Background: Exosome is a membrane vesicle released from several types of cells, including neurons.
Results: Neuronal exosomes accelerate Aβ fibril formation, and the exosome-associated Aβ is taken into microglia to degrade it.
Conclusion: Exosomes promote Aβ clearance.
Significance: These findings provide a new function of exosome in the brain and also suggest its involvement in the development of Alzheimer disease.
Keywords: Alzheimer Disease, Exosomes, Microglia, Neurons, Sphingolipid, Amyloid-beta
Abstract
Amyloid β-peptide (Aβ), the pathogenic agent of Alzheimer disease, is a physiological metabolite whose levels are constantly controlled in normal brain. Recent studies have demonstrated that a fraction of extracellular Aβ is associated with exosomes, small membrane vesicles of endosomal origin, although the fate of Aβ in association with exosome is largely unknown. In this study, we identified novel roles for neuron-derived exosomes acting on extracellular Aβ, i.e. exosomes drive conformational changes in Aβ to form nontoxic amyloid fibrils and promote uptake of Aβ by microglia. The Aβ internalized together with exosomes was further transported to lysosomes and degraded. We also found that blockade of phosphatidylserine on the surface of exosomes by annexin V not only prevented exosome uptake but also suppressed Aβ incorporation into microglia. In addition, we demonstrated that secretion of neuron-derived exosomes was modulated by the activities of sphingolipid-metabolizing enzymes, including neutral sphingomyelinase 2 (nSMase2) and sphingomyelin synthase 2 (SMS2). In transwell experiments, up-regulation of exosome secretion from neuronal cells by treatment with SMS2 siRNA enhanced Aβ uptake into microglial cells and significantly decreased extracellular levels of Aβ. Our findings indicate a novel mechanism responsible for clearance of Aβ through its association with exosomes. The modulation of the vesicle release and/or elimination may alter the risk of AD.
Introduction
Alzheimer disease (AD)2 is a late-onset neurological disorder with progressive loss of memory and cognitive ability as a result of neuronal impairment and death. AD is pathologically featured by extensive extraneuronal deposition of amyloid fibrils, which are composed of amyloid β protein (Aβ). The Aβ is generated by the processing of amyloid precursor protein (APP) as a physiological metabolite and is subsequently secreted to extracellular milieu. Steady-state levels of extracellular Aβ are controlled by the balance between its generation and its degradation/clearance. Several lines of evidence indicate that Aβ accumulation, attributable to an imbalance of its metabolism, is linked to the pathogenesis of AD (1). In the case of familial AD, genetic alterations of certain genes, such as APP and presenilin, appear to facilitate Aβ assembly through a marked enhancement in Aβ production (2). In contrast, in sporadic AD, a common form of the disease, the decreased level of Aβ elimination within the brains is apparent (3, 4). This suggests a perturbation of Aβ clearance through, for example, decreased catabolism via reduced proteolysis or impaired efflux across the blood-brain barrier into CSF. However, the precise clearance process, which appears damaged in AD, remains controversial.
In a recent report, a portion of extracellular Aβ was found to be associated with membrane vesicles called exosomes (5). Exosomes represent a specific subtype of secreted small vesicles (40–100 nm in diameter) derived from various types of cells, including neurons (6, 7). They correspond to the intraluminal vesicles of endosomal multivesicular bodies (MVBs) that fuse with the plasma membrane in an exocytic manner. A well known function of exosomes is to remove obsolete or misfolded proteins and to secrete them into a drainage system, such as the gut or urinary tract (8). In addition, accumulated evidence has indicated that exosomes act as shuttles for intercellular delivery of cargo, including specific proteins, lipids, and RNAs. The exosome marker Alix has been observed in Aβ plaques in the AD brain (5). Our previous study also demonstrated that PC12-derived exosomes potentially promote Aβ fibrillogenesis, under endocytic impairment, which is apparent with the early pathological changes in AD brains (9).
With these lines of evidence in mind, we investigated the fate of extracellular Aβ associated with exosomes. We demonstrated that exosomes were constitutively released from neuroblastoma N2a cells and mouse primary cortical neurons. These exosomes significantly accelerated amyloidogenesis of Aβ from its soluble form. Notably, the neuron-derived exosomes were incorporated into microglia, resulting in enhanced Aβ uptake and degradation by the microglia. Moreover, we determined that secretion of the exosomes was regulated by specific sphingolipid-metabolizing enzymes. Up-regulation of the exosome secretion, which is mediated by sphingomyelin synthase 2 (SMS2) siRNA, was sufficient for inducing the enhancement of Aβ uptake by microglia and resulted in a significant reduction of extracellular Aβ in transwell co-cultures of neuronal and microglial cells. These results imply the existence of novel machinery active in Aβ clearance mediated by exosomes.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Primary antibodies were obtained from the following suppliers: mouse monoclonal antibodies against Alix, BiP, GM130 (BD Biosciences); Aβ (Signet, Dedham, MA); rabbit polyclonal antibodies against Tsg-101 (Santa Cruz Biotechnology, Santa Cruz, CA); and Aβ oligomer (Invitrogen). Secondary antibodies were from GE Healthcare. Thioflavin T (ThT), cholera toxin B subunit (CTB), HRP-conjugated CTB, annexin V (AV), imipramine, GW4869, D609, bacterial SMase (Staphylococcus aureus), and endoglycoceramidase (EGCase) II with activator II were obtained from Sigma. AlexaFluor594-conjugated CTB, AlexaFluor488-conjugated AV, and LysoTracker Green DND-26 and Blue DND-22 were purchased from Invitrogen. N-Hexanoyl-d-erythrosphingosine (d18:1/6:0) was from Avanti Polar Lipids (Alabaster, AL). The synthetic Aβ peptides human Aβ(1–40) (Aβ40) and Aβ(1–42) (Aβ42) from Peptide Institute (Osaka, Japan) and FAM-conjugated human Aβ42 from AnaSpec (Fremont, CA) were used in this study.
Cell Cultures
Neuro2a mouse neuroblastoma cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum. The murine microglial cell line BV-2 was purchased from National Cancer Institute (Istituto Nazionale per la Ricerca sul Cancro, Genova, Italy) and cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum and l-glutamine.
Primary cultures of neurons were prepared from the cerebral cortices of mouse brains on embryonic day 15, according to the methods of Levi et al. (10). Briefly, neurons were prepared from the isolated cerebral cortices using a dissociation solution (Sumitomo Bakelite, Tokyo, Japan). The cells were plated onto a polyethyleneimine (PEI)-coated dish at a density of 5.0 × 105 cells/cm2 and cultured in neurobasal medium (Invitrogen) with 25 mm KCl, 2 mm glutamine, and B27 supplement (Invitrogen). After plating, the cells were cultured for 7 days and then used for assays. Primary microglia prepared from a newborn rat were purchased from Sumitomo Bakelite and maintained in a microglial culture medium (Sumitomo Bakelite), according to the manufacturer's protocol.
Exosome Isolation
Exosomes were prepared from culture supernatants of N2a cells and mouse primary cortical neurons as described previously (11). One day before preparation of exosomes, cell culture medium was replaced with serum-free medium. Cell culture supernatants were collected after 24 h and sequentially centrifuged at 3,000 × g for 10 min, 4,000 × g for 10 min, and 10,000 × g for 30 min to remove cells, dead cells, and debris and then spun again at 100,000 × g for 1 h to obtain exosomes as pellets.
For sucrose gradient analysis, each exosome pellet (correspond to the amount from 5 × 107 cells) was loaded onto 10 ml of sucrose gradient (0.25–2.3 m sucrose in 20 mm HEPES, 10 ml) and centrifuged at 100,000 × g for 18 h. After centrifugation, 1-ml fractions were collected, diluted with 20 mm HEPES, and precipitated by centrifugation for 1 h at 100,000 × g. The resulting pellets were resuspended in PBS and subjected to Western blot analysis.
Electron Microscopy
The 100,000 × g pellets purified from the culture supernatants of N2a cells and primary cortical neurons were resuspended in TBS and applied to a grid covered with collodion and then negatively stained with 2% phosphotungstic acid (Nisshin EM, Tokyo, Japan). Microphotographs were obtained using an HD-2000 scanning transmission electron microscope (Hitachi, Tokyo, Japan).
