (in 30 μm MK 212, % control NMDA response, 130 ± 8, n = 3, p < 0.05). These data demonstrate that Ca2+-related signaling is not involved in 5HT2C receptor enhancement of NMDA-induced motoneuronal depolarization.
5HT2C Receptor Activation and Potentiation of NMDA Motoneuron Depolarization Are Blocked by Protein Kinase Inhibition
To examine if activation of 5HT2C receptors and potentiation of NMDA-induced motoneuronal depolarization involves protein kinases, the broad range protein kinase inhibitor H-9 and the specific tyrosine kinase inhibitor genistein were tested in frog spinal cord preparations (+TTX, no Mg2+). In H-9 (50 μm), MK 212-evoked enhancement of NMDA-induced responses was blocked (Fig. 4A; 99 ± 1% control NMDA response, n = 3, p < 0.05); similarly, genistein (150 μm) blocked MK 212 potentiation of NMDA-induced responses (Fig. 4B; 87 ± 5% control NMDA response, n = 3, p < 0.05). Additionally, the Src-specific protein-tyrosine kinase inhibitor PP2 (66 μm) was examined under identical conditions, and it effectively prevented 5HT2C potentiation of NMDA-induced responses (Fig. 5C; 97 ± 9% control NMDA response, n = 3, p < 0.05). Taken together, these results indicate that Src tyrosine kinase mediates the effect that 5HT2C receptor activation has on NMDA-induced motoneuron depolarization.
FIGURE 4.
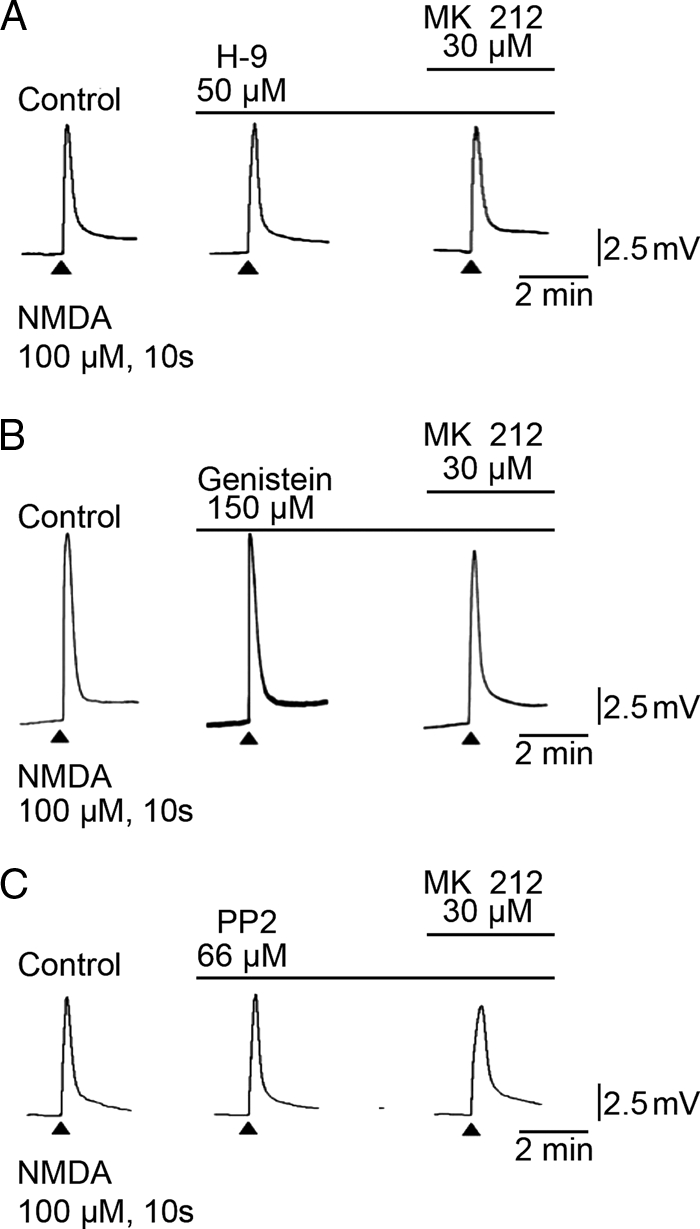
Effects of Src kinase blockers on MK 212 enhancement of NMDA-evoked motoneuronal depolarization (100 μm, 10 s). A, NMDA-induced depolarization of frog motoneurons in the presence of normal TTX-Ringer's with the broad spectrum kinase inhibitor H-9 (50 μm) added to the Ringer's and MK 212 (30 μm) added to H-9/TTX-Ringer's. B, NMDA-induced depolarization of frog motoneurons in the presence of normal TTX-Ringer's, with the tyrosine kinase inhibitor genistein (150 μm) added to the TTX/Ringer's and MK 212 (30 μm) added to the genistein/TTX-Ringer's. C, NMDA-induced depolarization of frog motoneurons in the presence of normal TTX-Ringer's, with the Src inhibitor PP2 (66 μm) added to the TTX/Ringer's, and MK 212 (30 μm) added to the PP2/TTX-Ringer's.
