Background: KLF4 is essential for VSMC differentiation induced by ATRA.
Results: RARα interacts with KLF4-Sp1-YB1 bound to the Klf4 promoter and transactivates Klf4 in VSMCs in a RARE-independent manner.
Conclusion: RARα functions as a co-activator of KLF4-Sp1-YB1 complex.
Significance: These results described a novel mechanism of regulation of Klf4 by ATRA and RARα.
Keywords: Gene Regulation, Kruppel-like Factor (KLF), Promoters, Retinoid, Vascular Smooth Muscle Cells, All-trans-retinoic Acid, Retinoic Acid Receptor Alpha
Abstract
The transcription factor Krüppel-like factor 4 (KLF4) plays a critical role in vascular smooth muscle cell (VSMC) differentiation induced by all-trans-retinoic acid (ATRA). Although it has been demonstrated that ATRA stimulation augments both KLF4 protein and mRNA levels in VSMCs, the molecular mechanisms by which ATRA regulates Klf4 transcription are unknown. In this study, we examined the roles of ATRA-selective nuclear retinoic acid receptors (RARs) in the transcriptional regulation of Klf4. The introduction of small interfering RNA and an RAR antagonist demonstrated that RARα, but not RARβ or RARγ, mediated ATRA-induced Klf4 expression. A luciferase assay for the Klf4 promoter showed that three GC boxes in the proximal Klf4 promoter were indispensible for ATRA-induced Klf4 transcription and that RARα enhanced Klf4 promoter activity in a GC box-dependent manner. Furthermore, chromatin immunoprecipitation and oligonucleotide pulldown assays demonstrated that the transcription factors KLF4, Sp1, and YB1 directly bound to the GC boxes of the proximal Klf4 promoter. Upon RARα agonist stimulation, RARα was recruited to the Klf4 promoter through its interaction with KLF4, Sp1, and YB1 to form a transcriptional activation complex on the three GC boxes of the Klf4 promoter. These results suggest that RARα serves as an essential co-activator for ATRA signaling and that the recruitment of RARα to the KLF4-Sp1-YB1 complex, which leads to Klf4 expression in VSMCs, is independent of a retinoic acid response element.
Introduction
Krüppel-like factor 4 (KLF4),2 a zinc finger-containing transcription factor, is expressed in a variety of tissues. KLF4 both activates and represses the transcription of different genes depending on the cellular context and thereby regulates numerous biological processes including proliferation, differentiation, development, inflammation, and apoptosis (1, 2). In addition, KLF4 plays an important role in reprogramming differentiated somatic cells into induced pluripotent stem cells in both mouse and human (1). The pleiotropic properties of KLF4 and its roles in human cancer and cardiovascular diseases are thus eliciting significant attention.
Previous studies have shown that the function of KLF4 may be regulated not only at the transcription level but also by post-translational modifications, such as phosphorylation, acetylation, sumoylation, and ubiquitylation (3). Furthermore, accumulating evidence suggests that KLF4 can be induced by a variety of stimuli, including serum starvation, oxidative stress, platelet-derived growth factor-BB, butyrate, cyclosporine, selenium, TGF-β, interferon-γ, cAMP, and all-trans-retinoic acid (ATRA) (4–14). We have found in our laboratory that in addition to induction of KLF4 expression, ATRA promotes KLF4 acetylation and phosphorylation, which subsequently transactivates SM22α and SM α-actin and promotes the differentiation process in vascular smooth muscle cells (VSMCs) (15, 16). Other studies also indicated that transcription factor stimulating protein-1 (Sp1) participates in the transcription regulation of Klf4 in VSMCs (17). Moreover, Yang and co-workers (18) showed that KLF4 is subjected to autoregulation by its own gene product.
ATRA, a metabolite of dietary vitamin A (retinol), is a major bioactive retinoid in the body (19). ATRA directly transactivates downstream target genes by binding to retinoic acid receptors (RARs) and retinoid X receptors (RXRs), which belong to the nuclear receptor superfamily and which promote VSMC differentiation (20–22). RARs bind to retinoic acid response elements (RAREs) and, when bound by ligands, recruit a protein complex to activate transcription (23); in the absence of ligands, RARs associate with a co-repressor complex that silences transcription (24). With other transcription factors, including Sp1/Sp3 and STAT5 (signal transducer and activator of transcription 5), RARs cooperatively transactivate the target genes (25–27). RARs are now considered to be an attractive research target for treatment of VSMC proliferation disease (28, 29). Clinical applications of ATRA have successfully been applied in human diseases such as leukemia, cancer, restenosis, and plaque formation (29, 30). Despite these advances, the mechanisms by which ATRA functions to induce Klf4 transcription are still largely unknown.
In this study, we aimed to elucidate the molecular mechanisms of ATRA signaling in the transactivation of Klf4 expression in VSMCs. We show that RARα, but not RARβ or RARγ, mediated ATRA-induced Klf4 expression in VSMCs. RARα was recruited to the Klf4 promoter via its interaction with KLF4, Sp1, and Y box-binding protein 1 (YB1), which are associated with GC boxes at the site, to cooperatively activate Klf4 transcription. ATRA promoted the interaction of RARα with KLF4, Sp1, and YB1. Accordingly, we reveal a novel mechanism by which ATRA-activated RARα, as a co-activator, promoted Klf4 transactivation in a RARE-independent manner in VSMCs.
EXPERIMENTAL PROCEDURES
Cells, Cell Culture, and Treatment
VSMCs were extracted from the thoracic aorta of male Sprague-Dawley rats (90–100 g) as described previously (31). The cells were maintained in DMEM supplemented with 10% FBS (HyClone, Logan, UT) in a humidified atmosphere with 5% CO2 at 37 °C; cells used in this study were passaged for three to six generations. Prior to ATRA stimulation, VSMCs were maintained in serum-free DMEM for 24 h. They were then cultured in DMEM containing 5% FBS and 10 μm ATRA (Sigma-Aldrich) for the indicated times. To introduce inhibitors, thet cells were pretreated with the indicated inhibitors at a final concentration of 20 μm for 2 h before the addition of 10 μm of ATRA. The A293 cells and CHO-K1 cells were purchased from the American Type Culture Collection (Manassas, VA) and maintained in high glucose DMEM supplemented with 10% FBS.
Adenovirus Expression Vector and Plasmid Construction
pEGFP-KLF4 and pCMV-RARα have been described previously (32). The RARα cDNA was amplified and subcloned into the pEGFP (Clontech), pCMV-FLAG (Sigma-Aldrich), and pGEX (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) vectors. For the adenovirus expression vector, the RARα cDNA was cloned into the pAD/CMV/V5-DEST vector (Invitrogen) to create the RARα adenovirus pAd-RARα. The resulting constructs were packaged in A293 cells by transfection with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Culture supernatants from A293 cells were used to infect VSMCs. The cells were passaged after 24 h and selected with 300 μg/ml G418 for 14 days. The expression plasmid for Sp1 (pPac-Sp1) was a generous gift from Dr. Tijan (University of California, Berkeley, CA). The full-length Sp1 cDNA was subcloned into the pEGFP and pGEX vectors. The pGEX-YB1 plasmid was kindly provided by Dr. Kiyoshi Higashi (Sumitomo Chemical, Konohana-ku, Osaka, Japan), and the YB1 cDNA was subcloned into the pEGFP vector. Full-length cDNA of mouse MEF2C was subcloned into the pGEX vector to generate pGEX-MEF2C. For the promoter assay, the luciferase reporter plasmid constructs bearing the mouse Klf4 promoter region was kindly provided by Dr. Walden Ai (University of South Carolina, Columbia, SC). Truncation promoter constructs were similarly generated using different 5′-primers.
Site-directed Mutagenesis
Site-directed mutagenesis was performed with a QuikChange site-directed mutagenesis kit (Agilent Technologies-Stratagene, La Jolla, CA) according to the manufacturer's instructions. Primers used to generate mutation in the putative GC box 1 (GC1) were 5′-GGGGGCTGCGGGAAGGAAAGGAGAAGAAAGGCAGG-3′ (sense) and 5′-CCTGCCTTTCTTCTCCTTTCCTTCCCGCAGCCCCC-3′ (antisense). Primers for mutation in the GC box 2 (GC2) were 5′-AGAAAGGCAGGGGTTTTGGCCTGGCGGCGG-3′ (sense) and 5′-CCGCCGCCAGGCCAAAACCCCTGCCTTTCT-3′ (antisense). The primers for mutation in the GC box 3 (GC3) were 5′-GCCACAGGGAGGAGGAAAGGAGCAAGCGAGCGAG-3′ (sense) and 5′-CTCGCTCGCTTGCTCCTTTCCTCCTCCCTGTGGC-3′ (antisense). All of the constructs were verified by sequencing.
siRNA Transfection
Small interfering RNA (siRNA) targeting Klf4 were synthesized by Sigma-Aldrich, as previously described (7). The siRNAs against the rat sequences RARα, RARβ, and RARγ were designed and synthesized by Sigma-Aldrich. The siRNA sequences against RARα (si-RARα) were, 5′-CUCAGAACAACGUGUCUCU-3′ and 5′-AGAGACACGUUGUUCUGAG-3′. The siRNA sequences against RARβ (si-RARβ) were 5′-GCCAUUUCAUGCCACCAGA-3′ and 5′-UCUGGUGGCAUGAAAUGGC-3′. The siRNA sequences against RARγ (si-RARγ) were 5′-GGUCUAUAAGCCGUGCUUU-3′ and 5′-AAAGCACGGCUUAUAGACC-3′. Nonspecific siRNA (si-NS) and the siRNAs specific for rat Sp1 and YB1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Transfection was performed using Lipofectamine reagent (Invitrogen) following the manufacturer's instructions. Twenty-four hours after transfection, VSMCs were treated with 10 μm of ATRA for indicated times. The cells were then harvested and lysed for Western blot.
