Background: Rtt106 is a histone H3-H4 chaperone.
Results: Rtt106 oligomerizes through its N terminus and binds (H3-H4)2 tetramers in vitro and in vivo.
Conclusion: Rtt106 likely deposits (H3-H4)2 tetramers to promote nucleosome assembly.
Significance: Determining the stoichiometry of H3-H4 molecules that associate with Rtt106 will increase our understanding on how nucleosomes are formed during S phase of the cell cycle.
Keywords: Chromatin Regulation, Chromatin Structure, Heterochromatin, Histone Chaperone, Histones, CAF-1, Rtt106, Chromatin Assembly, Nucleosome Assembly
Abstract
The yeast histone chaperone Rtt106 is involved in de novo assembly of newly synthesized histones into nucleosomes during DNA replication and plays a role in regulating heterochromatin silencing and maintaining genomic integrity. The interaction of Rtt106 with H3-H4 is modulated by acetylation of H3 lysine 56 catalyzed by the lysine acetyltransferase Rtt109. Using affinity purification strategies, we demonstrate that Rtt106 interacts with (H3-H4)2 heterotetramers in vivo. In addition, we show that Rtt106 undergoes homo-oligomerization in vivo and in vitro, and mutations in the N-terminal homodimeric domain of Rtt106 that affect formation of Rtt106 oligomers compromise the function of Rtt106 in transcriptional silencing and response to genotoxic stress and the ability of Rtt106 to bind (H3-H4)2. These results indicate that Rtt106 deposits H3-H4 heterotetramers onto DNA and provide the first description of a H3-H4 chaperone binding to (H3-H4)2 heterotetramers in vivo.
Introduction
In eukaryotic cells, genomic DNA is organized into chromatin that encodes epigenetic information and governs genome stability. The basic unit of chromatin is the nucleosome consisting of 147 bp of DNA wrapped around a histone octamer, containing a (H3-H4)2 tetramer and two H2A-H2B dimers (1–3). During S phase of the cell cycle, chromatin structures must be propagated to daughter cells to maintain gene expression states and genome integrity. DNA replication-coupled nucleosome assembly plays an important role in formation of nucleosomes, one of the early steps in the inheritance of higher chromatin structure (4–6). Because nucleosomal H2A-H2B dimers can exchange with free H2A-H2B dimers in cells, it is believed that the assembly of H3-H4 tetramers following DNA replication is the critical and rate-limiting step in nucleosome formation (7). Immediately following DNA replication both the transfer of parental H3-H4 and deposition of newly synthesized H3-H4 onto replicated DNA are important to promote nucleosome formation.
Early studies of parental histone transfer suggested that during DNA replication, parental nucleosomes tended to be distributed to nascent daughter DNA intact without mixing with newly synthesized H3-H4 (8, 9). However, later studies showed that parental and newly synthesized histones associate with each other, forming hybrid nucleosomes, such as parental H3-H4 heterotetramers forming a complex with newly synthesized H2A-H2B and vice versa (10). In addition, it has been shown that histone chaperone Asf1, which is involved in both replication-coupled nucleosome assembly as well as replication-independent nucleosome assembly, binds H3-H4 dimers (11, 12). Based on these results, it was hypothesized that a parental (H3-H4)2 tetramer could be split into two H3-H4 dimers, which would then associate with newly synthesized H3-H4 dimers or with other parental H3-H4 dimers to form nucleosomes with mixed (H3-H4)2 tetramers, respectively (13). More recently, work in mammalian cells demonstrated that during replication-coupled nucleosome assembly parental (H3-H4)2 tetramers were not split. Only a very small fraction of H3.3-H4 tetramers (which are in general deposited through the replication-independent nucleosome assembly pathway) did split and formed hybrid nucleosomes (14). The reason for the differences seen in these studies is unclear. Importantly, the form of the newly synthesized H3-H4 molecules (dimer or tetramer) deposited onto DNA was not addressed.
It is generally thought that newly synthesized H3-H4 dimers first form a complex with Asf1 (15). This view is supported by structural studies, which reveals that Asf1 binds a H3-H4 dimer through the H3 interface involved in the formation of (H3-H4)2 tetramers (11, 12). In addition, in vitro studies indicate that Asf1 disrupts (H3-H4)2 tetramers. Given these results it remained unclear how (H3-H4)2 tetramers could be formed in the presence of Asf1. We and others have made several observations suggesting that H3-H4 dimers are transferred from the Asf1-H3-H4 complex to CAF-1 and Rtt106, two other histone H3-H4 chaperones promoting assembly of newly synthesized H3-H4 into nucleosomes in budding yeast. First, genetic and biochemical evidence indicates that a major function of Asf1 is to regulate acetylation of histone H3 lysine 56 (H3K56Ac) by presenting new H3-H4 to the lysine acetyltransferase Rtt109 for acetylation (14, 16–18). Second, H3K56Ac enhances the binding of H3-H4 to Rtt106 and CAF-1 in vivo and in vitro. In addition, Rtt106 and CAF-1 (which also physically interact) are capable of depositing H3K56Ac-H4 onto replicating DNA in vitro (19). However, it is not known whether Rtt106 or CAF-1 binds a H3-H4 dimer like Asf1 or (H3-H4)2 tetramers.
