Background: Tangle distribution is largely overlapped with zinc-containing glutamatergic neurons. Synaptically released zinc may be involved in tau hyperphosphorylation.
Results: Increased synaptic activity induces tau hyperphosphorylation by synaptic zinc through PP2A inhibition.
Conclusion: Synaptic activity promotes tau hyperphosphorylation, and synaptically released zinc plays a central role.
Significance: Therapies targeted to maintaining zinc homeostasis and moderating synaptic activity may benefit AD by reducing tauopathy.
Keywords: Phosphorylation, PP2A, Synapses, Tau, Zinc
Abstract
Hyperphosphorylated tau is the major component of neurofibrillary tangles in Alzheimer disease (AD), and the tangle distribution largely overlaps with zinc-containing glutamatergic neurons, suggesting that zinc released in synaptic terminals may play a role in tau phosphorylation. To explore this possibility, we treated cultured hippocampal slices or primary neurons with glutamate or Bic/4-AP to increase the synaptic activity with or without pretreatment of zinc chelators, and then detected the phosphorylation levels of tau. We found that glutamate or Bic/4-AP treatment caused tau hyperphosphorylation at multiple AD-related sites, including Ser-396, Ser-404, Thr-231, and Thr-205, while application of intracellular or extracellular zinc chelators, or blockade of zinc release by extracellular calcium omission almost abolished the synaptic activity-associated tau hyperphosphorylation. The zinc release and translocation of excitatory synapses in the hippocampus were detected, and zinc-induced tau hyperphosphorylation was also observed in cultured brain slices incubated with exogenously supplemented zinc. Tau hyperphosphorylation induced by synaptic activity was strongly associated with inactivation of protein phosphatase 2A (PP2A), and this inactivation can be reversed by pretreatment of zinc chelator. Together, these results suggest that synaptically released zinc promotes tau hyperphosphorylation through PP2A inhibition.
Introduction
Alzheimer disease (AD)2 is one of the most common neurodegenerative disorders that cause adult dementia (1). It is characterized by the presence of extracellular amyloid plaques and intracellular neurofibrillary tangles (NFTs) within afflicted brains. Hyperphosphorylated and aggregated microtubule-associated protein tau is the major component of NFTs (2, 3). It is believed that hyperphosphorylated tau loses the ability to bind with and stabilize microtubules, dissociates from microtubules, and aggregates into paired helical filaments (PHF) (4, 5), the latter, precedes the formation of insoluble NFTs. Recently, researchers found that phosphorylation of tau promoted tau mislocalization to dendritic spines and disruption of synaptic function, as well as early memory deficits before overt synaptic or neuronal degeneration (6). Thus, tau phosphorylation seems to be an early event in the development of AD, although until now the mechanisms underlying tau hyperphosphorylation have not been fully elucidated.
In AD patients and many AD animal models, a high incidence of epilepsy was observed, indicating that abnormal synaptic activity is involved in AD pathogenesis (7–9). Glutamatergic neurons are the major excitatory neurons in AD-susceptible brain regions such as hippocampus, entorhinal, and temporal cortex, which are overloaded with NFTs. Recent studies suggested a positive role of glutamatergic excitatory neurotransmission in Aβ oligomer formation and accumulation at synapses (10), which is mediated by synaptically released zinc. Neurons with synapses which release zinc and glutamate together are called “gluzinergic” neurons. Because most of the gluzinergic neurons have their cell bodies located in either the cerebral cortex or the limbic structures of the forebrain (11), where tauopathy and neurodegeneration occur, we suspect that zinc released in excitatory neurotransmission may be also involved in tau hyperphosphorylation.
Our previous research has found that exogenous zinc application could activate extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), p38, and glycogen synthase kinase 3β (GSK-3β), which are consequently involved in changes in tau in cultured SH-SY5Y cells (12). Of all these kinases, GSK-3β is the most widely investigated kinase inducing tau hyperphosphorylation (13, 14). At the same time, protein phosphatase 2A (PP2A) is the most important phosphatase involved in tau dephosphorylation (15, 16). The relationship between synaptically released zinc and PP2A activity has not been clarified. In the present study, we used cultured competent brain slices and primary neurons as experimental models to explore the effects of synaptically released zinc on tau phosphorylation and the underlying mechanisms. Results showed that synaptic activity dramatically enhanced tau hyperphosphorylation both in cultured hippocampal slices and primary neurons. Hyperphosphorylation of tau was prevented by blockade of synaptic transmission or zinc release, or by zinc chelation. PP2A inhibition, but not GSK-3β activation, plays an important role in synaptic zinc-induced tau hyperphosphorylation.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Rabbit polyclonal antibodies (pAb) pS396, pS404, pT231, and pT205 (1:1000) against tau phosphorylated at corresponding sites were from Signalway Antibody (Pearland, TX). Polyclonal antibodies against PP2A C subunit (1:1000), total GSK-3 (1:1000), phosphorylated GSK-3β at Ser-9 (1:1000), total and phosphorylated Erk1/2 (1:1000) at Thr202/Tyr204, phosphorylated p38 (1:500) at Thr180/Tyr182, and total JNK (1:1000) were from Cell Signaling Technology (Beverly, MA). PAb against p-JNK (1:1000) at Thr183/Tyr185 was from BIOSOURCE (Camarillo, CA). PAb of Y307-PP2A and monoclonal antibody (mAb) of total p38 (1:1000) were from Abcam (Cambridge, UK). MAb against DM1A (1:2000) was purchased from Sigma, demethyl-PP2A C (1:500) was from Millipore (Billerica, MA), and AT8 PHF-tau (Ser202/Thr205) was from Innogenetics (Ghent, Belgium). Rabbit pAb R134d (1:1000) against total tau was a generous gift from Drs. K. Iqbal and I. Grundke-Iqbal (New York State Institute for Basic Research, Staten Island, NY). Clioquinol (5-chloro-7-iodo-8-hydroxyquinoline, CQ, 50 μm) was from Merck (KGaA, Darmstadt, Germany). Ca-EDTA (1 mm), glutamate (100 μm for brain slices and 20 μm for cultured neurons), bicucullin (50 μm), 4-aminopyridine (4-AP, 250 μm), APV (50 μm), nimodipine (10 μm), and SP600125 (10 μm) were all from Sigma. CNQX (10 μm) and SB216763 (10 μm) were from Tocris Bioscience (Bristol, UK). Tetrodotoxin (TTX) was purchased from Ruifang Bio-company (Dalian, Liaoning, China). d-Erythro-S (DES) was from Calbiochem (San Diego, CA). PD98059 (10 μm) was from Cell Signaling Technology, and SB202190 (10 μm) was from Millipore. Neurobasal and B27 were from Invitrogen (Grand Island, NY). The PP2A assay kit was from Promega (Fitchburg, WI).
