Background: How HEAT repeats contribute to PP2A assembly and function was unknown.
Results: A tumor-associated mutation within HEAT repeat 1 disrupts PP2A function.
Conclusion: HEAT repeat 1 is required for B56γ-PP2A assembly and tumor-suppressive function.
Significance: This work provides structural insights into HEAT repeats and illuminates a mechanism to inactivate B56γ-PP2A.
Keywords: Cell Proliferation, p53, Phosphatase, PP2A, Tumor Suppressor Gene, B56gamma, HEAT repeat
Abstract
Protein phosphatase 2A (PP2A) enzyme consists of a heterodimeric core (AC core) comprising a scaffolding subunit (A), a catalytic subunit (C), and a variable regulatory subunit (B). Earlier studies suggest that upon DNA damage, a specific B subunit, B56γ, bridges the PP2A AC core to p53, leading to dephosphorylation of p53 at Thr-55, induction of the p53 transcriptional target p21, and the inhibition of cell proliferation and transformation. In addition to dephosphorylation of p53, B56γ-PP2A also inhibits cell proliferation and transformation by an unknown mechanism. B56γ contains 18 α-helices that are organized into eight HEAT (Huntington-elongation-A subunit-TOR) repeat motifs. Although previous crystal structure study has revealed the residues of B56γ that directly contact the A and C subunits, the contribution of HEAT repeats to holoenzyme assembly and to B56γ-PP2A tumor-suppressive function remains to be elucidated. Here, we show that HEAT repeat 1 is required for the interaction of B56γ with the PP2A AC core and, more importantly, for B56γ-PP2A tumor-suppressive function. Within this region, we identified a tumor-associated mutation, C39R, which disrupts the interaction of B56γ with the AC core and thus was unable to mediate dephosphorylation of p53 by PP2A. Furthermore, due to its lack of AC interaction, C39R was also unable to promote the p53-independent tumor-suppressive function of B56γ-PP2A. This study provides structural insight into the PP2A holoenzyme assembly and emphasizes the importance of HEAT repeat 1 in B56γ-PP2A tumor-suppressive function.
Introduction
The protein phosphatase 2A (PP2A)2 family of serine/threonine phosphoprotein phosphatases is involved in a multitude of cell signaling pathways. The PP2A holoenzyme is a heterotrimeric complex that consists of scaffolding A subunit, catalytic C subunit, and regulatory B subunit. Each subunit is thought to have its own distinct function; the C subunit catalyzes the dephosphorylation of specific serine/threonine residues on target substrates, and the A subunit acts as a scaffold holding the complex together. Together, A and C subunits form a PP2A core enzyme (AC core). The AC core is associated with one of the variable B subunits, which determines the diverse cellular localization, substrate specificities, and enzymatic activity of PP2A holoenzyme (1, 2).
Recent evidence suggested that a subset of B56-containing PP2A holoenzyme (B56-PP2A) exhibits tumor-suppressive functions. The B56 family consists of five different genes, α (PPP2R5A), β (PPP2R5B), γ (PPP2R5C), δ (PPP2R5D), and ϵ (PPP2R5E) (3, 4). B56γ-PP2A has been reported to dephosphorylate tumor suppressor p53 at Thr-55, leading to p53 activation, induction of Cdk inhibitor p21, and inhibition of cell proliferation (5). B56α-PP2A has been reported to dephosphorylate the c-Myc oncogene, resulting in c-Myc inactivation (6, 7), and B56δ-PP2A has been reported to dephosphorylate Cdc25c, blocking cell cycle progression (8, 9). These studies suggest that B56-PP2A exerts its tumor-suppressive function by bridging the PP2A AC core to the substrate proteins involved in cell growth and proliferation. In support of this view, some viral oncoproteins have been shown to function by displacing the B56 subunit from AC core binding (10, 11). In addition, among seven cancer-derived mutations (E64D in lung carcinoma, E64G in breast carcinoma, R418W in malignant melanoma, Δ171–589 also in breast carcinoma, and R182W, R183W, and R183G in ovarian carcinoma) reported in PP2A Aα gene (PPP2R1A) to date, four of them have been characterized, and the unifying effect of them is loss of interaction with either C subunit or B56 subunits (12–14). We have also reported two tumor-associated mutations in B56γ gene that specifically block interaction with p53 and thus p53-dependent, but not p53-independent, tumor-suppressive activity of B56γ-PP2A (15). Together, those data suggest that interaction of the B56 subunit with either PP2A AC core or its substrate is critical for its tumor-suppressive function. However, no B56 mutation has been identified to specifically block PP2A AC core interaction in human cancer to date.