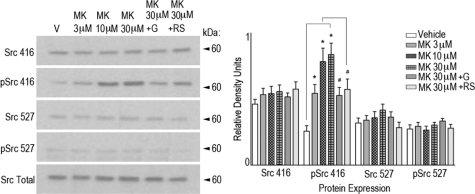
FIGURE 5.
5HT2C agonist MK 212 results in phosphorylation of Src416 in rat spinal neuronal culture. Immunoblot analysis of primary rat spinal neuronal culture treated with MK 212 (3–30 μm) for 20 min resulted in significant increases in Src416 phosphorylation over vehicle treatment (0.25% DMSO) at all doses tested. The tyrosine kinase inhibitor genistein (G) (100 μm) administered together with MK 212 (30 μm) significantly blocked the increase in Src416 phosphorylation observed with MK 212 alone. Similarly, the 5HT2C receptor antagonist RS 1022221 (RS) (10 μm) administered together with MK 212 (30 μm) significantly blocked the increases in Src416 phosphorylation observed with MK 212 alone. MK 212 (3–30 μm), MK 212 (30 μm) + genistein (100 μm), or MK 212 (30 μm) + RS 1022221 (10 μm) resulted in no significant difference in the expression of Src416, Src527, or phosphorylation of Src527. Total Src was used as an internal protein loading control. Quantification of immunoblots was according to data analysis methods described. p ≤ 0.05.
We next extended these findings to examine the effect of MK 212 administration and 5HT2C receptor activation on Src phosphorylation in rat spinal neuronal cultures. MK 212 (3–30 μm) induced significant increases in the amount of Src phosphorylation at tyrosine residue 416 compared with vehicle (DMSO) in a dose-dependent manner (Fig. 5). No significant change in the amount of Src phosphorylation at tyrosine residue 527 was detected. Treatment of cultures with the tyrosine kinase inhibitor genistein (100 μm) and the 5HT2C receptor antagonist RS 102221 (10 μm) significantly attenuated the amount of phosphorylated Src416 observed after MK 212 administration. These results support the idea that 5HT2C receptor activation induces Src416 phosphorylation and activation.
GLUN2A NMDA Channel Subunit, 5HT2C Receptors, and Src Tyrosine Kinase Form a Multiprotein Complex in Synaptosomes of Rat Cervical Spinal Cord
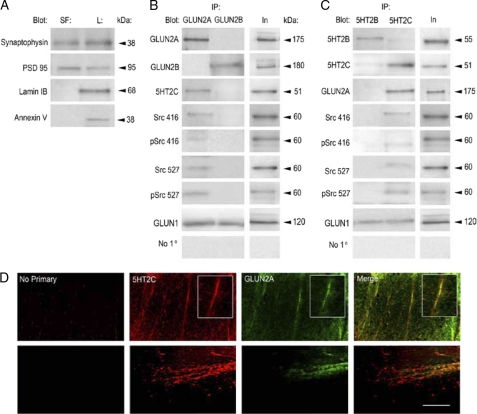
Protein-protein interactions between 5HT2C receptor subtype GluNs and Src tyrosine kinase were assessed using co-immunoprecipitation procedures of synaptosomes isolated from rat cervical spinal cord. The purity of synaptosome isolation was confirmed by immunoblotting samples with the pre-synaptic marker synaptophysin and the post-synaptic marker PSD 95 (Fig. 6A). The nonsynaptic peripheral membrane protein lamin IB and the nuclear membrane marker annexin V were not observed in synaptosome preparations, demonstrating specificity and purity in the biochemical isolation procedures. Co-immunoprecipitation of synaptosomal fractions using either anti-GluN2A or -GluN2B antisera showed that GluN2A, 5HT2C receptors, and Src tyrosine kinase form protein associations with each other (Fig. 6B). However GluN2B-containing GluNs did not associate with either 5HT2C receptors or Src tyrosine kinase. Reciprocal co-immunoprecipitation of synaptosomal fractions using either anti-5HT2B or -5HT2C revealed similar protein interactions (Fig. 6C). The omission of primary antibody did not immunoprecipitate any proteins examined, and thereby demonstrates antibody specificity and serves as a control. GluN1 receptor subunits were immunoprecipitated with both GluN2 subunits and 5HT2 receptors, providing evidence for assembled NMDA receptors. These findings reveal a novel multiprotein receptor complex consisting of GLUN2A-5HT2C-Src tyrosine kinase in synaptosomes isolated from rat cervical spinal cord tissue that may represent a synaptic organization regulating functional signaling.
FIGURE 6.