Seed-free Aβ Preparation
Seed-free Aβ solutions were prepared essentially according to a published report (12). Briefly, synthetic Aβ40 and Aβ42 were dissolved in 0.02% ammonia solution at 500 and 300 μm, respectively. To remove undissolved Aβ aggregates, which can act as pre-existing seeds, the prepared solutions were centrifuged at 540,000 × g for 3 h at 4 °C. The obtained supernatants were collected and stored at −80 °C until use.
EGCase Treatment and CTB Binding
N2a-derived exosome pellets were resuspended in PBS containing 20 mm HEPES (pH 7.4) and treated with 25 milliunits of EGCase for 15 h or 1 μm CTB for 1 h at 37 °C. Then the exosomes were precipitated again by ultracentrifugation at 100,000 × g for 1 h and used for ThT assay.
Thioflavin T Assay
Seed-free Aβ solutions (25 μm) were incubated at 37 °C in 100 μl of TBS containing 0, 1, or 10 μl of exosome solutions. One microliter of exosome solution was collected from culture supernatants of 1 × 106 cells. Fluorescence intensities of ThT in the mixtures were determined as described elsewhere (12), using an Appliskan spectrofluorophotometer (Thermo Fisher Scientific, Waltham, MA). The optimum fluorescence intensities of amyloid fibrils were measured at excitation and emission wavelengths of 446 and 490 nm, respectively, with the reaction mixtures containing 5 μm ThT in 50 mm glycine/NaOH at pH 8.5.
Dot Blot Analysis
Seed-free solutions of Aβ42 (25 μm) were incubated in 100 μl of TBS with or without exosomes (100,000 × g pellets) for various times at 37 °C. The mixtures were then dotted onto a nitrocellulose membrane, and the membrane was incubated with primary antibodies against Aβ oligomer (A11) and Aβ (6E10) and then with HRP-conjugated secondary antibodies. Chemiluminescence was detected and analyzed using a combination of an ECL Plus Kit (GE Healthcare) and a LAS4000 imager (Fuji Film, Tokyo, Japan).
Toxicity Assay
Seed-free Aβ solutions (25 μm) were incubated with or without exosomes for 5 h at 37 °C in 100 μl of neurobasal medium supplemented with 25 mm KCl, 2 mm glutamine, and B27 supplement. The preincubated mixtures were applied to the primary cortical neurons that had been plated on 24-well plates and incubated for 24 h. The cell viabilities were determined using WST-1 cell viability assay kit (Dojindo, Kumamoto, Japan) and LIVE/DEAD® cell viability kit (Invitrogen).
Drug Treatment
The treatment with imipramine (10 μm), GW4869 (10 μm), D609 (50 μm), N-hexanoyl-d-erythrosphingosine (50 μm), or bacterial SMase (100 microunits/ ml) was performed for 24 h in serum-free medium.
siRNA Delivery and Transfection
For RNA-mediated interference (RNAi) experiments, we used Stealth RNAiTM siRNA (Invitrogen) carrying the following sequences: 5′-AUACAUUGUAAUACACCGAUACAGG-3′ (sense) and 5′-CCUGUAUCGGUGUAUUACAAUGUAU-3′ (antisense) for SMS1; 5′-AUACAUAGUUAUACAGCGAUACAGG-3′ (sense) and 5′-CCUGUAUCGCUGUAUAACUAUGUAU-3′ (antisense) for SMS2; 5′-AUUGGUUUCCCUUUAUGAAGGGAGG-3′ (sense) and 5′-CCUCCCUUCAUAAAGGGAAACCAAU-3′ (antisense) for aSMase; 5′-AAUAGAACCACAUCUGCAUUCUUGG-3′ (sense) and 5′-CCAAGAAUGCAGAUGUGGUUCUAUU-3′ (antisense) for nSMase1; and 5′-AAUCGAUGUAGAUCUUGAUCUGAGG-3′ (sense) and 5′-CCUCAGAUCAAGAUCUACAUCGAUU-3′ (antisense) for nSMase2. StealthTM control RNA was obtained from Invitrogen. siRNA was delivered with LipofectamineTM transfection reagent (Invitrogen) according to the manufacturer's protocol.
The cDNA of human amyloid precursor protein (APP)770 was amplified from human brain cDNA (Clontech) by PCR with the selected primers as follows: 5′-ATGCTGCCCGGTTTGG-3′ and 5′-CTAGTTCTGCATCTGCTCAAAGAACTTG-3′. The cDNA was then cloned to a pENTRTMD-TOPO vector (Invitrogen) to finally construct p3×FLAG-APP770 using the Gateway® recombination system as described previously (13). Transient transfection was performed using a Lipofectamine2000 kit (Invitrogen) according to the manufacturer's protocol.
Analysis of Exosome Release
Exosome pellets purified from cell cultures (5 × 106 cells) were solubilized with Laemmli buffer (14) and subjected to SDS-PAGE and Western blotting. Bands were detected and analyzed using a combination of an ECL Plus kit (GE Healthcare) and a LAS4000 imager (Fuji Film).
Fluorescence Staining and Internalization Assay
Exosomes were fluorescent-stained with red dye PKH26 (Sigma) according to the manufacturer's protocol (15). Briefly, exosomes (100,000 × g pellet) were resuspended in diluent C (Sigma) and stained with PKH26 for 5 min, and the reaction was then stopped with 1% bovine serum albumin. The labeled exosomes were precipitated again by ultracentrifugation at 100,000 × g for 1 h.
The PKH26-labeled exosomes were administered to BV-2 cells on chamber slides (Thermo Fischer) several times in serum-free conditions. For inhibition experiments, the labeled exosomes were pretreated with AV or CTB (0, 0.5, or 1 μm) at 37 °C for 15 min. For detection of Aβ transfer into BV-2 cells by exosomes, the labeled exosomes were preincubated for 5 h at 37 °C with 25 μm of a fluorescence (FAM)-labeled Aβ42. The cells were then fixed with 4% paraformaldehyde, and confocal images were acquired using an Olympus Fluoview FV10i microscope. The fluorescence intensity was analyzed with ImageJ software.
Aβ Measurement
Aβ40 and Aβ42 levels in medium and cells were determined using a sandwich enzyme-linked immunosorbent assay (ELISA) kit from Wako. Aggregated Aβs both in medium and cells were solubilized with 4 m guanidine-HCl buffer for 2 h at room temperature and then were analyzed by ELISA. All samples were handled in duplicate.
Transwell Study
N2a cells were cultured on 24-well plate inserts (inner diameter 0.5 μm pore, Corning, NY) at 5 × 105 cells/cm2 and then transfected with the APP770 plasmid and siRNA for nSMase2 or SMS2, using a Lipofectamine2000 kit. After 24 h, inserts were placed onto wells containing BV-2 cells. After an additional 24 h of incubation, the levels of Aβs in the medium were determined by ELISA. Intracellular Aβ levels in BV-2 cells were also measured using ELISA following solubilization in guanidine HCl buffer, as described above.
RESULTS
Neuron-derived Exosomes Drive Aβ to Form Amyloid Fibrils
Culture medium from N2a cells or primary cortical neurons was subjected to successive centrifugation steps with increasing centrifugal forces, eventually providing a 100,000 × g pellet. Electron microscopy analysis revealed that the pellet, collected from the N2a cultures, mainly consisted of small membrane vesicles of ∼40–100 nm diameter (Fig. 1B), similar to previously described exosome preparations (16). The pellet was separated by a continuous sucrose density gradient, and the exosomal proteins, Alix and Tsg101, were detected in fractions 4 and 5 (corresponding to a sucrose density of 1.12 and 1.16 g/ml, Fig. 1A), similar to reports from others (6). Exosomes are reportedly enriched in the proteins and lipids, associated with lipid microdomains (17). The ganglioside GM1, a glycosphingolipid abundant in lipid microdomains, was also detected in high concentrations in the same fractions as Alix and Tsg101. In contrast, BiP and GM130, marker proteins for endoplasmic reticulum and for Golgi respectively, were not found in the 100,000 × g pellet. The pellets collected from the primary neuronal cultures also contained membrane vesicles of similar size and densities, and bearing Alix, Tsg101, and GM1 (data not shown). These data confirm that the 100,000 × g pellet mainly consists of exosomes and demonstrate that the exosomes are secreted from N2a and primary cortical neurons in a constitutive manner.