RNA Preparation and Quantitative RT-PCR
Total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Quantitative PCR of Klf4 was performed using Platinum SYBR Green quantitative PCR SuperMix UDG Kit (Invitrogen). As an internal control, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene primers were used for RNA template normalization. All of the PCRs were performed in triplicate. The relative expression level was calculated using the following equation: relative gene expression = 2−(ΔCt Sample − ΔCt Control). The following primers were used: Klf4, 5′-CGGGAAGGGAGAAGACACTGC-3′ (sense) and 5′-GCTAGCTGGGGAAGACGAGGA-3′ (antisense); and GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ (sense) and 5′-TTCACCACCCTGTTGCTGTA-3′ (antisense).
Western Blotting
Crude proteins were extracted from VSMCs as described previously (33), resolved by SDS-PAGE, and transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA). The membranes were blocked with 5% milk in Tris-buffered saline with Tween 20 for 2 h at 37 °C and then incubated overnight at 4 °C with the following primary antibodies purchased from Santa Cruz Biotechnology (Santa Cruz, CA): rabbit anti-KLF4, rabbit anti-RARα, rabbit anti-RARβ, rabbit anti-RARγ, rabbit anti-SP1, rabbit anti-GST, mouse anti-GFP, or mouse anti-β-actin. Additional primary antibodies were: rabbit anti-YB1 (Abcam, Cambridge, MA), rabbit anti-MEF2C (Proteintech, Chicago), or rabbit anti-FLAG (Sigma-Aldrich). After incubation with the appropriate secondary antibody, the immunoreactive signal of antibody antigens were visualized using the Chemiluminescence Plus Western blot analysis kit (Santa Cruz).
Luciferase Assay
CHO-K1 cells, maintained in high glucose DMEM supplemented with 10% FBS, were seeded in each well (3 × 104 cells/well) of a 24-well plate and grown for 24 h prior to transfection with reporter plasmids or the control reporter plasmid pRL-TK. The cells were transfected using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Twenty-four hours after treatment with ATRA, the cells were lysed, and luciferase assays were performed using a dual luciferase assay kit (Promega, Madison, WI). Specific promoter activity was expressed as the relative activity ratio of firefly luciferase to Renilla luciferase. All of the promoter constructs were evaluated in ≥3 separate wells/experiment.
Co-immunoprecipitation Assay
Co-immunoprecipitation was performed as described previously (15, 34). Briefly, cell lysates were first precleared with 25 μl of protein A-agarose (50% v/v, Santa Cruz). The supernatants were immunoprecipitated with 2 μg of anti-RARα antibody for 1 h at 4 °C, followed by incubation with protein A-agarose overnight at 4 °C. Protein A-agarose-antigen-antibody complexes were pelleted by centrifugation at 12,000 × g for 60 s at 4 °C. The pellets were washed five times with 1 ml of IPH buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 5 mm EDTA, 0.5% Nonidet P-40, 0.1 mm phenylmethylsulfonyl fluoride), for 20 min each time at 4 °C. Bound proteins were resolved by SDS-PAGE, followed by Western blot with antibodies against KLF4, Sp1, YB1, and RARα.
GST Pulldown Assay
GST, GST-KLF4, GST-Sp1, GST-YB1, and GST-MEF2C fusion proteins were produced by BL21 Escherichia coli under induction by isopropylthio-β-galactoside at 30 °C. Proteins were purified by affinity absorption using glutathione-Sepharose 4B beads (Amersham Biosciences, Uppsala, Sweden). The recombinant GST, GST-KLF4, GST-Sp1, GST-YB1, or GST-MEF2C proteins on the glutathione beads were incubated with total cell lysates of VSMCs at 4 °C overnight followed by extensive washing. Proteins on the beads were resolved by SDS-PAGE and probed by Western blot with anti-KLF4, anti-Sp1, anti-YB1, and anti-RARα antibodies.
Chromatin Immunoprecipitation Assay
The ChIP assay was carried out as described previously (15). Briefly, VSMCs were treated with 1% formaldehyde for 10 min to cross-link proteins with DNA. The cross-linked chromatin was then prepared and sonicated to an average size of 400–600 bp. The DNA fragments were immunoprecipitated overnight with the anti-RARα, anti-KLF4, anti-Sp1, or anti-YB1 antibodies. After reversal of cross-linking, the genomic region of Klf4 flanking the GC boxes (the region between −179 to +20) was amplified by PCR with the following primers (positive primers): 5′-CCACGTGCGCCGAGTTTGTTT-3′ (sense) and 5′-GCTCTTTCGGCCGGGGAACTG-3′ (antisense). A negative control region upstream of the Klf4 promoter (the region between −1063 to −821) was amplified with the following primers (negative primers): 5′-AACTGGAGAGTGCGAGTGCGT-3′ (sense) and 5′-GGACGGGTAAGAATCTCAGAAGC-3′ (antisense).
Oligonucleotide Pulldown Assay
The oligonucleotide pulldown assay was carried out as described previously (12). Oligonucleotides containing the wild-type or mutant GC1/2 or GC3 box sequence in the rat Klf4 promoter with biotin added to their 5′-end were as follows: GC1/2 (−128 to −84), biotin-5′-CTGCGGGAAGGCGGGGAGAAGAAAGGCAGGGGGCGGGGCCTGGCG-3′ (wild type) and biotin-5′-CTGCGGGAAGTTTTGGAGAAGAAAGGCAGGGGTTTTGGCCTGGCG-3′ (mutant); and GC3 (−70 to −34), biotin-5′-CGCCACAGGGAGGAGGCGGGGAGCGAACGAGCGAGAA-3′ (wild type) and biotin-5′-CGCCACAGGGAGGAGGAAAGGAGCGAACGAGCGAGAA-3′ (mutant). Each pair of oligonucleotides was annealed following standard protocols.
VSMCs treated with or without 10 μm of ATRA for 1 h were lysed in lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, and 0.1% Nonidet P-40) containing protease inhibitors. VSMCs lysates or purified recombinant proteins (GST, GST-KLF4, GST-Sp1, GST-YB1, and GST-RARα) were precleared with ImmunoPure streptavidin-agarose beads (20 μl/sample; Pierce) for 1 h at 4 °C. After centrifugation at 12,000 × g for 60 s at 4 °C, the supernatant was incubated with 100 pmol of biotinylated double-strand oligonucleotides and 10 μg of poly(deoxyinosinic-deoxycytidylic) for 16 h at 4 °C. DNA-bound proteins were enriched with 30 μl of immobilized streptavidin-agarose beads for 1 h at 4 °C. After washing with lysis buffer four times, bound proteins were separated by SDS-PAGE and subjected to Western blot.
Isolation and Identification of Factors Mediating ATRA-induced Klf4 Expression
Oligonucleotides containing the Klf4 proximal promoter region −179 to +20 sequence was amplified by PCR with 5′-biotinylated primers: biotin-5′-CCACGTGCGCGGAGTTTGTTT-3′ (sense) and biotin-5′-TCTCTTGGCCGGGGAACTG-3′ (antisense). The biotinylated oligonucleotide (−179/+20) was used as a probe. VSMCs treated with or without 10 μm ATRA for 1 h were lysed with lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, and 0.1% Nonidet P-40) containing protease inhibitors. The whole cell lysates were precleared and incubated with 200 pmol of biotinylated double-stranded oligonucleotide containing the Klf4-proximal promoter region (−179/+20). DNA-bound proteins were enriched with 50 μl of immobilized streptavidin-agarose beads, and the protein components were eluted with Laemmli loading buffer. After separation by SDS-PAGE following staining with Coomassie Brilliant Blue, target bands were excised and subjected to in-gel trypsin digestion. Peptides were analyzed by high capacity ion trap mass spectrometry. Spectra were formatted and searched against mammalian sequences in the Swiss Protein Database by using the Mascot search engine (Matrix Science, Boston, MA).