Here, we show that Rtt106 binds to a (H3-H4)2 tetramer in vivo. Furthermore, we demonstrate that Rtt106 undergoes homo-oligomerization in vivo, consistent with the homodimerization of Rtt106 N terminus demonstrated in vitro (20) and that homo-oligomerization of Rtt106 is likely important for Rtt106 to bind (H3-H4)2 tetramers efficiently and for its role in transcriptional silencing and conferring resistance to DNA-damaging agents.
EXPERIMENTAL PROCEDURES
Yeast Strains and Plasmid Constructions
Standard yeast genetics and molecular biology methods were utilized to generate yeast strains and plasmids listed in supplemental Tables 2 and 3. pET28MBP-RTT106 and pET28MBP-RTT106(1–301) were constructed by PCR-amplifying relevant regions using pZG175 and cloning into BamH1 and SalI sites of pET28MBP. RTT106 V21E, L28E, I30E, and F31E were generated by site-directed mutagenesis on pZG175 using the Gene Tailor site-directed mutagenesis system (Invitrogen). RTT106 V21E, L28E, I30E, and F31E ORFs were amplified by PCR and subcloned into pET28b. Either HHT1 or HHT2 was tagged with HA epitope in the W303 background, and haploid HA-tagged H3-expressing strains were back-crossed with parental W303 haploids two times (21). PCR-amplified HHT1–3HA-HHF1 DNA was digested with EcoRI and BglII and subcloned into the BamHI-EcoRI fragment of pRS416 (for primer list, see supplemental Table 1).
Protein Expression and Purification
Protein expressions were performed either in Sf9 cells using the Bac2Bac system (Invitrogen) or BL21 (DE3) bacterial strain. Plasmids were expressed in DH5α, and bacterial cultures were induced with 0.5 mm IPTG for 14–20 h at 27 °C. Following lysis in buffer A (PBS) recombinant His fusion proteins protein was purified by Ni-affinity chromatography. Following elution with a buffer B (PBS with a gradient of 0–500 mm imidazole), proteins were subjected to gel filtration analysis in a Superose 6 gel filtration column.
Sequential Affinity Purification and Western Blotting
Yeast cells with Rtt106-TAP3 were grown in liquid YPD medium up to 1.8–2.0 A600/ml, pelleted, and washed with water containing 10% glycerol and resuspended in IP buffer (25 mm Tris pH8, 100 mm NaCl, 1 mm EDTA, 10 mm MgCl2, 0.01% Nonidet P-40, 1 mm DTT, 1 mm PMSF, 1 mm benzamidine, 1 mm Pefabloc, 15 Kunitz units/ml DNase I). The TAP tag was fused to the C terminus of Rtt106 as described (22). To facilitate the expression of Rtt106-TAP, Rtt106-TAP containing the endogenous promoter of Rtt106 was cloned into a pRS313 plasmid. Cell suspension was flash frozen in liquid N2 and stored at −80 °C until use. Cells were lysed at cryogenic conditions using a 6770 Freezer/Mill grinder (SamplePrep). Powdered cell suspension was allowed to thaw on ice. Liquified cell lysate was spun at 20,000 × g on a JA-25.5 rotor on an Avanti J-E centrifuge (Beckman Coulter). Soluble portion was incubated on ice for 30 min with 75 μg/ml ethidium bromide. Lysate were spun at 14,000 × g on a Microfuge 22R centrifuge (Beckman Coulter). The cleared lysate were again spun at 14,000 × g. The cleared lysate was incubated with washed IgG beads for 2 h at 4 °C. Following extensive washing, proteins were eluted by digestion with TEV protease for 2 h at 16 °C. One third of the total volume of the eluate was precipitated with 10% TCA and boiled in sample buffer. The remainder of the TEV eluate (2/3 volume of original eluate) was allowed to incubate with HA beads for 2 h at 4 °C. Collected flow-through was TCA-precipitated and boiled in sample buffer. The HA beads were washed three times with IP buffer and boiled in the SDS sample buffer. Western blotting analysis was carried out with antibodies for calmodulin-binding peptide, H3K56Ac, HA, and H3. Antibodies against calmodulin-binding peptide were purchased from Upstate Biotechnology. Antibodies against H3K56Ac and H3 were made in the laboratory (23). Antibodies against HA were a gift from Dr. Stillman.
Co-purification of H3K56R with Rtt106-TAP in Presence of Wild-type H3
To determine how Rtt106 bound to H3K56R mutant in the presence of wild-type H3, yeast cells were grown to 1 A600/ml, pelleted, and washed with water containing 10% glycerol and resuspended in IP buffer. Cell suspension was flash frozen in liquid N2 and stored at −80 °C until use. Lysis was performed under cryogenic condition using the Freezer/Mill grinder, and tandem affinity purification was carried out as described previously (19). The assay to test in vivo oligomerization, H3-H4 binding ability of wild-type and mutant Rtt106 was performed with cells grown to 1.4 A600/ml, and IgG binding followed by TEV cleavage and binding to CaM beads were performed as described previously.