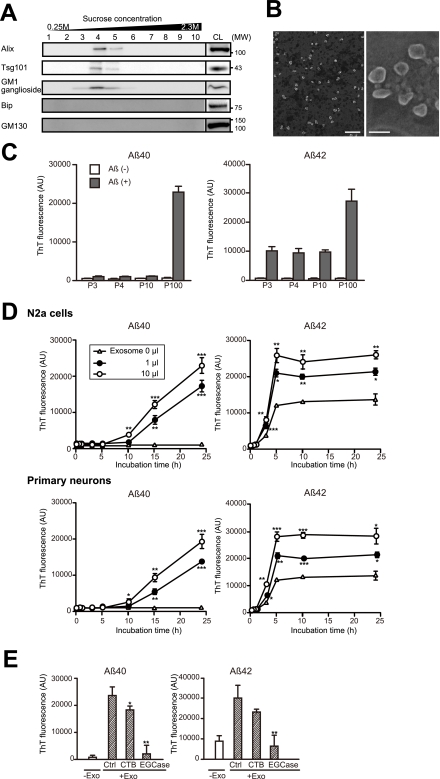
FIGURE 1.
Aβ amyloidogenesis by neuronal exosomes. A, exosomes were collected from the culture supernatant of Neuroblastoma N2a cells, by sequential centrifugation as indicated under “Experimental Procedures.” The 100,000 × g pellets were further subjected to sucrose gradient centrifugation, and the resulting fractions were analyzed for the exosomal proteins Alix and Tsg101 and for the GM1 ganglioside. B, purified exosomes (100,000 × g pellet) underwent negative staining with phosphotungstic acid and were examined by electron microscopy. Scale bars, right panel, 500 nm; left panel, 100 nm. C, culture medium from N2a cells was subjected to sequential centrifugation. The resulting pellets, 3,000 × g (P3), 4,000 × g (P4), 10,000 × g (P10), and 100,000 × g (P100), were mixed with soluble 25 μm seed-free soluble Aβ40 or Aβ42 and incubated for 24 h at 37 °C. Amyloid fibrils formed in the incubation mixtures were measured with a ThT assay. The indicated values for relative fluorescence (AU) are means ± S.E. D, after the indicated time of incubation, ThT fluorescence intensities were measured in mixtures containing 25 μm Aβ and the indicated amount of exosomes derived from the culture supernatant of N2a cells or cortical neurons. Values provided as the means ± S.E. are as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001; t test. E, after CTB or EGCase treatment, the exosomes (Exo) were mixed with 25 μm Aβ and incubated at 37 °C for 5 h. Ctrl, control. Values are the means ± S.E. *, p < 0.05; **, p < 0.01; t test.
To examine the effect of neuron-derived exosomes on Aβ conformational transition, we mixed the resulting pellets of the sequential centrifugations (P3, P4, P10, and P100) with soluble Aβ40 and Aβ42, two major species of Aβ, and incubated the mixture at 37 °C for 24 h. The amount of amyloid fibrils was then determined using ThT. As a result, ThT fluorescence intensities were significantly enhanced by only in the presence of P100, the exosome fraction (Fig. 1C). Both N2a- and primary neuron-derived exosomes significantly accelerated the fibril formations of Aβ40 and Aβ42 in a time-dependent fashion (Fig. 1D). The fluorescence of ThT follows a characteristic sigmoidal curve when Aβ was incubated at 37 °C (12). As Aβ42 has higher aggregate-prone property, Aβ42 reached a plateau more rapidly than Aβ40. In Aβ42, the amounts of amyloid Aβ significantly increased after reaching a plateau phase.
Accumulated lines of evidence indicate that glycosphingolipids (GSLs), especially gangliosides, serve as a template for Aβ assembly (18, 19). In addition, it has been found that the Aβ-bound ganglioside GM1 has been found in brains exhibiting early pathological changes of AD (20). We therefore examined whether GSLs in the exosome membranes are involved in acceleration of Aβ fibrillogenesis by the exosomes. Blocking of ganglioside GM1 by CTB partially but clearly prevented Aβ fibrillization, induced by N2a-derived exosomes (Fig. 1E). Moreover, glycan cleavage by EGCase treatment almost entirely inhibited the fibril formation. These findings suggest that the sugar chains of GSLs have a role to induce Aβ fibril formation on the surface of the exosomes.
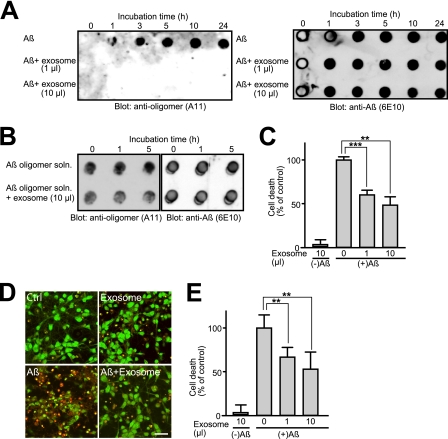
To further investigate the effect of exosomes on the formation of oligomeric Aβ, the mixtures of Aβ with or without N2a-derived exosomes were analyzed by dot blotting with the anti-oligomer antibody A11 (Fig. 2A). In the absence of exosomes, oligomeric Aβ was formed just after 1 h of incubation at 37 °C. In the presence of exosomes, however, oligomeric Aβ was not detected in the incubation mixtures during 24 h of incubation. In contrast, the exosomes could not change the amount of A11-positive oligomers, which had been already formed (Fig. 2B). These suggest that the exosomes might prevent oligomeric formation of Aβ from forming. Accumulated lines of evidence indicate that neurodegeneration and synaptic impairment in AD pathogenesis are directly caused by soluble Aβ oligomers (21, 22). Indeed, the Aβ solution that had been preincubated at 37 °C for 5 h without exosomes induced remarkable cell death in the primary neurons (Fig. 2, C–E). In contrast, addition of exosomes to the incubation mixtures dramatically prevented neuronal death. These findings suggest that neuronal exosomes facilitate rapid conformational transition of Aβ into nontoxic amyloid fibrils on its surface, resulting in a decline in the natural formation of toxic oligomeric species.
FIGURE 2.
Effects of exosomes on oligomerization and toxicity of Aβ. A, purified N2a-derived exosomes were mixed with soluble 25 μm Aβ42 and incubated at 37 °C for the indicated times. The incubation mixtures were subjected to dot blot analysis using anti-oligomer (A11) and anti-Aβ (6E10) antibodies. B, 25 μm Aβ42 was incubated at 37 °C for 5 h to form oligomeric Aβ. N2a-derived exosomes were added to the solution, which contained the oligomeric Aβ, and further incubated for the indicated times at 37 °C. The incubation mixtures were subjected to dot blotting. C–E, Aβ42 (25 μm) was incubated with or without N2a-derived exosomes at 37 °C for 5 h. The incubation mixtures were subsequently added to cortical neurons, and after 24 h, the cell viabilities were determined using a WST-1 assay (C) or LIVE/DEAD viability kit (D and E). Neurons were stained with SYTO10/RED DEADTM, showing green staining for all cells and red staining for dead cells. Scale bar, 20 μm. Data are represented as the means ± S.E. **, p < 0.01; ***, p < 0.001; t test.
Sphingolipid Metabolism Is Involved in the Exosome Secretion and Aβ Fibril Formation
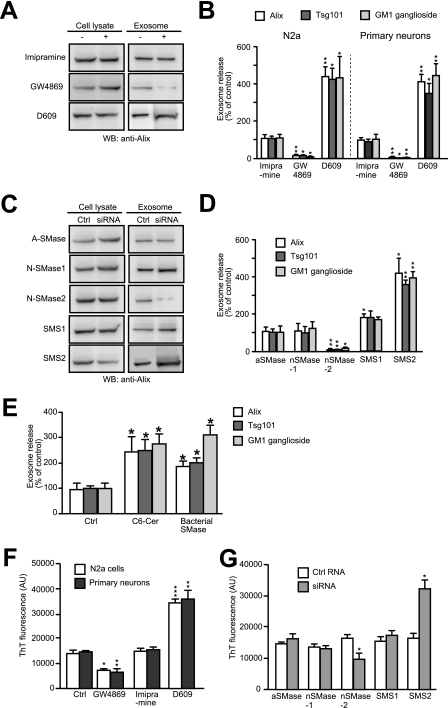
Exosomes originate from the budding of intraluminal vesicles into multivesicular endosomes. Trajkovic et al. (23) reported that sphingolipid ceramide triggers the intraluminal budding and leads to exosome release in oligodendrocytes. To investigate whether the secretion of the neuron-derived exosome can be modulated by sphingolipid metabolism, we first treated N2a cells and primary neurons with inhibitors for sphingolipid-metabolizing enzymes. The levels of released exosomes were determined by evaluating the amounts of the exosomal markers, Alix, Tsg101, and GM1, in the 100,000 × g pellets. GW4869 is an inhibitor of neutral sphingomyelinase (nSMase), a neutral pH-active form of the enzyme SMase, which converts sphingomyelin to ceramide (Cer). Treatment of either cell with GW4869 significantly decreased the levels of released exosomes (Fig. 3, A and B), consistent with the study of Trajkovic et al. (23). In contrast, the treatment with imipramine, which selectively inhibits acid sphingomyelinase (aSMase), did not affect the exosome release. D609 has been reported to inhibit sphingomyelin synthase (24), which catalyzes the conversion of Cer into SM, i.e. the opposite of SMase. Predictably, treatment with D609 significantly enhanced exosome secretion.
