Background: HSPG interacts with growth factors to influence growth and differentiation.
Results: ES cells lacking NDST1 and NDST2 show very limited differentiation potential. FGF and heparin rescued formation of neural progenitors.
Conclusion: HS-mediated FGF signaling is rate-limiting for commitment of primitive ectoderm to the neural lineage
Significance: This study shows the importance of the ratio between HSPG and FGF for neural differentiation.
Keywords: Glia, Heparin, Neurons, Neuroprogenitor Cell, Osteoblasts, NDST
Abstract
Heparan sulfate proteoglycans, present on cell surfaces and in the extracellular matrix, interact with growth factors and morphogens to influence growth and differentiation of cells. The sulfation pattern of the heparan sulfate chains formed during biosynthesis in the Golgi compartment will determine the interaction potential of the proteoglycan. The glucosaminyl N-deacetylase/N-sulfotransferase (NDST) enzymes have a key role during biosynthesis, greatly influencing total sulfation of the heparan sulfate chains. The differentiation potential of mouse embryonic stem cells lacking both NDST1 and NDST2 was studied using in vitro differentiation protocols, expression of differentiation markers, and assessment of the ability of the cells to respond to growth factors. The results show that NDST1 and NDST2 are dispensable for mesodermal differentiation into osteoblasts but necessary for induction of adipocytes and neural cells. Gene expression analysis suggested a differentiation block at the primitive ectoderm stage. Also, GATA4, a primitive endoderm marker, was expressed by these cells. The addition of FGF4 or FGF2 together with heparin rescued the differentiation potential to neural progenitors and further to mature neurons and glia. Our results suggest that the embryonic stem cells lacking both NDST1 and NDST2, expressing a very low sulfated heparan sulfate, can take the initial step toward differentiation into all three germ layers. Except for their potential for mesodermal differentiation into osteoblasts, the cells are then arrested in a primitive ectoderm and/or endoderm stage.
Introduction
Embryonic stem (ES)3 cells are pluripotent cells derived from the inner cell mass of the blastocyst-stage embryo (1, 2). In vitro differentiation of ES cells into many cell types has been shown to faithfully reproduce the developmental pathways normally followed in vivo (3). Differentiation by formation of free floating aggregates of ES cells (i.e. embryoid bodies (EB)) (4) is often used for derivation of mesodermal and endodermal cell types. The protocol to develop neural stem cells from ES cells has also relied on EB formation (5), but neural precursors are now more efficiently generated by adherent monolayer culture (6).
In the vertebrate embryo, formation of the neural plate is dependent on cell-cell interactions and is orchestrated by a number of molecules, involving signaling pathways dependent on, for example, Wnt, TGFβ, and FGF (7). Bone morphogenetic proteins (BMPs) are required to activate epidermis-specific gene expression (8). Expression of the BMP antagonists, noggin and chordin, by the dorsal mesoderm is needed for formation of ectoderm (9). Mice lacking chordin and/or noggin are able to form a nervous system but display severe defects in forebrain development (10). In the chick, BMP inhibition is not sufficient for neural induction (11). FGF signaling is necessary but not sufficient for the repression of BMP and subsequent neural fate (12, 13). More recent studies show that inhibition of FGF-induced ERK signaling impedes neural induction of ES cells (14, 15).
Heparan sulfate (HS) proteoglycans are present on cell surfaces and in basement membranes, where they interact with a large number of physiologically important macromolecules, thereby influencing biological processes (16–18). The HS polysaccharide chains of the proteoglycans, covalently attached to different core proteins, carry negatively charged sulfate groups. The positioning of these sulfate groups on the two monosaccharide building blocks of the polysaccharide (N-acetylglucosamine and hexuronic acid) contributes to the specificity of the interactions. Many growth factors and cytokines bind to HS present on cell surface proteoglycans, which act as co-receptors for the signaling molecules (19). In addition, HS has been shown to be important during embryonic development by creating and maintaining gradients of morphogens and cytokines (20).
The structure of the HS chains produced is determined during biosynthesis, which takes place in the Golgi compartment of the cells. The N-deacetylase/N-sulfotransferases (NDSTs), which initiate the modification reactions, have a key role in designing the sulfation pattern because subsequent modifications, such as O-sulfation at various positions, occur in the vicinity of N-sulfate groups (21). Of the four vertebrate NDSTs, NDST1 and NDST2 are widely spread in various tissues both during the embryonic stage and in adult mice, whereas NDST3 and NDST4 show a more restricted mRNA expression (22, 23). A complete lack of NDST1 results in perinatal lethality, forebrain defects, skeletal malformation, and lung hypoplasia (24–26). Vascular development, endothelial cell function, lipid metabolism, lacrimal gland induction, lens development, and neural tube fusion have also been shown to be impaired in NDST1-deficient animals (27). In contrast, NDST2-deficient mice are healthy and fertile, but their connective tissue type mast cells lack sulfated heparin and contain reduced levels of histamine and mast cell-specific proteases (28, 29). Also, mice with a targeted deletion of NDST3 develop normally with only subtle symptoms, such as lowered cholesterol and HDL levels (30). No knock-out strain carrying a targeted deletion of NDST4 has yet been described.
A lack of both NDST1 and NDST2 results in early embryonic lethality (31). ES cells isolated from double knock-out blastocysts are unable to develop blood capillary structures (32) and synthesize HS that lacks N-sulfate groups and contains low levels of 6-O-sulfate groups (31). The NDST1−/−NDST2−/− ES cells have also been used to study the role of heparan sulfate in regulating FGF receptor signaling, which in turn is important for ES cell pluripotency (33)
In this paper, we investigated the ability of NDST1−/−NDST2−/− ES cells to develop into different lineages. We show that mesodermal differentiation into osteoblasts can occur, whereas adipocytes, which are also of mesodermal origin, do not form in the mutant cultures. Based on expression of differentiation markers, we show that NDST1−/−NDST2−/− ES cells can take on a primitive ectodermal fate. Rescue experiments with the addition of heparin with or without FGF2 or FGF4 supported the hypothesis that heparan sulfate-mediated FGF signaling is an early rate-limiting step in the commitment of primitive ectoderm to the neural cell lineage.
EXPERIMENTAL PROCEDURES
Embryonic Stem Cell Culture
Mouse ES cells were routinely cultured in DMEM with Glutamax (Invitrogen) supplemented with 20% ES cell qualified FBS (Invitrogen), 1× nonessential amino acids (Invitrogen), 0.1 mm β-mercaptoethanol (Sigma), and 1000 units/ml leukemia inhibitory factor (ESGRO, Millipore) on feeder cells (irradiated mouse embryonic fibroblasts). Medium was changed every day, and ES cells were passaged every second day. NDST1−/−NDST2−/− ES cells were derived as described previously (31). One double knock-out ES cell line (A3) was used for the majority of the experiments, but two additional lines (A1 and B5) were also tested and showed similar results as A3. All three mutant ES cell lines had normal chromosome counts. GSI-1 (Genome Systems Inc., St Louis, MO) ES cells were used as wild type (WT) control.