Functional Assays
Measurement of the expression of the GFP transgene integrated at the HMR locus using FACS was used to assay for silencing at the HMR locus. Exponentially growing cells expressing wild-type or mutant Rtt106-TAP in cac1Δ rtt106Δ double mutant background as well as a strain deleted for sir3 (Δsir3) were used in this analysis as described previously (24, 25). The DNA damage sensitivity assays were performed using 10-fold serial dilutions of exponentially growing cells that were spotted on camptothecin (CPT) containing HIS dropout (−HIS SCM) plates as described previously (19).
RESULTS
Rtt106 Binds H3-H4 Tetramers
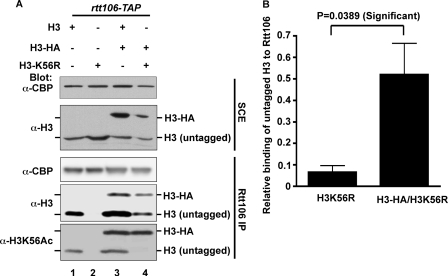
Rtt106 has been shown to bind to H3-H4 in vivo and in vitro (19, 24, 25). However, the molecular form (heterotetramer or heterodimer) of the H3-H4 that binds to Rtt106 was not known. Because Rtt106 appears to bind a surface of H3 that is distinct from the surface bound by Asf1 and Asf1 is known to bind a H3-H4 dimer (19), we set out to determine whether Rtt106 bound to a H3-H4 heterodimer (H3-H4) or a heterotetramer (H3-H4)2, using sequential affinity purification. The budding yeast Saccharomyces cerevisiae has two histone genes, HHT1 and HHT2, that encode identical proteins. In a strain expressing Rtt106-TAP, either HHT1 or HHT2 was modified to encode H3 C-terminally tagged with three HA epitopes (H3-HA) whereas the other genes remained unmodified. This resulted in the expression of both the tagged H3-HA and untagged H3 in each strain. The tagged and untagged H3 species were distinguished from each other in Western blots by the difference in migration because of the added molecular mass of the HA tags (Fig. 1A). The sequential affinity purification procedure involved an initial purification of Rtt106-TAP using IgG beads followed by affinity purification of H3-HA using anti-HA antibody-conjugated beads (Fig. 1B). If the sequential affinity purification of Rtt106-TAP and H3-HA resulted in purification of only H3-HA, that would suggest that Rtt106 binds to an H3-H4 heterodimer. However, if untagged H3 were co-purified with tagged H3-HA, that would be consistent with Rtt106 binding to a (H3-H4)2 heterotetramer (see “Discussion”). As expected, the initial IgG purification resulted in co-purification of both tagged and untagged H3 with Rtt106 (Fig. 1C). This eluate was then subjected to affinity purification of H3-HA using HA antibody-conjugated beads (Fig. 1C). Untagged H3 as well as Rtt106 co-purified with H3-HA (Fig. 1C), suggesting that H3-H4 co-purified with Rtt106 may be a (H3-H4)2 heterotetramer.
FIGURE 1.
Sequential affinity purification of Rtt106-TAP and histone H3-HA. A, Western blot analysis of soluble cell extracts used in the affinity purification of Rtt106-TAP with IgG-Sepharose beads. B, schematic outline of the key steps in the experimental procedure. C, untagged H3 co-purified with H3-HA and Rtt106 during sequential affinity purification. Rtt106-TAP was affinity-purified using IgG-Sepharose and eluted with TEV protease. About one-third of the eluate was precipitated with TCA (lanes 1–4) and the remainder was immune-precipitated using antibodies against the HA epitope (lanes 5–8). The material not bound to HA beads is shown in lanes 9–12. The proteins were analyzed by Western blotting using antibodies against indicated proteins. SCE, soluble cell extracts of S. cerevisiae cells.
Because Rtt106 forms an oligomer (see below), another possible explanation for co-purification of H3-H4 with H3-HA-H4 shown in Fig. 1 was that the H3-H4 dimer and the H3-HA-H4 dimer bound to different subunits of an Rtt106 oligomer. To test this possibility, we asked whether Rtt106 bound to H3K56R mutant in the presence or absence of a wild-type H3. We have shown that H3K56Ac increases the binding of H3-H4 to Rtt106 in vivo and in vitro. Mutating the lysine 56 of H3 to arginine (H3K56R) significantly reduces the binding of H3-H4 to Rtt106 (19). If a H3-H4 heterodimer and a H3-HA-H4 heterodimer bound to different subunit of an Rtt106 oligomer, one would not expect Rtt106 to bind to H3K56R even in the presence of wild-type H3. However, if Rtt106 bound a H3-H4 heterotetramer, H3K56R that was associated with wild-type H3 as part of a tetramer could co-purify with Rtt106 in the presence of a wild-type of H3. Therefore, H3-HA was expressed in cells co-expressing either untagged wild-type H3 or H3K56R. Rtt106-TAP was purified from these strains, and co-purified H3 was detected by Western blotting using antibodies against H3 or H3K56Ac. As reported, Rtt106 binding to H3K56R was barely detectable when H3K56R was the only copy of H3 in cells. Remarkably, significantly more H3K56R was co-purified with Rtt106 in the presence of wild-type H3-HA (Fig. 2). These results strongly support the conclusion that Rtt106 binds a (H3-H4)2 heterotetramer in vivo.