Statistical Analyses
The data presented as bar graphs are the means ± S.E. of at least three independent experiments. Statistical analyses were performed using Student's t test. The results were considered statistically significant at p < 0.05.
RESULTS
RARα-mediated ATRA-induced Klf4 Expression
Previous studies showed that in VSMCs ATRA induces KLF4 expression and its post-translational modifications, including phosphorylation and acetylation (15, 16, 35). However, the underlying mechanism by which ATRA regulates Klf4 transcription in VSMCs remains largely unknown. ATRA functions by binding to RARs and RXRs, which act as transcription factors upon ligand binding (20). There are three types of RARs: RARα, RARβ, and RARγ, each of which is encoded by their respective genes.
To explore the actual relationship between KLF4 and RARs in ATRA-stimulated VSMCs, we first examined the expression of KLF4 and RARs in response to ATRA signaling. ATRA increased KLF4 and RARα expressions in a concentration- (supplemental Fig. S1) and time-dependent manner (Fig. 1A). As shown in Fig. 1A, 6 h after 10 μm of ATRA treatment, KLF4 and RARα levels were elevated, and this increase was maintained until 24 h after treatment. However, unlike RARα, the expressions of RARβ and RARγ were little changed by the ATRA treatment.
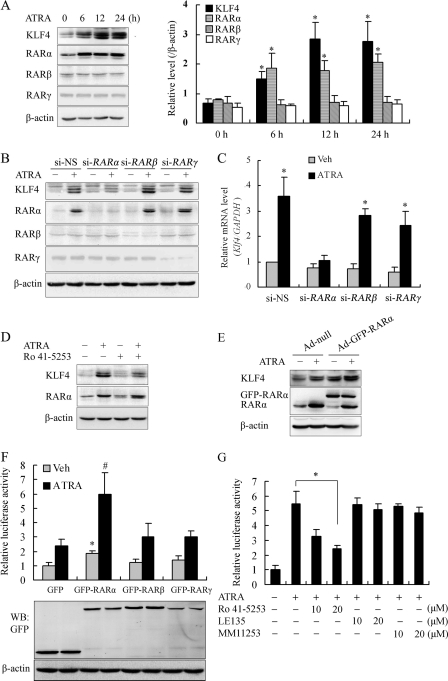
FIGURE 1.
RARα mediated ATRA-induced Klf4 expression. A, VSMCs were treated with 10 μm of ATRA for 0, 6, 12, or 24 h. Crude proteins were extracted from the treated cells and then subjected to Western blot with antibodies against KLF4, RARα, RARβ, or RARγ. β-Actin was used as a control for equal protein loading. Left, blots from a representative experiment. Right, densitometry; results were normalized to β-actin. The bars represent the means ± S.E. from three independent experiments. *, p < 0.05 versus ATRA-free group. B, VSMCs were transfected with si-RARα, si-RARβ, si-RARγ, or si-NS for 24 h and then treated with 10 μm of ATRA for 24 h. The cell lysates were analyzed by Western blot with antibodies against KLF4, RARα, RARβ, or RARγ. β-Actin was the loading control. C, VSMCs were transfected with si-RARα, si-RARβ, si-RARγ, or si-NS for 24 h and then treated with 10 μm of ATRA for 24 h. Total RNA was isolated and subjected to quantitative RT-PCR. The bars represent the means ± S.E. from three independent experiments. *, p < 0.05 versus si-NS-treated and ATRA-free group (first bar). D, VSMCs were pretreated with 20 μm of Ro 41-5253 for 2 h prior to exposure to ATRA (10 μm) for 24 h. Crude proteins from cell lysates were analyzed by Western blot with antibodies against KLF4 and RARα. β-Actin was the loading control. E, VSMCs were infected with Ad-null or Ad-GFP-RARα for 24 h prior to the exposure to ATRA (10 μm) for 24 h. Crude proteins from cell lysates were analyzed by Western blot with antibodies against KLF4 or RARα. β-Actin was the loading control. F, CHO-K1 cells were co-transfected with a Klf4 promoter-reporter construct and RARα, RARβ, or RARγ expression plasmid (GFP-RARα, GFP-RARβ, or GFP-RARγ) for 24 h and then treated with 10 μm of ATRA for 24 h. Cell lysates were subjected to luciferase activity assays using the dual luciferase reporter assay system, and the luciferase activity was normalized to pRL-TK activity. The bars represent the means ± S.E. from three independent experiments. * and #, p < 0.05 versus the respective control group. Expression level of GFP, GFP-RARα, GFP-RARβ, and GFP-RARγ was assessed by Western blot analysis. β-Actin was used as a control for equal protein loading. G, CHO-K1 cells were pretreated with Ro 41-5253, LE135, or MM11253 (antagonists of RARα, RARβ, or RARγ, respectively) for 2 h and then stimulated with 10 μm of ATRA for 24 h. Luciferase activity of the Klf4 promoter-reporter construct was measured as described above. *, p < 0.05 versus ATRA-treated group (second bar).
To further define which RAR isoform mediated the induction of KLF4 by ATRA, we knocked down endogenous RARs by transfecting VSMCs with siRNAs against RARα (si-RARα), RARβ (si-RARβ), RARγ (si-RARγ), or a control (si-NS), respectively. Transfection of si-RARα, si-RARβ, and si-RARγ down-regulated endogenous RARα, RARβ, and RARγ level, respectively. Although ATRA was able to induce both KLF4 and RARα in the cells with RARβ and RARγ knockdown, the induction of KLF4 by ATRA was significantly reduced in mRNA and protein levels in the cells with RARα knockdown (Fig. 1, B and C). Furthermore, when VSMCs were treated with a RARα antagonist (Ro 41-5253) prior to the addition of ATRA, the blockade of RARα signaling partially reduced the response of KLF4 to ATRA (Fig. 1D).
To validate the roles of RARα in ATRA-induced KLF4 expression, RARα overexpression was introduced by infection of the adenovirus vector pAd-GFP-RARα. RARα overexpression further increased the induction of KLF4 by ATRA (Fig. 1E). To examine whether the RARα-mediated KLF4 induction was dependent on its elevated promoter activity, CHO-K1 cells were transiently co-transfected with a Klf4 promoter-reporter construct and RARα, RARβ, or RARγ expression plasmid (GFP-RARα, GFP-RARβ, or GFP-RARγ). The luciferase activity assay showed that RARα overexpression significantly increased the activation of the Klf4 promoter under ATRA treatment (Fig. 1F). The inductive effect of ATRA was partially compromised by the presence of increasing amounts of RARα antagonist Ro 41-5253, whereas pretreated with RARβ antagonist LE135 or RARγ antagonist MM11253 did not affect ATRA-induced Klf4 transcription (Fig. 1G). Collectively, these results suggest that RARα, but not RARβ or RARγ, is essential for induction of Klf4 transcription by ATRA.
RARα-mediated ATRA-induced Klf4 Expression via Three Proximal GC Boxes in Klf4 Promoter
To identify the RARα-responsive elements in the Klf4 promoter region, we constructed progressive 5′-deletion constructs of the Klf4 promoter fused to the luciferase reporter gene. Using transfection of these constructs, we examined the ability of ATRA to activate the expression of each, in CHO-K1 cells. The results showed that the Klf4 promoter-proximal region −179 to +20 exhibited the greatest increase in luciferase activity, compared with control, in response to ATRA treatment (Fig. 2A). Sequence analysis revealed that there were three GC boxes in the Klf4 promoter region within this region (Fig. 2B).
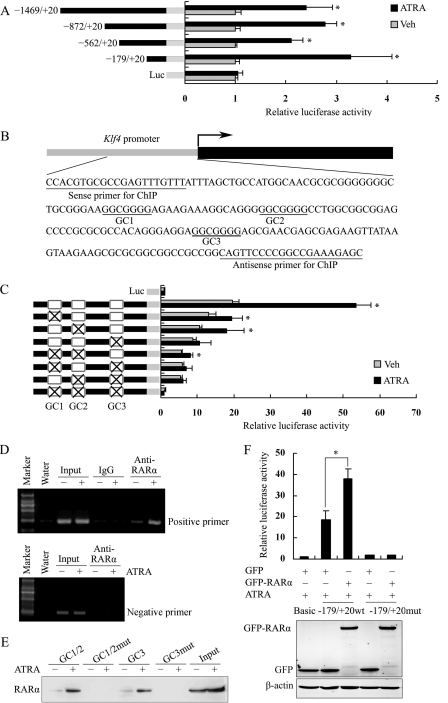
FIGURE 2.