In Vitro Differentiation of ES Cells
Before onset of differentiation experiments, the ES cell cultures were depleted of feeder cells by incubating trypsinized cells in ES cell medium on culture dishes for 30 min, whereby feeder cells attached to the dish. ES cells were thereafter plated on gelatin-coated dishes, cultured overnight, and feeder-depleted again before they were counted and plated according to the various differentiation protocols. For differentiation to adipocytes (34) and osteoblasts (35), embryoid bodies were formed by incubating hanging drops containing 1000 cells/drop for 2 days, after which published protocols were followed. ES cells were differentiated toward the neural lineage using the adherent monolayer protocol published by Ying et al. (6). Rescue of neural differentiation of NDST1−/−NDST2−/− ES cells with conditioned medium from WT ES cells undergoing neural differentiation was performed by plating ES cells at 11,000 cells/cm2 in N2B27 medium. 48 h later, the medium of the mutated cells was exchanged for conditioned, sterile filtered medium from the differentiating WT ES cells mixed 3:1 with fresh N2B27. The medium was changed in this way every day. For testing the effect of various factors on rescue of neural differentiation of mutated ES cells, cells were plated at 11,000 cells/cm2 in N2B27 medium; 18 h, later the medium was exchanged for N2B27 medium containing the factors indicated in each experiment; and cultivation continued with medium change every other day. For differentiation of mutant cells to neurons and astrocytes, neural precursor cells expanded in N2B27 with FGF4 and heparin were plated on coverslips coated with polyornithine and fibronectin. The day after plating, medium was changed to N2B27 without FGF4 and heparin, and cells were differentiated for another 5 days. Noggin, BMP4, FGF2, FGF4, and FGF8b were from R&D Systems. PDGF-BB and EGF were from Pepro Technologies. Heparin from pig intestinal mucosa (Inolex, Park Forest South, IL), purified as described (36), was a kind gift from Prof. U. Lindahl (Uppsala University).
Alkaline Phosphatase Assay and Immunofluorescence
Alkaline phosphatase activity was measured using the alkaline phosphatase detection kit (Chemicon International). For immunofluorescence, cells were fixed in 4% paraformaldehyde for 10 min, permeabilized in 0.2% Triton X-100 15 min, and blocked in 20% goat serum 30 min, with PBS washes between each step. Antibodies were diluted in blocking solution.
Primary antibodies were Oct3/4 (1:100; Santa Cruz Biotechnology, Inc.), nestin (1:200; Chemicon), β-III-tubulin (Tuj1, 1:500; Covance), and glial fibrillary acidic protein (GFAP) (1:200; Sigma-Aldrich). Appropriate secondary antibodies (Cy3-conjugated 1:200, FITC-conjugated 1:100) were from Jackson ImmunoResearch.
RNA Preparations and RT-PCR
Total RNA was prepared from cells using the RNeasy kit (Qiagen). cDNA was synthesized from 3 μg of total RNA with Superscript II reverse transcriptase (Invitrogen) using random primers (Invitrogen). For semiquantitative RT-PCR, 5-fold serial dilutions of the cDNA were used. Real-time RT-PCR in Fig. 1 was performed using KAPA SYBR FAST quantitative PCR (KM4101, Kapabiosystems), and in Fig. 3, it was performed using iQ SYBR Green Supermix (Bio-Rad).
FIGURE 1.
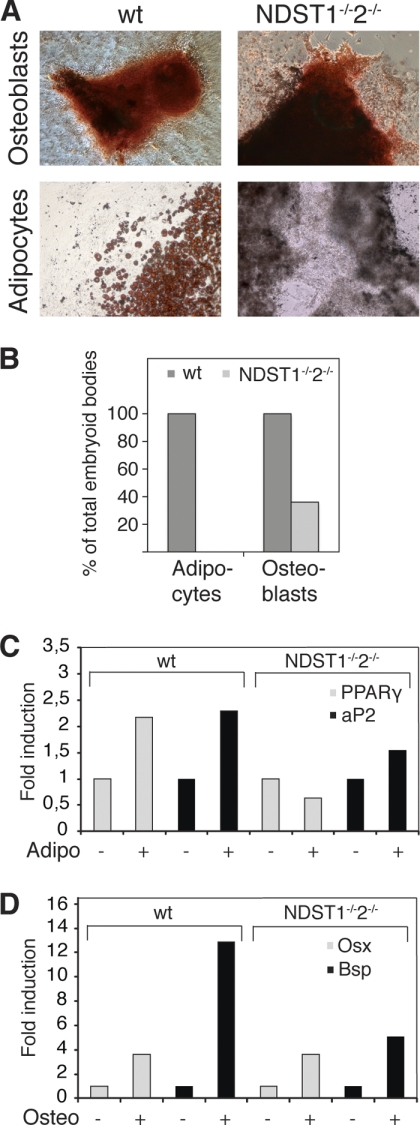
NDST1 and NDST2 are required for adipocyte but not for osteoblast differentiation. A, WT and NDST1−/−NDST2−/− ES cells were subjected to in vitro differentiation to the osteoblast and adipocyte lineages. Top panels, osteoblast-differentiated ES cells stained with Alizarin Red at day 26. Bottom panels, adipocyte-differentiated ES cells stained with Oil Red O at day 27. B, quantification of the fraction of embryoid bodies, plated one per well, containing osteoblasts and adipocytes, using the respective differentiation protocols. C and D, real-time RT-PCR of WT and mutant ES cells differentiated without (−) or with (+) cell type-specific promoting additives. For each individual gene, the value for cells differentiated without additives was set to 1. C, expression of PPARγ and Bsp after 27 days of differentiation with or without adipocyte-promoting additives. D, expression of Osx and Bsp after 26 days of differentiation with or without osteoblast-promoting additives.
FIGURE 3.
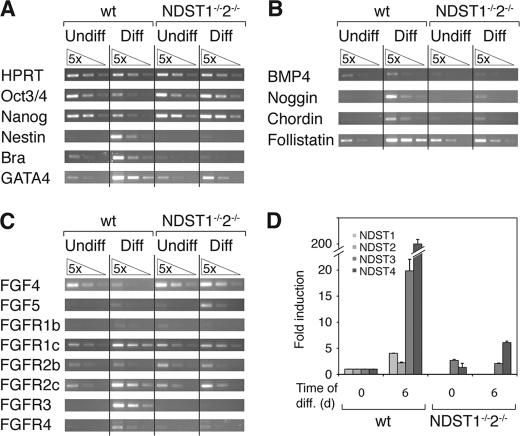
Gene expression analysis of WT and NDST1−/−NDST2−/− ES cells, before and after 6 days of neural differentiation. WT and mutant ES cells were cultured in ES cell medium (Undiff) or for 6 days in N2B27 medium to promote neural differentiation (Diff). Hypoxanthine-guanine phosphoribosyltransferase (HPRT) was used as an internal control (A, top). A–C, semiquantitative RT-PCR analysis. A, expression of the undifferentiated markers Oct3/4 and nanog, neural precursor protein nestin, mesoderm marker Brachyury, and primitive endoderm marker GATA4. B, expression of BMP4 and the BMP inhibitors noggin, chordin, and follistatin. C, expression of FGF4, FGF5, and the FGF receptors 1b, 1c, 2b, 2c, 3, and 4. D, real-time RT-PCR analysis of NDST1, -2, -3, and -4 transcripts. For each individual gene, the value for undifferentiated WT ES cells was set to 1. NDST1 and -2 were not assayed for in the mutant cells. Error bars, S.D.
N-Sulfotransferase Assay
N-Sulfotransferase activity was analyzed by measuring incorporation of 35S from the sulfate donor [35S]3′-phosphoadenosine 5′-phosphosulfate into N-deacetylated Escherichia coli capsular K5 polysaccharide as described previously (58). Briefly, solubilized cells (40 μg of protein) were incubated with substrate and 1 μCi of [35S]PAPS in 50 mm Hepes (pH 7.4), 10 mm MgCl2, 10 mm MnCl2, 5 mm CaCl2, 3.5 μm NaF, and 1% Triton X-100 in 0.1 ml for 30 min at 37 °C. The polysaccharide was precipitated with ethanol for 4 h, separated from excess [35S]PAPS by Sephadex G25 superfine (GE Healthcare) gel filtration, and quantified by scintillation counting. Three parallel determinations of enzyme activity in duplicate of WT and mutant cells were analyzed. The results are given as mean values ± S.D.