FIGURE 2.
Additional evidence that Rtt106 binds a (H3-H4)2 tetramer. A, increased association of H3K56R with Rtt106 in the presence of wild-type H3. Rtt106-TAP was purified from strains expressing wild-type H3 (lanes 1 and 3) or H3K56R mutant (lanes 2 and 4) with an empty plasmid pRS416 (lanes 1 and 2) or a plasmid expressing H3-HA (lanes 3 and 4). Co-purified proteins as well as proteins in soluble cell extracts of S. cerevisiae cells (SCE) were analyzed by Western blotting using indicated antibodies. B, quantification of relative amounts H3K56R binding to Rtt106 in the absence (A, lane 2) or presence (B, lane 4) of H3-HA performed using ImageQuant software.
Rtt106 Homo-oligomerizes in Vivo and in Vitro
In the course of recombinant Rtt106 purification, full-length Rtt106 appeared to migrate through a gel filtration column at an apparent molecular mass (>450 kDa) much larger than the calculated molecular mass of 52 kDa. This led us to hypothesize that Rtt106 might form oligomers and/or exist with an elongated shape in solution. To discern between these possibilities further, a recombinant Rtt106 protein (amino acids 1–301) lacking its C terminus was subjected to gel filtration chromatography. From multiple purifications, the migration of Rtt106(1–301) consistently corresponds to a molecular mass intermediate between that of a dimer and a trimer (Fig. 3A and data not shown). This observation agrees well with a homodimer of elongated shape as expected from structural studies of N-terminal and middle domains of Rtt106. In addition, dynamic light-scattering analysis shows that Rtt106(1–301 and 1–315) are close to a dimerized state (data not shown). When a Rtt106 mutant (containing residues 67–301) lacking both N-terminal 66 residues and its C terminus was subjected to the same analysis, the migration was consistent with the expected molecular mass of ∼27 kDa (Fig. 3A). This result supports the conclusion that Rtt106 undergoes oligomerization in vitro. The apparently much higher than expected molecular mass of full-length Rtt106 may be due to further oligomerization through the C terminus. Alternatively, the disordered nature of Rtt106 C terminus may cause anomalous migration in gel filtration chromatography (Fig. 3A).
FIGURE 3.
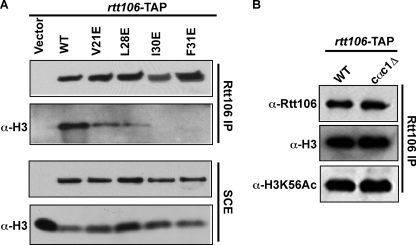
Rtt106 undergoes homo-oligomerization in vivo and in vitro. A, N terminus and C terminus of Rtt106 contribute to an apparent molecular mass higher than expected for a monomer. Recombinant full-length Rtt106 as well as two Rtt106 mutants, indicated on the left, were analyzed by Superose 6 gel filtration chromatography. The positions of protein standards are indicated. B, Rtt106 undergoes homo-oligomerization in vivo and the N terminus of Rtt106 is important for this process and the C terminus is dispensable. Plasmid for expression of Rtt106-TAP was transformed into a strain expressing Rtt106-FLAG or rtt106Δ. Alternatively, Rtt106-TAP lacking C terminus(1–315) was expressed in the strain containing Rtt106-FLAG. Rtt106-TAP was purified as described under “Experimental Procedures,” and co-purified proteins were detected using indicated antibodies. C, mutations at the N terminus of Rtt106 compromise Rtt106 oligomerization in vivo. Rtt106-TAP and four TAP-tagged Rtt106 mutants, V21E, L28E, I30E, and F31E were expressed in wild-type cells from a plasmid and analyzed by Western blotting using antibodies against Rtt106 (left). Wild-type or mutant proteins were also purified by tandem affinity, and co-purified proteins as well as proteins in soluble cell extracts of S. cerevisiae cells (SCE) were analyzed by Western blotting. D, association of Rtt106 with H3-H4 is not required for Rtt106 homo-oligomerization. Rtt106-TAP was expressed from a plasmid in cells containing wild-type H3 or H3K56R mutant and purified. Co-purified endogenous Rtt106 was detected by antibodies against Rtt106. As controls, Rtt106-TAP was purified from cells lacking endogenous Rtt106 (rtt106Δ) or from wild-type cells transformed with an empty vector. Pon S, Ponceau S staining of nitrocellulose membrane. * indicates nonspecific protein detected by Rtt106 antibodies. E, mutations at N terminus of Rtt106 compromise the formation of Rtt106 oligomers in vitro. Recombinant wild-type and Rtt106 site-specific mutants, V21E, L28E, I30E, and F31E, were analyzed on a Superose 6 gel filtration column. The apparent molecular masses of wild-type and Rtt106 mutants were estimated based on the protein standards shown in A.