RARα mediated ATRA-induced Klf4 expression via the proximal three GC boxes in the Klf4 promoter. A, CHO-K1 cells were transfected with the Klf4 promoter-reporter plasmid containing various 5′ deletion fragments for 24 h and then treated with 10 μm of ATRA for 24 h. The cell lysates were subjected to luciferase activity assay. The data represent relative Klf4 promoter activity normalized to pRL-TK activity. The bars indicate the means ± S.E. from three independent experiments. *, p < 0.05 versus the respective control group. B, a schematic map of the Klf4 promoter region −179 to +20 showing position of GC boxes. The underlined sequences were primers for the ChIP assay. C, CHO-K1 cells were transfected with the Klf4 proximal promoter-reporter constructs (−179/+20) containing various GC box mutations for 24 h and then treated with 10 μm of ATRA for 24 h. × indicates the mutation of GC boxes. Luciferase activity assay was performed with total cell lysates as described above. *, p < 0.05 versus the respective control group. D, VSMCs were exposed to ATRA (10 μm) for 1 h. ChIP assay was then performed with antibody against RARα. Nonimmune IgG was used as negative control for immunoprecipitation. Immunoprecipitated DNA was amplified by PCR using primers spanning the proximal region of rat Klf4 promoter containing the three GC boxes (positive primers) or using primers spanning the distal Klf4 promoter region −1063 to −821 (negative primers). E, VSMCs were treated with or without ATRA (10 μm) for 1 h, and the whole cell lysates were subjected to oligonucleotide pulldown assay with biotinylated double-stranded oligonucleotides containing wild-type GC1 and GC2 box sequences (GC1/2), mutated GC1 and GC2 (GC1/2mut), wild-type GC3 (GC3), or mutated GC3 (GC3mut) as probes. DNA-bound proteins were collected with streptavidin-agarose beads and analyzed by Western blot with anti-RARα antibody. F, CHO-K1 cells were co-transfected with a GFP-RARα expression vector and the Klf4-proximal promoter-reporter plasmid containing the three wild-type or mutated GC boxes for 24 h. The cells were then treated with vehicle or ATRA (10 μm) for 24 h. The cell lysates were prepared for luciferase assay as described above. *, p < 0.05 versus GFP-RARα plasmid-untransfected group. Expression level of GFP and GFP-RARα was assessed by Western blot analysis. β-Actin was used as a control for equal protein loading. Veh, vehicle; WB, Western blot.
To assess whether these GC boxes were required for ATRA-induced Klf4 expression, we transfected the proximal promoter constructs (−179/+20) encoding various GC box mutations with ATRA stimulation. We observed that the mutation of a single GC box reduced the ATRA-stimulated activation of Klf4 promoter activity by 64–80%. The simultaneous mutation of two GC boxes decreased ATRA-induced Klf4 promoter activity by ∼85%. Furthermore, the elimination of all three GC boxes completely abrogated the response of the Klf4 promoter to ATRA (Fig. 2C). This implies that the response to ATRA by the three GC boxes in the proximal region of the Klf4 promoter was synergistic.
To investigate whether RARα directly bound to the three GC boxes in ATRA-stimulated VSMCs, we carried out the ChIP assay. The results showed that ATRA stimulation significantly promoted the binding of RARα to the proximal region of the Klf4 promoter (Fig. 2D); no binding of RARα was detected when distal Klf4 promoter region was amplified. Consistent with the results of the ChIP assay, the oligonucleotide pulldown assay showed that the binding of RARα to the GC1/2 or GC3 box was increased by ATRA stimulation, whereas mutation in the GC boxes interrupted binding (Fig. 2E), indicating that the binding of RARα to the GC boxes is specific.
To further determine whether the binding of RARα to GC boxes affected Klf4 promoter activity, CHO-K1 cells were co-transfected with GFP-RARα and the Klf4 promoter-reporter construct (−179/+20 wt) or with GFP-RARα and the Klf4 promoter-reporter mutated construct (−179/+20 mutant). As shown in Fig. 2F, RARα overexpression significantly elevated the activities of the Klf4-proximal promoter with three integrative GC boxes in response to ATRA stimulation. However, when all three GC boxes were mutated, the response of the Klf4 promoter to RARα overexpression and ATRA was almost completely abolished. Taken together, these results indicate that RARα-mediated ATRA-induced Klf4 expression in a GC box-dependent manner.
Interaction of RARα with Sp1, YB1, or KLF4 Cooperatively Activated Klf4 Promoter Activity
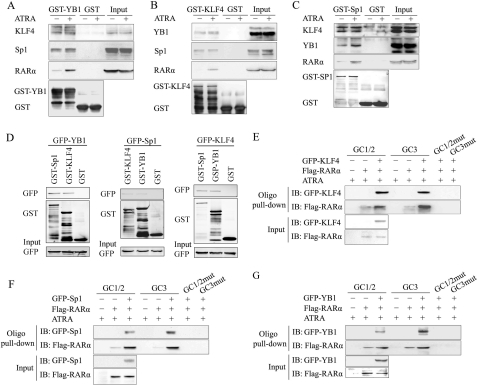
Several recent studies have reported that RARs can exert their effect via RARE-independent regulatory mechanisms by interacting with other transcription factors (26, 36). We reasoned that, as a nuclear receptor mediating the effect of ATRA, RARα could interact with one or more GC box-binding factors to regulate KLF4 expression. Several transcription factors, including Sp1 and KLF4, have been described as binding with the proximal Klf4 promoter (17, 18).
To find new factors mediating ATRA-stimulated KLF4 expression, we affinity-purified DNA-binding transcription factors from VSMC extracts using as a probe a biotinylated double-stranded DNA fragment harboring nucleotides −179 to +20 of the Klf4 promoter. Fig. 3A shows that one protein of 36 kDa was pulled down by the Klf4 (−179 to +20) promoter fragment after ATRA stimulation. High capacity ion trap mass spectrometry analysis with a database search and further confirmation of the amino acid sequence by post-source decay peptide sequencing showed that this protein was YB1 (supplemental Fig. S2).
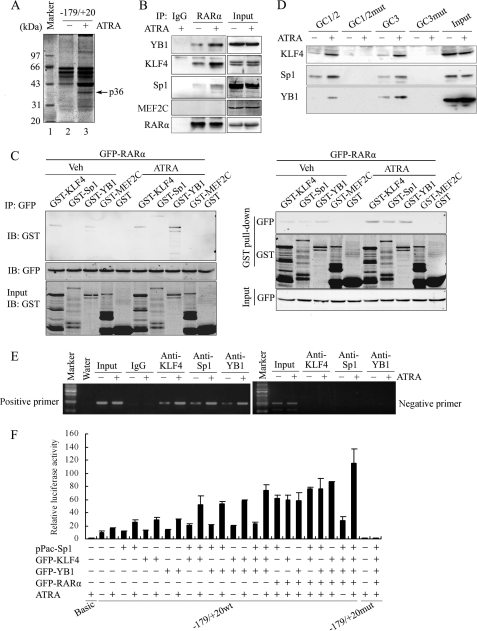
FIGURE 3.
Interaction of RARα with Sp1, YB1, or KLF4 cooperatively activated Klf4 promoter activity. A, YB1 associated with the Klf4-proximal promoter region in response to ATRA stimulation. VSMCs were treated with ATRA (10 μm) for 1 h. Oligonucleotide pulldown was carried out with biotinylated double-stranded oligonucleotide containing Klf4 promoter region −179 to +20 as a probe. DNA-bound proteins were collected with streptavidin-agarose beads and analyzed by SDS-PAGE and Coomassie Brilliant Blue staining. p36, 36-kDa band. B, VSMCs were treated with 10 μm of ATRA for 1 h. The cell lysates were immunoprecipitated with anti-RARα antibody and analyzed by Western blotting using anti-YB1, anti-KLF4, anti-Sp1, anti-MEF2C, or anti-RARα antibodies, respectively. Nonimmune IgG was used as negative control. C, GST, GST-KLF4, GST-Sp1, GST-YB1, and GST-MEF2C fusion proteins were purified from isopropylthio-β-galactoside-induced BL21 E. coli using glutathione-Sepharose 4B beads. CHO-K1 cells were transfected to express GFP-RARα protein for 24 h and then treated with 10 μm of ATRA for 1 h. Left, CHO-K1 cell lysates were incubated with the recombinant GST, GST-KLF4, GST-Sp1, GST-YB1, and GST-MEF2C proteins at 4 °C for 2 h, followed by immunoprecipitation with anti-GFP antibody. Proteins on the beads were eluted and detected by Western blot with anti-GST antibody. Right, the whole cell lysates of CHO-K1 cells overexpressing exogenous GFP-RARα were also used to perform GST pulldown assay. The recombinant GST, GST-KLF4, GST-Sp1, GST-YB1, and GST-MEF2C proteins on the glutathione beads were incubated with the cell lysates, respectively, overnight at 4 °C, followed by extensive washing. Proteins on the beads were eluted and detected by Western blot with anti-GFP antibodies. D, VSMCs were treated with 10 μm of ATRA for 1 h. Oligonucleotide pulldown assay was performed with biotinylated double-stranded oligonucleotides containing wild-type GC1 and GC2 box sequences (GC1/2), mutated GC1 and GC2 (GC1/2mut), wild-type GC3 (GC3), and mutated GC3 (GC3mut) as probes. DNA-bound proteins were collected with streptavidin-agarose beads and analyzed by Western blot with anti-KLF4, anti-Sp1, or anti-YB1 antibodies. E, VSMCs were treated with 10 μm of ATRA for 1 h, and ChIP assays were performed using antibodies against KLF4, Sp1, or YB1. Primers spanning the proximal region of the Klf4 promoter, which contains the three GC boxes (positive primers), or the distal region of the Klf4 promoter −1063 to −821 (negative primers) were used to amplify the precipitated DNA by PCR. F, CHO-K1 cells were co-transfected with the Klf4 proximal promoter-reporter construct containing the three wild-type or mutated GC boxes, along with various combinations of expression plasmids for KLF4, Sp1, YB1, and RARα as indicated. Luciferase activity assay was performed on total cell lysates as described above. IB, immunoblot; IP, immunoprecipitation; Veh, vehicle.