ERK Phosphorylation Assay
Two days prior FGF induction, cells were feeder-depleted and plated on tissue culture plates coated with 0.1% gelatin at a density of 1.2 × 105 cells/cm2. The next day, cells underwent another round of feeder depletion and were plated in gelatin-coated 6-well tissue culture plates at a density of 7.3 × 104 cells/cm2 and cultured in N2B27 medium for 18 h. As control, cells were also cultured in ES medium. To induce FGF signaling, FGF4 (10 ng/ml) and heparin (5 μg/ml) was added to the cells, either alone or in combination. After an incubation period of 15 min, the cells were lysed in 150 μl of SDS sample buffer (50 mm Tris-HCl, pH 8.8, 18% sucrose, 2.5% SDS, 0.01% bromphenol blue) containing 40 mm dithiothreitol, boiled, and aspirated through a 27-gauge injection needle to shear the DNA. Equivalent amounts of the samples were separated on 10% SDS-PAGE and transferred to a nitrocellulose membrane (Hybond ECL, Amersham Biosciences). The membrane was blocked in 5% milk, Tris-buffered saline supplemented with 0.1% Tween 20 (TBST) for 1 h and washed three times in TBST before incubation with anti-phospho-ERK1/2 antibody (mouse anti-phospho-p44/42 MAPK (ERK1/2) (Thr-202/Tyr-204), Cell Signaling Technology, catalog no. 9106), diluted 1:1000 in 5% bovine serum albumin, TBST overnight at 4 °C. After three washes in TBST, the membrane was incubated with ECL anti-mouse IgG horseradish peroxidase-linked whole antibody (NA931V, Amersham Biosciences) diluted 1:2500 in 5% milk, TBST for 45 min at room temperature and developed by an ECL detection system as described by the manufacturer (RPN2132, GE Healthcare). For detection of total ERK, the membrane was stripped with 0.1 m glycine/HCl, pH 2.5, and reprobed with anti-total ERK1/2 antibody (rabbit anti-p44/42 MAPK (ERK1/2), Cell Signaling Technology, catalog no. 9102). After three washes in TBST, the membrane was incubated with ECL anti-rabbit IgG horseradish peroxidase-linked whole antibody (NA934V, Amersham Biosciences) diluted 1:2500 in 5% milk, TBST for 45 min at room temperature, and developed as above. Quantification of the Western blot was performed using the freeware ImageJ (National Institutes of Health, Bethesda, MD). The blackness of each band was measured, and the ratio of phosphorylated ERK/total ERK was calculated.
RESULTS
NDST1 and NDST2 Are Required for Adipocyte but Not for Osteoblast Differentiation
We have previously derived three different ES cell lines (A1, A3, and B5) from mouse blastocysts deficient in the two heparan sulfate-modifying enzymes NDST1 and NDST2 (31). The mutant cells produce normal amounts of HS, but the polysaccharide is devoid of N-sulfate groups, and except for a low level of 6-O-sulfation, the mutant HS is devoid of other modifications (31). These ES cells were now tested for their ability to differentiate into various mature cell types in vitro. Using the method of EB formation and subsequent differentiation in culture media promoting the development of specific cell types, we performed in vitro differentiation protocols for two types of mesodermal cells, the generation of osteblasts (35) and adipocytes (34). Formation of osteblasts and adipocytes was detected by staining with Alizarin Red and Oil Red O, respectively. Whereas wild type ES cells readily differentiated into both osteoblasts and adipocytes, the mutant cells were able to differentiate into osteoblasts but could not form adipocytes (Fig. 1A). By seeding one EB per well in 24-well plates, we scored the number of EB differentiating to the expected cell type. Although the mutant cells could make osteoblasts, the fraction of EB-containing osteoblasts was reduced compared with WT cells (100% of WT EB and 38% of mutant EB) (Fig. 1B). Real-time RT-PCR analysis of cultures differentiated in the presence or absence of adipocyte- or osteoblast-promoting additives supported these findings. The markers of terminal adipocyte differentiation PPARγ and aP2 were induced in WT cells, but not in mutant, subjected to the adipocyte differentiation protocol (Fig. 1C). Expression of the late stage osteoblast genes osx and bsp was increased in both WT and mutant cells using the osteoblast protocol (Fig. 1D). ES cells lacking properly modified HS chains can thus differentiate into at least one kind of mesodermal cells in vitro, although less efficiently than WT ES cells.
NDST1 and NDST2 Double Knock-out Cells Are Deficient in Neural Differentiation
To further test the differentiation potential of the NDST1/NDST2 double knock-out cells, we applied the method of adherent monolayer culture to produce neural cells (6). This protocol enables the conversion of ES cells to neural precursor cells within 5–7 days of culture in the neural promoting medium N2B27 and the consecutive maturation of the precursors to neurons and glial cells. Whereas the wild type cells readily formed neural precursor cells after 5–7 days in culture, the double knock-out cells showed no morphological signs of differentiating to neural precursor cells (Fig. 2A). During the first 2–5 days of neural monolayer differentiation, extensive cell death takes place due to the increased differentiation/selection pressure on the cells. After 4 days of differentiation, the cell death in the wild type cultures was followed by increased proliferation of the appearing neural precursor cells. However, in the mutant culture, the initial cell death was not followed by formation of neural precursor; instead, the majority of the cells appeared morphologically similar to undifferentiated ES cells. These cells proliferated very slowly, and after 9 days, there were approximately 5 times more cells in the wild type cultures compared with the mutant cultures (Fig. 2B). Similar results were observed for all three mutant ES cell lines. After 6 days of differentiation, a majority of the cells in the wild type cultures expressed nestin, an intermediate filament present in neural precursor cells (Fig. 2C). Among the mutant cells, ∼85% of the few remaining cells lacked nestin expression. The nestin-expressing cells were found in small clusters where the cells initially had been most dense, usually in the center of the plates. The initial cell density is of great importance for the differentiation ability of ES cells. However, seeding the mutant cells at 3 times higher initial density (not shown) did not affect their differentiation potential, and thus the impairment in neural cell formation seemed inherent to the mutant cells and not due to the cell density.
FIGURE 2.
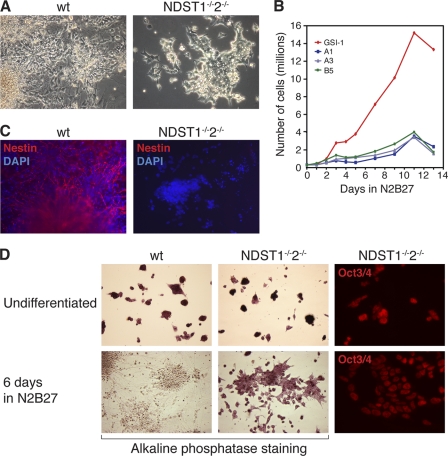
NDST1−/−NDST2−/− ES cells are blocked in differentiation to the neural lineage. A, WT ES cells differentiate to neural cells, whereas mutant ES cells appear morphologically undifferentiated after 6 days in N2B27 medium. B, growth curve comparing WT and mutant cell numbers during 13 days of differentiation in N2B27 medium. Three different mutant ES cell lines were tested, A1, A3, and B5. C, immunofluorescent staining of WT and mutant cells for the neural precursor marker nestin (red) and nucleus DAPI (blue) after 6 days of differentiation. D, undifferentiated ES cells (top panels) and cells differentiated for 6 days (bottom panels) were stained for alkaline phosphatase (purple, WT cells (left panels) and mutant ES cells (middle panels)). Right panels, immunofluorescent staining of mutant ES cells for Oct3/4 (red).