The crystal structure of the B56γ subunit revealed that it has an elongated, superhelical structure comprising 18 α-helices that are organized into eight HEAT-like (Huntington-elongation-A subunit-TOR-like) repeat motifs (16, 17). Each HEAT repeat consists of two antiparallel α-helices connected by an intra-loop, and adjacent HEAT repeats are connected by short inter-repeat turns. Upon holoenzyme assembly, B56γ is placed into a position close to the active site of the C subunit and forms the substrate docking site of the holoenzyme, supporting the view that B56γ controls PP2A specificity by bridging the PP2A AC core and phosphorylated protein substrates. The structure of the B56γ-PP2A holoenzyme reveals a number of conserved amino acid residues located mainly on the intra-loops, which mediate interaction of B56γ with either A or C subunit. However, how each HEAT repeat modulates holoenzyme formation and, importantly, contributes to B56γ-PP2A activity remains unknown.
In this study, we show that deletion of HEAT repeat 1, although distant from the A and C subunits, prevents B56γ from binding to the AC core, suggesting that this motif is critical for the assembly of the B56γ-PP2A holoenzyme. To further investigate the importance of HEAT repeat 1, we characterized a mutation, C39R, within this motif that was previously identified from a pooled glandular tumor sample. Our data reveal that like the HEAT repeat 1 deletion mutants, the C39R mutant was unable to interact with the PP2A A and C subunits. Importantly, we show that although retaining binding to p53, all HEAT repeat 1 mutants tested fail to promote p53 Thr-55 dephosphorylation and transcriptional activation of the p21 gene. As a consequence, they abolished the p53-dependent tumor-suppressive function of PP2A. Furthermore, due to their missing interaction with the A and C subunit, those mutants also lost the p53-independent tumor-suppressive function of B56γ-PP2A. This study thus provides structural insight into the PP2A holoenzyme assembly and suggests an additional mechanism to inactivate tumor-suppressive function of B56γ-PP2A.
EXPERIMENTAL PROCEDURES
Cell Culture and Plasmids
U2OS and HCT116 cells were cultured in McCoy's 5A medium supplemented with 10% fetal calf serum. The B56γ deletion mutants, ΔN40, ΔN53, and ΔN73, were generated by PCR from the wild type B56γ3 gene. The B56γ point mutants, C39R, C39A, and C39S, were generated using the QuikChange site-directed mutagenesis kit (Stratagene). All plasmids were verified by sequencing.
Western Blot and Immunoprecipitation
Whole-cell extract was prepared by lysing the cells in a buffer containing 50 mm Tris-HCl (pH 8.0), 120 mm NaCl, 0.5% Nonidet P-40, 1 mm dithiothreitol, 2 μg/ml aprotinin, and 2 μg/ml leupeptin. Cell lysates were subjected to SDS-PAGE followed by Western blot analysis with anti-p53 (DO1, Santa Cruz Biotechnology), anti-PP2A A subunit (Upstate Biotech Millipore), anti-PP2A C subunit (1D6, Upstate Biotech Millipore), anti-p21 (Santa Cruz Biotechnology), anti-PP2A B56γ (18), anti-ERK (Santa Cruz Biotechnology), anti-cyclin G (Santa Cruz Biotechnology), anti-HA (12CA5), or anti-vinculin (VIN-11-5, Sigma) antibodies. For Thr-55 dephosphorylation, the cell lysate was immunoprecipitated with phospho-specific antibody for Thr-55 (Ab202) and immunoblotted with anti-p53 antibody (5). For interaction of PP2A A and C subunits, p53, ERK, and cyclin G with B56γ3 proteins, U2OS cells were transfected with various B56γ plasmids using FuGENE (Roche Applied Science) or BioT (Bioland Scientific) and lysed 28 h after transfection. Immunoprecipitation was performed using anti-HA monoclonal antibody. The amounts of co-precipitated proteins were determined by immunoblotting. For microcystin binding assay, U2OS cell lysate was incubated with microcystin agarose beads (Upstate Biotech Millipore).
Identification of Cancer-derived Mutation
The National Center for Biotechnology Information (NCBI) AceView program (www.ncbi.nlm.nih.gov/IEB/Research/Acembly) provides a comprehensive sequence of the human transcriptome and genes of all quality-filtered human complementary DNA data from GenBankTM, RefSeq, dbEST, and Trace in a strictly complementary DNA-supported manner. Using this program, we looked for B56γ mutations within HEAT repeat 1 region from the annotated sequencing data taken from samples of tumor sample and cancer cell lines. C39R mutation was identified from a glandular tumor sample, and the corresponding gene accession number is CB956287.
Circular Dichroism (CD) Analysis
GST fusion proteins were expressed in BL21 bacteria and purified using glutathione-Sepharose beads (GE Healthcare) in 50 mm Tris-HCl (pH 8) and 100 mm NaCl. CD measurements were performed on a JASCO J-815 CD spectrophotometer (Essex, UK).