Rat Brain Slice Culture and Treatment
Rat hippocampal slices were prepared following the procedures previously described (17). Sprague-Dawley rats (male, 150–200 g) were supplied by Experiment Animal Center of Tongji Medical College, Huazhong University of Science and Technology. Rats were decapitated when they were deeply anesthetized. Brains were rapidly removed and put into oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (aCSF) containing 126 mm NaCl, 3.5 mm KCl, 1.2 mm NaH2PO4, 1.3 mm MgCl2, 2.0 mm CaCl2, 11 mm d(+)-glucose, and 25 mm NaHCO3, pH 7.4, for 7–8 min at 4 °C. The hippocampus was separated and mounted on a vibratome holder. Coronal hippocampal slices (400 μm thick) were sectioned with a Mcllwain Tissue Chopper (The Mickle Laboratory Engineering Co. Ltd, Gomshall, Surrey, UK). After 30 min of equilibration at room temperature, the brain slices were transferred into incubating chambers with aCSF bubbled with 95% O2 and 5% CO2 at 33 °C for different treatments. At the end of the incubation, brain slices were harvested and homogenized in buffer containing 50 mm Tris-HCl, pH 7.0, 1.0 mm PMSF, 1.0 mm EDTA, 10 mm β-mercaptoethanol, 1:200 protease inhibitors mixture at a ratio 1:9 (g/ml). Homogenates were divided into two parts. One was centrifuged at 16,000 × g for 10 min at 4 °C, and the supernatant was used for activity assays. For the other half, equal volumes of phosphate inhibitor mixture (2.0 mm Na3VO4 and 100 mm NaF, pH 7.0) were immediately added, and the homogenates were stored at −80 °C for Western blotting.
Primary Neuronal Culture and Immunofluorescence
Primary hippocampal neurons were isolated from embryonic E18 Sprague-Dawley rats. Briefly, embryonic hippocampus were dissected, dissociated, and incubated with 5 ml of D-Hanks containing 0.25% trypsin for 5 min, centrifuged at 1000 × g for 5 min after addition of 4 ml of the neuronal plating medium containing DMEM/F12 with 10% fetal bovine serum. Then the cells were resuspended and plated onto 12-well plates for Western blotting or glass cover slips for cell imaging. Both the plates and the glass cover slips were previously coated with poly-d-lysine. The neurons were then put into a humidified incubator with 5% CO2 at 37 °C. The medium was changed to Neurobasal medium supplemented with 2% B27 (maintenance medium) after 2–4 h. The cells were cultured for 9–11 days for treatment. During the culture, the medium was half-changed every 3 days with fresh maintenance medium.
After treatment, the primary hippocampal neurons were quickly fixed with 4% paraformaldehyde for 15 min, permeabilized in 0.1% Triton X-100 for 15 min, followed by incubation with 3% bovine serum albumin (BSA) to block nonspecific sites. Primary antibody against AT8 PHF-tau (Ser202/Thr205, 1:200) incubation was performed overnight at 4 °C. Alexa 488-conjugated anti-mouse secondary antibody (1:200) was used for fluorescence labeling. The imaging was observed with the LSM710 confocal microscope (Zeiss, Germany).
Western Blotting
For Western blotting, samples were boiled at 100 °C for 5 min in the loading buffer (50 mm Tris-HCl, pH 7.6, 2% SDS, 10% glycerol, 10 mm DTT, and 0.2% bromphenol blue). The proteins were electrophoresed in 10% SDS-polyacrylamide gel and the separated proteins transferred to nitrocellulose membranes (Amersham Biosciences). The membranes were then blocked with 5% nonfat milk dissolved in TBS Tween-20 (50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 0.2% Tween-20) for 1 h and probed with primary antibody at 4 °C overnight. Then the blots were detected using anti-rabbit or anti-mouse IgG conjugated to IRDyeTM (800CW; Licor Biosciences, Lincoln, NE) for 1 h at room temperature and visualized using the Odyssey Infrared Imaging System (LicorBiosciences, Lincoln, NE). The protein bands were quantitatively analyzed by Kodak Digital Science 1D software (Eastman Kodak Company, New Haven, CT). The levels of the phosphorylated proteins were normalized by the corresponding total protein levels.
Detection of Zinc Concentration in the aCSF
The zinc concentration in the aCSF was analyzed by atomic absorption spectrophotometry. At the end of brain slice incubation, 3 ml of aCSF were collected for each sample; three replicates (1 ml per sample) were analyzed using atomic absorption spectrophotometer AA-240FS from Varian (Palo Alto, CA).
PP2A Activity Assay
PP2A activity was measured according to the protocol provided by the manufacturer (V2460 kit, Promega). First, endogenous free phosphate was removed from the supernatants, and then the extracts were normalized for protein content, 5 μg of protein samples in triplicates were incubated with a chemically synthesized phosphopeptide (RRA(pT)VA), an optimal substrate for PP2A, PP2B, and PP2C, not for PP1 in the buffer optimized for PP2A activity while cation-dependent PP2B and PP2C were inhibited for 30 min at 33 °C. Phosphate released from the substrate was detected by measuring the absorbance of a molybdate-malachite green-phosphate complex at 630 nm. PP2A activity was calculated by the release of phosphate per μg of protein and per minute (pmol/μg/min).
Statistical Analysis
Values are presented as mean ± S.D. All individual experiments were repeated at least three times. Changes were analyzed by ANOVA followed by post hoc comparisons using Tukey's test or Student's t test. The significance was assessed at p < 0.05. All results shown correspond to individual representative experiments.
RESULTS
Increased Synaptic Activity Induces Tau Hyperphosphorylation at Multiple AD-related Sites in Hippocampal Slices
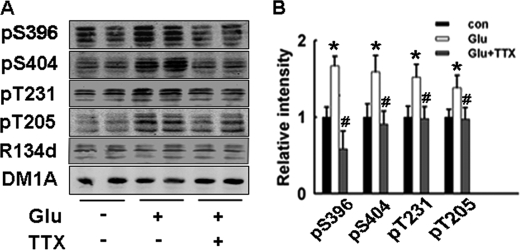
First we explored the effect of increased synaptic activity on tau phosphorylation in cultured rat hippocampal brain slices. The hippocampal brain slices were incubated with glutamate (100 μm) (10) for 20 min to increase the synaptic activity, with or without the pretreatment of TTX (3 μm, 20 min). Glutamate induced tau hyperphosphorylation at several AD-like tau hyperphosphorylation sites such as Ser-396, Ser-404, Thr-205, and Thr-231. No significant change of the total tau level was observed (R134d). Preincubation of the slices with 3 μm TTX to block the synaptic activity before the addition of glutamate completely blocked tau hyperphosphorylation (Fig. 1). Prolonged incubation with glutamate for up to 1 h still resulted in strong tau phosphorylation at most studied sites (supplemental Fig. S1). These results indicate that the tau phosphorylation state is modulated by synaptic activity; increased activity of glutamatergic neurons may induce tau hyperphosphorylation.