One hallmark of undifferentiated ES cells is their intrinsic alkaline phosphatase activity, which we could detect in the undifferentiated WT and mutant ES cells (Fig. 2D). Whereas the WT cells lost the alkaline phosphatase activity after 6 days of differentiation in N2B27 medium, the majority of the mutant cells remaining at the same time point were still positive for alkaline phosphatase activity (Fig. 2D). In addition, the NDST1/NDST2 knock-out cells still expressed Oct3/4 (Fig. 2D), a transcription factor present in the nucleus of undifferentiated ES cells, after 6 days in N2B27. The mutant cells continued to express markers specific for undifferentiated ES cells despite being cultured in a medium usually promoting neural cell formation, suggesting a blockage in their capacity to differentiate.
Gene Expression in NDST1/NDST2-deficient ES Cells Cultured in Neural Promoting Medium Indicates Early Block in Differentiation
To investigate the developmental status of the NDST1/NDST2 knock-out ES cells, we analyzed the expression of a core set of genes in their undifferentiated state when cells were feeder-depleted and cultured in ES cell medium and after 6 days of culture in neural differentiation medium compared with WT ES cells. The gene expression was measured by semiquantitative RT-PCR, using hypoxanthine-guanine phosphoribosyltransferase as an internal control. For the genes examined, we found that the undifferentiated WT and undifferentiated mutant ES cells showed a similar expression pattern (Fig. 3, A–C). Transcripts normally expressed in undifferentiated ES cells, such as Oct3/4 and nanog, were present, whereas nestin was absent (Fig. 3A). The primitive endoderm marker GATA4 and the mesodermally expressed Brachyury were present at low levels (Fig. 3A). This reflects that some cells in the culture have lost their pluripotency, something commonly experienced during cultivation of ES cells. Both GATA4 and Brachyury were up-regulated in the WT cells after 6 days of differentiation, when 70–80% of the ES cells had turned into neural cells, and the remaining cells had differentiated into other cell types. GATA4 expression was about 5 times increased in the mutant cells in N2B27 medium compared with undifferentiated cells, whereas Brachyury expression was unaltered. The increased expression of GATA4 indicates that some mutant cells can take on an endodermal fate. After 6 days of neural differentiation, the expression of Oct3/4 and nanog had decreased in the WT population, and nestin was now strongly expressed. In contrast, the mutant cells continued to express the undifferentiated markers and showed no apparent sign of activating the nestin gene. BMP4 was slightly more expressed in the WT cells compared with the mutant cells, but no change was seen during differentiation in either cell type (Fig. 3B). Interestingly, the BMP inhibitors noggin, chordin, and follistatin were strongly induced in WT cells during neural differentiation, but no apparent induction was observed in the mutant cells. Because FGF signaling was previously indicated to play a key role in neural induction, we assayed for expression of FGF4, FGF5, and FGF receptors (Fig. 3C). The undifferentiated ES cells of both genotypes showed similar expression of FGF receptors; FGFR1c, -2b, and -2c were weakly expressed, whereas FGFR1b and -4 showed an even lower expression. After induction of WT neural precursors FGFR1c, -2c, -3, and -4 were clearly up-regulated, whereas the mutant cells showed no change in receptor expression. The mutant cells also expressed similar levels of FGF4 as WT pluripotent ES cells. FGF4 expression was down-regulated in differentiated WT cells but not in mutant cells. The only gene examined that was noticeably up-regulated in NDST1/NDST2 mutant ES cells after 6 days in neural differentiation medium was FGF5, indicating that the mutant cells had taken a small step toward differentiation to early ectoderm. We also analyzed expression of the four NDST genes by real-time PCR (Fig. 3D), using actin as a control. NDST1 and NDST2 were weakly induced during differentiation of WT ES cells. A more dramatic change was seen for NDST3 and NDST4 levels in WT cells; expression was very low in undifferentiated cells, but both genes were strongly induced after differentiation of the cells to neural precursors. The initial level of NDST4 is barely detectable, and this must be taken into account when viewing the -fold change in induction. In the mutant cells, the levels of NDST3 remained unchanged, whereas NDST4 was slightly induced after 6 days in differentiation medium. NDST1 and NDST2 levels were not analyzed in the mutant cells that lack these genes. To investigate if the small increase in NDST4 transcript in the mutant cells resulted in expression of NDST enzyme activity, N-sulfotransferase activity was measured in cultures after 6 days in neural differentiation medium. In this assay, the incorporation of [35S]sulfate from the sulfate donor [35S]PAPS into a polysaccharide substrate is quantified. Although no 35S radioactivity was found in the substrate incubated with the mutant cell extracts (47 ± 13 cpm), 18.6 ± 0.6 × 103 cpm was incorporated into the substrate in the WT cell extracts. Taken together, the gene expression analyses show that NDST1−/−NDST2−/− ES cells continue to express markers characteristic of undifferentiated ES cells and early primitive ectoderm after being exposed to a neural differentiation protocol.
NDST1 and NDST2 Double Knock-out Cells Can Respond to BMP and Noggin Signaling
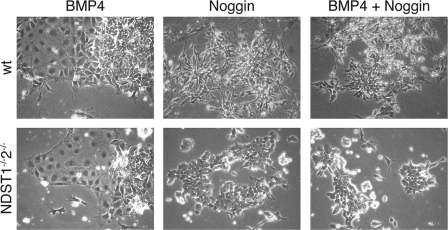
Inhibition of BMP signaling is considered to be necessary for neural differentiation. The failure of the NDST1/NDST2 mutant ES cells to form neural cells might be due to an inability to respond to BMP inhibitors, such as noggin. We therefore investigated if the BMP and noggin responses were intact in the mutant cells. It has been shown that WT ES cells cultured in N2B27 form non-neural cells in response to the addition of BMP4 (6). In our hands, both WT and mutant ES cells similarly formed non-neural cells after the addition of 8 ng/ml BMP4 to the medium, demonstrating that the BMP signaling pathways were fully functional in the mutant cells (Fig. 4). The addition of only noggin (0.1 μg/ml) had no visible morphological effect, neither on the WT nor on the mutant cells (Fig. 4); the cell morphology was indistinguishable from controls where no noggin was added. When BMP4 and noggin were added simultaneously, the effect of BMP4 could be reversed by noggin for both WT and mutant cells, showing that the BMP-signaling circuits were intact in the mutant cells and that they could give a proper biological response to BMP inhibitors (Fig. 4). These results show that the NDST1/NDST2 mutant cells were able to respond to both BMP and BMP inhibitors and that the cause of the differentiation block was not lack of noggin production. In this model system, inhibition of BMP signaling only was not enough to promote neural differentiation of the mutant cells.
FIGURE 4.
The BMP and noggin pathways are functional in NDST1−/−NDST2−/− ES cells. WT and NDST1−/−NDST2−/− ES cells cultured in N2B27 medium in the presence of 8 ng/ml BMP4 for 5 days form non-neural cells. The addition of 0.1 μg/ml noggin for 6 days has no visible morphological effect. Noggin counteracts the effect of BMP4 for WT and mutant cells when both factors are present during 6 days of culture in N2B27.