FIGURE 3.
Effect of sphingolipid metabolism on exosome secretion and Aβ amyloidogenesis. A and B, N2a cells or cortical neurons were treated with imipramine, GW4869, D609, or their respective diluent for 24 h. Exosomes were then collected from the medium of each culture (5 × 106 cells) and were subjected to SDS-PAGE, followed by Western blotting (WB) to detect Alix, Tsg101, and GM1 ganglioside. A, representative blots illustrating the amount of Alix in N2a cell lysates (2.5 × 105 cells) and 100,000 × g pellets (Exosome). B, quantification of staining in Western blots. Results shown are the means ± S.E. from two independent experiments (n = 4). *, p < 0.05; **, p < 0.01; t test. C and D, small interfering RNAs (siRNA) active against aSMase, nSMase1, nSMase2, SMS1, and SMS2 were delivered into N2a cells. Exosomes were purified from the media of the siRNA-treated cells as in A, and the amounts of exosome markers in the resulting pellets were determined by Western blotting. C, Alix was detected in the cell lysates and in the exosomes as in A. D, band intensities of exosomal markers were analyzed. Data are presented as the means ± S.E. from two independent experiments (n = 4). *, p < 0.05; **, p < 0.01; t test. E, N2a cells were treated with N-hexanoyl-d-erythrosphingosine (50 μm) or bacterial SMase (100 microunits/ ml) for 24 h. The level of released exosomes were evaluated by Western blotting. Results are expressed as means ± S.E. (n = 3). *, p < 0.05; t test. F, exosomes isolated from the cultures of N2a cells or primary neurons, that had been treated with the indicated inhibitors, were incubated at 37 °C with 25 μm soluble Aβ42. Aβ amyloid fibrils formed in the mixtures were measured by ThT assay. The indicated values for relative fluorescence (AU) are means ± S.E. from two independent experiments (n = 4). *, p < 0.05; **, p < 0.01; ***, p < 0.001; t test. G, exosomes isolated from the cultures of N2a cells that had been treated with the indicated siRNA were mixed with 25 μm Aβ42. After 5 h of incubation, ThT fluorescence was measured. Data are represented as the mean ± S.E. (n = 4). *, p < 0.05; t test. Ctrl, control.
To further explore the role of sphingolipid metabolism in exosome secretion, we employed an RNA interference approach to knock down the expression of endogenous SMases and SMSs in N2a cells. We employed siRNAs against SMS1, SMS2, aSMase, nSMase1, and nSMase2, which reduced expression of the target genes efficiently in N2a cells (∼85% reduction in cells transfected with each siRNA). Again in agreement with the findings of Trajkovic et al. (23), treatment with siRNA for nSMase2 reduced exosome release from N2a cells (Fig. 3, C and D). However, following treatment with siRNA against aSMase or nSMase1, no similar reduction was observed. Conversely, knockdown of either SMS1 or SMS2 with siRNA induced significant increases in exosome secretion. Increases were especially remarkable following SMS2 knockdown compared with SMS1 knockdown (Fig. 3D). These results indicate that Cer production affects the exosome secretion, and modulations of nSMase2 and SMS can alter the levels of released exosomes from N2a cells. Indeed, exogenously added Cer and Cer production following exogenously added SMase significantly increased the release levels of the exosomes (Fig. 3E).
Next, we examined the role of sphingolipid metabolism on exosome-mediated Aβ fibrillogenesis. As described above, we collected exosomes from the culture supernatants of the cells that had been treated with inhibitors or siRNAs, and we measured the ThT fluorescence in the mixtures of the exosomes and Aβ42 after a 5-h incubation at 37 °C. When exosomes purified from GW4869- or nSMase2 siRNA-treated cultures were included in the mixture, Aβ fibrillogenesis was significantly reduced (Fig. 3, F and G). Conversely, in the presence of exosomes from D609- or SMS2 siRNA-treated cultures, amyloid formation was increased, compared with that in controls. From these combined data, we consider that the potential of the exosomes to modulate Aβ fibril formation is closely related to their relative amount. In addition, the release of exosomes can be modulated by activities of enzymes responsible for sphingolipid synthesis.
Microglia Engulf Exosomes in a Phosphatidylserine (PS)-dependent Manner
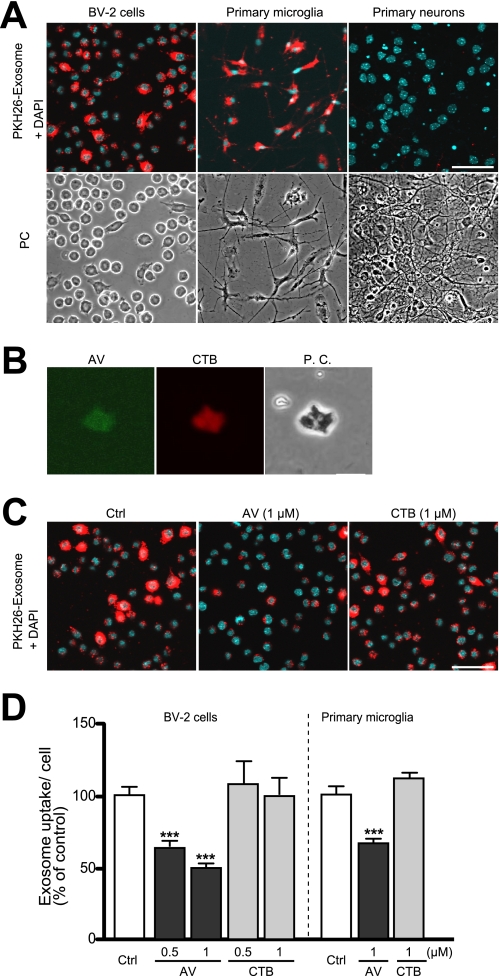
Microglia are the resident phagocytes in the central nervous system. It is now widely accepted that these cells are derived from macrophages and contribute to the removal of dead cells and debris in the brain (25). Several reports have revealed that macrophages also take up exosomes secreted from several different cell types to transduce inflammatory signals or its elimination (26). Recently, Fitzner et al. (27) reported that oligodendrocyte-derived exosomes are preferentially internalized by microglia in brain. To evaluate whether microglia also engulf neuronal exosomes, we applied N2a-derived exosomes labeled with the fluorescent dye PKH26 to BV-2 microglial cells, primary microglia, or primary cortical neurons. After a 3-h incubation with the labeled exosomes at 37 °C, the cells were fixed, stained with DAPI, and analyzed by confocal microscopy. We observed significant fluorescence in both BV2 cells and primary microglia (Fig. 4A). These results suggest that exosomes are efficiently internalized into microglia. In contrast, fluorescent signals were rarely detected in primary neurons, further demonstrating the selective transfer of neuron-derived exosomes into microglia.
FIGURE 4.
Transfer of exosomes into microglia. A, exosomes purified from N2a cell cultures were labeled with the dye PKH26 (red) and added to BV-2 microglial cells or primary cultures of microglia or cortical neurons. After 3 h of incubation, cells were fixed, stained with DAPI, and analyzed by confocal microscopy. B, N2a-derived exosomes were bound to AlexaFluor-conjugated AV or CTB to detect surface-exposed PS and GM1 ganglioside (GM1), respectively. Fluorescence labeling was visualized by confocal microscopy. The far right panel shows the same field in phase contrast (PC). Scale bar, 200 nm. C and D, exosomes collected from N2a cultures were labeled with the red dye PKH26 and subsequently treated with nothing, AV, or CTB. Labeled exosomes were applied to BV-2 cells and incubated for 3 h. Cells were subsequently fixed and stained with DAPI. C, confocal images of internalized exosomes are shown. D, fluorescence intensities per cell were determined by image analysis. Exosome internalization was quantified from three independent experiments. Values are means ± S.E. ***, p < 0.001; t test. Ctrl, control.