FIGURE 1.
Increased synaptic activity induces tau hyperphosphorylation at multiple AD-related sites in cultured hippocampal slices. A, rat hippocampal brain slices were incubated with glutamate (100 μm, to increase synaptic activity) for 20 min, with or without the pretreatment of TTX (3 μm, synaptic transmission blocker). Tau phosphorylation levels at Ser-396, Ser-404, Thr-205, and Thr-231 were detected by Western blotting. B, quantitative analysis of the blots in A. Tau phosphorylation levels at different sites were normalized by total tau level recognized by R134d.*, p < 0.05 versus control slices; #, p < 0.05 versus glutamate-treated slices.
Synaptic Activity-induced Tau Hyperphosphorylation Is Dependent on Zinc Release
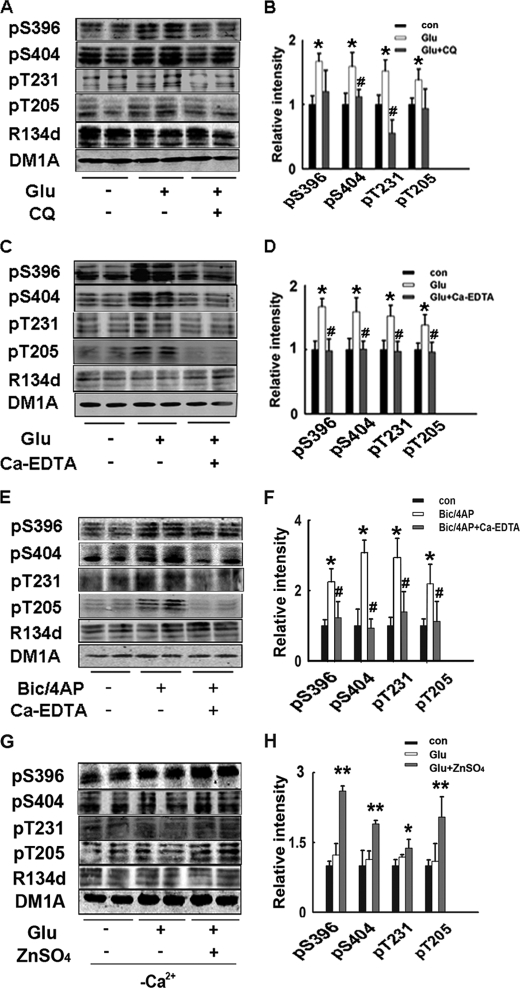
Zinc is enriched in synaptic vesicles together with glutamate; multiple research studies have demonstrated that zinc is co-released with glutamate in the synaptic cleft (18, 19). Zinc can also accelerate the aggregation of a tau peptide (20). Thus, we hypothesized that a local increase in zinc concentration as a result of synaptic activity may promote tau hyperphosphorylation at the excited neurons. To test this hypothesis, we used the zinc-binding 8OH-quinoline clioquinol (CQ), a membrane-permeable zinc chelator, which has been shown to decrease Aβ deposition and improve cognitive deficits in AD animal models (21). Preincubation of the slices with 50 μm CQ for 30 min before the addition of glutamate blocked the increase in tau phosphorylation (Fig. 2, A and B), indicating that endogenous zinc is involved in the synaptic activity-induced tau hyperphosphorylation.
FIGURE 2.
Synaptic activity-induced tau hyperphosphorylation is dependent on endogenously released zinc. Rat hippocampal brain slices were incubated with glutamate (100 μm) for 20 min, with or without the pretreatment of membrane-permeable zinc chelator CQ (50 μm) (A) or membrane-impermeable zinc chelator Ca-EDTA (1 mm) (C), tau phosphorylation levels were detected by Western blotting. B and D, quantitative analysis of the blots in A and C. Tau phosphorylation levels were normalized by total tau levels recognized by R134d, *, p < 0.05 versus control slices; #, p < 0.05 versus glutamate-treated slices. E, rat hippocampal brain slices were incubated with Bic/4-AP (50 μm/250 μm, another experimental model to increase synaptic activity) for 3 h, with or without the pretreatment of Ca-EDTA (1 mm). Tau phosphorylation levels were detected by Western blotting. F, quantitative analysis of blots in E, *, p < 0.05 control versus slices; #, p < 0.05 versus Bic/4-AP-treated slices. G, rat hippocampal brain slices were incubated with glutamate (100 μm) for 20 min in aCSF without Ca2+, with or without addition of ZnSO4 (100 μm). Tau phosphorylation levels were detected by Western blotting. H, quantitative analysis of blots in G, *, p < 0.05; **, p < 0.01 versus control slices.
To further identify whether the synaptically released zinc or the motivated intracellular zinc mediates tau hyperphosphorylation, we used another membrane-impermeable zinc chelator Ca-EDTA (1 mm) to treat the slices 10 min before the administration of glutamate. As is shown in Fig. 2, C and D, Ca-EDTA at a concentration that only chelates extracellular zinc (22) completely blocked tau hyperphosphorylation induced by glutamate, suggesting that increased extracellular zinc by synaptic activity up-regulates tau phosphorylation.
To confirm that the tau phosphorylation state is modulated by synaptically released zinc in neurotransmission, we used another established model of enhanced synaptic activity by using the GABAA receptor antagonist bicuculline (Bic). This treatment results in synchronized bursts of action potentials (APs), which are associated with activation of NMDA receptors, as well as zinc release from synaptic vesicles. A weak potassium channel blocker 4-aminopyridine (4-AP) was co-applied with bicuculline to enhance burst frequency (23), herein labeled Bic/4-AP. Consistent with previous findings, treatment of Bic/4-AP for 3 h induced tau hyperphosphorylation at the same phosphorylation sites. Preincubation of the slices with Ca-EDTA completely blocked the increase of the tau phosphorylation level (Fig. 2, E and F).