GluN2A, 5HT2C, and Src tyrosine kinase multiprotein association in synaptosomes and GluN2A/5HT2C localization in rat spinal cord tissue and spinal neuronal culture. A, synaptosomal membranes were isolated from rat spinal cord tissue as described. Synaptosomal fractions (SF) and total lysate (L) contained the pre-synaptic protein synaptophysin and the post-synaptic protein PSD 95. The nonsynaptic peripheral membrane protein annexin V and the nuclear membrane protein lamin IB were not detected in synaptosomal fractions but were present in total lysate. B, co-immunoprecipitation (IP) from synaptosomes using GluN2A antisera precipitated its cognate receptor subunit, the 5HT2C receptor, phosphorylated and nonphosphorylated forms of Src416 and Src527, as well as the GluN1 receptor subunit. GluN2A did not precipitate GluN2B. Co-immunoprecipitation using GluN2B antisera precipitated its cognate receptor subunit, as well as the GluN1 receptor subunit. GluN2B did not precipitate either 5HT2C or any form of Src examined. C, co-immunoprecipitation from synaptosomes using 5HT2C antisera precipitated its cognate receptor, GluN2A and GluN1 receptor subtypes, and both phosphorylated and nonphosphorylated forms of Src416 and Src527. Co-immunoprecipitation using 5HT2B antisera precipitated its cognate receptor and the GluN1 receptor subtype, but it did not precipitate either 5HT2C or any form of Src examined. Omission of primary antibody did not immunoprecipitate any proteins examined. D, 5HT2C receptors and GluN2A are expressed and co-localize in processes of cervical rat spinal cords and cultured spinal neurons. Confocal images show spinal neuronal cultures immunostained for the 5HT2C receptor (red) and GluN2A (green). 5HT2C and GluN2A immunoreactivity is observed in processes in the medial aspect of the ventral horn (lamina IX) in spinal cord tissue. 5HT2C and GluN2A immunoreactivity also co-localize in distinct regions in processes of cultured spinal neurons. Omission of primary antibody did not result in 5HT2C or GluN2A immunoreactivity in spinal cord tissue or spinal neuronal cultures. Scale bar, 100 μm.
5HT2C and GLUN2A Co-localize in Rat Spinal Cord Tissue and Spinal Neuronal Cultures
Fig. 6D shows confocal images of the cellular expression and distribution of 5HT2C and GluN2A in rat cervical spinal cord sections and spinal neurons. Spinal cord sections were immunostained with anti-5HT2C (Fig. 6D, red, top panels) and anti-GluN2A (green, top panels). 5HT2C and GluN2A immunoreactivity were seen in processes within the medial aspect of the ventral horn and co-localized to regionally similar areas (Fig. 6D, merge, top panel). Moreover, spinal neuronal cultures were immunostained with anti-5HT2C (Fig. 6D, red, bottom panel) and anti-GluN2A (green, bottom panel), illustrating similar immunoreactivity and co-localization in distinct regions in processes (Fig. 6D, merge, bottom panel). These results suggest that 5HT2C and GluN2A co-localize in spinal neuronal cultures in areas distinct from the soma and support the idea of their cellular localization in axodendritic processes.
DISCUSSION
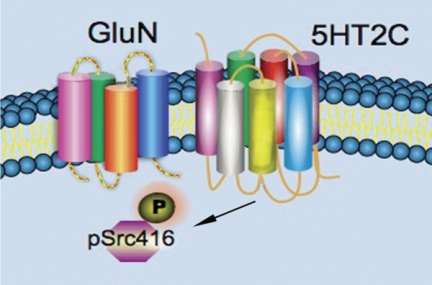
In this study, we show in electrophysiology experiments on metabolically active adult R. pipiens motoneurons in situ that the 5HT2C agonist MK 212 enhances NMDA-induced motoneuronal depolarization. In addition, in mammalian spinal neuronal cultures, we observe 5H2C agonist MK 212 dose-dependently increases phosphorylation of SrcTyr-416. The electrophysiological and immunological effects found in frog and rat, respectively, were blocked by 5HT2C antagonists and tyrosine kinase inhibitors. In synaptosomal fractions from rat spinal cord, NMDA GluN2A subunit antisera co-immunoprecipitated the 5HT2C receptor and also phosphorylated and nonphosphorylated SrcTyr-416 and SrcTyr-527. Reciprocal experiments using 5HT2C antisera gave similar results. Immunohistochemistry in cervical rat spinal cord sections and immunocytochemistry in cultured spinal neurons show GluN2A and 5HT2C localized on axodendritic processes. These data provide molecular biochemical, pharmacological, and electrophysiological evidence that activation of Src tyrosine kinase is induced by activation of 5HT2C receptors and mediates increases in motoneuronal depolarization produced by NMDA (Fig. 7).
FIGURE 7.
Model of 5HT2C modulation of the NMDA channel. The 5HT2C receptor and NMDA channel are shown in close proximity. We hypothesize activation of 5HT2C receptors results in phosphorylation of SrcTyr-416, which then modifies the GluN2A subunit of the NMDA channel to increase channel open times in response to an agonist.
Selectivity of the agents we used has been previously demonstrated in experimental preparations using similar electrophysiological methods. Pharmacological specificity of the 5HT2C antagonists was demonstrated in previous studies where the 5HT2B/2C antagonist SB206553, but not the selective 5HT2C antagonist RS 102221, blocked 5HT2B-mediated enhancement of NMDA-induced motoneuronal depolarization (15). Thapsigargin inhibition of Ca2+-ATPase as well as nominally Ca2+-free medium have previously been shown to reduce G-protein-mediated increases in NMDA responses produced by trans-(±)-1-amino-1,3-cyclopentane dicarboxylic acid (41).