Various cells produce exosomes expressing PS on their surface, and PS exposed on the outer leaflet of the plasma membrane of apoptotic cells is often used as a recognition signal by macrophages and microglia (15, 28). We stained N2a-derived 100,000 × g pellets with fluorescently labeled AV or CTB subunit, which specifically recognizes PS and GM1, respectively. Significant fluorescence corresponding to both AV and CTB was observed (Fig. 4B), suggesting PS was located on the outer leaflet of N2a-derived exosomes. To examine the mechanism for exosome uptake by microglia, we exposed exosomes preincubated with AV or CTB to microglial cultures. We found that treatment with AV significantly suppressed uptake of exosomes into BV-2 cells or primary microglia, although treatment with CTB had no effect (Fig. 4, C and D). These results suggest that PS facilitate for the recognition and internalization of neuronal exosomes by microglia.
Exosomes Facilitate Aβ Clearance by Microglia
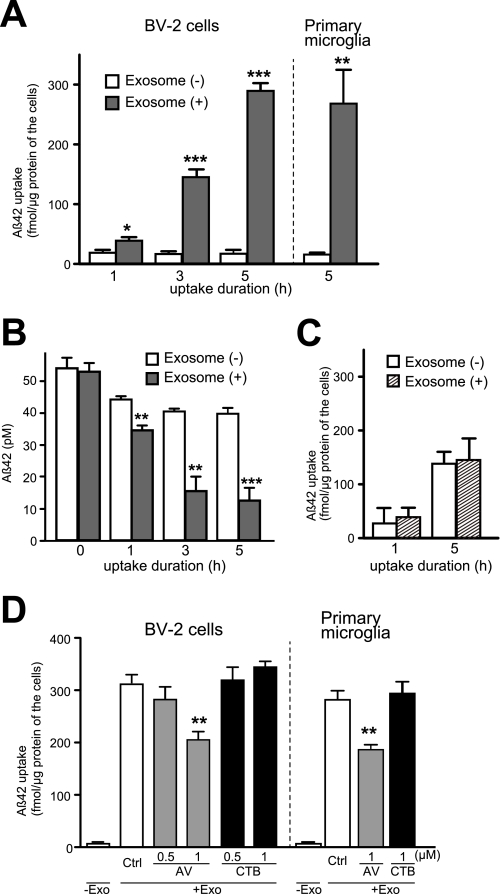
The interaction between the exosomes and Aβ leads to accelerated Aβ fibril formation (Fig. 1, C and D), suggesting that Aβ amyloid fibrils accumulate surrounding exosomes. Indeed, the exosomal marker Alix was observed to be concentrated in the senile plaque, an extracellular deposition of Aβ fibrils, found in AD brain (5). We also confirmed that exosomes are internalized by microglia (Fig. 4A). Based on these findings, we hypothesized that the exosomes may have roles in the uptake of Aβ amyloid by microglia, aiding Aβ degradation. To test this hypothesis, we added Aβ42, preincubated with or without exosomes, to BV-2 cells or primary microglia. After incubating at 37 °C for up to 5 h, we determined the intra- and extracellular levels of Aβ42. Both BV-2 and primary microglia internalized the Aβ much more dramatically in the presence of exosomes than without (Fig. 5A). Correspondingly, the levels of Aβ in the medium gradually decreased with significant difference between the presence and absence of exosomes (Fig. 5B)m 76.5 ± 2.6 and 26.6 ± 5.4% reduction of Aβ in the presence and absence of exosomes after 5 h of incubation, respectively. In contrast, the exosome could not affect the uptake of the amyloid fibrils, which had been already formed (Fig. 5C). To further examine whether the prevention of exosome uptake might affect Aβ incorporation by microglia, we blocked PS on the outer surface of exosomes by AV. As shown in Fig. 5D, the Aβ uptake was significantly suppressed when exosomes had been preincubated with AV and not when preincubated with CTB. These results suggest that exosomes can, at least partially, mediate Aβ uptake in a PS-dependent manner.
FIGURE 5.
Acceleration of Aβ uptake into microglia by exosome. A and B, N2a-derived exosomes were incubated with 25 μm Aβ42 at 37 °C for 5 h. The preincubated mixtures were then added to BV-2 cells or primary microglia (final concentration of Aβ, 0.5 μm) and further incubated for the indicated times. Levels of Aβ42 in BV-2 cells (A) and in conditioned media (B) were quantified by ELISA. Values are means ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001; t test. C, 50 μm Aβ42 was incubated at 37 °C for 5 days to form amyloid fibrils. The fibrils were incubated with or without N2a-derived exosomes at 37 °C for 5 h. The incubation mixtures were added to BV-2 cells (final concentration of Aβ, 0.5 μm) and incubated for the additional times. The levels of intracellular Aβ in BV-2 cells were measured by ELISA. Values are means ± S.E. D, N2a-derived exosomes (Exo) were incubated with 25 μm Aβ42 at 37 °C for 5 h, then with AV or CTB for an additional 15 min at 37 °C. The mixtures were applied to BV-2 cells or primary microglia and incubated for 3 h. The intracellular levels of Aβ were measured using ELISA. Values represent as the mean ± S.E. **, p < 0.01; t test.
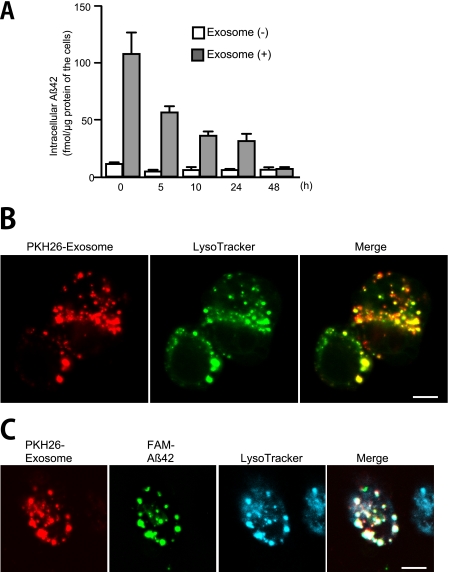
Next, to assess whether the Aβ internalized together with exosomes is degraded in microglia, we administered Aβ42, preincubated with or without the exosomes, to BV-2 cells, incubated them for 3 h, and then washed the cells. After additional culture time, cells were harvested, and the Aβ levels in the cell lysates were determined. The intracellular Aβ levels in the BV-2 cells gradually decreased in a time-dependent manner and were nearly depleted by 48 h (Fig. 6A). To gain insight into the degradation pathway of the internalized exosomes and Aβ, we investigated their localization by staining with LysoTracker, a fluorescence marker of late endosome/lysosomes. We incubated PKH26-labeled, N2a-derived exosomes with BV-2 cells for 3 h at 37 °C and examined the cells by fluorescence microscopy. Punctate fluorescence was observed in the cells and portions of the exosome fluorescence co-localized with the lysosomal compartments (Fig. 6B). We next applied a preincubated mixture of FAM-Aβ42 and labeled exosomes to BV-2 cells. Together with the exosome fluorescence, the signal corresponding to Aβ also co-localized with the LysoTracker signal (Fig. 6C). These data demonstrated that the Aβ internalized in an exosome-mediated manner was delivered to lysosomes within BV-2 cells to be degraded via the endocytic pathway.
FIGURE 6.
Degradation of Aβ in microglia. A, Aβ42 (25 μm) was incubated in the presence or absence of N2a-derived exosomes at 37 °C for 5 h. The incubation mixtures were subsequently administered to BV-2 cells (final concentration of Aβ, 0.5 μm) for 3 h. After removal of free Aβ and exosomes by washing in medium, the cells were further cultured for up to 48 h. Intracellular levels of Aβ42 were determined at the indicated times by ELISA. B, exosomes were labeled with PKH26 (red) and added to cultures of BV-2 cells. After a 3-h incubation, cells were stained with LysoTracker Green and analyzed by confocal microscopy. Scale bar, 5 μm. C, PKH26-labeled exosomes were mixed with the fluorescent FAM-coupled Aβ42 (25 μm). After a 5-h incubation, the mixtures were administered to cultures of BV-2 cells for 3 h and stained with LysoTracker Blue. Scale bar, 5 μm.
Does Up-regulation of Exosome Secretion Affect Aβ Clearance?