Zinc release is calcium-dependent; omission of Ca2+ from bathing slices reduced zinc release by more than 70% (24), so we explored the effect of glutamate on tau phosphorylation in slices cultured in aCSF without Ca2+. In the aCSF withdrawal of Ca2+ glutamate treatment no longer induced marked tau hyperphosphorylation. With the addition of exogenous zinc sulfate (100 μm), tau hyperphosphorylation was re-observed (Fig. 2, G and H). This result further confirmed that tau hyperphosphorylation was mediated by synaptic released zinc.
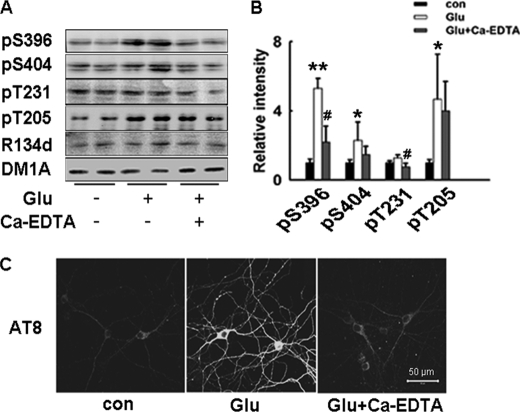
Last, we used the cultured hippocampal primary neurons as an experimental model to identify the effect of synaptically released zinc on tau phosphorylation. Glutamate treatment (20 μm, with addition of 10 μm glycine, dose-dependent effects are showed in supplemental Fig. S2) induced a similar change of tau hyperphosphorylation in primary neurons; while Ca-EDTA preincubation restored the tau phosphorylation to control levels at most studied sites (Fig. 3, A and B). Compared with cultured brain slices, tau phosphorylation levels at some sites (Ser-404, Thr-205) in primary neurons showed a larger variation upon the treatment, possibly because tau phosphorylation in developing neurons in different stages is not as steady as that in mature neurons (3, 25). This big variation may also explain that Ca-EDTA showed no significant blocking to the effect of Glu on pT205, pS404 in primary neurons, even though there was such a tendency. In normal control neurons, weak staining of phosphorylated tau (AT8, recognizes PHF-tau at Ser-202/Thr-205) was observed in the cell body and a few axon-like neurites, while glutamate treatment induced enhanced tau staining in the cell body and tau hyperphosphorylation both in axons and dendrites. Extracellular zinc chelating by Ca-EDTA reversed tau hyperphosphorylation (Fig. 3C).
FIGURE 3.
Increased synaptic activity induces zinc-dependent tau hyperphosphorylation in cultured hippocampal neurons. A, rat primary hippocampal neurons were incubated with glutamate (20 μm, with 10 μm glycine) for 20 min, with or without the pretreatment of extracellular zinc chelator Ca-EDTA (1 mm). Tau phosphorylation levels were detected by Western blotting. B, quantitative analysis of blots in A, *, p < 0.05; **, p < 0.01 versus untreated neurons, #, p < 0.05 versus glutamate-treated neurons. C, hippocampal neurons were treated as described in A, then cells were fixed and immunostained with AT-8 (phosphorylated tau at Ser202/Thr205). Scale bar: 50 μm.
Increased Synaptic Activity Induces Zinc Release and Translocation in Cultured Brain Slices
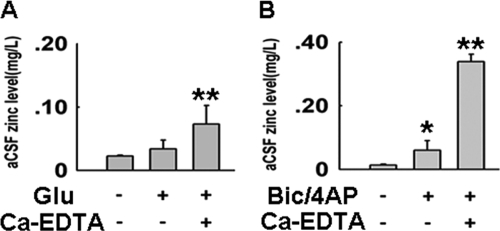
To confirm that zinc is released during excitatory synaptic neurotransmission in our experimental models, we measured total zinc level in the aCSF by atomic absorption spectrophotometry after glutamate or Bic/4-AP incubation of the brain slices. Glutamate treatment for 20 min induced a slight increase in zinc concentration (Fig. 4A), while Bic/4-AP incubation for 3 h resulted in a higher increase of zinc concentration in the aCSF (Fig. 4B), possibly due to the longer incubation with the drugs. A dramatic zinc level increase was observed in both groups when Ca-EDTA was added into the aCSF before glutamate or Bic/4-AP treatment (Fig. 4). Because the detected zinc level is the extracellular total zinc, which includes free zinc ions and Ca-EDTA-chelated zinc, the results strongly indicate that large amounts of zinc ions are released into the aCSF in neurotransmission. When there is no Ca-EDTA chelating, most of the zinc ions undergo re-uptake or influx into the postsynaptic neurons through membrane zinc channels (voltage-gated calcium channels/NMDA/AMPA receptors), Ca-EDTA captures the zinc ions released into the aCSF, then prevents the downstream effects induced by zinc, including tau hyperphosphorylation.
FIGURE 4.
Increased synaptic activity induces zinc release and translocation in cultured hippocampal slices. Zinc levels in aCSF after the hippocampal slices incubation were detected by atomic absorption spectrophotometry. A, zinc levels in aCSF after glutamate treatment with or without preincubation of Ca-EDTA (1 mm, membrane impermeable zinc chelator, which prevents translocation of the released zinc); B, zinc levels in aCSF after Bic/4-AP treatment with or without preincubation of Ca-EDTA. *, p < 0.05; **, p < 0.01 versus control group.
Exogenous Zinc Induces Tau Hyperphosphorylation Directly
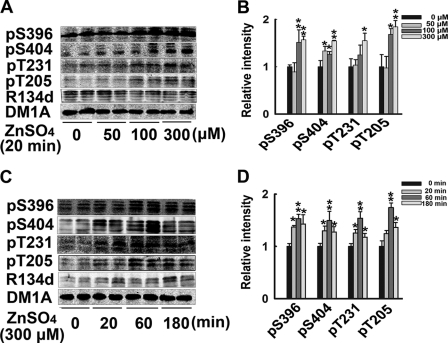
Zinc is released during synaptic activity, reaching extracellular concentrations close to 300 μm (18). To further confirm that synaptic-released zinc mediates tau hyperphosphorylation, we treated the brain slices with different concentrations of ZnSO4 for different times to observe the direct effect of zinc on tau phosphorylation. Zinc treatment induced a time- and dosage-dependent tau phosphorylation at the Ser/Thr sites, which were hyperphosphorylated in neurotransmission. 100 μm ZnSO4 for 20 min was sufficient to induce tau phosphorylation at all the detected sites; concentrations higher than 100 μm induced similar tau hyperphosphorylation (Fig. 5). These results indicated that zinc promotes tau hyperphosphorylation directly. Extracellular zinc may enter into the cells through voltage-gated calcium channels (VGCC), Ca2+-permeable AMPA and NMDA receptor channels (26, 27). We further explored the possible route mediating zinc influx in our experimental model, and found that VGCC was the most implicated channel that mediated the zinc-induced tau hyperphosphorylation (supplemental Fig. S3).