Neural Differentiation of NDST1/NDST2 Mutant ES Cells Is Rescued by Heparin and FGF
Because inhibition of BMP signaling was not enough to rescue the neural differentiation of NDST1/NDST2 knock-out ES cells, we concluded that other signaling molecules were needed. WT ES cells undergoing neural differentiation could produce such molecules, and to test this hypothesis, we added conditioned medium from differentiating WT cells to the mutant cells. Culturing the mutant ES cells in conditioned medium resulted in rescue of the capacity of the ES cells to differentiate to neural cells (Fig. 5A). The majority of HS proteoglycans (HSPG) produced by cells are found on the cell surface or in the extracellular matrix, but cells also secrete HS proteoglycans to the cell culture medium. To elucidate if the rescue was caused by secreted HS proteoglycans, growth factors, or a combination of both, we added various candidate growth factors and/or heparin to mutant cells cultured in N2B27. Heparin is an easily accessible highly sulfated HS chain that shares with HS the ability to interact with a number of growth factors (16). The addition of EGF (20 ng/ml) or PDGF (10 ng/ml) had no effect on the differentiation of the mutant cells; nor did FGF2 (not shown), FGF4 (Fig. 5A), or FGF8 (all FGFs were tested at both 10 and 100 ng/ml). However, the addition of heparin (0.1 or 5 μg/ml) together with either FGF2 or FGF4 (10 ng/ml) completely rescued the neural differentiation of the NDST1/NDST2 mutant ES cells (Fig. 5A).
FIGURE 5.
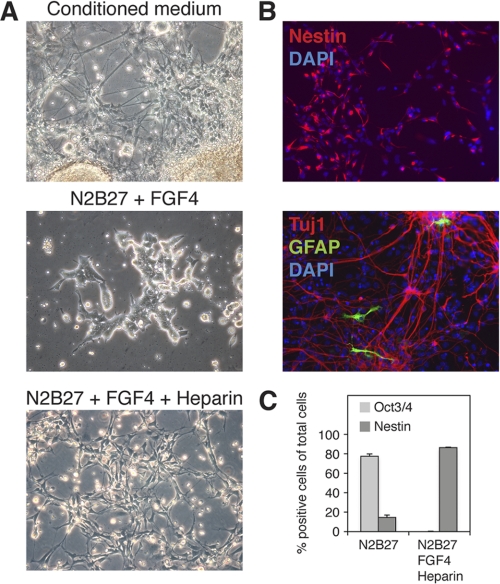
FGF4 and heparin rescue neural differentiation of NDST1−/−NDST2−/− ES cells. A, the addition of conditioned medium from WT ES cells subjected to neural differentiation to the NDST1−/−NDST2−/− cells rescued neural differentiation of the mutant cells (top, day 13). The addition of 10 ng/ml FGF4 only for 7 days had no effect on mutant cells in N2B27 (middle), whereas 10 ng/ml FGF4 in combination with 0.1 μg/ml heparin rescued neural differentiation (bottom, day 9). B, immunofluorescence staining of mutant cells. Shown is neural differentiation in the presence of FGF4 and heparin (top) and staining for nestin (red) and DAPI (blue). Mutant neural cells were plated on a polyornithine/fibronectin-coated surface 5 days after withdrawal of FGF4 and heparin and stained for the neuronal marker Tuj1 (red), the astrocyte marker GFAP (green), and DAPI (blue). C, quantification of Oct3/4 and nestin-expressing NDST1−/−NDST2−/− cells subjected to 6 days of neural differentiation in N2B27 medium with and without the addition of FGF4 and heparin. Error bars, S.D.
Initially, the cells differentiated to nestin-expressing neural precursors that, after removal of FGF and heparin from the medium, further matured into neurons and astrocytes (Fig. 5B). Quantification of the number of Oct3/4- and nestin-expressing cells showed that after rescue, more than 80% of the mutant cells expressed nestin (Fig. 5C), a number similar to what is seen for WT cells using the monolayer protocol (6). Surprisingly, heparin alone at a concentration of 5 μg/ml caused lethality (supplemental Fig. 1A), whereas, at low doses, it allowed formation of neural progenitors in the absence of exogenous FGF4 (supplemental Fig. 1B). Inhibition of FGF signaling in the presence of heparin has previously been shown to inhibit neural differentiation of EXT1−/− cells, totally devoid of heparan sulfate (42). It thus appears likely that the FGF4 produced by the mutant NDST1/NDST2 knock-out cells (Fig. 3), together with added heparin at low concentrations, stimulates the cells to differentiate. However, also the lethal effect of heparin at higher concentrations was modulated by FGF4. The addition of increasing amounts of the growth factor together with 5 μg/ml heparin demonstrated that 1 ng/ml of FGF4 rescued cell survival (supplemental Fig. 1C).
ERK Signaling Is Activated by FGF and Heparin in Mutant Cells
FGF4 is a strong activator of ERK signaling, shown to be essential for the ability of naive ES cells to exit the self-renewal program (14, 37). Because the NDST1−/−NDST2−/− ES cells did not respond to neural differentiation cues when cultured in N2B27 medium, their ability to activate the ERK signaling pathway was investigated. WT and mutant ES cells were cultured in serum-containing ES cell medium or N2B27 overnight. Cells in N2B27 were then stimulated for 15 min with FGF4 and/or heparin. Protein lysates were prepared and assayed by Western blotting for the presence of phosphorylated ERK1/2, using total ERK protein as a loading control (Fig. 6). The addition of only FGF4 in N2B27 medium had a barely detectable stimulating effect on the phosphorylation of ERK in both cell types. However, the simultaneous addition of FGF4 and heparin increased phosphorylation of ERK1/2 in WT and in particular in NDST1/2-deficient cells. Intriguingly, in both WT and mutant ES cells, the short time addition of heparin alone also strongly activated ERK1/2 phosphorylation to a similar level as that seen with FGF4 and heparin together.
FIGURE 6.
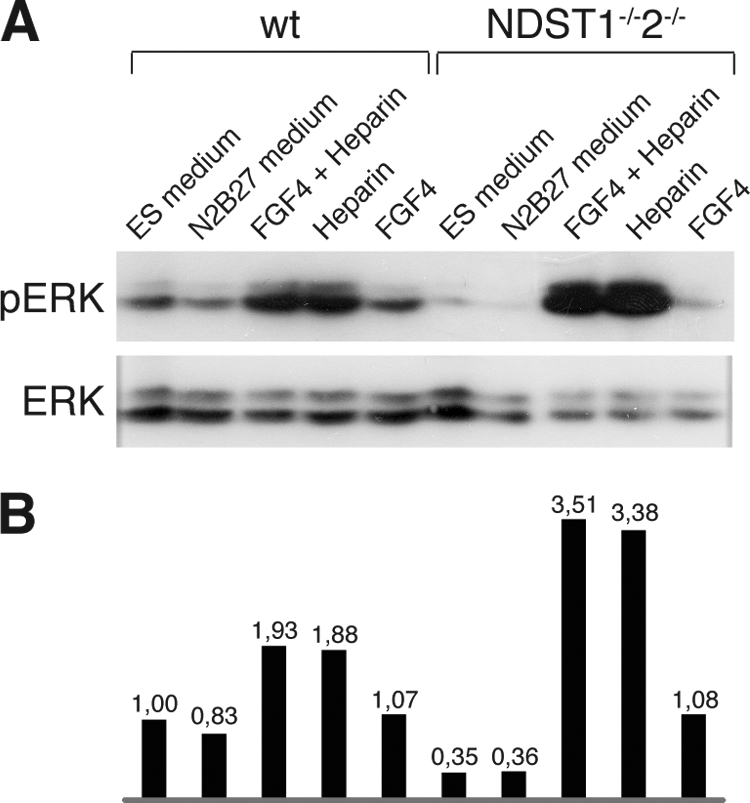
Heparin and FGF4 stimulate ERK phosphorylation in WT and NDST1−/−NDST2−/− ES cells. A, WT and NDST1−/−NDST2−/− ES cells were cultured in ES cell medium or N2B27 overnight. Cells in N2B27 were subsequently treated with FGF and/or heparin for 15 min. Cell lysates were prepared, and levels of phosphorylated ERK and total ERK were analyzed by Western blotting. B, quantification of the ERK phosphorylation assay shown in A. The ratio of phosphorylated ERK versus total ERK was calculated. The value for WT cells in ES medium was set to 1.