A co-immunoprecipitation assay showed that ATRA stimulation significantly increased the interaction of RARα with KLF4, Sp1, and YB1 in VSMCs in vivo (Fig. 3B). To reveal whether RARα directly interacted with YB1, Sp1, and KLF4 in vitro, GST pulldown with overexpressed GFP-RARα was performed in vitro. Apparently, GFP-RARα physically bound to GST-KLF4, GST-Sp1, or GST-YB1 in vitro but not GST alone; ATRA treatment increased their binding (Fig. 3C). The binding of RARα to MEF2C, a negative control, could not be detected in vivo and in vitro (Fig. 3, B and C). In addition, the interactions of RARβ and RARγ with these transcription factors were also examined by GST pulldown assay. As shown in supplemental Fig. S3, the interaction of GFP-RARβ and GFP-RARγ with KLF4, Sp1, and YB1 could hardly be detected.
Furthermore, the oligonucleotide pulldown assay demonstrated that the binding affinity of KLF4, Sp1, and YB1 to GC boxes (GC1/2 or GC3) in the proximal Klf4 promoter were increased in ATRA-stimulated VSMCs, whereas the mutation in GC boxes abrogated the binding (Fig. 3D). The ChIP assay also showed that ATRA promoted the association of KLF4, Sp1, and YB1 with the proximal Klf4 promoter in VSMCs (Fig. 3E).
Finally, to evaluate the additive effects of the interaction of RARα with KLF4, Sp1, or YB1 on Klf4 promoter activity, CHO-K1 cells were co-transfected with a Klf4 promoter reporter, along with various combinations of expression plasmids for KLF4, Sp1, YB1, and RARα and then treated with or without 10 μm of ATRA and followed by a luciferase assay. As shown in Fig. 3F, transient expression of KLF4, Sp1, or YB1 alone, as well as two or three combinations of these expression plasmids, increased Klf4 promoter activity to a certain extent. The strongest activation was observed when all four expression plasmids for KLF4, Sp1, YB1, and RARα were co-transfected in the presence of ATRA (Fig. 3F). Altogether, these results demonstrated that the interaction of RARα with KLF4, Sp1, or YB1 in the Klf4 promoter region facilitated promoter activity.
Association of KLF4, Sp1, or YB1 with GC Boxes Facilitated Klf4 Promoter Activities
To examine the physical binding of KLF4-Sp1-YB1 to the GC boxes, we performed an oligonucleotide pulldown assay using GST-KLF4, GST-Sp1, GST-YB1, or GST-RARα with biotinylated double-stranded oligonucleotides containing the wild-type or mutant GC1/2 or GC3 box. As shown in Fig. 4 (A and B), GST-KLF4 or GST-Sp1 directly bound to the GC1/2 or GC3 boxes, but not their mutants, in a concentration-dependent manner. Importantly, GST-YB1 was also associated with biotinylated GC1/2 or GC3 probes, in that a higher dose of the probe caused a stronger binding of YB1 to the GC box, and the mutation of the GC box sequence interrupted its binding activity (Fig. 4C). These results strongly suggested that, in vitro, YB1 bound to the GC box directly. However, we did not observe the physical binding of GST-RARα to biotinylated probes containing GC boxes in vitro (Fig. 4D).
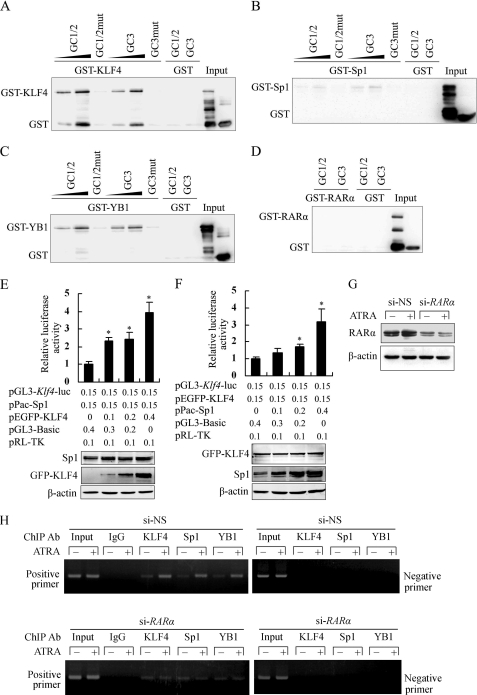
FIGURE 4.
Association of KLF4, Sp1, or YB1 with GC boxes facilitated Klf4 promoter activities. A–D, oligonucleotide pulldown assay was performed to analyze the binding of recombinant KLF4 (A), Sp1 (B), YB1 (C), or RARα (D) to the Klf4 promoter in vitro. DNA-bound proteins were collected with streptavidin-agarose beads and analyzed by Western blot with anti-GST antibody. E, CHO-K1 cells were co-transfected with a constant amount of pPac-Sp1 and increasing amounts of pEGFP-KLF4, along with Klf4 promoter-reporter construct pGL3-Klf4-luc. Luciferase activity was measured on total cell lysates as described above. *, p < 0.05 versus pEGFP-KLF4-untransfected group (first bar). Expression level of Sp1 and GFP-KLF4 was assessed by Western blot analysis. β-Actin was used as a control for equal protein loading. F, CHO-K1 cells were co-transfected with the indicated constructs, and then luciferase activity of Klf4 promoter-reporter construct was analyzed as described above. *, p < 0.05 versus the pPac-Sp1-untransfected-group (first bar). The expression level of GFP-KLF4 and Sp1 was assessed by Western blot analysis. β-Actin was used as a control for equal protein loading. G, VSMCs were transfected with si-RARα or si-NS for 24 h and then treated with 10 μm of ATRA for 1 h. Expression of RARα was assessed by Western blot analysis. β-Actin was used as a control for equal protein loading. H, VSMCs were transfected with si-RARα or si-NS for 24 h and then treated with 10 μm of ATRA for 1 h. ChIP assays were performed using antibodies against KLF4, Sp1, or YB1. Primers spanning the proximal region of the Klf4 promoter containing the three GC boxes (positive primers) or the distal region of the Klf4 promoter −1063 to −821 (negative primers) were used to amplify the precipitated DNA by PCR.
To further investigate the effect of KLF4, Sp1, and YB1 on Klf4 promoter activity, CHO-K1 cells were co-transfected with a constant amount of pPac-Sp1 plasmid and increasing amounts of pEGFP-KLF4 plasmid, along with the Klf4 promoter-reporter construct pGL3-Klf4-luc. As shown in Fig. 4E, the stimulatory effect of Sp1 on the Klf4 promoter gradually increased with increasing amounts of pEGFP-KLF4. Likewise, when CHO-K1 cells were co-transfected with a constant amount of pEGFP-KLF4 and increasing amounts of pPac-Sp1, Sp1 enhanced the stimulatory effect of KLF4 on the Klf4 promoter in a concentration-dependent manner (Fig. 4F). These results suggested that both KLF4 and Sp1 bound to the Klf4 promoter to co-operatively activate its transcription. In addition, as shown in supplemental Fig. S4, both Sp1 and YB1, as well as both KLF4 and YB1, also cooperated with each other to activate the Klf4 promoter.
Next, we knocked down endogenous RARα by transfecting VSMCs with si-RARα (Fig. 4G). The ChIP assay showed that ATRA promoted the binding of KLF4, Sp1, and YB1 to the GC box region of the Klf4 promoter in si-NS-treated VSMCs; down-regulation of endogenous RARα by si-RARα reduced ATRA-induced recruitments of these factors to the Klf4 promoter (Fig. 4H). The results suggest that the presence of RARα or ATRA stimulation facilitated the association of KLF4, Sp1, or YB1 with the GC box region of the Klf4 promoter.