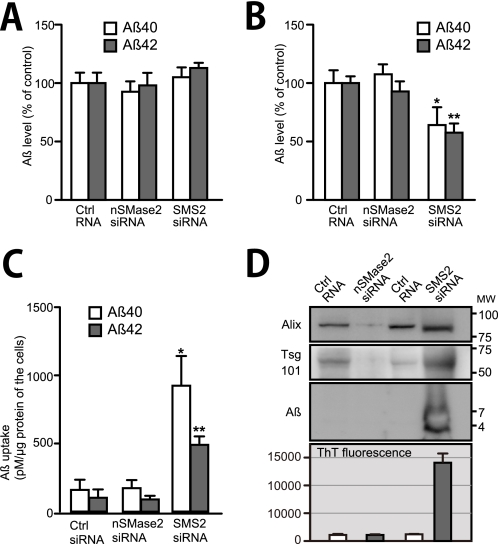
Finally, we investigated whether modulating exosome secretion can affect Aβ clearance by microglia. We plated N2a cells on transwell inserts and treated with siRNA for SMase2 or SMS2 to modulate the amount of exosomes released from the cells. We also concurrently transfected with APP to overexpress Aβ. Twenty four hours after the transfection, we set the inserts into the 24-well multiplates, on which BV-2 cells had been seeded. Under this experimental setting, we presumed that exosomes and Aβ, secreted from N2a cells, would be able to interact with the BV-2 cells through medium shared between the cells. After 24 h of co-incubation, the levels of Aβ in the medium were determined. RT-PCR showed that either nSMase2 or SMS2 siRNA efficiently decreased its target mRNA expression in N2a-APP cells (81 ± 2.7 and 86 ± 4.3% reduction in cells with nSMase2 and SMS2 siRNA treatment, respectively). When there were no BV-2 cells in the lower wells, the level of extracellular Aβ remained unchanged even in N2a-APP cells that were treated with nSMase2 or SMS2 siRNA (Fig. 7A). Several studies have been reported that sphingolipid metabolism is involved in the APP processing for generating Aβ (29). However, the knockdown of nSMase2 or SMS2 with siRNA did not affect the levels of Aβ secreted from N2a cells. In contrast, in the presence of BV-2 cells on the lower wells, the levels of both Aβ40 and Aβ42 in the culture media were significantly decreased following SMS2 siRNA treatment (Fig. 7B). In addition, the Aβ levels in the BV-2 cells were significantly increased in the SMS2 siRNA-treated cultures compared with control RNA treatment (Fig. 7C).
FIGURE 7.
Enhancement of Aβ clearance by SMS2 knockdown. A–C, N2a cells seeded in inserts were transfected with the APP770 and siRNA as indicated. After 24 h, the media were removed and the inserts with the N2a cells were placed into wells with (B) or without (A) BV-2 cells and cultured for another 24 h. The levels of Aβ in the medium (A and B) and in the BV-2 cells (C) were measured by ELISA. Values are means ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001; t test. D, N2a cells were transfected with APP and siRNA for N-SMase2 or SMS2 for 24 h. The media were changed and the cells incubated for an additional 24 h. The exosomes were then collected from the medium of each culture (5 × 106 cells) and were solubilized with guanidine HCl buffer to be analyzed by Western blot or resuspended in TBS to perform ThT assay. Ctrl, control.
To examine levels of Aβ associated with exosomes in siRNA-treated N2a-APP cell cultures, we analyzed the amounts of Alix, Tsg101, and Aβ in the exosome pellets collected from culture media of the N2a-APP cells, transfected with nSMase2 or SMS2 siRNA. Consistent with the previous data (Fig. 3, C and D), the levels of released exosomes, estimated by the amounts of Alix and Tsg101, were obviously decreased or increased by nSMase2 or SMS2 knockdown, respectively (Fig. 7D). In addition, Aβ was detected in the exosomes from SMS2 siRNA-treated N2a cultures, although not in those from control or N-SMase2 siRNA-treated cultures. Furthermore, we performed ThT assay to examine whether the exosomes induce Aβ fibrillization under physiological conditions. We collected the exosomes from the medium of nSMase2 or SMS2 siRNA- or control RNA-treated N2a-APP cells. We then diluted the exosomes in TBS at 1,000-fold higher density than those in the medium and measured ThT fluorescence of the exosome solutions. The increased fluorescence of ThT was observed only in the solution of exosomes derived from the medium of SMS2 siRNA-treated cells (Fig. 7D). These findings suggest that acceleration of exosome secretion increases Aβ fibrils associated with the exosomes, enhancing Aβ uptake by microglia and eventually resulting in the reduction of extracellular Aβ.
DISCUSSION
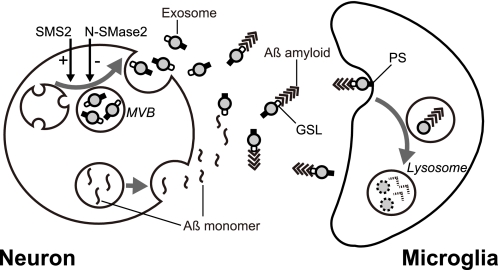
In the study presented here, we found that exosomes are constitutively secreted by neurons, and the exosomes dramatically promote Aβ amyloidogenesis. Furthermore, the assembled Aβ, in association with exosomes, was further taken up by microglia for degradation. We also demonstrated that up-regulation of exosome secretion, which was induced by SMS2 knockdown, efficiently reduced extracellular levels of Aβ in a co-culture of neuronal and microglial cells. In CNS, neurons are surrounded by microglia that survey to remove damaged structures such as apoptotic cells and obsolete synaptic connections (30). This study provides new insight into the coordinating machinery between neurons and neighboring microglia using exosomes for the clearance of Aβ (see the proposed scheme, Fig. 8).
FIGURE 8.
Schematic representation of the role of exosome in Aβ metabolism. Both exosomes and Aβs are generated and released from neurons into the extracellular space. Exosome secretion is modulated by the sphingolipid-metabolizing enzymes, N-SMase2 and SMS2, bidirectionally. Exosomes enhance Aβ amyloidogenesis by GSLs on its surface and, subsequently, incorporation of Aβ fibrils into microglia in a PS-dependent manner to degrade Aβ. Neuronal exosomes likely promote Aβ clearance.
Regarding the formation of ordered Aβ aggregates, the seeding polymerization theory was previously proposed (31). In this theory, the transition of the monomeric Aβ to its polymer requires a conformational change of Aβ to act as a seed, as might be provided by condensation or interaction with other molecules (28). We found that the neuron-derived exosomes accelerate Aβ amyloidogenesis from monomeric Aβ (Fig. 1, C and D). Furthermore, cleavage of GSL glycans by EGCase inhibited acceleration of Aβ fibrillogenesis by exosomes. These data suggest that interaction between Aβ and GSL glycans leads the conformational change of Aβ. GSL glycans are also localized at the plasma membrane of the cells. The mechanism why only GSL glycans at the exosome surface can lead Aβ assembly has remained unclear. The GSLs are well known as the components of lipid microdomains. Lipid microdomain-associated proteins are preferentially sorted into exosomes and enriched in exosome membranes (17). Furthermore, high density clustering of GM1 has been reported to promote Aβ assembly (32). Further detailed characterization of the exosome is needed; however, these findings raise a possibility that GSL accumulation and clustering at the exosome membrane might serves as a template for Aβ assembly. In a recent report, Aβ fibrils formed by GM1-containing membranes exhibited toxicity toward PC12 cells (33). However, we demonstrated that addition of an exosome/Aβ mixture to primary cortical cells significantly suppressed neuronal toxicity. This suppression was inversely correlated with the amount of oligomeric Aβ (Fig. 2), but it had no correlation with the amount of exosome-mediated Aβ fibrils (Fig. 1D). It is well known that Aβ fibrils exhibit polymorphism, which depends on the differences in the first step of amyloidogenesis (34, 35). Further examination is needed to identify the mechanism behind exosome-mediated Aβ fibrillogenesis.
In this study, we collected released exosomes and assessed their ability to facilitate Aβ amyloidogenesis in TBS (Fig. 1, C and D) or in the culture medium (data not shown). The results suggest that the exosomes would be able to efficiently promote the formation of Aβ fibrils in extracellular space. However, β-site cleavage of APP has been reported to occur in MVBs (36). In addition, Aβ42 has been found to localize predominantly to MVBs in normal mouse and human brain. In addition, in a mouse model of AD and in human AD brain, Aβ42 progressively accumulates in MVBs with age (37). Notably, GM1-bound Aβ, an amyloid seed, is preferentially observed in endosomes of neurons from aged monkey brain (38). Thus, additional careful examinations will be required to investigate the possibility that the intraluminal space of MVB might provide another cellular milieu for Aβ assembly prior to the release of exosomes and Aβ.