In addition, the broad spectrum G-protein antagonist GMP-PNP irreversibly activates G-proteins by saturating GTP-binding sites but did not block the effects of 5HT2C activation in this study. However, in other studies, GMP-PNP blocked the activation of Gq by 5HT2B (15) and Gi/o by mGluR group 1 receptors (41). Because 5HT2C is in the class of 5HT receptors that activate Gq, it is unlikely that GMP-PNP would not block an effect coupled to Gq. Similarly, in an additional experiment, MK 212 potentiation of NMDA responses was not blocked by the Gq-protein antagonist GP2-antagonist, which previously blocked Gq-mediated effects of 5HT2B (15).
Src activation has been linked to a number of G-protein-coupled receptor signaling pathways, including M1 muscarinic receptors (42, 43), P2Y2 purinergic receptors (44), β2 (45)- and β3-adrenergic receptors (46). Furthermore, in transfected HEK cells, Src co-localizes β2-adrenergic receptor and β-arrestin 1 after agonist stimulation of the β2-adrenergic receptor (45) indicating that activation of a G-protein may be involved in mobilizing these proteins. Interestingly, although our electrophysiological data showed that activation of the 5HT2C receptor mediates Src potentiation of NMDA responses, we found that the increases produced by activation of 5HT2C were not blocked by the G-protein antagonist GMP-PNP. A similar result was reported by Barthet et al. (47), where 7-transmembrane 5HT4 receptor activation of ERK signaling was mediated by Src but did not involve a G-protein.
A growing body of literature has shown that the 5HT2C receptor pre-mRNA is a substrate for base modification (39, 48) that alters the amino acid coding potential within the second intracellular loop of the receptor (49, 50). RNA editing of 5HT2C effects receptor binding, G-protein coupling, constitutive activity, and trafficking (49–52). 5HT2C receptor transcripts can be edited at up to five sites, potentially generating 24 different receptor versions and hence a diverse receptor population (53). Unedited INI has been identified as the most efficient in G-protein coupling (54), although fully edited VGV is the least efficient in this regard (49, 50, 55). We identified mRNA for VNV, VSV and VGV 5HT2C isoforms in the rat spinal cord but significantly less of the unedited INI isoform. These data support our results showing that activation of spinal cord 5HT2C receptor mediates an increase in NMDA-induced motoneuronal depolarization that is not blocked by G-protein antagonists.
Co-immunoprecipitation experiments show 5HT2C receptors, Src and GluN2A subunits, are in a multiprotein complex in synaptosomes. Correspondingly, we find that GluN2A subunits and 5HT2C receptors co-localize to processes in the medial aspect of the ventral horn of cervical rat spinal cord sections, as well as rat spinal neuronal cultures. Co-immunoprecipitation of Src from GluN1 (56) and protein associations linking Src to NMDA channels have been shown (57). Isolated NMDA-PSD 95 complexes also pull down Src in immunoprecipitation experiments (8). In our experiments, activation of 5HT2C receptors renders Src in the active conformation where SrcTyr-416 is phosphorylated (58–60). Our findings illustrate that GluN1 is immunoprecipitated with both 5HT2C and GluN2A antisera, providing further evidence that 5HT2C, GluN2A, and Src multiprotein complexes involve functionally assembled NMDA receptors. Interestingly, 5HT2B and GluN2B antisera also immunoprecipitated GluN1, suggesting that these receptor subtypes also form distinct multiprotein complexes with functionally assembled NMDA receptors and may affect NMDA receptor-induced motoneuronal responses. Previously reported electrophysiological and pharmacological data has shown that NMDA-induced depolarization of motor neurons is modulated by activation of a Gq-protein-coupled 5HT2B receptor and the entry of extracellular Ca2+ through voltage-dependent L-type Ca2+ channels (15). We show distinct multiprotein interactions of GluN2A and 5HT2C in subcellular microdomains, shown to localize NMDA channel modulators and intracellular signaling (8, 9, 31, 57, 61–63). Taken together, these findings support that NMDA receptor functional activity is regulated through distinct subcellular localization and receptor subtype-mediated intracellular signaling.
Neuronal output depends on highly specialized local intracellular environments and subcellular microdomains, including dendritic spines and synapses (64, 65). Several distinct pathways for receptor interaction and signal transduction mechanisms may contribute to differential and controlled neuronal output. Multiple functional responses have been reported for 5HT receptor subtypes (66), and NMDA-specific subunit activation has been shown to mediate very different effects on neuronal excitation and activation of intracellular signals (67–69). Our findings extend previous studies on 5HT2C receptors in the spinal cord (29, 30) and support the hypothesis that 5HT2C receptors positively modulate NMDA-mediated motoneuronal depolarization, through non-G-protein-mediated mechanisms, by increasing Src416 tyrosine kinase phosphorylation. It is well established that Src functions to regulate NMDA receptor ion channels (60, 70, 71) and directly phosphorylates GluN2A subunits (73, 74). GluN2A phosphorylation may regulate the protein composition of NMDA receptor complexes, NMDA receptor trafficking, or directly affect gating (60), resulting in the enhancement of NMDA receptor-mediated motoneuronal responses.