ATRA Promoted Binding of RARα to Klf4 Promoter in KLF4-Sp1-YB1-dependent Manner
To reveal the roles of ATRA in the interactions between RARα and KLF4, Sp1, or YB1, we next performed a GST pulldown assay in vitro. We observed that although KLF4, Sp1, and YB1 formed a complex without ATRA treatment, the addition of ATRA stimulation promoted the binding of RARα to the KLF4-Sp1-YB1 complex. (Fig. 5, A–C). Furthermore, to analyze the interactions between KLF4, Sp1, and YB1, GST pulldown assays with overexpressed GFP-KLF4, GFP-Sp1, or GFP-YB1 were performed in vitro. Apparently, GFP-KLF4, GFP-Sp1, and GFP-YB1 physically bound to any of the other factors (Fig. 5D). Simultaneously, we also analyzed the effect of ATRA on some other target genes of KLF4, Sp1, or YB1. As shown in supplemental Fig. S5, the protein level of SM22α, p53, and p21, the target genes of KLF4, YB1, and Sp1, significantly increased in VSMCs after ATRA stimulation for 24 h.
FIGURE 5.
ATRA promoted the binding of RARα to the Klf4 promoter in a KLF4-Sp1-YB1-dependent manner. A, the purified recombinant GST and GST-YB1 proteins on the glutathione beads were incubated with total cell lysates of VSMCs treated with 10 μm ATRA for 1 h, followed by extensive washing. Proteins on the beads were subjected to Western blot with anti-KLF4, anti-Sp1, or anti-RARα antibodies. B, GST pulldown assay of recombinant GST-KLF4 or GST with lysates from VSMCs treated with ATRA for 1 h. Proteins on the beads were subjected to Western blot with anti-YB1, anti-Sp1, and anti-RARα antibodies. C, GST pulldown assay of recombinant GST-Sp1 or GST with lysates from VSMCs treated with ATRA for 1 h. Proteins on the beads were subjected to Western blot with anti-KLF4, anti-YB1, or anti-RARα antibodies. D, CHO-K1 cells were transfected for 24 h to express GFP-YB1 (left), GFP-Sp1 (middle), or GFP-KLF4 (right) protein and then treated with 10 μm of ATRA for 1 h. The whole cell lysates of CHO-K1 cells were used to perform GST pulldown assay. The recombinant GST, GST-KLF4, GST-Sp1, and GST-YB1 proteins on the glutathione beads were incubated with the cell lysates, respectively, overnight at 4 °C, followed by extensive washing. Proteins on the beads were eluted and detected by Western blot with anti-GFP antibody. E–G, CHO-K1 cells were co-transfected to express indicated factors, with a FLAG tag attached to RARα and with a GFP tag attached to KLF4 (E), Sp1 (F), or YB1 (G). The cell lysates were subjected to an oligonucleotide pulldown assay with biotinylated double-stranded oligonucleotides containing wild-type GC1 and GC2 box sequences (GC1/2), mutated GC1 and GC2 (GC1/2mut), wild-type GC3 (GC3), and mutated GC3 (GC3mut) as probes. The precipitates were analyzed by Western blot with antibodies against the FLAG tag or GFP tag.
To further examine whether RARα recruitment to the proximal Klf4 promoter depended on the binding of the KLF4-Sp1-YB1 complex to GC boxes on the Klf4 promoter, CHO-K1 cells were co-transfected with expression constructs for KLF4, Sp1, YB1, or RARα, and then oligonucleotide pulldown assays were performed using GC1/2 or GC3 box sequence as probes. The results showed that RARα bound to GC1/2 or GC3 box at the lower level, but the co-expression of KLF4, Sp1, or YB1 significantly increased the binding of RARα to GC boxes when ATRA was present (Fig. 5, E–G). These results suggested that the binding of RARα to the Klf4 promoter was KLF4-Sp1-YB1 complex-dependent and that ATRA stimulation promoted the interaction of RARα with KLF4, Sp1, and YB1.
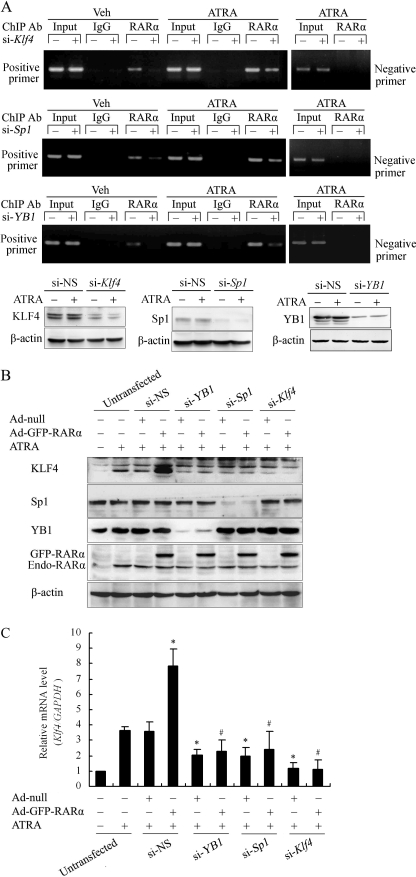
Knockdown of Endogenous KLF4, Sp1, and YB1 Disrupted Recruitment of RARα to Klf4 Promoter
To further test whether the binding of RARα to the Klf4 promoter depended on KLF4, Sp1, and YB1, we performed a ChIP assay in VSMCs where KLF4, Sp1, or YB1 was knocked down by RNA interference (siRNA). As shown in Fig. 6A, higher occupancy of RARα on the Klf4 promoter was observed under ATRA stimulation. Knockdown of endogenous KLF4, Sp1, or YB1 by siRNA significantly attenuated the binding of RARα to the Klf4 promoter; no binding of RARα was detected when one pair of negative control primers was used (Fig. 6A).
FIGURE 6.
Knockdown of endogenous KLF4, Sp1, and YB1 disrupted the recruitment of RARα to the Klf4 promoter. A, VSMCs were transfected with siRNA targeting KLF4 (si-Klf4), Sp1 (si-Sp1), or YB1 (si-YB1) for 24 h and then treated with 10 μm of ATRA for 1 h. ChIP assay was performed using anti-RARα antibody. Primers spanning the proximal region of the Klf4 promoter containing the three GC boxes (positive primers) or the distal Klf4 promoter region −1063 to −821 (negative primers) were used to amplify the precipitated DNA by PCR. Knockdown of level of KLF4, Sp1, or YB1 in VSMCs was assessed by Western blot analysis. β-Actin was used as a control for equal protein loading. B, VSMCs were transfected with siRNA targeting KLF4 (si-Klf4), Sp1 (si-Sp1), YB1 (si-YB1), or NS-siRNA (si-NS) and infected with Ad-null or Ad-GFP-RARα for 24 h. The cells were treated with 10 μm of ATRA for 24 h and then lysed. Western blot was performed with anti-KLF4, anti-Sp1, anti-YB1, or anti-RARα antibodies. β-Actin was the loading control. Endo-RARα indicated endogenous RARα in VSMCs. C, VSMCs were transfected with si-Klf4, si-Sp1, si-YB1, or si-NS for 24 h and infected with Ad-null or Ad-GFP-RARα for 24 h. The cells were then treated with 10 μm of ATRA for 24 h. Total RNA was isolated and subjected to quantitative RT-PCR. The bars represent the means ± S.E. from three independent experiments. *, p < 0.05 versus si-NS and Ad-null-infected group (third bar). #, p < 0.05 versus si-NS and Ad-GFP-RARα-infected group (fourth bar).
To further validate the above results, we next transfected VSMCs with siRNAs targeting Klf4, Sp1, or YB1 or with an adenovirus expression vector for RARα, followed by ATRA treatment. Overexpression of RARα significantly enhanced KLF4 expression induced by ATRA. Knockdown of endogenous KLF4, Sp1, or YB1, together with RARα overexpression, abrogated the induction of KLF4 expression by ATRA in VSMCs (Fig. 6, B and C). Altogether, these results confirmed that KLF4, Sp1, and YB1 were indispensible for the recruitment of RARα to the Klf4 promoter.
DISCUSSION
KLFs form a large family of transcription factors that share in common a transcriptional activation/repression domain, a nuclear localization signal, and three Krüppel-like zinc fingers (37). To date, at least 20 KLFs have been identified in mammals. Each individually has important biological functions in cell proliferation, apoptosis, development, and oncogenic processes (3, 38). KLF4 has been shown to play a key role in pathological vascular processes and is considered a molecular switch, regulating the function of VSMCs under pathophysiological conditions (3).
Recently, we demonstrated that KLF4 is required for VSMC differentiation induced by ATRA (16). Because the effect of ATRA is mediated by RARs and RXRs, we then speculated that the role of RARs in up-regulating KLF4 expression may be important. In the present study, we characterized the regulation of Klf4 by ATRA and RARα. We found that ATRA regulated Klf4 expression through the three GC boxes in the proximal promoter of the gene and that this regulation required interaction between its nuclear receptor RARα and the KLF4-Sp1-YB1 complex. These results therefore provide evidence for the first time that RARα activates the Klf4 promoter in an RARE-independent manner in VSMCs.