We found that selective inhibition of nSMase2 activity reduced the exosome secretion, but inhibiting SMS2 activity increased the secretion (Fig. 3, C and D). nSMase2 is especially abundant in mammalian brains (39). It has two putative transmembrane domains at the N terminus and is mainly localized in the plasma membrane (40). SMS2 also has predicted six membrane-spanning regions, and it contributes to sphingomyelin production at the plasma membrane (41). We also found that exogenously added synthetic Cer and bacterial SMase increase exosome secretion (Fig. 3E). SMS2 apparently contributes more to exosome secretion than does SMS1, which is actually responsible for the bulk of sphingomyelin generation (Fig. 3, C and D) but is localized in the Golgi apparatus (42). Altogether, this information suggests that the elevations in the local levels of Cer, especially at the plasma membranes, including endocytosed membrane regions, would be important for the promotion of exosome generation.
Our results here support a role for Cer in exosome generation. One possible mechanism would be Cer inducing a physical alternation in the endosomal membrane that preferentially promotes the budding of intraluminal vesicles. Indeed, it has been reported that Cer can induce a coalescence of small microdomains into lager microdomains, thereby promoting domain-induced budding of plasma membranes (43). Treatment with bacterial SMase is known to induce intraluminal membrane budding from SM-containing synthetic giant liposomes (23). Alternatively, several lines of evidence suggest that Cer can also affect endocytic transport. Cer production, induced by exocytosis of aSMase, reportedly promotes endocytic transport (44). Exogenously added bacterial SMase is also known to induce ATP-independent endocytosis (45). These studies suggest another possibility that Cer might promote exosome generation by enhancing the rate of endocytosis.
Previous studies have shown that microglia can directly take up Aβ itself and degrade it in lysosomal compartments (46). Thus, the functional significance of Aβ incorporation together with exosomes remains unclear. One conceivable purpose would be to increase the efficiency of the Aβ uptake. Aβ rapidly forms amyloid fibrils in the presence of exosomes (Fig. 1, C and D), and microglia can take up Aβ more promptly after the excessive production of Aβ, in the presence of exosomes (Fig. 5). Furthermore, addition of exosomes suppressed the formation of toxic oligomers (Fig. 2); this would be highly effective for avoiding impairment of neurons. Another conceivable benefit of microglia incorporating Aβ with exosomes would be a decrease in immunological reactions by the microglia. Accumulated evidence indicates that fibril Aβ facilitates inflammatory responses in microglia, including the release of proinflammatory cytokines and reactive oxygen species (47). Activated microglia surrounding senile plaque, an excessive deposition of Aβ fibrils, contribute to chronic inflammation in the AD brain. In general, it is well understood that PS-dependent ingestion of apoptotic bodies by microglia is associated with anti-inflammatory reactions (48). In addition, it has recently been shown that oligodendrocyte-derived exosomes are taken up by microglia in an immunologically silent manner (27). This study also found that the exosome internalization was preferentially associated with inflammatory unresponsive microglia, which presented with low levels of MHCII. Moreover, we found that mRNA expression of IL-1β and TNF-α did not change in BV-2 cells after N2a-derived exosome uptake (data not shown). Further studies are needed; however, these data suggest that the neuron-derived exosomes can aide in clearing Aβ by preventing proinflammatory reactions by microglia.
Other aggregate-prone proteins, including α-synuclein and prion protein, which cause Parkinson and Creutzfeldt-Jakob diseases, respectively, are also associated with neuronal exosomes (49, 50). A challenging subject of studies in the future will be determining whether exosomes are involved in the assembly of these proteins and in their clearance. We presume that when uptake/clearance activity of microglia decreases, secretion of exosomes bearing these proteins might provoke the pathological events, which substantially occur in the extracellular space. Indeed, in the absence of exosome-removing cells, exosomes associating with both normal and abnormally folded species of prion proteins are infectious, resulting in their spreading between neuronal cells (51, 52). Furthermore, secreted α-synuclein in association with exosomes causes cell death of recipient neuronal cells (50). Aβ plaques might also be pathological structures built under lack of glial activity for removing exosomes. Actually, decreased numbers of microglia in a mouse model of AD result in increased Aβ deposition (53).
Improvement of Aβ clearance is a potent strategy for AD therapy (3). This study might provide a new approach using exosomes to aid in Aβ elimination. Modulation of exosome secretion by selective regulation of the Cer-metabolizing pathway is likely therapeutically useful. In addition, delivery technology of exosomes, including the targeting of intravenously injected exosomes into the brain, has been developed for therapeutic applications (54). It might also be useful for exosome-mediated Aβ clearance in AD with some advantages, such as treatment with engineered exosomes or with required quantities of exosomes.
Footnotes
- AD
- Alzheimer disease
- Aβ
- amyloid β protein
- APP
- amyloid precursor protein
- nSMase
- neutral sphingomyelinase
- aSMase
- acid sphingomyelinase
- Cer
- ceramide
- ThT
- thioflavin T
- D609
- tricyclodecan-9-xanthogenate
- AV
- annexin V
- MVB
- multivesicular body
- CTB
- cholera toxin B
- EGCase
- endoglycoceramidase
- PS
- phosphatidylserine
- GSL
- glycosphingolipid
- FAM
- carboxyfluorescein-conjugated human Aβ.
REFERENCES
- 1. Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer disease. Progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 2. Selkoe D. J. (1997) Alzheimer disease. Genotypes, phenotypes, and treatments. Science 275, 630–631 [DOI] [PubMed] [Google Scholar]
- 3. Mawuenyega K. G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J. C., Yarasheski K. E., Bateman R. J. (2010) Decreased clearance of CNS β-amyloid in Alzheimer disease. Science 330, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bateman R. J., Munsell L. Y., Morris J. C., Swarm R., Yarasheski K. E., Holtzman D. M. (2006) Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat. Med. 12, 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajendran L., Honsho M., Zahn T. R., Keller P., Geiger K. D., Verkade P., Simons K. (2006) Alzheimer disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 103, 11172–11177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simons M., Raposo G. (2009) Exosomes. Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581 [DOI] [PubMed] [Google Scholar]
- 7. Fauré J., Lachenal G., Court M., Hirrlinger J., Chatellard-Causse C., Blot B., Grange J., Schoehn G., Goldberg Y., Boyer V., Kirchhoff F., Raposo G., Garin J., Sadoul R. (2006) Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 31, 642–648 [DOI] [PubMed] [Google Scholar]
- 8. Keller S., Rupp C., Stoeck A., Runz S., Fogel M., Lugert S., Hager H. D., Abdel-Bakky M. S., Gutwein P., Altevogt P. (2007) CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 72, 1095–1102 [DOI] [PubMed] [Google Scholar]
- 9. Yuyama K., Yamamoto N., Yanagisawa K. (2008) Accelerated release of exosome-associated GM1 ganglioside (GM1) by endocytic pathway abnormality. Another putative pathway for GM1-induced amyloid fibril formation. J. Neurochem. 105, 217–224 [DOI] [PubMed] [Google Scholar]
- 10. Levi G., Aloisi F., Ciotti M. T., Thangnipon W., Kingsbury A., Balazs R. (1989) A Dissection and Tissue Culture Manual of Nervous System (Shahar A., de Vellis J., Vernadakis A., Haken B., eds.) pp. 154–186, Alan R. Liss, Inc., New York [Google Scholar]
- 11. Thery C., Amigorena S., Raposo G., Clayton A. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Chapter 3, Unit 3.22 [DOI] [PubMed] [Google Scholar]
- 12. Naiki H., Gejyo F. (1999) Kinetic analysis of amyloid fibril formation. Methods Enzymol. 309, 305–318 [DOI] [PubMed] [Google Scholar]