NMDA channels and 5HT receptors are integral in the regulation of spinal reflexes and rhythmic motor pathways (13, 14, 75–77). Activation of 5HT2C receptors induces long lasting reflexes (78), and after spinal cord injury, locomotion in rats requires constitutive activity of 5HT2C receptors (79). In addition, 5HT2C receptors mediate post-injury recovery of the large inward persistent L-type Ca2+ channel in motoneurons and the development of spasticity (29, 30). 5HT2C receptors are also involved in maturation (80), plasticity (81), and neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS) (72). Understanding the protein associations involved in 5HT2C receptor signaling and NMDA, channels may provide insight into their regulation and control to target specific therapeutic intervention in pathologies affecting spinal cord motor pathways.
Acknowledgments
We greatly appreciate the help and expertise of Doris Nonner with culturing spinal neurons and Jessie S. Truettner with the development of primers and RT-PCR experiments. We also thank John C. Hackman, W. Dalton Dietrich, and The Miami Project to Cure Paralysis for their support.
The work was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Program.
A. M. Holohean and J. C. Hackman, unpublished results.
- NMDA
- N-methyl-d-aspartate
- 5HT
- 5-hydroxytryptamine
- TTX
- tetrodotoxin
- GMP-PNP
- guanylyl-5′-imidodiphosphate, tetralithium salt
- FAM
- 6-carboxyfluorescein
- BAPTA-AM
- 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester).
REFERENCES
- 1. Ziskind-Conhaim L. (1990) NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. J. Neurosci. 10, 125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rekling J. C., Funk G. D., Bayliss D. A., Dong X. W., Feldman J. L. (2000) Synaptic control of motoneuronal excitability. Physiol. Rev. 80, 767–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hägglund M., Borgius L., Dougherty K. J., Kiehn O. (2010) Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat. Neurosci. 13, 246–252 [DOI] [PubMed] [Google Scholar]
- 4. Nakanishi N., Axel R., Shneider N. A. (1992) Alternative splicing generates functionally distinct N-methyl-d-aspartate receptors. Proc. Natl. Acad. Sci. U.S.A. 89, 8552–8556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hollmann M., Heinemann S. (1994) Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31–108 [DOI] [PubMed] [Google Scholar]
- 6. Wenthold R. J., Prybylowski K., Standley S., Sans N., Petralia R. S. (2003) Trafficking of NMDA receptors. Annu. Rev. Pharmacol. Toxicol. 43, 335–358 [DOI] [PubMed] [Google Scholar]
- 7. Nagy G. G., Watanabe M., Fukaya M., Todd A. J. (2004) Synaptic distribution of the NR1, NR2A, and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur. J. Neurosci. 20, 3301–3312 [DOI] [PubMed] [Google Scholar]
- 8. Husi H., Ward M. A., Choudhary J. S., Blackstock W. P., Grant S. G. (2000) Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat. Neurosci. 3, 661–669 [DOI] [PubMed] [Google Scholar]
- 9. Husi H., Grant S. G. (2001) Proteomics of the nervous system. Trends Neurosci. 24, 259–266 [DOI] [PubMed] [Google Scholar]
- 10. Traynelis S. F., Wollmuth L. P., McBain C. J., Menniti F. S., Vance K. M., Ogden K. K., Hansen K. B., Yuan H., Myers S. J., Dingledine R. (2010) Glutamate receptor ion channels. Structure, regulation, and function. Pharmacol. Rev. 62, 405–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smart T. G. (1997) Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Curr. Opin. Neurobiol. 7, 358–367 [DOI] [PubMed] [Google Scholar]
- 12. Holohean A. M., Hackman J. C., Davidoff R. A. (1990) Changes in membrane potential of frog motoneurons induced by activation of serotonin receptor subtypes. Neuroscience 34, 555–564 [DOI] [PubMed] [Google Scholar]
- 13. Holohean A. M., Hackman J. C., Shope S. B., Davidoff R. A. (1992) Serotonin1A facilitation of frog motoneuron responses to afferent stimuli and to N-methyl-d-aspartate. Neuroscience 48, 469–477 [DOI] [PubMed] [Google Scholar]
- 14. Holohean A. M., Hackman J. C., Shope S. B., Davidoff R. A. (1992) Activation of 5-HT1C/2 receptors depresses polysynaptic reflexes and excitatory amino acid-induced motoneuron responses in frog spinal cord. Brain Res. 579, 8–16 [DOI] [PubMed] [Google Scholar]
- 15. Holohean A. M., Hackman J. C. (2004) Mechanisms intrinsic to 5-HT2B receptor-induced potentiation of NMDA receptor responses in frog motoneurones. Br. J. Pharmacol. 143, 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mengod G., Pompeiano M., Martínez-Mir M. I., Palacios J. M. (1990) Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 524, 139–143 [DOI] [PubMed] [Google Scholar]