RARα has been shown to distinctly mediate the growth inhibitory effect of retinoids and therefore is a potential target of research for preventive treatment of vascular proliferation disease (24). In VSMCs, we discovered that treatment with ATRA induced the expressions of KLF4 and RARα. Silencing of the RARα gene or inhibiting RARα with its antagonist Ro 41-5253 abrogated the ATRA-induced Klf4 transcription, indicating that RARα plays a critical role in the induction of Klf4 by ATRA and cannot be substituted by other RARs.
Although retinoid signaling is considered responsible for the direct binding of RARs/RXRs to RAREs in target gene promoters, other evidence has shown that RARs exert their effect by interacting with other general transcription factors, such as Sp1, Ets-1, cAMP response element-binding protein, and KLF5 (24, 26, 36, 39). There are three GC boxes in the proximal region of the Klf4 promoter, which may play a vital role in Klf4 transactivation (9, 18). Yang and co-workers (40) reported that KLF4 activates its own gene promoter, whereas KLF5 suppresses the Klf4 promoter through the same three closely spaced GC boxes within the Klf4 promoter. Owens and co-workers (17) demonstrated that Sp1 regulates the expression of KLF4 in platelet-derived growth factor-BB-stimulated VSMCs via the GC boxes in the proximal Klf4 promoter. Their results demonstrate that GC boxes in the Klf4 promoter execute important roles in Klf4 transcriptional activities.
Our data showed that ATRA stimulation increased RARα binding to the proximal Klf4 promoter. Luciferase reporter gene assays revealed that when all three of the GC boxes were mutated, ATRA-dependent Klf4 promoter transactivation was abrogated, and overexpression of RARα failed to activate Klf4 expression. These results suggest that the three GC boxes in the proximal Klf4 promoter are required for RARα-dependent Klf4 transcription.
Interestingly, using the oligonucleotide pulldown assay, we found that YB1 specifically binds to the Klf4 promoter region in response to ATRA stimulation. ChIP and oligonucleotide pulldown assays demonstrated that YB1 interacted with DNA in the Klf4 proximal promoter region with the GC boxes motif (−179 to +20). It is well known that YB1 belongs to the evolutionarily conserved group of CCAAT-binding proteins that control the expression of a large number of gene products. It classically binds to DNA containing a Y box or inverted CCAAT box (CTGATTGGCCAA) and activates genes associated with proliferation and cancer such as cyclin A, cyclin B1, DNA polymerase α, and the multidrug resistance 1 gene (41–44). Importantly, the present data for the first time provide in vivo and in vitro evidence that YB1 directly binds to the GC box (GGCGGGG) and transactivates the Klf4 promoter in ATRA-stimulated VSMCs. Therefore, the novel binding motif of YB1 implies an unrevealed signaling pathway involving YB1 in VSMCs in response to ATRA stimulation.
Because RARα has been shown to synergistically interact with other transcription factors, it is conceivable that besides a direct activation, RARα might also cooperate with other transcription factors in the regulation of Klf4 gene transcription. The present study showed that KLF4, Sp1, and YB1 formed a protein complex without ATRA treatment. When cells were treated with the RAR-selective antagonist, RARα was recruited to the three GC boxes in the Klf4 promoter by its association with KLF4, Sp1, and YB1. Knockdown of endogenous YB1 led to a significant decrease of RARα occupancy in the Klf4 promoter in ATRA-stimulated VSMCs. YB1 transactivates CC chemokine ligand 5 gene transcription by binding to the CCAAT box on the chemokine ligand 5 promoter in VSMCs and contributes to neointimal hyperplasia (45). This implicates YB1 as a critical factor in the regulation of the differentiation and proliferation of VSMCs, and our findings further suggest that YB1 may serve as a key mediator of VSMC differentiation. This might be due to selective binding of YB1 to different specific DNA elements in a cell context-dependent manner.
In summary, we showed that ATRA signaling up-regulated Klf4 gene transcription through functional interaction of RARα with KLF4, Sp1, and YB1 bound to the GC boxes of the Klf4 promoter. The present results thus describe a novel mechanism of regulation of Klf4 by ATRA and RARα, which is critical toward the understanding of the biological functions of retinoids during VSMC phenotypic modulation.
Supplementary Material
This work is supported by National Natural Science Foundation of China Grants 30971457, 90919035, 30871272, and 31071003 and National Basic Research Program of China Grant 2012CB518601.

This article contains supplemental Figs. S1–S5.
- KLF
- Krüppel-like factor
- ATRA
- all-trans-retinoic acid
- RAR
- retinoic acid receptor
- RARE
- retinoic acid response element
- RXR
- retinoid X receptor
- si-NS
- nonspecific siRNA
- Sp1
- transcription factor stimulating protein-1
- VSMC
- vascular smooth muscle cell
- YB1
- Y box-binding protein 1.
REFERENCES
- 1. Evans P. M., Liu C. (2008) Roles of Krüpel-like factor 4 in normal homeostasis, cancer and stem cells. Acta Biochim. Biophys. Sin. 40, 554–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McConnell B. B., Ghaleb A. M., Nandan M. O., Yang V. W. (2007) The diverse functions of Krüppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays 29, 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zheng B., Han M., Wen J. K. (2010) Role of Krüppel-like factor 4 in phenotypic switching and proliferation of vascular smooth muscle cells. IUBMB Life 62, 132–139 [DOI] [PubMed] [Google Scholar]
- 4. Chen Z. Y., Wang X., Zhou Y., Offner G., Tseng C. C. (2005) Destabilization of Krüppel-like factor 4 protein in response to serum stimulation involves the ubiquitin-proteasome pathway. Cancer Res. 65, 10394–10400 [DOI] [PubMed] [Google Scholar]
- 5. Shields J. M., Christy R. J., Yang V. W. (1996) Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J. Biol. Chem. 271, 20009–20017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cullingford T. E., Butler M. J., Marshall A. K., Tham el L., Sugden P. H., Clerk A. (2008) Differential regulation of Krüppel-like factor family transcription factor expression in neonatal rat cardiac myocytes. Effects of endothelin-1, oxidative stress and cytokines. Biochim. Biophys. Acta 1783, 1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang C., Han M., Zhao X. M., Wen J. K. (2008) Kruppel-like factor 4 is required for the expression of vascular smooth muscle cell differentiation marker genes induced by all-trans-retinoic acid. J. Biochem. 144, 313–321 [DOI] [PubMed] [Google Scholar]
- 8. Liu Y., Sinha S., McDonald O. G., Shang Y., Hoofnagle M. H., Owens G. K. (2005) Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J. Biol. Chem. 280, 9719–9727 [DOI] [PubMed] [Google Scholar]
- 9. Chen Z. Y., Rex S., Tseng C. C. (2004) Krüppel-like factor 4 is transactivated by butyrate in colon cancer cells. J. Nutr. 134, 792–798 [DOI] [PubMed] [Google Scholar]
- 10. Garvey S. M., Sinden D. S., Schoppee Bortz P. D., Wamhoff B. R. (2010) Cyclosporine up-regulates Krüppel-like factor-4 (KLF4) in vascular smooth muscle cells and drives phenotypic modulation in vivo. J. Pharmacol. Exp. Ther. 333, 34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu S., Zhang H., Zhu L., Zhao L., Dong Y. (2008) Kruppel-like factor 4 is a novel mediator of selenium in growth inhibition. Mol. Cancer Res. 6, 306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li H. X., Han M., Bernier M., Zheng B., Sun S. G., Su M., Zhang R., Fu J. R., Wen J. K. (2010) Krüppel-like factor 4 promotes differentiation by transforming growth factor-β receptor-mediated Smad and p38 MAPK signaling in vascular smooth muscle cells. J. Biol. Chem. 285, 17846–17856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Z. Y., Shie J., Tseng C. (2000) Up-regulation of gut-enriched Krüppel-like factor by interferon-γ in human colon carcinoma cells. FEBS Lett. 477, 67–72 [DOI] [PubMed] [Google Scholar]
- 14. Birsoy K., Chen Z., Friedman J. (2008) Transcriptional regulation of adipogenesis by KLF4. Cell Metab. 7, 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meng F., Han M., Zheng B., Wang C., Zhang R., Zhang X. H., Wen J. K. (2009) All-trans-retinoic acid increases KLF4 acetylation by inducing HDAC2 phosphorylation and its dissociation from KLF4 in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 387, 13–18 [DOI] [PubMed] [Google Scholar]