FIGURE 5.
Exogenous zinc induces tau hyperphosphorylation. A, rat hippocampal brain slices were incubated with 0, 50, 100, or 300 μm ZnSO4 for 20 min, tau phosphorylation levels at different sites were detected by Western blotting. B, quantitative analysis of blots in A, *, p < 0.05; **, p < 0.01 versus untreated control slices. C, rat hippocampal brain slices were incubated with 300 μm ZnSO4 for 0, 20 min, 60 min, and 180 min. Tau phosphorylation levels at different sites were detected by Western blotting. D, quantitative analysis of blots in C, *, p < 0.05; **, p < 0.01 versus untreated control slices.
Synaptically Released Zinc Induces Tau Hyperphosphorylation through PP2A Inhibition
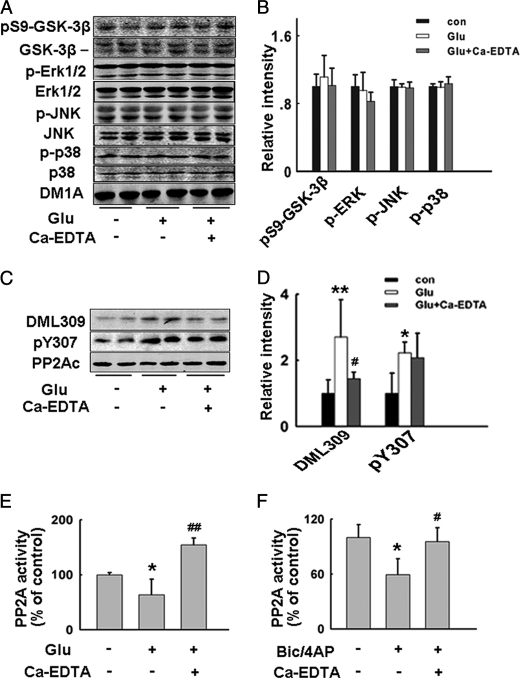
To identify the mechanisms for synaptically released zinc-induced tau hyperphosphorylation, we explored the possible involved tau kinase and phosphatase. Zinc has been reported to modulate the activity of several kinases that may phosphorylate tau, including ERK1/2, JNK, p38, and GSK-3β (12), so we measured the levels of total and activated/inactivated forms of these kinases. No changes of GSK-3β, ERK1/2, JNK, and p38 were observed in brain slices treated with glutamate for 20 min (Fig. 6, A and B). Preincubation of kinase inhibitors: SB216763 (inhibitor of GSK-3), PD98059 (inhibitor of Erk1/2), SP600125 (inhibitor of JNK), and SB202190 (inhibitor of p38) for 30 min did not prevent tau hyperphosphorylation by glutamate (supplemental Fig. S4). These results suggested that tau hyperphosphorylation induced by synaptically released zinc may be mediated by mechanisms other than kinase activation.
FIGURE 6.
Synaptically released zinc inhibits PP2A with no effects on GSK-3β, ERK1/2, JNK, and p38. Rat hippocampal brain slices were incubated with glutamate, with or without the pretreatment of Ca-EDTA. A, total protein levels and active/inactive forms of GSK-3β, ERK1/2, JNK, and p38 were detected by Western blotting. B, quantitative analysis of the blots in A. The phosphorylation levels were normalized by corresponding total protein levels. C, two inactive forms of PP2A (Tyr-307 phosphorylated and Leu-309 demethylated PP2A) levels were detected by Western blotting. D, quantitative analysis of blots in C. Phosphorylation and demethylation levels of PP2A were normalized by total PP2A level. E, PP2A activities in glutamate-treated slices with or without preincubation of Ca-EDTA. *, p < 0.05; **, p < 0.01 versus control slices; #, p < 0.05; ##, p < 0.01 versus glutamate-treated slices. F, rat hippocampal brain slices were incubated with Bic/4-AP, with or without the pretreatment of Ca-EDTA. PP2A activities in slices were detected. *, p < 0.05 versus control slices; #, p < 0.05 versus Bic/4-AP-treated slices.
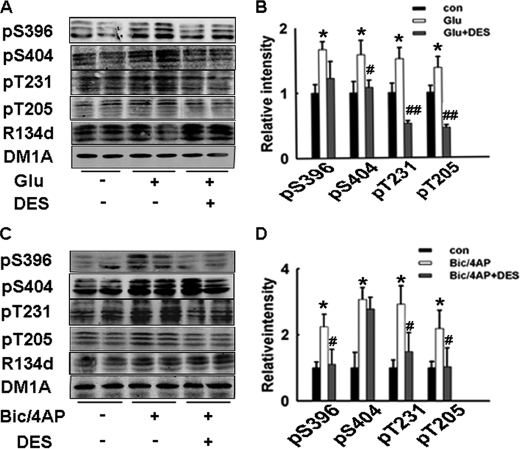
We further explored whether the activity of PP2A changed during glutamatergic neurotransmission, since PP2A accounted for more than 70% dephosphorylation of tau in all identified tau phosphatases (15). To our surprise, a dramatic inhibition of PP2A was induced in brain slices by glutamate incubation. At the same time, the levels of two inactive forms of PP2A (Tyr-307 phosphorylated PP2A and Leu-309 demethylated PP2A) were significantly increased (Fig. 6, C and D), which was consistent with the activity assay result. Ca-EDTA preincubation restored the PP2A activity (Fig. 6E). PP2A inhibition by synaptic zinc was further confirmed in Bic/4-AP-incubated slices (Fig. 6F). These results suggest that synaptically released zinc may induce tau hyperphosphorylation through inhibition of PP2A. To confirm this hypothesis, we used d-erythro-S (DES) to activate PP2A (28) in glutamate- or Bic/4-AP-treated brain slices. We found that preincubation of DES (10 nm, 1 h) could reverse tau hyperphosphorylation at all the Ser/Thr sites detected (Fig. 7). These results suggest that PP2A inhibition plays an important role in synaptic zinc-induced tau hyperphosphorylation.
FIGURE 7.
PP2A agonist DES reverses synaptic activity-induced tau hyperphosphorylation. Rat hippocampal brain slices were incubated with glutamate (100 μm) for 20 min (A), or Bic/4-AP (50 μm/250 μm) for 3 h (C), with or without the pretreatment of DES (10 nm, PP2A activator). Tau phosphorylation levels were detected by Western blotting. B and D, quantitative analysis of the blots in A and C. Tau phosphorylation levels were normalized by total tau levels recognized by R134d. *, p < 0.05 versus control slices; #, p < 0.05; ##, p < 0.01 versus glutamate- or Bic/4-AP-treated slices.