- 17. Hellendall R. P., Schambra U. B., Liu J. P., Lauder J. M. (1993) Prenatal expression of 5-HT1C and 5-HT2 receptors in the rat central nervous system. Exp. Neurol. 120, 186–201 [DOI] [PubMed] [Google Scholar]
- 18. Helton L. A., Thor K. B., Baez M. (1994) 5-Hydroxytryptamine2A, 5-hydroxytryptamine2B, and 5-hydroxytryptamine2C receptor mRNA expression in the spinal cord of rat, cat, monkey, and human. Neuroreport 5, 2617–2620 [DOI] [PubMed] [Google Scholar]
- 19. Ridet J. L., Tamir H., Privat A. (1994) Direct immunocytochemical localization of 5-hydroxytryptamine receptors in the adult rat spinal cord. A light and electron microscopic study using an anti-idiotypic antiserum. J. Neurosci. Res. 38, 109–121 [DOI] [PubMed] [Google Scholar]
- 20. Okabe S., Mackiewicz M., Kubin L. (1997) Serotonin receptor mRNA expression in the hypoglossal motor nucleus. Respir. Physiol. 110, 151–160 [DOI] [PubMed] [Google Scholar]
- 21. Alvarez F. J., Pearson J. C., Harrington D., Dewey D., Torbeck L., Fyffe R. E. W. (1998) Distribution of 5-hydroxytryptamine-immunoreactive boutons on α-motoneurons in the lumbar spinal cord of adult cats. J. Comp. Neurol. 393, 69–83 [PubMed] [Google Scholar]
- 22. Hentall I. D., Pinzon A., Noga B. R. (2006) Spatial and temporal patterns of serotonin release in the rat's lumbar spinal cord following electrical stimulation of the nucleus raphe magnus. Neuroscience 142, 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berg K. A., Maayani S., Goldfarb J., Scaramellini C., Leff P., Clarke W. P. (1998) Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors. Evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol. 54, 94–104 [PubMed] [Google Scholar]
- 24. Lan J. Y., Skeberdis V. A., Jover T., Grooms S. Y., Lin Y., Araneda R. C., Zheng X., Bennett M. V., Zukin R. S. (2001) Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 4, 382–390 [DOI] [PubMed] [Google Scholar]
- 25. Banes A., Florian J. A., Watts S. W. (1999) Mechanisms of 5-hydroxytryptamine(2A) receptor activation of the mitogen-activated protein kinase pathway in vascular smooth muscle. J. Pharmacol. Exp. Ther. 291, 1179–1187 [PubMed] [Google Scholar]
- 26. Barnes N. M., Sharp T. (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152 [DOI] [PubMed] [Google Scholar]
- 27. Lu R., Alioua A., Kumar Y., Kundu P., Eghbali M., Weisstaub N. V., Gingrich J. A., Stefani E., Toro L. (2008) c-Src tyrosine kinase, a critical component for 5-HT2A receptor-mediated contraction in rat aorta. J. Physiol. 586, 3855–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holmes G. M. (2005) 5-Hydroxytryptamine2C receptors on pudendal motoneurons innervating the external anal sphincter. Brain Res. 1057, 65–71 [DOI] [PubMed] [Google Scholar]
- 29. Murray K. C., Nakae A., Stephens M. J., Rank M., D'Amico J., Harvey P. J., Li X., Harris R. L., Ballou E. W., Anelli R., Heckman C. J., Mashimo T., Vavrek R., Sanelli L., Gorassini M. A., Bennett D. J., Fouad K. (2010) Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat. Med. 16, 694–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murray K. C., Stephens M. J., Ballou E. W., Heckman C. J., Bennett D. J. (2011) Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J. Neurophysiol. 105, 731–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bécamel C., Alonso G., Galéotti N., Demey E., Jouin P., Ullmer C., Dumuis A., Bockaert J., Marin P. (2002) Synaptic multiprotein complexes associated with 5-HT(2C) receptors. A proteomic approach. EMBO J. 21, 2332–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pluder F., Mörl K., Beck-Sickinger A. G. (2006) Proteome analysis to study signal transduction of G protein-coupled receptors. Pharmacol. Ther. 112, 1–11 [DOI] [PubMed] [Google Scholar]
- 33. Hackman J. C., Holohean A. M., Wohlberg C. J., Davidoff R. A. (1987) After-hyperpolarizations produced in frog motoneurons by excitatory amino acid analogues. Brain Res. 407, 94–101 [DOI] [PubMed] [Google Scholar]
- 34. Shope S. B., Hackman J. C., Holohean A. M., Davidoff R. A. (1993) Activation of α-adrenoceptors indirectly facilitates sodium pumping in frog motoneurons. Brain Res. 630, 207–213 [DOI] [PubMed] [Google Scholar]
- 35. Lanfranco M. F., Seitz P. K., Morabito M. V., Emeson R. B., Sanders-Bush E., Cunningham K. A. (2009) An innovative real time PCR method to measure changes in RNA editing of the serotonin 2C receptor (5-HT(2C)R) in brain. J. Neurosci. Methods 179, 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 37. Kaufman L. M., Barrett J. N. (1983) Serum factor supporting long term survival of rat central neurons in culture. Science 220, 1394–1396 [DOI] [PubMed] [Google Scholar]