Cell Proliferation and Anchorage-independent Growth Assays
To generate proliferation curves for HCT116 cells, cells were transfected with wild type, C39R, ΔN40, and ΔN73 mutant B56γ or a control CMV empty vector using BioT, seeded in triplicate, and counted at 120 h after seeding. For anchorage-independent growth assays of HCT116 cells, cells were transfected with wild type, C39R, and ΔN73 mutant B56γ or a control CMV empty vector seeded in triplicate in 0.35% Noble agar (Fisher), and colony numbers were counted 4 weeks after seeding.
RESULTS
HEAT Repeat 1 of B56γ Is Required for B56γ-PP2A Holoenzyme Assembly
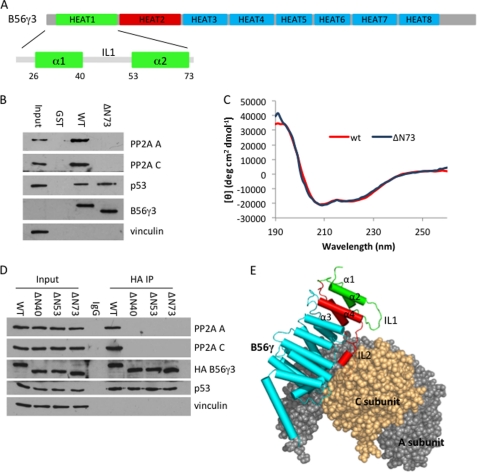
Previously, we have mapped the p53-binding domain at a varying region in the C terminus of B56γ and shown that this domain is required for the p53-dependent tumor-suppressive function of B56γ-PP2A (15). To better understand the role of the N-terminal domain in B56γ function, we first constructed a HEAT repeat 1 deletion mutant (ΔN73; Fig. 1A) as a glutathione S-transferase fusion protein and assayed its ability to interact with the PP2A AC core and p53 in U2OS cell lysates (Fig. 1B). The assay shows that although the wild type B56γ protein was able to interact with both AC core and p53, the ΔN73 mutant lost interaction with the PP2A A and C subunits. This is a surprising result because, according to B56γ-PP2A crystal structure data, the HEAT repeat 1 motif is distant from the AC core (Fig. 1E) and thus should be less likely to affect the interaction. To exclude the possibility that our result could be explained by a loss of proper conformation because of the deletion, we conducted CD analysis (Fig. 1C and supplemental Fig. S1). The wild type B56γ protein is expected to have characteristic CD spectra of α-helical proteins with negative bands at 222 and 208 nm and a positive band at 193 nm. The CD spectrum of our bacterially expressed and purified B56γ protein indeed displays this feature. Importantly, the CD spectrum of purified ΔN73 protein was identical to that of the wild type protein over the entire recorded spectrum. These data, taken together with the ΔN73-p53 interaction result (Fig. 1B), suggest that mutation of HEAT repeat 1 is unlikely to cause overall conformational change of the protein.
FIGURE 1.
HEAT repeat 1 motif of B56γ is required for B56γ-PP2A holoenzyme assembly. A, diagram of amino acid sequence and eight HEAT repeats of B56γ. B, bacterially expressed and purified GST wild type B56γ (WT) or HEAT repeat 1 deletion mutant ΔN73 was incubated with U2OS cell lysates, and bound proteins were analyzed by Western blot using PP2A A and C, p53, B56γ, and vinculin antibodies. C, comparison of the CD spectra of wild type B56γ and ΔN73. D, lysates of U2OS cells transfected with HA-tagged wild type B56γ or HEAT repeat 1 deletion mutants were immunoprecipitated with anti-HA antibody (HA IP) and then analyzed by Western blot using PP2A A and C, HA, p53, and vinculin antibodies. All experiments were repeated three times, and representative data are shown. E, the crystal structure of the B56γ-PP2A holoenzyme (adapted from Protein Data Bank, accession code 2NYM) was prepared using PyMOL. Parts of PP2A are displayed as spheres with subunit A in gray and subunit C in orange. Helices of B56γ are displayed are colored cylinders with HEAT repeat 1 in green, HEAT repeat 2 in red, and the rest of the HEAT repeats in blue.