DISCUSSION
Zinc is one of the most abundant essential elements in the adult human brain. Nearly half of the glutamatergic synapses are “gluzinergic” in the cerebral cortex and limbic nuclei (29). These gluzinergic neurons release glutamate and zinc simultaneously upon excitation in neurotransmission. Synaptically released Zn2+ interacts with various neuronal ion channels, receptors, and transporters and therefore modulates synaptic transmission and plasticity (30, 31). Most of the released zinc ions are re-uptaked by the presynaptic neurons and further sequestered into the synaptic vesicles by zinc transporter-3 (ZnT-3) (32). In AD brains, zinc levels are elevated in degenerated brain regions such as hippocampus, amygdala, and cortex (33, 34). ZnT-3 is significantly down-regulated, together with the altered expression of other zinc transporters such as ZnT-1, -4, and -6 (35–37), indicating an impaired zinc homeostasis. High concentrations of zinc are observed in the neuritic plaques and amyloid deposits (38), suggesting the possible roles of zinc in the development of AD pathology. Recently, a role of synaptic zinc in Aβ oligomer formation and accumulation at excitatory synapses was observed and reported (10). In the present study, we explored the effect of synaptic zinc on tauopathy, and found that endogenously released zinc in excitatory neurotransmission promoted AD-like tau hyperphosphorylation through PP2A inhibition.
In cultured rat hippocampal brain slices, we used two classic strategies to increase the synaptic activity, glutamate and Bic/4-AP treatments (10, 23). Exogenous glutamate may activate glutamate receptors directly, while Bic/4-AP increased the intrinsic synaptic activity by inhibiting GABAA receptors (23). The hippocampal slices were chosen because of the highest concentration of zinc in this region of mammalian brains (39). Both glutamate and Bic/4-AP treatments induced tau phosphorylation at several AD-like tau hyperphosphorylation sites, indicating that synaptic activity may modulate the phosphorylation state of tau. TTX, a potent blocker of sodium channels, completely blocked tau hyperphosphorylation, underscoring the role of neuronal activity in tau phosphorylation modification. Hyperphosphorylation of tau induced by glutamate was completely reversed by preincubation of CQ, a membrane-permeable zinc chelator, which has been shown to significantly reduce the Aβ oligomer formation at synapses in neurotransmission (10). This result suggests zinc is involved in intracellular tau hyperphosphorylation.
During the activation and neurotransmission of excitatory synapses, large amounts of zinc ions are released into the synaptic cleft, reaching an extracellular concentration close to 300 μm. Previous studies have identified the zinc release and translocation in different models with increased synaptic activity, which include physiological stimulations such as electrical stimulation and KCl treatment (24), and pathologic conditions such as oxygen and glucose deprivation (40), hypoglycemia (41), and global cerebral ischemia (42). The routes responsible for zinc influx were also widely studied and identified as voltage-gated calcium channels (VGCC), Ca2+- permeable AMPA and NMDA receptor channels (26, 27). To confirm that zinc ions are released and translocated during glutamate or Bic/4-AP incubation, we detected the zinc concentrations in the aCSF after the slice culture. Results showed that extracellular zinc levels were increased by synaptic activity. The increase of zinc level in the glutamate treatment group was not statistically significant, possibly due to the short incubation time. Preincubation of the slices with Ca-EDTA, a membrane-impermeable zinc chelator, resulted in dramatic elevation of detected zinc levels in aCSF, indicating that most of the released zinc are rapidly re-uptaked by presynaptic neurons or entered into postsynaptic neurons when there is no extracellular zinc chelator. The observation that Ca-EDTA also completely prevented tau hyperphosphorylation by increased synaptic activity favors an external origin of zinc that induces modifications of tau. In other words, it is the synaptically released zinc but not the intrinsic zinc in cells which mediate tau hyperphosphorylation. This idea was further confirmed by omission of calcium from the aCSF, since synaptic zinc release is calcium-dependent. In the absence of extracellular Ca2+, zinc release may be reduced by more than 70% (24). When calcium was withdrawn from the aCSF, glutamate treatment no longer induced marked tau hyperphosphorylation, while addition of exogenous zinc sulfate into the calcium-free aCSF resulted in tau hyperphosphorylation in glutamate-treated slice. When we incubated brain slices with zinc directly, we also observed tau hyperphosphorylation at the same Ser/Thr sites seen in glutamate or Bic/4-AP incubation, which was mediated by zinc influx through VGCC. All these data strongly indicated that synaptic zinc promoted tau hyperphosphorylation.
Several protein kinases and phosphatases are involved in regulating tau phosphorylation. To explore the mechanism of synaptic zinc-induced tau hyperphosphorylation, we measured the activities of possible involved tau kinases and phosphatases. GSK-3β is the most important kinase tau phosphorylates tau in the brain (13, 14). Our previous research also showed that exogenous zinc may activate GSK-3β, ERK1/2, JNK, and p38 in SH-SY5Y cells, which are all tau kinases (12). In a recently published report, zinc was found to promote tau phosphorylation at Ser-214 in human wild-type tau1–441-expressing SH-SY5Y cells through ERK activation (43). But in the present study, we did not observe any change of active or inactive forms of GSK-3β, ERK1/2, JNK, and p38. Preincubation of the slices with specific inhibitors of these kinases also failed to prevent tau hyperphosphorylation by glutamate-induced synaptic activity, suggesting that these kinases may not be the most implicated kinase in tau hyperphosphorylation in our experimental system. Lee et al. (44) have reported that GSK-3β showed undulating phosphorylation/dephosphorylation after membrane depolarization by KCl, indicating that the time point chosen for detection may influence the results of kinase activity. In addition, the zinc-regulated pathways may be different in primary neuron cultures from that in neuroblastoma cells.
Because the kinases detected did not account for tau hyperphosphorylation induced by synaptically released zinc, we further examined the activity of PP2A, which is the most important tau phosphatase in human brain. We were surprised to find that PP2A is significantly inhibited by synaptically released zinc; an activator of PP2A can reverse tau hyperphosphorylation. These results indicate that PP2A is a key regulator in synaptic zinc-promoted tau hyperphosphorylation. It has been reported that zinc at concentrations as low as 10 μm could completely inhibit a membrane-associated phosphotyrosyl-protein phosphatase in vitro (45). So, it is possible that zinc entered into the cells directly inhibits PP2A. Recent studies showed that crystal structure of the core domain of small T antigen of DNA tumor viruses SV40 (SV40 ST, an inhibitor of PP2A, which bind to the scaffolding subunit of human PP2A) has a novel zinc-binding fold (46), implying that zinc may also indirectly inhibit PP2A through regulating the function of SV40 ST. The underlying mechanisms for synaptic zinc inhibition of PP2A and the physiological roles of PP2A inhibition in glutamatergic neurotransmission need to be further investigated.