- 38. Keane R. W., Srinivasan A., Foster L. M., Testa M. P., Ord T., Nonner D., Wang H. G., Reed J. C., Bredesen D. E., Kayalar C. (1997) Activation of CPP32 during apoptosis of neurons and astrocytes. J. Neurosci. Res. 48, 168–180 [DOI] [PubMed] [Google Scholar]
- 39. Burns C. M., Chu H., Rueter S. M., Hutchinson L. K., Canton H., Sanders-Bush E., Emeson R. B. (1997) Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 387, 303–308 [DOI] [PubMed] [Google Scholar]
- 40. Werry T. D., Loiacono R., Sexton P. M., Christopoulos A. (2008) RNA editing of the serotonin 5HT2C receptor and its effects on cell signaling, pharmacology, and brain function. Pharmacol. Ther. 119, 7–23 [DOI] [PubMed] [Google Scholar]
- 41. Holohean A. M., Hackman J. C., Davidoff R. A. (1999) Mechanisms involved in the metabotropic glutamate receptor-enhancement of NMDA-mediated motoneurone responses in frog spinal cord. Br. J. Pharmacol. 126, 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marino M. J., Rouse S. T., Levey A. I., Potter L. T., Conn P. J. (1998) Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-d-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc. Natl. Acad. Sci. U.S.A. 95, 11465–11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lu W. Y., Xiong Z. G., Lei S., Orser B. A., Dudek E., Browning M. D., MacDonald J. F. (1999) G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat. Neurosci. 2, 331–338 [DOI] [PubMed] [Google Scholar]
- 44. Liu J., Liao Z., Camden J., Griffin K. D., Garrad R. C., Santiago-Pérez L. I., González F. A., Seye C. I., Weisman G. A., Erb L. (2004) Src homology 3-binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J. Biol. Chem. 279, 8212–8218 [DOI] [PubMed] [Google Scholar]
- 45. Luttrell L. M., Ferguson S. S., Daaka Y., Miller W. E., Maudsley S., Della Rocca G. J., Lin F., Kawakatsu H., Owada K., Luttrell D. K., Caron M. G., Lefkowitz R. J. (1999) β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 283, 655–661 [DOI] [PubMed] [Google Scholar]
- 46. Cao W., Luttrell L. M., Medvedev A. V., Pierce K. L., Daniel K. W., Dixon T. M., Lefkowitz R. J., Collins S. (2000) Direct binding of activated c-Src to the β3-adrenergic receptor is required for MAP kinase activation. J. Biol. Chem. 275, 38131–38134 [DOI] [PubMed] [Google Scholar]
- 47. Barthet G., Framery B., Gaven F., Pellissier L., Reiter E., Claeysen S., Bockaert J., Dumuis A. (2007) 5-hydroxytryptamine 4 receptor activation of the extracellular signal-regulated kinase pathway depends on Src activation but not on G protein or β-arrestin signaling. Mol. Biol. Cell 18, 1979–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakae A., Nakai K., Tanaka T., Takashina M., Hagihira S., Shibata M., Ueda K., Mashimo T. (2008) Serotonin2C receptor mRNA editing in neuropathic pain model. Neurosci. Res. 60, 228–231 [DOI] [PubMed] [Google Scholar]
- 49. Herrick-Davis K., Grinde E., Niswender C. M. (1999) Serotonin 5-HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J. Neurochem. 73, 1711–1717 [DOI] [PubMed] [Google Scholar]
- 50. Niswender C. M., Copeland S. C., Herrick-Davis K., Emeson R. B., Sanders-Bush E. (1999) RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J. Biol. Chem. 274, 9472–9478 [DOI] [PubMed] [Google Scholar]
- 51. Moro O., Lameh J., Högger P., Sadée W. (1993) Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J. Biol. Chem. 268, 22273–22276 [PubMed] [Google Scholar]
- 52. Pin J. P., Joly C., Heinemann S. F., Bockaert J. (1994) Domains involved in the specificity of G protein activation in phospholipase C-coupled metabotropic glutamate receptors. EMBO J. 13, 342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seeburg P. H., Hartner J. (2003) Regulation of ion channel/neurotransmitter receptor function by RNA editing. Curr. Opin. Neurobiol. 13, 279–283 [DOI] [PubMed] [Google Scholar]
- 54. Gurevich I., Englander M. T., Adlersberg M., Siegal N. B., Schmauss C. (2002) Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J. Neurosci. 22, 10529–10532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gardiner K., Du Y. (2006) A-to-I editing of the 5HT2C receptor and behavior. Brief Funct. Genomic Proteomic. 5, 37–42 [DOI] [PubMed] [Google Scholar]
- 56. Yu X. M., Askalan R., Keil G. J., 2nd, Salter M. W. (1997) NMDA channel regulation by channel-associated protein-tyrosine kinase Src. Science 275, 674–678 [DOI] [PubMed] [Google Scholar]
- 57. Dai S., Hall D. D., Hell J. W. (2009) Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol. Rev. 89, 411–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. (1981) Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-Src) and its normal cellular homologue (pp60c-Src). Proc. Natl. Acad. Sci. U.S.A. 78, 6013–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roskoski R. (2004) Src protein-tyrosine kinase structure and regulation. Biochem. Biophys. Res. Commun. 324, 1155–1164 [DOI] [PubMed] [Google Scholar]