DISCUSSION
Targeted deletion of either of the HS polymerases EXT1 or EXT2 results in early embryonic lethality (38, 39), as does lack of both NDST1 and NDST2 (31). EXT1−/− ES cells have previously been derived and studied (40, 41). These cells do not express any HS and fail to transit to differentiation upon leukemia inhibitory factor withdrawal (41) and are not able to differentiate in vitro into neural cells (40). NDST1−/−NDST2−/− ES cells synthesize HS lacking N-sulfate groups but with a low degree of 6-O-sulfation (31). Lack of both NDST1 and NDST2 has been shown to result in an inability of the cells to respond to VEGF, preventing angiogenic sprouting (32). In a recent study by Lanner et al. (33), it was suggested that NDST1−/−NDST2−/− ES cells display a general failure to differentiate upon embryoid body formation. However, the methods used by us for in vitro differentiation are designed to yield specific cell fates, including stepwise formation of intermediate progenitors, enabling primitive cells of ectodermal and endodermal type to appear. Using this approach, we can show that NDST1−/−NDST2−/− mouse ES cells can take on a mesodermal fate and differentiate into osteoblasts, albeit with a lower efficiency (Fig. 1). In addition, the expression of FGF5 in NDST1−/−NDST2−/− ES cells cultured under neural inducing conditions (Fig. 3) indicates that the cells can enter a primitive ectoderm-like state (37). FGF5 expression was recently demonstrated also for the EXT1−/− ES cells (42). Thus, HS appears not to be necessary for the formation of primitive ectoderm.
BMP signaling is essential for bone formation (43). Our finding that functional BMP pathways exist in the double knock-out cells (Fig. 4) may thus explain why osteoblasts can form. Adipocyte differentiation from ES cells also involves BMP-4 (44), but contrary to calcified bone, fat would not form in the NDST1−/−NDST2−/− ES cell cultures (Fig. 1). It is currently unknown where the differentiation block toward adipocytes lies, but terminal markers of adipogenesis are lacking. HS is implied in transport of fatty acids over the plasma membrane (45), and a possible explanation for the failure to stain for Oil Red O could be a defect in lipid accumulation.
The vast majority (more than 90%) of the mutant ES cells were unable to differentiate into neural progenitors; nor did they show neural specific nestin expression (Fig. 2). In combination with the reduced proliferation in FGF-containing medium and inability to down-regulate markers of pluripotency, our findings highlight the need for functional HS in neural commitment. In the embryo, FGF5 marks the transition from inner cell mass to epiblast (46), and when its expression declines, neural cells can form. Because NDST1−/−NDST2−/− ES cells show expression of FGF5 during neural inducing conditions, we suggest that they become blocked at the stage of primitive ectoderm and cannot proceed to neural differentiation. Similarly, expression of GATA4, a key marker of early endoderm (47) in mutant ES cells undergoing differentiation, shows an ability of the cells to form primitive endoderm. However, the inability of NDST1−/−NDST2−/− embryoid bodies to differentiate into endothelial cells shows that this capacity is limited (32). As previously demonstrated for WT ES cells (40), NDST3 and in particular NDST4 became up-regulated during neural differentiation (Fig. 3). In mutant cells, a less pronounced increase in NDST4 expression was noted (Fig. 3). However, no N-sulfotransferase activity was detected in mutant cells, suggesting that HS structure in these cells was unaltered after 6 days in neural differentiation medium.
Inhibition of BMP signaling is needed for neural differentiation (48), and we therefore investigated if the failure of mutant ES cells to form neural progenitors was due to an inability to properly respond to BMP inhibitors. However, the addition of BMP4 to NDST1−/−NDST2−/− ES cells induced a non-neural fate that in turn could be blocked by concomitant noggin addition, excluding a defective BMP pathway (Fig. 4). There are many examples in the literature of cell surface HS proteoglycans as critical determinants of the biological activity of BMPs and their endogenous antagonists in vivo and in vitro (49). For example, chordin binding in mouse embryonic tissues was shown to be dependent upon its interaction with cell surface HSPG (50). Our finding that functional HS is not needed to modulate the signaling efficiency of BMP was therefore somewhat unexpected but was in line with a recent study where HSPG were found to play no role in BMP signaling in the early Drosophila embryo (51).
Undifferentiated ES cells are known to produce FGF4 (52), also evident in our cells where both WT and mutant cells expressed the growth factor (Fig. 3). According to the literature, exposure to FGF signaling via the ERK1/MAPK pathway is required before the cells can respond to the anti-neural action of BMP (37, 53). Hence, it is possible that the low level of FGF4 signaling taking place in mutant cells is sufficient to make the cells sensitive to BMP but not for differentiation into neural progenitors.
Neural differentiation of the mutant ES cells could be rescued by the addition of conditioned medium from WT cells (Fig. 5), thus showing that soluble factors were enough to restore the capacity to form neural progenitors. WT ES cells readily formed neural progenitors (Fig. 5), despite a quite modest ERK phosphorylation in response to added FGF4 (Fig. 6). When instead FGF was added together with heparin, massive ERK phosphorylation was seen, in particular in the mutant ES cells (Fig. 6). Somewhat surprisingly, the addition of heparin alone had a similar effect on ERK phosphorylation in both WT and mutant cells (Fig. 6). It has been reported that heparin, in its role as co-factor for FGF receptors, can activate FGFR-4 in the absence of ligand (54). This effect was enhanced in cells lacking heparan sulfate, which goes well together with our finding that ERK activation was stronger in KO cells than in WT ES cells.
We found that heparin could either cause rescue or cell death depending on the dose. The high degree of sulfation of heparin compared with that of endogenous heparan sulfate may explain the lethal effect of heparin at high concentration. The deleterious effect may be related to the recently recognized ability of heparin to induce apoptosis in cancer cells (55, 56). The mechanism has not been elucidated, but it may be related to the capacity of the highly negatively charged polysaccharide to interfere with transcription factor activity. Another possibility is that heparin in the extracellular space captures survival factors produced by non-neural cells in the cultures (57). Adding FGF4 may balance the potentially negative effects of heparin and thus enable differentiation.
Heparin at lower concentrations, on the other hand, was not lethal but induced neural differentiation in NDST1−/−NDST2−/− ES cells. These cells produce FGF4 (Fig. 3), but the concentration of growth factor may be too low to induce differentiation. The rescuing effect of low doses of heparin may thus be to enhance FGF signaling to levels sufficient for differentiation. Taken together, our data thus suggest that the molar ratio of HS to FGF4 is essential for neural differentiation.