Zinc concentration undergoes highly dynamic changes during neurotransmission, which implies that the phosphorylation state of tau is also dynamically changed in zinc-rich areas. In the human brain, zinc-releasing neurons located in hippocampus, amygdala, olfactory bulb, and cerebral cortex (38), which are all brain regions susceptible to NFTs formation. The observations in our study disclosed a mechanism for the region-specific development and distribution of NFTs. A recent study showed that phosphorylation of tau in dendritic spines correlated well with the early impairment of synaptic function and memory deficit before obvious neurodegeneration occurs (6). Since the synaptic-released zinc ions first enter the dendrites though membrane channels, we suspect that zinc also plays a role in early dendritic tau phosphorylation and synaptic dysfunction. This hypothesis requires further exploration.
In summary, we show that synaptic activity promotes tau hyperphosphorylation, and synaptically released zinc plays a central role. Therapies targeted on maintaining zinc homeostasis and moderating synaptic activity may benefit the AD patients not only through reducing amyloidosis but also through reducing tauopathy.
Supplementary Material
Acknowledgment
We thank Drs. K. Iqbal and I. Grundke-Iqbal of New York State Institute for Basic Research (Staten Island, NY) for the antibody R134d.
This work was supported by Fundamental Research Funds for the Central Universities, HUST, (No: 2010MS050), the National Natural Science Foundation of China (No: 81130079), and Alzheimer's Association (IIRG-09-133433).

This article contains supplemental Figs. S1–S4.
- AD
- Alzheimer Disease
- NFT
- neurofibrillary tangle
- AP
- aminopyridine
- Bic
- bicuculline
- aCSF
- artificial cerebrospinal fluid
- PP2A
- protein phosphatase 2A
- TTX
- Tetrodotoxin.
REFERENCES
- 1. Mount C., Downton C. (2006) Alzheimer disease: progress or profit? Nat. Med. 12, 780–784 [DOI] [PubMed] [Google Scholar]
- 2. Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 261, 6084–6089 [PubMed] [Google Scholar]
- 3. Wang J. Z., Liu F. (2008) Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog. Neurobiol. 85, 148–175 [DOI] [PubMed] [Google Scholar]
- 4. Bancher C., Brunner C., Lassmann H., Budka H., Jellinger K., Wiche G., Seitelberger F., Grundke-Iqbal I., Iqbal K., Wisniewski H. M. (1989) Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 477, 90–99 [DOI] [PubMed] [Google Scholar]
- 5. Köpke E., Tung Y. C., Shaikh S., Alonso A. C., Iqbal K., Grundke-Iqbal I. (1993) Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J. Biol. Chem. 268, 24374–24384 [PubMed] [Google Scholar]
- 6. Hoover B. R., Reed M. N., Su J., Penrod R. D., Kotilinek L. A., Grant M. K., Pitstick R., Carlson G. A., Lanier L. M., Yuan L. L., Ashe K. H., Liao D. (2010) Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hauser W. A., Morris M. L., Heston L. L., Anderson V. E. (1986) Seizures and myoclonus in patients with Alzheimer disease. Neurology 36, 1226–1230 [DOI] [PubMed] [Google Scholar]
- 8. LaFerla F. M., Tinkle B. T., Bieberich C. J., Haudenschild C. C., Jay G. (1995) The Alzheimer's Aβ peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat. Genet. 9, 21–30 [DOI] [PubMed] [Google Scholar]
- 9. Minkeviciene R., Rheims S., Dobszay M. B., Zilberter M., Hartikainen J., Fulöp L., Penke B., Zilberter Y., Harkany T., Pitkänen A., Tanila H. (2009) Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci. 29, 3453–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deshpande A., Kawai H., Metherate R., Glabe C. G., Busciglio J. (2009) A role for synaptic zinc in activity-dependent Aβ oligomer formation and accumulation at excitatory synapses. J. Neurosci. 29, 4004–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slomianka L., Danscher G., Frederickson C. J. (1990) Labeling of the neurons of origin of zinc-containing pathways by intraperitoneal injections of sodium selenite. Neuroscience 38, 843–854 [DOI] [PubMed] [Google Scholar]
- 12. An W. L., Bjorkdahl C., Liu R., Cowburn R. F., Winblad B., Pei J. J. (2005) Mechanism of zinc-induced phosphorylation of p70 S6 kinase and glycogen synthase kinase 3β in SH-SY5Y neuroblastoma cells. J. Neurochem. 92, 1104–1115 [DOI] [PubMed] [Google Scholar]
- 13. Takashima A. (2006) GSK-3 is essential in the pathogenesis of Alzheimer's disease. J. Alzheimers Dis. 9, 309–317 [DOI] [PubMed] [Google Scholar]
- 14. Ishiguro K., Takamatsu M., Tomizawa K., Omori A., Takahashi M., Arioka M., Uchida T., Imahori K. (1992) Tau protein kinase I converts normal tau protein into A68-like component of paired helical filaments. J. Biol. Chem. 267, 10897–10901 [PubMed] [Google Scholar]
- 15. Liu F., Grundke-Iqbal I., Iqbal K., Gong C. X. (2005) Contributions of protein phosphatases PP1, PP2A, PP2B, and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 22, 1942–1950 [DOI] [PubMed] [Google Scholar]
- 16. Gong C. X., Singh T. J., Grundke-Iqbal I., Iqbal K. (1993) Phosphoprotein phosphatase activities in Alzheimer disease brain. J. Neurochem. 61, 921–927 [DOI] [PubMed] [Google Scholar]
- 17. Liu R., Pei J. J., Wang X. C., Zhou X. W., Tian Q., Winblad B., Wang J. Z. (2005) Acute anoxia induces tau dephosphorylation in rat brain slices and its possible underlying mechanisms. J. Neurochem. 94, 1225–1234 [DOI] [PubMed] [Google Scholar]
- 18. Frederickson C. J., Bush A. I. (2001) Synaptically released zinc: physiological functions and pathological effects. Biometals 14, 353–366 [DOI] [PubMed] [Google Scholar]
- 19. Ketterman J. K., Li Y. V. (2008) Presynaptic evidence for zinc release at the mossy fiber synapse of rat hippocampus. J. Neurosci. Res. 86, 422–434 [DOI] [PubMed] [Google Scholar]