- 60. Salter M. W., Kalia L. V. (2004) Src kinases. A hub for NMDA receptor regulation. Nat. Rev. Neurosci. 5, 317–328 [DOI] [PubMed] [Google Scholar]
- 61. Bécamel C., Gavarini S., Chanrion B., Alonso G., Galéotti N., Dumuis A., Bockaert J., Marin P. (2004) The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J. Biol. Chem. 279, 20257–20266 [DOI] [PubMed] [Google Scholar]
- 62. Bockaert J., Roussignol G., Bécamel C., Gavarini S., Joubert L., Dumuis A., Fagni L., Marin P. (2004) GPCR-interacting proteins (GIPs). Nature and functions. Biochem. Soc. Trans. 32, 851–855 [DOI] [PubMed] [Google Scholar]
- 63. Labasque M., Reiter E., Becamel C., Bockaert J., Marin P. (2008) Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol. Biol. Cell 19, 4640–4650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hotulainen P., Hoogenraad C. C. (2010) Actin in dendritic spines. Connecting dynamics to function. J. Cell Biol. 189, 619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pozo K., Goda Y. (2010) Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66, 337–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barnes N. M., Sharp T. (1999) Neuropharmacology 524, 171–174 [DOI] [PubMed] [Google Scholar]
- 67. Takadera T., Sakamoto Y., Ohyashiki T. (2004) NMDA receptor 2B-selective antagonist ifenprodil-induced apoptosis was prevented by glycogen synthase kinase-3 inhibitors in cultured rat cortical neurons. Brain Res. 1020, 196–203 [DOI] [PubMed] [Google Scholar]
- 68. Hetman M., Kharebava G. (2006) Survival signaling pathways activated by NMDA receptors. Curr. Top. Med. Chem. 6, 787–799 [DOI] [PubMed] [Google Scholar]
- 69. Liu Y., Wong T. P., Aarts M., Rooyakkers A., Liu L., Lai T. W., Wu D. C., Lu J., Tymianski M., Craig A. M., Wang Y. T. (2007) NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 27, 2846–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang Y. T., Salter M. W. (1994) Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature 369, 233–235 [DOI] [PubMed] [Google Scholar]
- 71. Ali D. W., Salter M. W. (2001) NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr. Opin. Neurobiol. 3, 336–342 [DOI] [PubMed] [Google Scholar]
- 72. Sandyk R. (2006) Serotonergic mechanisms in amyotrophic lateral sclerosis. Int. J. Neurosci. 116, 775–826 [DOI] [PubMed] [Google Scholar]
- 73. Lau L. F., Huganir R. L. (1995) Differential tyrosine phosphorylation of N-methyl-d-aspartate receptor subunits. J. Biol. Chem. 270, 20036–20041 [DOI] [PubMed] [Google Scholar]
- 74. Yang M., Leonard J. P. (2001) Identification of mouse NMDA receptor subunit NR2A C-terminal tyrosine sites phosphorylated by coexpression with v-Src. J. Neurochem. 77, 580–588 [DOI] [PubMed] [Google Scholar]
- 75. Beato M., Nistri A. (1998) Serotonin-induced inhibition of locomotor rhythm of the rat isolated spinal cord is mediated by the 5-HT1 receptor class. Proc. Biol. Sci. 265, 2073–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wallis D. I., Elliott P., Foster G. A., Stringer B. M. (1998) Synaptic activity, induced rhythmic discharge patterns, and receptor subtypes in enriched primary cultures of embryonic rat motoneurones. Can. J. Physiol. Pharmacol. 76, 347–359 [PubMed] [Google Scholar]
- 77. Maclean J. N., Schmidt B. J. (2001) Voltage sensitivity of motoneuron NMDA receptor channels is modulated by serotonin in the neonatal rat spinal cord. J. Neurophysiol. 86, 1131–1138 [DOI] [PubMed] [Google Scholar]
- 78. Shay B. L., Sawchuk M., Machacek D. W., Hochman S. (2005) Serotonin 5-HT2 receptors induce a long lasting facilitation of spinal reflexes independent of ionotropic receptor activity. J. Neurophysiol. 94, 2867–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fouad K., Rank M. M., Vavrek R., Murray K. C., Sanelli L., Bennett D. J. (2010) Locomotion after spinal cord injury depends on constitutive activity in serotonin receptors. J. Neurophysiol. 104, 2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pflieger J. F., Clarac F., Vinay L. (2002) Postural modifications and neuronal excitability changes induced by a short term serotonin depletion during neonatal development in the rat. J. Neurosci. 22, 5108–5117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li Q. H., Nakadate K., Tanaka-Nakadate S., Nakatsuka D., Cui Y., Watanabe Y. (2004) Unique expression patterns of 5-HT2A and 5-HT2C receptors in the rat brain during postnatal development. Western blot and immunohistochemical analyses. J Comp. Neurol. 469, 128–140 [DOI] [PubMed] [Google Scholar]