The addition of heparin to ES cells lacking the HS polymerase EXT1 results in a partial rescue of neural differentiation also in the absence of FGF (40), and a restoration of FGF2 induced ERK phosphorylation (41), but it was not studied if this was the case also for phosphorylation in response to FGF4. In experiments performed by Lanner et al. (33), FGF2 induced ERK phosphorylation in NDST1−/−NDST2−/− ES cells without the addition of heparin, whereas FGF4 did not have this effect. It is possible that factors in addition to FGF4, stimulated by the added heparin, were responsible for the rescue of the neural differentiation of the NDST1−/−NDST2−/− ES cells (Fig. 5). However, Pickford et al. (42) could recently show that heparin cannot support neural differentiation of EXT1−/− ES cells when FGF signaling is inhibited, demonstrating the importance of FGF in this process.
In summary, we show that NDST1−/−NDST2−/− ES cells, which synthesize HS with a very low sulfate content, can take on a mesodermal fate and differentiate into primitive ectodermal cells. The cells express FGF4, and autocrine FGF4 signaling in these cells appears to be sufficient to render the cells sensitive to BMP signaling. However, to undergo neural differentiation, the ratio between heparin (or HSPG) and FGF appears to be the crucial factor determining if the cells will die (too much heparin/HSPG), survive but not differentiate (too little of either heparin/HSPG or FGF), or differentiate into neural cells (optimal ratio between heparin/HSPG and FGF).
Supplementary Material
Acknowledgments
We thank Inger Eriksson for assistance with N-sulfotransferase assays. Differentiation of ES cells was performed at the Uppsala University Transgenic Facility with support from the Wallenberg Consortium North.
This work was supported by grants from the Swedish Research Council (Medicine) (to L. K. and K. F.-N.), the Swedish Board for Agriculture (K. F.-N. and M. F.), the Swedish Childhood Cancer Foundation (to K. F.-N.), the Swedish Cancer Foundation (to K. F.-N. and L. K.), and Polysackaridforskning AB (to L. K.).

This article contains supplemental Fig. 1.
- ES
- embryonic stem
- BMP
- bone morphogenic protein
- EB
- embryoid body/bodies
- GFAP
- glial fibrillary acidic protein
- HS
- heparan sulfate
- HSPG
- heparan sulfate proteoglycan(s)
- NDST
- N-deacetylase/N-sulfotransferase.
REFERENCES
- 1. Evans M. J., Kaufman M. H. (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 [DOI] [PubMed] [Google Scholar]
- 2. Martin G. R. (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U.S.A. 78, 7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keller G. (2005) Embryonic stem cell differentiation. Emergence of a new era in biology and medicine. Genes Dev. 19, 1129–1155 [DOI] [PubMed] [Google Scholar]
- 4. Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. (1985) The in vitro development of blastocyst-derived embryonic stem cell lines. Formation of visceral yolk sac, blood islands, and myocardium. J. Embryol. Exp. Morphol. 87, 27–45 [PubMed] [Google Scholar]
- 5. Okabe S., Forsberg-Nilsson K., Spiro A. C., Segal M., McKay R. D. (1996) Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech. Dev. 59, 89–102 [DOI] [PubMed] [Google Scholar]
- 6. Ying Q. L., Stavridis M., Griffiths D., Li M., Smith A. (2003) Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183–186 [DOI] [PubMed] [Google Scholar]
- 7. Bronner-Fraser M., Fraser S. E. (1997) Differentiation of the vertebrate neural tube. Curr. Opin. Cell Biol. 9, 885–891 [DOI] [PubMed] [Google Scholar]
- 8. De Robertis E. M., Kuroda H. (2004) Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 20, 285–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moreau M., Leclerc C. (2004) The choice between epidermal and neural fate. A matter of calcium. Int. J. Dev. Biol. 48, 75–84 [DOI] [PubMed] [Google Scholar]
- 10. Bachiller D., Klingensmith J., Kemp C., Belo J. A., Anderson R. M., May S. R., McMahon J. A., McMahon A. P., Harland R. M., Rossant J., De Robertis E. M. (2000) The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403, 658–661 [DOI] [PubMed] [Google Scholar]
- 11. Linker C., Stern C. D. (2004) Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development 131, 5671–5681 [DOI] [PubMed] [Google Scholar]
- 12. Streit A., Berliner A. J., Papanayotou C., Sirulnik A., Stern C. D. (2000) Initiation of neural induction by FGF signaling before gastrulation. Nature 406, 74–78 [DOI] [PubMed] [Google Scholar]
- 13. Wilson S. I., Graziano E., Harland R., Jessell T. M., Edlund T. (2000) An early requirement for FGF signaling in the acquisition of neural cell fate in the chick embryo. Curr. Biol. 10, 421–429 [DOI] [PubMed] [Google Scholar]
- 14. Kunath T., Saba-El-Leil M. K., Almousailleakh M., Wray J., Meloche S., Smith A. (2007) FGF stimulation of the Erk1/2 signaling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 134, 2895–2902 [DOI] [PubMed] [Google Scholar]
- 15. Ying Q. L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. (2008) The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esko J. D., Selleck S. B. (2002) Order out of chaos. Assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 71, 435–471 [DOI] [PubMed] [Google Scholar]
- 17. Bishop J. R., Schuksz M., Esko J. D. (2007) Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037 [DOI] [PubMed] [Google Scholar]
- 18. Bülow H. E., Hobert O. (2006) The molecular diversity of glycosaminoglycans shapes animal development. Annu. Rev. Cell Dev. Biol. 22, 375–407 [DOI] [PubMed] [Google Scholar]
- 19. Grobe K., Ledin J., Ringvall M., Holmborn K., Forsberg E., Esko J. D., Kjellén L. (2002) Heparan sulfate and development. Differential roles of the N-acetylglucosamine N-deacetylase/N-sulfotransferase isozymes. Biochim. Biophys. Acta 1573, 209–215 [DOI] [PubMed] [Google Scholar]
- 20. Kreuger J., Perez L., Giraldez A. J., Cohen S. M. (2004) Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev. Cell 7, 503–512 [DOI] [PubMed] [Google Scholar]
- 21. Esko J. D., Lindahl U. (2001) Molecular diversity of heparan sulfate. J. Clin. Invest. 108, 169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kusche-Gullberg M., Eriksson I., Pikas D. S., Kjellén L. (1998) Identification and expression in mouse of two heparan sulfate glucosaminyl N-deacetylase/N-sulfotransferase genes. J. Biol. Chem. 273, 11902–11907 [DOI] [PubMed] [Google Scholar]
- 23. Aikawa T., Segre G. V., Lee K. (2001) Fibroblast growth factor inhibits chondrocytic growth through induction of p21 and subsequent inactivation of cyclin E-Cdk2. J. Biol. Chem. 276, 29347–29352 [DOI] [PubMed] [Google Scholar]
- 24. Ringvall M., Ledin J., Holmborn K., van Kuppevelt T., Ellin F., Eriksson I., Olofsson A. M., Kjellen L., Forsberg E. (2000) Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J. Biol. Chem. 275, 25926–25930 [DOI] [PubMed] [Google Scholar]
- 25. Fan G., Xiao L., Cheng L., Wang X., Sun B., Hu G. (2000) Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett. 467, 7–11 [DOI] [PubMed] [Google Scholar]
- 26. Grobe K., Inatani M., Pallerla S. R., Castagnola J., Yamaguchi Y., Esko J. D. (2005) Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development 132, 3777–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ringvall M., Kjellén L. (2010) Mice deficient in heparan sulfate N-deacetylase/N-sulfotransferase 1. Prog. Mol. Biol. Transl Sci. 93, 35–58 [DOI] [PubMed] [Google Scholar]