To determine whether Rtt106 undergoes oligomerization in vivo, we asked whether exogenously expressed Rtt106-TAP bound to endogenous Rtt106. Rtt106-TAP expressed from a plasmid was purified from cells containing endogenous Rtt106 and co-purified proteins was detected by antibodies against Rtt106. Endogenous Rtt106 co-purified with Rtt106-TAP, indicating that Rtt106 can form oligomers in vivo (Fig. 3B and data not shown). In addition, Rtt106 mutant(1–315) lacking the C terminus bound to endogenous Rtt106 to a similar degree as wild-type Rtt106-TAP (Fig. 3B). These results strongly suggest that the N-terminal region of Rtt106 is sufficient to mediate oligomerization of Rtt106, presumably dimerization, in vivo. These data also suggest that Rtt106 does not oligomerize through its C terminus in vivo and that the C terminus contributes to the anomalous migration in gel filtration chromatography as indicated above.
The NMR structure of the Rtt106 N terminus (residues 1–67) shows this region forms a tight homodimer (20), consistent with the results above indicating that the N terminus of Rtt106 is involved in Rtt106 oligomerization. Based on structural information, four residues (Val21, Leu28, Ile30, and Phe31) at the dimer interface were mutated to glutamic acid. These four Rtt106 N-terminal point mutants were expressed from plasmids as a Rtt106-TAP fusion in cells containing endogenous Rtt106 and subjected to tandem affinity purification to test for their ability to bind endogenous Rtt106. Co-purified proteins were detected by Western blotting using antibodies against Rtt106. Like wild-type Rtt106-TAP, two Rtt106 V21E and L28E mutants bound to endogenous Rtt106. In contrast, the Rtt106 I30E mutant showed significantly diminished association with endogenous Rtt106 compared with the wild type, and the binding of Rtt106 F31E mutant to endogenous Rtt106 was not detectable (Fig. 3C). These results strongly support the idea that Rtt106 can undergo homo-oligomerization in vivo, most likely homodimer, and the C terminus of Rtt106 appears not to have a role in promoting further oligomerization.
Because Rtt106 I30E and F31E mutants also lost their ability to bind H3-H4 (see below), it is possible that the reduced association of these Rtt106 mutants with endogenous Rtt106 was due to a loss of the interaction between Rtt106 and H3-H4. To test this idea, we determined whether the association of Rtt106 with H3-H4 is required for Rtt106 oligomerization. Rtt106-TAP, expressed from a plasmid, was purified from cells expressing wild-type H3 and H3K56R. Rtt106-TAP bound to endogenous Rtt106 irrespective of the status of H3 in cells (Fig. 3D). These results suggest that the ability of Rtt106 to bind H3-H4 is not a prerequisite for Rtt106 to form oligomers and that inability of Rtt106 I30E and F31E mutants to bind endogenous Rtt106 is not due to loss of H3-H4 binding. Consistent with these in vivo data, full-length recombinant Rtt106 V21E and L28E mutant proteins migrated at apparent molecular masses comparable with the full-length wild-type Rtt106. In contrast, both I30E and F31E mutant Rtt106 proteins migrated at a molecular mass corresponding to between half and one third of that of wild-type Rtt106, consistent with loss or diminished dimerization through the N-terminal domain (Fig. 3E). Again, the molecular masses appear larger than expected most likely due to the anomalous migration caused by the Rtt106 C terminus. These results support that conclusion that both I30E and F31E mutations at the Rtt106 dimer interface compromise the formation of Rtt106 oligomers in vitro and in vivo.
Mutations at Rtt106 Dimer Interface Affect Ability of Rtt106 to Bind H3-H4
Rtt106 is a histone H3-H4 chaperone and binds CAF-1 (24, 25). To understand the functional significance of Rtt106 oligomerization, we first determined how Rtt106 mutations at the N-terminal dimerization domain affect the ability of Rtt106 to bind H3-H4. To do this, wild-type Rtt106-TAP and N-terminal point mutants were expressed in rtt106Δ mutant cells. Tandem affinity purification was then performed, and purified proteins were detected by Western analysis. As expected, wild-type Rtt106 was able to bind H3-H4 efficiently. In addition, the V21E and L28E mutants retained the ability to bind H3-H4. However, the I30E and F31E mutants, which exhibited significant defects in oligomerization, were defective for H3-H4 binding. Finally, deletion of Cac1, the large subunit of CAF-1, had no apparent effect on the ability of Rtt106 to bind H3-H4 (Fig. 4B), suggesting that the Rtt106-CAF-1 interaction is not required for Rtt106 to bind H3-H4. Together, these results indicate that the N terminus of Rtt106, potentially through homodimerization, is important for its ability to bind H3-H4 in vivo (Fig. 4).
FIGURE 4.
Rtt106 N-terminal point mutants that perturb homo-oligomerization in vivo are defective for H3-H4 binding. A, wild type Rtt106-TAP and TAP-tagged Rtt106 mutants (V21E, L28E, I30E, and F31E) were expressed in rtt106Δ mutant cells. Following TAP purification, proteins in soluble cell extracts (SCE) as well as purified proteins were analyzed by Western blotting using indicated antibodies. B, Cac1 is dispensable for Rtt106 to bind H3-H4. Rtt106-TAP was purified from wild type (WT) or cells lacking Cac1 (cac1Δ). Co-purified H3 or H3K56Ac was detected by Western blotting.