The HEAT repeat 1 motif consists of two antiparallel α-helices (helix-1 and -2) connected by an intra-loop (IL1) (Fig. 1A). To study their role in the interaction with the PP2A AC core, we generated two smaller deletion mutants, ΔN40 for deleting helix-1 only and ΔN53 for deleting helix-1 plus IL1, and tested their ability to interact with the PP2A AC core in vivo (Fig. 1D). Although the wild type B56γ protein binds to both PP2A AC core and p53 effectively in U2OS cells, all HEAT repeat 1 mutants lost their interaction with the PP2A A and C subunits, suggesting that the entire HEAT repeat 1 domain is required for PP2A AC core interaction. All deletion mutants were able to bind to p53 in vivo. Together, these data suggest that the HEAT repeat 1 motif, although distant from the A and C subunits on the crystal structure, is required for B56γ-PP2A holoenzyme assembly.
A Cancer-associated HEAT Repeat 1 Mutant, C39R, Is Unable to Form B56γ-PP2A Holoenzyme
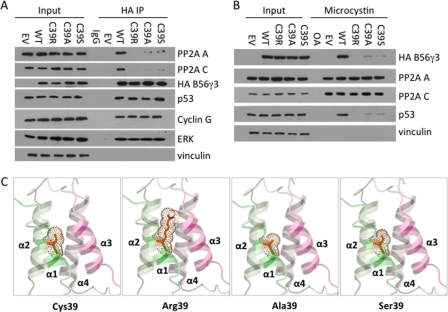
Because B56γ-PP2A functions as a tumor suppressor (5, 10), our finding that HEAT repeat 1 is required for PP2A holoenzyme assembly prompted us to search for cancer-associated mutation within this region. We analyzed annotated complementary DNA sequences in public databases that were derived from human cancer cell lines and tumor samples and identified a mutation of a cysteine residue at amino acid position 39 that was mutated to arginine in a pooled glandular tumor sample. Next, we tested whether the C39R mutation could affect interaction of B56γ with the PP2A A and C subunits as well as with several reported substrates for B56γ-PP2A including p53 (5), ERK (19), and cyclin G2 (20) by either immunoprecipitation (Fig. 2A) or microcystin bead pulldown (Fig. 2B). Both assays indicate that the C39R mutant, although retaining binding to all substrates tested, lost interaction with the PP2A A and C subunits (Fig. 2, A and B). The CD analysis of C39R confirms that the cysteine-to-arginine mutation has no effect on overall protein conformation (supplemental Fig. S1). Together, these results suggest that the cancer-associated C39R mutant disrupts B56γ-PP2A holoenzyme assembly.
FIGURE 2.
C39R mutation disrupts interaction of B56γ with A and C subunits. A, lysates of U2OS cells transfected with empty vector control (EV), HA-tagged wild type B56γ (WT), C39R, C39A, or C39S were immunoprecipitated with anti-HA antibody (HA IP) and then analyzed by Western blot using PP2A A and C, HA, p53, cyclin G, ERK, and vinculin antibodies. B, lysates of U2OS cells transfected with empty vector control, HA-tagged wild type B56γ, C39R, C39A, or C39S were bound to microcystin beads and analyzed by Western blot using HA, PP2A A and C, p53, and vinculin antibodies. All experiments were repeated three times, and representative data are shown. C, stereo view of the interface along the first four helices of B56γ (adapted from Protein Data Bank accession code 2NYM) in which Cys-39 (red) is mutated to arginine, alanine, or serine, respectively. The dots represent van der Waals spheres of residue 39. The figure was prepared using PyMOL.
We consider the possibility that cysteine-to-arginine mutation results in a larger side chain at the position of residue 39, which may clash with nearby helixes and disrupt the HEAT repeat 1 structure. As a consequence, structural change in HEAT repeat 1 may affect the stacking of the rest of the HEAT repeats and thus prevent the interaction of B56γ with the PP2A A and C subunits (Fig. 2C), To test this hypothesis, we generated two mutations with smaller side chains, C39S and C39A, and tested whether these changes could rescue the interaction of B56γ with the AC core in vivo (Fig. 2, A and B). The assays show that both C39S and C39A mutants only slightly rescued the PP2A core interaction in U2OS cells, suggesting that the cysteine residue, but not just the size of its side chain, is specifically required for maintenance of proper HEAT repeat 1 conformation and for the PP2A AC core interaction. Because Cys-39 is located in a hydrophobic region and is not involved in any S–S bridges, it may play a role in holding adjacent helices together through van der Waals interactions with hydrophobic residues from helix-2 (Leu-63 and Met-66), -3 (Val-85 and Met-88), and -4 (Val-127 and Phe-130) (Fig. 2C). Nevertheless, these data further support our finding of the importance of HEAT repeat 1 in PP2A holoenzyme.