- 28. Forsberg E., Pejler G., Ringvall M., Lunderius C., Tomasini-Johansson B., Kusche-Gullberg M., Eriksson I., Ledin J., Hellman L., Kjellén L. (1999) Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 400, 773–776 [DOI] [PubMed] [Google Scholar]
- 29. Humphries D. E., Wong G. W., Friend D. S., Gurish M. F., Qiu W. T., Huang C., Sharpe A. H., Stevens R. L. (1999) Heparin is essential for the storage of specific granule proteases in mast cells. Nature 400, 769–772 [DOI] [PubMed] [Google Scholar]
- 30. Pallerla S. R., Lawrence R., Lewejohann L., Pan Y., Fischer T., Schlomann U., Zhang X., Esko J. D., Grobe K. (2008) Altered heparan sulfate structure in mice with deleted NDST3 gene function. J. Biol. Chem. 283, 16885–16894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holmborn K., Ledin J., Smeds E., Eriksson I., Kusche-Gullberg M., Kjellén L. (2004) Heparan sulfate synthesized by mouse embryonic stem cells deficient in NDST1 and NDST2 is 6-O-sulfated but contains no N-sulfate groups. J. Biol. Chem. 279, 42355–42358 [DOI] [PubMed] [Google Scholar]
- 32. Jakobsson L., Kreuger J., Holmborn K., Lundin L., Eriksson I., Kjellén L., Claesson-Welsh L. (2006) Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev. Cell 10, 625–634 [DOI] [PubMed] [Google Scholar]
- 33. Lanner F., Lee K. L., Sohl M., Holmborn K., Yang H., Wilbertz J., Poellinger L., Rossant J., Farnebo F. (2010) Heparan sulfation-dependent fibroblast growth factor signaling maintains embryonic stem cells primed for differentiation in a heterogeneous state. Stem Cells 28, 191–200 [DOI] [PubMed] [Google Scholar]
- 34. Rosen E. D., Sarraf P., Troy A. E., Bradwin G., Moore K., Milstone D. S., Spiegelman B. M., Mortensen R. M. (1999) PPAR γ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617 [DOI] [PubMed] [Google Scholar]
- 35. Phillips B. W., Belmonte N., Vernochet C., Ailhaud G., Dani C. (2001) Compactin enhances osteogenesis in murine embryonic stem cells. Biochem. Biophys. Res. Commun. 284, 478–484 [DOI] [PubMed] [Google Scholar]
- 36. Lindahl U., Cifonelli J. A., Lindahl B., Roden L. (1965) The Role of Serine in the Linkage of Heparin to Protein. J. Biol. Chem. 240, 2817–2820 [PubMed] [Google Scholar]
- 37. Stavridis M. P., Lunn J. S., Collins B. J., Storey K. G. (2007) A discrete period of FGF-induced Erk1/2 signaling is required for vertebrate neural specification. Development 134, 2889–2894 [DOI] [PubMed] [Google Scholar]
- 38. Lin X., Wei G., Shi Z., Dryer L., Esko J. D., Wells D. E., Matzuk M. M. (2000) Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev. Biol. 224, 299–311 [DOI] [PubMed] [Google Scholar]
- 39. Stickens D., Zak B. M., Rougier N., Esko J. D., Werb Z. (2005) Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development 132, 5055–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson C. E., Crawford B. E., Stavridis M., Ten Dam G., Wat A. L., Rushton G., Ward C. M., Wilson V., van Kuppevelt T. H., Esko J. D., Smith A., Gallagher J. T., Merry C. L. (2007) Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein-expressing neural progenitor cells. Stem Cells 25, 1913–1923 [DOI] [PubMed] [Google Scholar]
- 41. Kraushaar D. C., Yamaguchi Y., Wang L. (2010) Heparan sulfate is required for embryonic stem cells to exit from self-renewal. J. Biol. Chem. 285, 5907–5916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pickford C. E., Holley R. J., Rushton G., Stavridis M. P., Ward C. M., Merry C. L. (2011) Specific glycosaminoglycans modulate neural specification of mouse embryonic stem cells. Stem Cells 29, 629–640 [DOI] [PubMed] [Google Scholar]
- 43. Hoffmann A., Gross G. (2001) BMP signaling pathways in cartilage and bone formation. Crit. Rev. Eukaryot. Gene Expr. 11, 23–45 [PubMed] [Google Scholar]
- 44. Taha M. F., Valojerdi M. R., Mowla S. J. (2006) Effect of bone morphogenetic protein-4 (BMP-4) on adipocyte differentiation from mouse embryonic stem cells. Anat. Histol. Embryol. 35, 271–278 [DOI] [PubMed] [Google Scholar]
- 45. Wilsie L. C., Chanchani S., Navaratna D., Orlando R. A. (2005) Cell surface heparan sulfate proteoglycans contribute to intracellular lipid accumulation in adipocytes. Lipids Health Dis. 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haub O., Goldfarb M. (1991) Expression of the fibroblast growth factor-5 gene in the mouse embryo. Development 112, 397–406 [DOI] [PubMed] [Google Scholar]
- 47. Rojas A., Schachterle W., Xu S. M., Martín F., Black B. L. (2010) Direct transcriptional regulation of Gata4 during early endoderm specification is controlled by FoxA2 binding to an intronic enhancer. Dev. Biol. 346, 346–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Levine A. J., Brivanlou A. H. (2007) Proposal of a model of mammalian neural induction. Dev. Biol. 308, 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Irie A., Habuchi H., Kimata K., Sanai Y. (2003) Heparan sulfate is required for bone morphogenetic protein-7 signaling. Biochem. Biophys. Res. Commun. 308, 858–865 [DOI] [PubMed] [Google Scholar]
- 50. Jasuja R., Allen B. L., Pappano W. N., Rapraeger A. C., Greenspan D. S. (2004) Cell surface heparan sulfate proteoglycans potentiate chordin antagonism of bone morphogenetic protein signaling and are necessary for cellular uptake of chordin. J. Biol. Chem. 279, 51289–51297 [DOI] [PubMed] [Google Scholar]
- 51. Bornemann D. J., Park S., Phin S., Warrior R. (2008) A translational block to HSPG synthesis permits BMP signaling in the early Drosophila embryo. Development 135, 1039–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ma Y. G., Rosfjord E., Huebert C., Wilder P., Tiesman J., Kelly D., Rizzino A. (1992) Transcriptional regulation of the murine k-FGF gene in embryonic cell lines. Dev. Biol. 154, 45–54 [DOI] [PubMed] [Google Scholar]
- 53. Stavridis M. P., Collins B. J., Storey K. G. (2010) Retinoic acid orchestrates fibroblast growth factor signalling to drive embryonic stem cell differentiation. Development 137, 881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao G., Goldfarb M. (1995) Heparin can activate a receptor tyrosine kinase. EMBO J. 14, 2183–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Berry D., Lynn D. M., Sasisekharan R., Langer R. (2004) Poly(β-amino ester)s promote cellular uptake of heparin and cancer cell death. Chem. Biol. 11, 487–498 [DOI] [PubMed] [Google Scholar]
- 56. Bae K. H., Mok H., Park T. G. (2008) Synthesis, characterization, and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials 29, 3376–3383 [DOI] [PubMed] [Google Scholar]
- 57. Jordan P. M., Cain L. D., Wu P. (2008) Astrocytes enhance long-term survival of cholinergic neurons differentiated from human fetal neural stem cells. J. Neurosci. Res. 86, 35–47 [DOI] [PubMed] [Google Scholar]
- 58. Pettersson I., Kusche M., Unger E., Wlad H., Nylund L., Lindahl U., Kjellén L. (1991) Biosynthesis of heparin. Purification of a 110-kDa mouse mastocytoma protein required for both glucosaminyl N-deacetylation and N-sulfation. J. Biol. Chem. 266, 8044–8049 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.