Mutations at Rtt106 Dimer Interface Compromise Function of Rtt106 in Heterochromatin Silencing and Response to DNA Damage
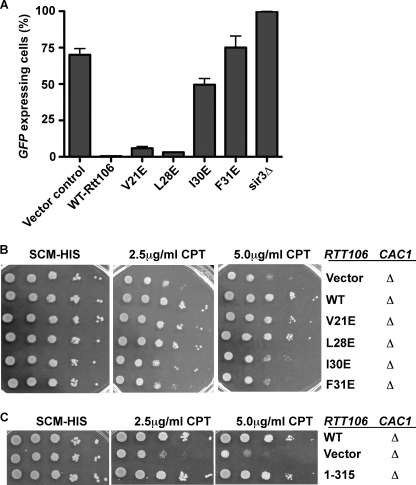
Rtt106 functions in parallel with CAF-1 in transcriptional silencing (24). Cells lacking Rtt106 and Cac1 exhibit significant defects in transcriptional silencing at the HMR locus compared with cells lacking either Rtt106 or Cac1 alone. Therefore, we also tested whether the N terminus of Rtt106 was important for its role in transcriptional silencing. Wild-type and N-terminal point mutants were expressed in rtt106Δ cac1Δ double deletion cells carrying a GFP transgene integrated near the silent mating type locus HMR. The rtt106Δ cac1Δ double deletion resulted in the loss of silencing in the HMR locus and thus allowing increased expression of the GFP transgene. The reintroduction of wild-type Rtt106-TAP into this strain restored transcriptional silencing as measured by the percentage of GFP-expressing cells. Expression of Rtt106 mutants, V21E and L28E, also restored heterochromatin silencing, to a level similar to wild-type Rtt106. In contrast, expression of Rtt106 I30E and F31E in rtt106Δ cac1Δ double mutant cells failed to correct the silencing defects of these double mutant cells (Fig. 5A). Together, these results demonstrate that mutations at the dimer interface of the N terminus Rtt106 result in defects in transcriptional silencing at the HMR locus.
FIGURE 5.
Mutations at the N terminus of Rtt106 result in defects in transcriptional silencing and response to DNA damage. A, N terminus of Rtt106 is required for HMR silencing. Wild type and Rtt106 mutants V21E, L28E, I30E, and F31E were expressed in rtt106Δ cac1Δ strain containing the GFP transgene integrated near the HMR locus. Percentage of GFP-positive cells was measured using FACS through the percentage of cells expressing GFP. B, N terminus of Rtt106 is required to maintain viability following exposure to genotoxic stress. Wild-type Rtt106 or site-specific mutants were expressed in rtt106Δ cac1Δ cells. 10-fold serial dilutions of these cells were spotted on medium containing indicated amounts of the DNA-damaging agent CPT. C, C terminus of Rtt106 is dispensable for Rtt106 role in response to CPT. The experiment was performed as described above.
Similarly, deletion of both rtt106 and cac1 together caused a decrease in viability following exposure to the DNA-damaging agent CPT that was much more severe than deletions of either gene individually (19, 24). We evaluated the sensitivity to genotoxic stress of either wild-type or mutant Rtt106 when expressed in cac1Δ rtt106Δ double mutant cells. Expression of the mutants V21E and L28E restored viability to rtt106Δ cac1Δ exposed to CPT to a level similar to that of expression of wild-type Rtt106. In contrast, expression of the I30E and F31E mutants continued to show increased sensitivity to CPT (Fig. 5B). Moreover, expression of Rtt106 mutant(1–315) lacking the C terminus also restored CPT sensitivity (Fig. 5C) and transcriptional silencing defects of cac1Δ rtt106Δ double mutant (data not shown). Thus, mutations at the N terminus of Rtt106 result in defects in transcriptional silencing and response to DNA-damaging agents, whereas deletion of the C terminus of Rtt106 has no apparent effect. These results are consistent with the results that the C terminus of Rtt106 is not required for the ability of Rtt106 to oligomerize in vivo (Fig. 3B). Taken together, we suggest that oligomerization of Rtt106, most likely a homodimer mediated by its N terminus, is important for the Rtt106 role in genotoxic stress and transcriptional silencing.
DISCUSSION
Here, we present biochemical evidence that Rtt106 binds to a H3-H4 heterotetramer (H3-H4)2. This represents the first biochemical demonstration of a H3-H4 histone chaperone binding to a heterotetramer (H3-H4)2 in vivo. In addition, we have shown that Rtt106 is capable of undergoing homo-oligomerization in vivo and in vitro. Furthermore, we show that oligomerization of Rtt106 is required for its efficient association with H3-H4 in vivo and its function in transcriptional silencing and resistance to the DNA-damaging agent CPT. It is known that the N terminus (residues 1–67) of Rtt106 forms a homo-dimer in solution (20). We show here that the C terminus of Rtt106 is dispensable for the ability of Rtt106 to homo-oligomerize and for the Rtt106 function in transcriptional silencing and DNA damage response. Therefore, we suggest that Rtt106 functions as a homo-dimer in nucleosome assembly, transcriptional silencing, and DNA damage response.