B56γ HEAT Repeat 1 Mutants Abate B56γ-PP2A Tumor-suppressive Function
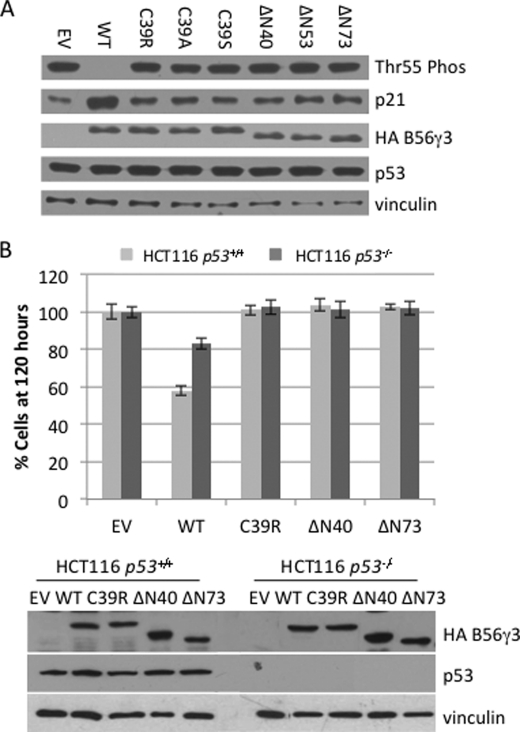
Next, we tested the impact of HEAT repeat 1 mutants on B56γ-PP2A tumor-suppressive function. We previously showed that B56γ-PP2A dephosphorylates p53 at Thr-55, leading to p53 activation, induction of p53 transcriptional target p21, and cell growth arrest (5). We thus first assessed the ability of the C39R mutant to promote p53 Thr-55 dephosphorylation and p21 induction. As shown in Fig. 3A, overexpression of wild type B56γ led to an efficient dephosphorylation of p53 at Thr-55. As a consequence of this dephosphorylation, p21 induction was clearly observed. Overexpression of C39R, however, was unable to promote p53 Thr-55 dephosphorylation and p21 induction, indicating that this mutant lost its ability to direct PP2A phosphatase activity toward substrate p53. Similarly, none of the HEAT repeat 1 deletion mutants tested were able to promote Thr-55 dephosphorylation or p21 induction, suggesting the importance of HEAT repeat 1 in directing B56γ-PP2A phosphatase activity toward p53. Because the C39R mutation was identified in a tumor sample, those results also indicated that this mutant might potentially contribute to a cancer phenotype by blocking B56γ-PP2A tumor-suppressive function.
FIGURE 3.
HEAT repeat 1 mutants of B56γ fail to inhibit cell proliferation. A, lysates of U2OS cells transfected with HA-tagged wild type B56γ (WT), C39R, C39A, C39S, ΔN40, ΔN53 and ΔN73, or a control empty vector (EV) were analyzed by Western blot for p53 Thr-55 dephosphorylation (Thr55 Phos), p21, HA-B56γ, p53, and vinculin. B, representative data of cell proliferation of the HCT116 human colon cancer cell lines transfected with HA-tagged wild type B56γ, C39R, ΔN40, or ΔN73, or a control empty vector. The number of cells present at the 120 h time point was normalized against the empty vector controls and plotted in a bar graph. Error bars show average ± S.E. from triplicate plates in one representative experiment. Cell lysates were analyzed by immunoblotting of transfected HA-B56γ and p53 proteins.
To test this directly, we assessed the effect of C39R on cell proliferation. Overexpression of wild type B56γ has been previously shown to inhibit cell proliferation in HCT116 cells in either p53−/− or p53+/+ background (5). We thus transfected those cells with the C39R construct and compared its ability to inhibit cell growth with the wild type protein. As shown in Fig. 3B and supplemental Fig. S2, overexpression of wild type B56γ in the presence of p53 led to an ∼40% decrease in cell number as compared with control empty vector after 120 h of cell growth, whereas in the absence of p53, overexpression of wild type B56γ had a decreased effect on cell proliferation, with a 20% decrease in cell number. By comparison, overexpression of C39R showed no significant difference from control (p = 0.75 and p = 0.57, respectively), suggesting that this mutant had no effect on blocking cell proliferation in both p53−/− and p53+/+ background. The presence of B56γ and p53 at the analysis was verified by immunoblotting (Fig. 3B). These results indicate that the C39R mutant was no longer able to inhibit cell proliferation regardless of p53 status. To further assess the role of the HEAT repeat 1 domain in inhibition of cell proliferation, we also overexpressed the HEAT repeat 1 deletion mutant ΔN40 and ΔN73 in HCT116 cells (Fig. 3B and supplemental Fig. S2). Our results show that overexpression of those mutants also led to no inhibition of cell proliferation, suggesting the importance of the HEAT repeat 1 domain in PP2A tumor-suppressive function.