- 20. Mo Z. Y., Zhu Y. Z., Zhu H. L., Fan J. B., Chen J., Liang Y. (2009) Low micromolar zinc accelerates the fibrillization of human tau via bridging of Cys-291 and Cys-322. J. Biol. Chem. 284, 34648–34657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cherny R. A., Atwood C. S., Xilinas M. E., Gray D. N., Jones W. D., McLean C. A., Barnham K. J., Volitakis I., Fraser F. W., Kim Y., Huang X., Goldstein L. E., Moir R. D., Lim J. T., Beyreuther K., Zheng H., Tanzi R. E., Masters C. L., Bush A. I. (2001) Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30, 665–676 [DOI] [PubMed] [Google Scholar]
- 22. Lavoie N., Peralta M. R., 3rd, Chiasson M., Lafortune K., Pellegrini L., Seress L., Tóth K. (2007) Extracellular chelation of zinc does not affect hippocampal excitability and seizure-induced cell death in rats. J. Physiol. 578, 275–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardingham G. E., Arnold F. J., Bading H. (2001) Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat. Neurosci. 4, 261–267 [DOI] [PubMed] [Google Scholar]
- 24. Li Y., Hough C. J., Suh S. W., Sarvey J. M., Frederickson C. J. (2001) Rapid translocation of Zn(2+) from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J. Neurophysiol. 86, 2597–2604 [DOI] [PubMed] [Google Scholar]
- 25. Riederer B. M. (1992) Differential phosphorylation of some proteins of the neuronal cytoskeleton during brain development. Histochem. J. 24, 783–790 [DOI] [PubMed] [Google Scholar]
- 26. Frederickson C. J., Koh J. Y., Bush A. I. (2005) The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 6, 449–462 [DOI] [PubMed] [Google Scholar]
- 27. Sheline C. T., Ying H. S., Ling C. S., Canzoniero L. M., Choi D. W. (2002) Depolarization-induced 65zinc influx into cultured cortical neurons. Neurobiol. Dis. 10, 41–53 [DOI] [PubMed] [Google Scholar]
- 28. Dobrowsky R. T., Kamibayashi C., Mumby M. C., Hannun Y. A. (1993) Ceramide activates heterotrimeric protein phosphatase 2A. J. Biol. Chem. 268, 15523–15530 [PubMed] [Google Scholar]
- 29. Sindreu C. B., Varoqui H., Erickson J. D., Pérez-Clausell J. (2003) Boutons containing vesicular zinc define a subpopulation of synapses with low AMPAR content in rat hippocampus. Cereb. Cortex 13, 823–829 [DOI] [PubMed] [Google Scholar]
- 30. Karakas E., Simorowski N., Furukawa H. (2009) Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 28, 3910–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takeda A., Iwaki H., Ando M., Itagaki K., Suzuki M., Oku N. (2010) Zinc differentially acts on components of long-term potentiation at hippocampal CA1 synapses. Brain Res. 1323, 59–64 [DOI] [PubMed] [Google Scholar]
- 32. Wenzel H. J., Cole T. B., Born D. E., Schwartzkroin P. A., Palmiter R. D. (1997) Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc. Natl. Acad. Sci. U.S.A. 94, 12676–12681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deibel M. A., Ehmann W. D., Markesbery W. R. (1996) Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer's disease: possible relation to oxidative stress. J. Neurol. Sci. 143, 137–142 [DOI] [PubMed] [Google Scholar]
- 34. Religa D., Strozyk D., Cherny R. A., Volitakis I., Haroutunian V., Winblad B., Naslund J., Bush A. I. (2006) Elevated cortical zinc in Alzheimer disease. Neurology 67, 69–75 [DOI] [PubMed] [Google Scholar]
- 35. Lovell M. A., Smith J. L., Xiong S., Markesbery W. R. (2005) Alterations in zinc transporter protein-1 (ZnT-1) in the brain of subjects with mild cognitive impairment, early, and late-stage Alzheimer's disease. Neurotox. Res. 7, 265–271 [DOI] [PubMed] [Google Scholar]
- 36. Smith J. L., Xiong S., Markesbery W. R., Lovell M. A. (2006) Altered expression of zinc transporters-4 and -6 in mild cognitive impairment, early and late Alzheimer's disease brain. Neuroscience 140, 879–888 [DOI] [PubMed] [Google Scholar]
- 37. Adlard P. A., Parncutt J. M., Finkelstein D. I., Bush A. I. (2010) Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer's disease? J. Neurosci. 30, 1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bitanihirwe B. K., Cunningham M. G. (2009) Zinc: the brain's dark horse. Synapse 63, 1029–1049 [DOI] [PubMed] [Google Scholar]
- 39. Frederickson C. J., Danscher G. (1990) Zinc-containing neurons in hippocampus and related CNS structures. Prog. Brain Res. 83, 71–84 [DOI] [PubMed] [Google Scholar]
- 40. Yin H.Z., Sensi S.L., Ogoshi F., Weiss J.H. (2002) Blockade of Ca2+-permeable AMPA/kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J. Neurosci. 22, 1273–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suh S. W., Garnier P., Aoyama K., Chen Y., Swanson R. A. (2004) Zinc release contributes to hypoglycemia-induced neuronal death. Neurobiol. Dis. 16, 538–545 [DOI] [PubMed] [Google Scholar]
- 42. Koh J. Y., Suh S. W., Gwag B. J., He Y. Y., Hsu C. Y., Choi D. W. (1996) The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 272, 1013–1016 [DOI] [PubMed] [Google Scholar]
- 43. Kim I., Park E. J., Seo J., Ko S. J., Lee J., Kim C. H. (2011) Zinc stimulates tau S214 phosphorylation by the activation of Raf/mitogen-activated protein kinase-kinase/extracellular signal-regulated kinase pathway. Neuroreport. 22, 839–844 [DOI] [PubMed] [Google Scholar]
- 44. Lee Y. I., Seo M., Kim Y., Kim S. Y., Kang U. G., Kim Y. S., Juhnn Y. S. (2005) Membrane depolarization induces the undulating phosphorylation/dephosphorylation of glycogen synthase kinase 3β, and this dephosphorylation involves protein phosphatases 2A and 2B in SH-SY5Y human neuroblastoma cells. J. Biol. Chem. 280, 22044–22052 [DOI] [PubMed] [Google Scholar]
- 45. Brautigan D. L., Bornstein P., Gallis B. (1981) Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J. Biol. Chem. 256, 6519–6522 [PubMed] [Google Scholar]
- 46. Chen Y., Xu Y., Bao Q., Xing Y., Li Z., Lin Z., Stock J. B., Jeffrey P. D., Shi Y. (2007) Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nat. Struct. Mol. Biol. 14, 527–534 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.