During DNA replication, H3-H4 molecules are assembled first followed by a rapid assembly of H2A-H2B. It is thought that deposition of H3-H4 onto DNA is a rate-limiting step during nucleosome assembly (3). Moreover, it is known that deposition of newly synthesized H3-H4 onto DNA requires histone chaperones including CAF-1, Asf1, and Rtt106 (4). Biochemical and structural studies of Asf1 have shown that Asf1 binds to an H3-H4 heterodimer and is essential for acetylation of H3 lysine 56 (a mark for newly synthesized H3), suggesting that newly synthesized H3-H4 are heterodimers and associated with Asf1 (17). Therefore, there are at least two possible models to explain how H3-H4 tetramers, the first building block of nucleosomes, are formed during de novo nucleosome assembly. H3-H4 tetramers are formed either through the sequential deposition of H3K56 acetylated H3-H4 heterodimers onto DNA or through assembly of H3-H4 heterotetramers on histone chaperones prior to deposition on DNA. Our observation that Rtt106 binds H3-H4 heterotetramers supports the hypothesis that newly synthesized H3-H4 heterotetramers are assembled on Rtt106 prior to deposition on the DNA. These observations also have implications on how parental H3-H4 molecules are segregated. Deposition of newly synthesized H3-H4 as a heterotetramer effectively prevents formation of mixed H3-H4 tetramers each containing a newly synthesized H3-H4 heterodimer and a parental H3-H4 heterodimer. Therefore, parental H3-H4 must also be deposited as heterotetramers during nucleosome assembly. It follows that for chromatin regions or nucleosome assembly processes where Rtt106 serves as the histone H3-H4 chaperone, it is likely that splitting of H3-H4 tetramers is not going to occur.
Our results also show that Rtt106 undergoes homo-oligomerization in vivo. Noticeably, the truncated form of Rtt106(1–315), which retains full function in vivo, homo-oligomerizes via the very N-terminal region of Rtt106. Previous studies have shown that the middle domain of Rtt106, which shares sequence homology with the PH domain, is the site of H3-H4 binding in vivo and in vitro (19). The fact that the inability of I30E and F31E mutations at the homo-dimer interface to bind H3-H4 in vivo correlates with the defects observed in oligomerization strongly supports the view that oligomerization of Rtt106, presumably dimerization, is required for Rtt106 to bind H3-H4 in vivo (Fig. 4). Finally, cells expressing these mutants display defects in transcriptional silencing at the HMR locus and sensitivity toward DNA-damaging agents. These results suggest that the physiologically relevant functional form of Rtt106 in vivo is the oligomeric form.
Although it is not clear whether CAF-1, another histone H3-H4 chaperone, binds a H3-H4 heterodimer or a H3-H4 heterotetramer, it is known that the large subunit of human CAF-1 p150 dimerizes, and the dimerization is critical for the ability of CAF-1 to assemble nucleosomes (26). The dimerization of p150 is also regulated by protein phosphorylation (26, 27). Therefore, it is not unprecedented that a H3-H4 chaperone forms homo-oligomers. While our studies were under review, it was shown that two members of the NAP (nucleosome assembly protein) family, Nap1 and Vps75, bind histone H3-H4 in a tetramer fashion (28). Both Nap1 and Vps75 form dimers. Nap1 in cells is a H2A-H2B chaperone (29), and Vps75 is a component of Rtt109-Vps75 complex (16). Although the significance of Nap1 and Vps75 binding H3-H4 as tetramers remains to be investigated further, these results suggest that Rtt106, Nap1, and Vps75 represent a group of histone chaperones that are different from Asf1.
Supplementary Material
Acknowledgment
We thank Dr. Brian Davies for help in purifications of various recombinant Rtt106 mutants.
This work is supported, in whole or in part, by National Institutes of Health Grants GM72719 and GM81838.

This article contains supplemental Tables 1–3.
- TAP
- tandem affinity purification
- TEV
- tobacco etch virus
- CPT
- camptothecin.