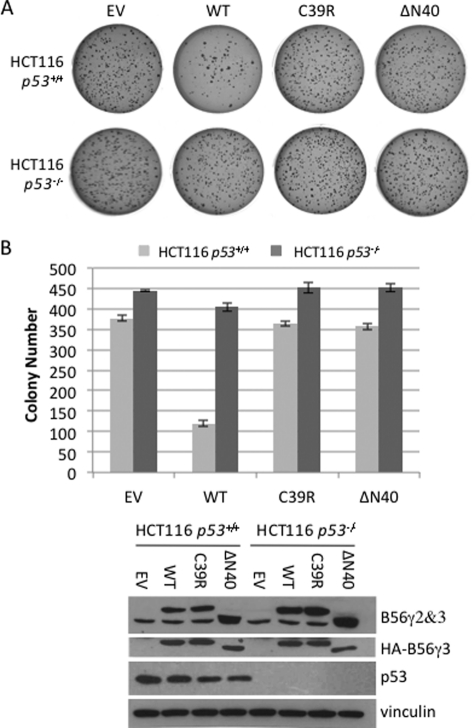
In addition to blocking cell proliferation, overexpression of wild type B56γ has also been shown to inhibit anchorage-independent cell growth (5). We thus tested the effect of C39R on this activity of B56γ. As shown in Fig. 4 and supplemental Fig. S2, overexpression of wild type B56γ3 in HCT116 cells with p53 significantly decreased the number of colonies from ∼375 colonies to ∼120 colonies, whereas in HCT116 cells lacking p53, overexpression of wild type B56γ3 decreased the number of colonies from 450 to 400 on the agar. By comparison, overexpression of C39R showed no significant difference from control empty vector (p = 0.28 and p = 0.65 respectively), suggesting that this mutant had no effect on colony formation in both cell lines. The presence of B56γ and p53 at the analysis was verified by immunoblotting (Fig. 4B). These results indicate that C39R lost both p53-dependent and p53-independent tumor-suppressive activity. Further, overexpression of the smallest HEAT repeat 1 deletion mutant ΔN40 also had no effect on colony formation in both cell lines (Fig. 4 and supplemental Fig. S2). Together, these data confirm the importance of the HEAT repeat 1 domain in B56γ-PP2A tumor-suppressive function. Our results of the functional consequences of a cancer-associated mutation in the B56γ gene also provide an additional mechanism to inactivate B56γ-PP2A in cancer.
FIGURE 4.
HEAT repeat 1 mutants of B56γ fail to inhibit anchorage-independent cell growth. A, representative data of anchorage-independent growth of HCT116 human colon cancer cell lines transfected with HA-tagged wild type B56γ (WT), C39R, or ΔN40 or a control empty vector (EV). B, colony numbers for anchorage-independent growth were counted and represented on a bar graph. The values are the averages ± S.E. from three experiments. Cell lysates were analyzed by immunoblotting of transfected or endogenous B56γ, p53, and vinculin proteins.
DISCUSSION
In this study, we show that the HEAT repeat 1 motif of B56γ plays a critical role in assembly of the B56γ-PP2A holoenzyme. Importantly, we identified a tumor-associated missense mutation, C39R, within the HEAT repeat 1 motif. Although this mutation was found in a pooled glandular tumor sample, its effect on B56γ-PP2A tumor-suppressive function had not been previously determined. We showed that the mutant protein, although retaining binding to its substrates, is no longer able to interact with the AC core. As a consequence, C39R lost its ability to support both p53-dependent and p53-independent B56γ-PP2A tumor-suppressive function. These results suggest a novel mechanism behind a cancer-associated loss of function mutation in the PP2A B56γ subunit gene and provide evidence for the importance of the HEAT repeat 1 motif in B56γ-PP2A tumor-suppressive function.