- 13. Mitsutake S., Zama K., Yokota H., Yoshida T., Tanaka M., Mitsui M., Ikawa M., Okabe M., Tanaka Y., Yamashita T., Takemoto H., Okazaki T., Watanabe K., Igarashi Y. (2011) Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J. Biol. Chem. 286, 28544–28555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 15. Morelli A. E., Larregina A. T., Shufesky W. J., Sullivan M. L., Stolz D. B., Papworth G. D., Zahorchak A. F., Logar A. J., Wang Z., Watkins S. C., Falo L. D., Jr., Thomson A. W. (2004) Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104, 3257–3266 [DOI] [PubMed] [Google Scholar]
- 16. Théry C., Zitvogel L., Amigorena S. (2002) Exosomes. Composition, biogenesis, and function. Nat. Rev. Immunol. 2, 569–579 [DOI] [PubMed] [Google Scholar]
- 17. de Gassart A., Geminard C., Fevrier B., Raposo G., Vidal M. (2003) Lipid raft-associated protein sorting in exosomes. Blood 102, 4336–4344 [DOI] [PubMed] [Google Scholar]
- 18. Yanagisawa K., Odaka A., Suzuki N., Ihara Y. (1995) GM1 ganglioside-bound amyloid β-protein (Aβ). A possible form of preamyloid in Alzheimer disease. Nat. Med. 1, 1062–1066 [DOI] [PubMed] [Google Scholar]
- 19. Ariga T., McDonald M. P., Yu R. K. (2008) Role of ganglioside metabolism in the pathogenesis of Alzheimer disease. A review. J. Lipid Res. 49, 1157–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayashi H., Kimura N., Yamaguchi H., Hasegawa K., Yokoseki T., Shibata M., Yamamoto N., Michikawa M., Yoshikawa Y., Terao K., Matsuzaki K., Lemere C. A., Selkoe D. J., Naiki H., Yanagisawa K. (2004) A seed for Alzheimer amyloid in the brain. J. Neurosci. 24, 4894–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Selkoe D. J. (2002) Alzheimer disease is a synaptic failure. Science 298, 789–791 [DOI] [PubMed] [Google Scholar]
- 22. Haass C., Selkoe D. J. (2007) Soluble protein oligomers in neurodegeneration. Lessons from the Alzheimer amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 [DOI] [PubMed] [Google Scholar]
- 23. Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 [DOI] [PubMed] [Google Scholar]
- 24. Luberto C., Hannun Y. A. (1998) Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? J. Biol. Chem. 273, 14550–14559 [DOI] [PubMed] [Google Scholar]
- 25. Napoli I., Neumann H. (2009) Microglial clearance function in health and disease. Neuroscience 158, 1030–1038 [DOI] [PubMed] [Google Scholar]
- 26. Ransohoff R. M. (2007) Microgliosis. The questions shape the answers. Nat. Neurosci. 10, 1507–1509 [DOI] [PubMed] [Google Scholar]
- 27. Fitzner D., Schnaars M., van Rossum D., Krishnamoorthy G., Dibaj P., Bakhti M., Regen T., Hanisch U. K., Simons M. (2011) Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 124, 447–458 [DOI] [PubMed] [Google Scholar]
- 28. Miyanishi M., Tada K., Koike M., Uchiyama Y., Kitamura T., Nagata S. (2007) Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439 [DOI] [PubMed] [Google Scholar]
- 29. Haughey N. J., Bandaru V. V., Bae M., Mattson M. P. (2010) Roles for dysfunctional sphingolipid metabolism in Alzheimer disease neuropathogenesis. Biochim. Biophys. Acta 1801, 878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kreutzberg G. W. (1996) Microglia. A sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 [DOI] [PubMed] [Google Scholar]
- 31. Harper J. D., Lansbury P. T., Jr. (1997) Models of amyloid seeding in Alzheimer disease and scrapie. Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 66, 385–407 [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto N., Matsubara T., Sato T., Yanagisawa K. (2008) Age-dependent high density clustering of GM1 ganglioside at presynaptic neuritic terminals promotes amyloid β-protein fibrillogenesis. Biochim. Biophys. Acta 1778, 2717–2726 [DOI] [PubMed] [Google Scholar]
- 33. Okada T., Ikeda K., Wakabayashi M., Ogawa M., Matsuzaki K. (2008) Formation of toxic Aβ(1–40) fibrils on GM1 ganglioside-containing membranes mimicking lipid rafts: polymorphisms in Aβ(1–40) fibrils. J. Mol. Biol. 382, 1066–1074 [DOI] [PubMed] [Google Scholar]
- 34. Goldsbury C., Frey P., Olivieri V., Aebi U., Müller S. A. (2005) Multiple assembly pathways underlie amyloid-β fibril polymorphisms. J. Mol. Biol. 352, 282–298 [DOI] [PubMed] [Google Scholar]
- 35. Petkova A. T., Leapman R. D., Guo Z., Yau W. M., Mattson M. P., Tycko R. (2005) Self-propagating, molecular-level polymorphism in Alzheimer's β-amyloid fibrils. Science 307, 262–265 [DOI] [PubMed] [Google Scholar]
- 36. Sharples R. A., Vella L. J., Nisbet R. M., Naylor R., Perez K., Barnham K. J., Masters C. L., Hill A. F. (2008) Inhibition of γ-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 22, 1469–1478 [DOI] [PubMed] [Google Scholar]
- 37. Takahashi R. H., Milner T. A., Li F., Nam E. E., Edgar M. A., Yamaguchi H., Beal M. F., Xu H., Greengard P., Gouras G. K. (2002) Intraneuronal Alzheimer αβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 161, 1869–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kimura N., Yanagisawa K. (2007) Endosomal accumulation of GM1 ganglioside-bound amyloid β-protein in neurons of aged monkey brains. Neuroreport 18, 1669–1673 [DOI] [PubMed] [Google Scholar]
- 39. Liu B., Hassler D. F., Smith G. K., Weaver K., Hannun Y. A. (1998) Purification and characterization of a membrane-bound neutral pH optimum magnesium-dependent and phosphatidylserine-stimulated sphingomyelinase from rat brain. J. Biol. Chem. 273, 34472–34479 [DOI] [PubMed] [Google Scholar]
- 40. Karakashian A. A., Giltiay N. V., Smith G. M., Nikolova-Karakashian M. N. (2004) Expression of neutral sphingomyelinase-2 (NSMase-2) in primary rat hepatocytes modulates IL-β-induced JNK activation. FASEB J. 18, 968–970 [DOI] [PubMed] [Google Scholar]
- 41. Huitema K., van den Dikkenberg J., Brouwers J. F., Holthuis J. C. (2004) Identification of a family of animal sphingomyelin synthases. EMBO J. 23, 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tafesse F. G., Ternes P., Holthuis J. C. (2006) The multigenic sphingomyelin synthase family. J. Biol. Chem. 281, 29421–29425 [DOI] [PubMed] [Google Scholar]
- 43. Gulbins E., Kolesnick R. (2003) Raft ceramide in molecular medicine. Oncogene 22, 7070–7077 [DOI] [PubMed] [Google Scholar]
- 44. Tam C., Idone V., Devlin C., Fernandes M. C., Flannery A., He X., Schuchman E., Tabas I., Andrews N. W. (2010) Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell Biol. 189, 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zha X., Pierini L. M., Leopold P. L., Skiba P. J., Tabas I., Maxfield F. R. (1998) Sphingomyelinase treatment induces ATP-independent endocytosis. J. Cell Biol. 140, 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Majumdar A., Cruz D., Asamoah N., Buxbaum A., Sohar I., Lobel P., Maxfield F. R. (2007) Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol. Biol. Cell 18, 1490–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cameron B., Landreth G. E. (2010) Inflammation, microglia, and Alzheimer disease. Neurobiol. Dis. 37, 503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Magnus T., Chan A., Grauer O., Toyka K. V., Gold R. (2001) Microglial phagocytosis of apoptotic inflammatory T cells leads to down-regulation of microglial immune activation. J. Immunol. 167, 5004–5010 [DOI] [PubMed] [Google Scholar]
- 49. Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., Raposo G. (2004) Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 101, 9683–9688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Emmanouilidou E., Melachroinou K., Roumeliotis T., Garbis S. D., Ntzouni M., Margaritis L. H., Stefanis L., Vekrellis K. (2010) Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30, 6838–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Février B., Raposo G. (2004) Exosomes. Endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 16, 415–421 [DOI] [PubMed] [Google Scholar]
- 52. Vella L. J., Sharples R. A., Lawson V. A., Masters C. L., Cappai R., Hill A. F. (2007) Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J. Pathol. 211, 582–590 [DOI] [PubMed] [Google Scholar]
- 53. El Khoury J., Toft M., Hickman S. E., Means T. K., Terada K., Geula C., Luster A. D. (2007) Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 13, 432–438 [DOI] [PubMed] [Google Scholar]
- 54. Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M. J. (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 [DOI] [PubMed] [Google Scholar]