REFERENCES
- 1. Allis C. D., Jenuwein T., Reinberg D. (2007) Epigenetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 2. van Holde K. E. (1989) Chromatin, Elsevier, New York [Google Scholar]
- 3. Chodaparambil J. V., Edayathumangalam R. S., Bao Y., Park Y. J., Luger K. (2006) Nucleosome structure and function. Ernst Schering Res. Found Workshop 57, 29–46 [DOI] [PubMed] [Google Scholar]
- 4. Ransom M., Dennehey B. K., Tyler J. K. (2010) Chaperoning histones during DNA replication and repair. Cell 140, 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith S., Stillman B. (1989) Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 58, 15–25 [DOI] [PubMed] [Google Scholar]
- 6. Stillman B. (1986) Chromatin assembly during SV40 DNA replication in vitro. Cell 45, 555–565 [DOI] [PubMed] [Google Scholar]
- 7. Das C., Tyler J. K., Churchill M. E. (2010) The histone shuffle: histone chaperones in an energetic dance. Trends Biochem. Sci. 35, 476–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leffak I. M., Grainger R., Weintraub H. (1977) Conservative assembly and segregation of nucleosomal histones. Cell 12, 837–845 [DOI] [PubMed] [Google Scholar]
- 9. Jackson V., Chalkley R. (1981) A new method for the isolation of replicative chromatin: selective deposition of histone on both new and old DNA. Cell 23, 121–134 [DOI] [PubMed] [Google Scholar]
- 10. Russev G., Hancock R. (1981) Formation of hybrid nucleosomes containing new and old histones. Nucleic Acids Res. 9, 4129–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. English C. M., Adkins M. W., Carson J. J., Churchill M. E., Tyler J. K. (2006) Structural basis for the histone chaperone activity of Asf1. Cell 127, 495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. English C. M., Maluf N. K., Tripet B., Churchill M. E., Tyler J. K. (2005) ASF1 binds to a heterodimer of histones H3 and H4: a two-step mechanism for the assembly of the H3-H4 heterotetramer on DNA. Biochemistry 44, 13673–13682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Probst A. V., Dunleavy E., Almouzni G. (2009) Epigenetic inheritance during the cell cycle. Nat. Rev. Mol. Cell Biol. 10, 192–206 [DOI] [PubMed] [Google Scholar]
- 14. Han J., Zhou H., Horazdovsky B., Zhang K., Xu R. M., Zhang Z. (2007) Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science 315, 653–655 [DOI] [PubMed] [Google Scholar]
- 15. Jasencakova Z., Scharf A. N., Ask K., Corpet A., Imhof A., Almouzni G., Groth A. (2010) Replication stress interferes with histone recycling and predeposition marking of new histones. Mol. Cell 37, 736–743 [DOI] [PubMed] [Google Scholar]
- 16. Han J., Zhou H., Li Z., Xu R. M., Zhang Z. (2007) The Rtt109-Vps75 histone acetyltransferase complex acetylates non-nucleosomal histone H3. J. Biol. Chem. 282, 14158–14164 [DOI] [PubMed] [Google Scholar]
- 17. Recht J., Tsubota T., Tanny J. C., Diaz R. L., Berger J. M., Zhang X., Garcia B. A., Shabanowitz J., Burlingame A. L., Hunt D. F., Kaufman P. D., Allis C. D. (2006) Histone chaperone Asf1 is required for histone H3 lysine 56 acetylation, a modification associated with S phase in mitosis and meiosis. Proc. Natl. Acad. Sci. U.S.A. 103, 6988–6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsubota T., Berndsen C. E., Erkmann J. A., Smith C. L., Yang L., Freitas M. A., Denu J. M., Kaufman P. D. (2007) Histone H3-K56 acetylation is catalyzed by histone chaperone-dependent complexes. Mol. Cell 25, 703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Q., Zhou H., Wurtele H., Davies B., Horazdovsky B., Verreault A., Zhang Z. (2008) Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell 134, 244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su D., Hu Q., Li Q., Thompson J. R., Cui G., Fazly A., Davies B. A., Botuyan M. V., Zhang Z., Mer G. (2012) Structural basis for recognition of H3K56-acetylated histone H3-H4 by the chaperone RH106. Nature 483, 104–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 22. Rigaut G., Shevchenko A., Rutz B., Wilm M., Mann M., Séraphin B. (1999) A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17, 1030–1032 [DOI] [PubMed] [Google Scholar]
- 23. Zhou H., Madden B. J., Muddiman D. C., Zhang Z. (2006) Chromatin assembly factor 1 interacts with histone H3 methylated at lysine 79 in the processes of epigenetic silencing and DNA repair. Biochemistry 45, 2852–2861 [DOI] [PubMed] [Google Scholar]
- 24. Huang S., Zhou H., Katzmann D., Hochstrasser M., Atanasova E., Zhang Z. (2005) Rtt106p is a histone chaperone involved in heterochromatin-mediated silencing. Proc. Natl. Acad. Sci. U.S.A. 102, 13410–13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang S., Zhou H., Tarara J., Zhang Z. (2007) A novel role for histone chaperones CAF-1 and Rtt106p in heterochromatin silencing. EMBO J. 26, 2274–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quivy J. P., Grandi P., Almouzni G. (2001) Dimerization of the largest subunit of chromatin assembly factor 1: importance in vitro and during Xenopus early development. EMBO J. 20, 2015–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gérard A., Koundrioukoff S., Ramillon V., Sergère J. C., Mailand N., Quivy J. P., Almouzni G. (2006) The replication kinase Cdc7-Dbf4 promotes the interaction of the p150 subunit of chromatin assembly factor 1 with proliferating cell nuclear antigen. EMBO Rep. 7, 817–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bowman A., Ward R., Wiechens N., Singh V., El-Mkami H., Norman D. G., Owen-Hughes T. (2011) The histone chaperones Nap1 and Vps75 bind histones H3 and H4 in a tetrameric conformation. Mol. Cell 41, 398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ito T., Bulger M., Kobayashi R., Kadonaga J. T. (1996) Drosophila NAP-1 is a core histone chaperone that functions in ATP-facilitated assembly of regularly spaced nucleosomal arrays. Mol. Cell. Biol. 16, 3112–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.