Study of the crystal structure has revealed that the regulatory B56γ subunit has 18 α-helices with pairs of antiparallel α-helices forming eight pseudo-HEAT repeat motifs stacking against each other. Previous studies have shown that the B56γ subunit is involved in extensive interactions with both A and C subunits mediated by a number of conserved residues mainly located in the loops connecting these HEAT repeats (16, 17). In this study, we examined the role of HEAT repeat 1 in PP2A holoenzyme formation. Although the crystal structures have shown that HEAT repeat 1 is not directly involved in binding with either the A or the C subunits, deletion of this region prevented the holoenzyme assembly both in vivo and in vitro (Fig. 1). The importance of HEAT repeat 1 is also supported by the result that a single point mutation, C39R, within this region has also lost the AC core interaction. One possible explanation for this disruption is that HEAT repeat 1 (helix-1 and -2) may play a role in stabilizing HEAT repeat 2 (helix-3 and -4), which contains loop IL2 that is involved in interacting with the A and C subunits. Examining the helices of the B56γ subunit indicates interactions among the helices within each HEAT repeat and between two adjacent HEAT repeats including HEAT repeat 1 and HEAT repeat 2. Therefore, when HEAT repeat 1 is disrupted by mutations, the stacking of HEAT repeat 1 with HEAT repeat 2 may change, leading to a position shift of the interacting motif at the tip of IL2 in HEAT repeat 2 and therefore affecting the interaction of B56γ with the PP2A AC core. In the case of C39R mutation, the cysteine residue at position 39 may play a role in holding adjacent helices together so that HEAT repeat 1 is in a proper position to stack with HEAT repeat 2 through van der Waals interactions with residues from helix-3 and -4. This cysteine-specific interaction network seems to be indispensable for the proper coordination of HEAT repeat 2 as mutation of cysteine to another residue also caused significant weakening of the interaction with the AC core. Indeed, our study reveals that neither alanine nor serine substitution at residue 39 could rescue the binding deficiency despite their side-chain size being comparable with cysteine. These results suggest that point mutation at Cys-39 rearranges the alignment of the helices between HEAT repeats 1 and 2, resulting in the position shift of the interacting motif at the tip of the extended IL2 loop and therefore weakening its interaction with the AC core. Naturally, arginine substitution introduces two dramatic changes as the arginine side chain is larger in size and is positively charged. This has likely led to a much more significant repositioning of helix-3, forcing the tip of the extended IL2 to be pushed out of position. Together, those results suggest that Cys-39 is important for stable HEAT repeat 1 and the proper positioning of HEAT repeat 2 helices, allowing B56γ to form a stable complex with the PP2A AC core. Perhaps it is worthwhile to mention that the cysteine residue is conserved among all B56 family members, suggesting its importance in maintaining the rigid alignment of the helices for all B56-PP2A holoenzyme assembly.
B56γ has been described to inhibit cell transformation in both a p53-dependent and a p53-independent manner (5). Previously, we identified two B56γ mutations, F395C from lung cancer and A383G from intestinal tumor, which specifically disrupted B56γ-p53 interaction and thus specifically inactivated the p53-dependent tumor-suppressive function of B56γ (15). However, because these mutants retain their ability to interact with the PP2A AC core, they continue to support the p53-independent tumor-suppressive function of B56γ (15). In this study, we identified a new cancer-associated mutant in the B56γ gene, C39R, which, unlike F395C and A383G, prevented B56γ from binding to the PP2A AC core. As a consequence, this mutant is no longer able to mediate any B56γ-mediated PP2A dephosphorylation and thus inactivated both p53-dependent and p53-independent tumor-suppressive function of B56γ-PP2A. These data thus provide a new, and perhaps more important, molecular basis for inactivating tumor suppressor B56γ in cancer. Given the fact that p53 is highly mutated in human cancers, this mechanism may have a broader impact on tumor-suppressive function of B56γ. Interestingly, several mutations in the PP2A A subunit have been reported to abolish interaction with the B56 subunit in human carcinoma (12, 14). Furthermore, several viral proteins such as SV40 virus small t antigen as well as polyoma virus small t and middle T antigen have been reported to cause cancer by dissociating B56 subunits from the PP2A AC core (10, 11). These data suggest that displacement of the B56γ subunit from PP2A holoenzyme probably represents a more common mechanism for inactivation of tumor-suppressive function B56γ in cancer. It will be interesting to further investigate the role of HEAT repeat 1 in the displacement of B56γ from PP2A by oncoproteins.
Supplementary Material
Acknowledgments
We are very grateful to Dr. J. A. Traugh, Dr. F. M. Sladek, and members of our laboratory for valuable suggestions and discussions. We thank Bob Zhao for assistance on constructing C39A plasmid and Dr. D. Borchardt for assistance on performing CD analysis.
This work was supported, in whole or in part, by National Institutes of Health Grant CA0751 (to X. L.).

This article contains supplemental Figs. S1 and S2.
- PP2A
- protein phosphatase 2A
- HEAT
- Huntington-elongation-A subunit-TOR
- TOR
- target of rapamycin.