- 16. Yu K., Zheng B., Han M., Wen J. K. (2011) ATRA activates and PDGF-BB represses the SM22α promoter through KLF4 binding to, or dissociating from, its cis-DNA elements. Cardiovasc. Res. 90, 464–474 [DOI] [PubMed] [Google Scholar]
- 17. Deaton R. A., Gan Q., Owens G. K. (2009) Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 296, H1027–H1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahatan C. S., Kaestner K. H., Geiman D. E., Yang V. W. (1999) Characterization of the structure and regulation of the murine gene encoding gut-enriched Krüppel-like factor (Krüppel-like factor 4). Nucleic Acids Res. 27, 4562–4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blomhoff R., Blomhoff H. K. (2006) Overview of retinoid metabolism and function. J. Neurobiol. 66, 606–630 [DOI] [PubMed] [Google Scholar]
- 20. Miano J. M., Topouzis S., Majesky M. W., Olson E. N. (1996) Retinoid receptor expression and all-4-retinoic acid-mediated growth inhibition in vascular smooth muscle cells. Circulation 93, 1886–1895 [DOI] [PubMed] [Google Scholar]
- 21. Miano J. M., Kelly L. A., Artacho C. A., Nuckolls T. A., Piantedosi R., Blaner W. S. (1998) all-Trans-retinoic acid reduces neointimal formation and promotes favorable geometric remodeling of the rat carotid artery after balloon withdrawal injury. Circulation 98, 1219–1227 [DOI] [PubMed] [Google Scholar]
- 22. Axel D. I., Frigge A., Dittmann J., Runge H., Spyridopoulos I., Riessen R., Viebahn R., Karsch K. R. (2001) All-trans-retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells. Cardiovasc. Res. 49, 851–862 [DOI] [PubMed] [Google Scholar]
- 23. Bastien J., Rochette-Egly C. (2004) Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 328, 1–16 [DOI] [PubMed] [Google Scholar]
- 24. Zheng B., Han M., Shu Y. N., Li Y. J., Miao S. B., Zhang X. H., Shi H. J., Zhang T., Wen J. K. (2011) HDAC2 phosphorylation-dependent Klf5 deacetylation and RARα acetylation induced by RAR agonist switch the transcription regulatory programs of p21 in VSMCs. Cell Res. 21, 1487–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loudig O., Babichuk C., White J., Abu-Abed S., Mueller C., Petkovich M. (2000) Cytochrome P450RAI(CYP26) promoter. A distinct composite retinoic acid response element underlies the complex regulation of retinoic acid metabolism. Mol. Endocrinol. 14, 1483–1497 [DOI] [PubMed] [Google Scholar]
- 26. Wu J. B., Chen K., Ou X. M., Shih J. C. (2009) Retinoic acid activates monoamine oxidase B promoter in human neuronal cells. J. Biol. Chem. 284, 16723–16735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Si J., Collins S. J. (2002) IL-3-induced enhancement of retinoic acid receptor activity is mediated through Stat5, which physically associates with retinoic acid receptors in an IL-3-dependent manner. Blood 100, 4401–4409 [DOI] [PubMed] [Google Scholar]
- 28. Wiegman P. J., Barry W. L., McPherson J. A., McNamara C. A., Gimple L. W., Sanders J. M., Bishop G. G., Powers E. R., Ragosta M., Owens G. K., Sarembock I. J. (2000) All-trans-retinoic acid limits restenosis after balloon angioplasty in the focally atherosclerotic rabbit. A favorable effect on vessel remodeling. Arterioscler. Thromb. Vasc. Biol. 20, 89–95 [DOI] [PubMed] [Google Scholar]
- 29. Herdeg C., Oberhoff M., Baumbach A., Schroeder S., Leitritz M., Blattner A., Siegel-Axel D. I., Meisner C., Karsch K. R. (2003) Effects of local all-trans-retinoic acid delivery on experimental atherosclerosis in the rabbit carotid artery. Cardiovasc. Res. 57, 544–553 [DOI] [PubMed] [Google Scholar]
- 30. Charoensit P., Kawakami S., Higuchi Y., Yamashita F., Hashida M. (2010) Enhanced growth inhibition of metastatic lung tumors by intravenous injection of ATRA-cationic liposome/IL-12 pDNA complexes in mice. Cancer Gene Ther. 17, 512–522 [DOI] [PubMed] [Google Scholar]
- 31. Han M., Wen J. K., Zheng B., Cheng Y., Zhang C. (2006) Serum deprivation results in redifferentiation of human umbilical vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 291, C50–C58 [DOI] [PubMed] [Google Scholar]
- 32. Zheng B., Han M., Bernier M., Zhang X. H., Meng F., Miao S. B., He M., Zhao X. M., Wen J. K. (2009) Krüppel-like factor 4 inhibits proliferation by platelet-derived growth factor receptor β-mediated, not by retinoic acid receptor α-mediated, phosphatidylinositol 3-kinase and ERK signaling in vascular smooth muscle cells. J. Biol. Chem. 284, 22773–22785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X. H., Zheng B., Han M., Miao S. B., Wen J. K. (2009) Synthetic retinoid Am80 inhibits interaction of KLF5 with RAR α through inducing KLF5 dephosphorylation mediated by the PI3K/Akt signaling in vascular smooth muscle cells. FEBS Lett. 583, 1231–1236 [DOI] [PubMed] [Google Scholar]
- 34. Han M., Li A. Y., Meng F., Dong L. H., Zheng B., Hu H. J., Nie L., Wen J. K. (2009) Synergistic co-operation of signal transducer and activator of transcription 5B with activator protein 1 in angiotensin II-induced angiotensinogen gene activation in vascular smooth muscle cells. FEBS J. 276, 1720–1728 [DOI] [PubMed] [Google Scholar]
- 35. Zhang R., Han M., Zheng B., Li Y. J., Shu Y. N., Wen J. K. (2010) Krüppel-like factor 4 interacts with p300 to activate mitofusin 2 gene expression induced by all-trans-retinoic acid in VSMCs. Acta Pharmacol. Sin. 31, 1293–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Makkonen K. M., Pasonen-Seppänen S., Törrönen K., Tammi M. I., Carlberg C. (2009) Regulation of the hyaluronan synthase 2 gene by convergence in cyclic AMP response element-binding protein and retinoid acid receptor signaling. J. Biol. Chem. 284, 18270–18281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghaleb A. M., Yang V. W. (2008) The pathobiology of Krüppel-like factors in colorectal cancer. Curr. Colorectal Cancer Rep. 4, 59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nemer M., Horb M. E. (2007) The KLF family of transcriptional regulators in cardiomyocyte proliferation and differentiation. Cell Cycle 6, 117–121 [DOI] [PubMed] [Google Scholar]
- 39. Kumar P., Garg R., Bolden G., Pandey K. N. (2010) Interactive roles of Ets-1, Sp1, and acetylated histones in the retinoic acid-dependent activation of guanylyl cyclase/atrial natriuretic peptide receptor-A gene transcription. J. Biol. Chem. 285, 37521–37530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dang D. T., Zhao W., Mahatan C. S., Geiman D. E., Yang V. W. (2002) Opposing effects of Krüppel-like factor 4 (gut-enriched Krüppel-like factor) and Krüppel-like factor 5 (intestinal-enriched Krüppel-like factor) on the promoter of the Krüppel-like factor 4 gene. Nucleic Acids Res. 30, 2736–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ting J. P., Painter A., Zeleznik-Le N. J., MacDonald G., Moore T. M., Brown A., Schwartz B. D. (1994) YB-1 DNA-binding protein represses interferon γ activation of class II major histocompatibility complex genes. J. Exp. Med. 179, 1605–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jurchott K., Bergmann S., Stein U., Walther W., Janz M., Manni I., Piaggio G., Fietze E., Dietel M., Royer H. D. (2003) YB-1 as a cell cycle-regulated transcription factor facilitating cyclin A and cyclin B1 gene expression. J. Biol. Chem. 278, 27988–27996 [DOI] [PubMed] [Google Scholar]
- 43. En-Nia A., Yilmaz E., Klinge U., Lovett D. H., Stefanidis I., Mertens P. R. (2005) Transcription factor YB-1 mediates DNA polymerase α gene expression. J. Biol. Chem. 280, 7702–7711 [DOI] [PubMed] [Google Scholar]
- 44. Sengupta S., Mantha A. K., Mitra S., Bhakat K. K. (2011) Human AP endonuclease (APE1/Ref-1) and its acetylation regulate YB-1-p300 recruitment and RNA polymerase II loading in the drug-induced activation of multidrug resistance gene MDR1. Oncogene 30, 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krohn R., Raffetseder U., Bot I., Zernecke A., Shagdarsuren E., Liehn E. A., van Santbrink P. J., Nelson P. J., Biessen E. A., Mertens P. R., Weber C. (2007) Y-box binding protein-1 controls CC chemokine ligand 5 (CCL5) expression in smooth muscle cells and contributes to neointima formation in atherosclerosis-prone mice. Circulation 116, 1812–1820 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.