REFERENCES
- 1. Eichhorn P. J., Creyghton M. P., Bernards R. (2009) Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta 1795, 1–15 [DOI] [PubMed] [Google Scholar]
- 2. Virshup D. M., Shenolikar S. (2009) From promiscuity to precision: protein phosphatases get a makeover. Mol. Cell 33, 537–545 [DOI] [PubMed] [Google Scholar]
- 3. Csortos C., Zolnierowicz S., Bakó E., Durbin S. D., DePaoli-Roach A. A. (1996) High complexity in the expression of the B′ subunit of protein phosphatase 2A. J. Biol. Chem. 271, 2578–2588 [DOI] [PubMed] [Google Scholar]
- 4. McCright B., Brothman A. R., Virshup D. M. (1996) Assignment of human protein phosphatase 2A regulatory subunit genes b56alpha, b56beta, b56gamma, b56delta, and b56epsilon (PPP2R5A-PPP2R5E), highly expressed in muscle and brain, to chromosome regions 1q41, 11q12, 3p21, 6p21.1, and 7p11.2→p12. Genomics 36, 168–170 [DOI] [PubMed] [Google Scholar]
- 5. Li H. H., Cai X., Shouse G. P., Piluso L. G., Liu X. (2007) A specific PP2A regulatory subunit, B56γ, mediates DNA damage-induced dephosphorylation of p53 at Thr-55. EMBO J. 26, 402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold H. K., Sears R. C. (2006) Protein phosphatase 2A regulatory subunit B56α associates with c-Myc and negatively regulates c-Myc accumulation. Mol. Cell Biol. 26, 2832–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeh E., Cunningham M., Arnold H., Chasse D., Monteith T., Ivaldi G., Hahn W. C., Stukenberg P. T., Shenolikar S., Uchida T., Counter C. M., Nevins J. R., Means A. R., Sears R. (2004) A signaling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat. Cell Biol. 6, 308–318 [DOI] [PubMed] [Google Scholar]
- 8. Forester C. M., Maddox J., Louis J. V., Goris J., Virshup D. M. (2007) Control of mitotic exit by PP2A regulation of Cdc25C and Cdk1. Proc. Natl. Acad. Sci. U.S.A. 104, 19867–19872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Margolis S. S., Perry J. A., Forester C. M., Nutt L. K., Guo Y., Jardim M. J., Thomenius M. J., Freel C. D., Darbandi R., Ahn J. H., Arroyo J. D., Wang X. F., Shenolikar S., Nairn A. C., Dunphy W. G., Hahn W. C., Virshup D. M., Kornbluth S. (2006) Role for the PP2A/B56δ phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell 127, 759–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen W., Possemato R., Campbell K. T., Plattner C. A., Pallas D. C., Hahn W. C. (2004) Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5, 127–136 [DOI] [PubMed] [Google Scholar]
- 11. Chen Y., Xu Y., Bao Q., Xing Y., Li Z., Lin Z., Stock J. B., Jeffrey P. D., Shi Y. (2007) Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nat. Struct. Mol. Biol. 14, 527–534 [DOI] [PubMed] [Google Scholar]
- 12. Chen W., Arroyo J. D., Timmons J. C., Possemato R., Hahn W. C. (2005) Cancer-associated PP2A Aα subunits induce functional haploinsufficiency and tumorigenicity. Cancer Res. 65, 8183–8192 [DOI] [PubMed] [Google Scholar]
- 13. Jones S., Wang T. L., Shih IeM., Mao T. L., Nakayama K., Roden R., Glas R., Slamon D., Diaz L. A., Jr., Vogelstein B., Kinzler K. W., Velculescu V. E., Papadopoulos N. (2010) Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 330, 228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ruediger R., Pham H. T., Walter G. (2001) Disruption of protein phosphatase 2A subunit interaction in human cancers with mutations in the Aα subunit gene. Oncogene 20, 10–15 [DOI] [PubMed] [Google Scholar]
- 15. Shouse G. P., Nobumori Y., Liu X. (2010) A B56γ mutation in lung cancer disrupts the p53-dependent tumor suppressor function of protein phosphatase 2A. Oncogene 29, 3933–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho U. S., Xu W. (2007) Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature 445, 53–57 [DOI] [PubMed] [Google Scholar]
- 17. Xu Y., Xing Y., Chen Y., Chao Y., Lin Z., Fan E., Yu J. W., Strack S., Jeffrey P. D., Shi Y. (2006) Structure of the protein phosphatase 2A holoenzyme. Cell 127, 1239–1251 [DOI] [PubMed] [Google Scholar]
- 18. Shouse G. P., Cai X., Liu X. (2008) Serine 15 phosphorylation of p53 directs its interaction with B56γ and the tumor suppressor activity of B56γ-specific protein phosphatase 2A. Mol. Cell Biol. 28, 448–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Letourneux C., Rocher G., Porteu F. (2006) B56-containing PP2A dephosphorylate ERK, and their activity is controlled by the early gene IEX-1 and ERK. EMBO J. 25, 727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bennin D. A., Don A. S., Brake T., McKenzie J. L., Rosenbaum H., Ortiz L., DePaoli-Roach A. A., Horne M. C. (2002) Cyclin G2 associates with protein phosphatase 2A catalytic and regulatory B′ subunits in active complexes and induces nuclear aberrations and a G1/S phase cell cycle arrest. J. Biol. Chem. 277, 27449–27467 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.