Abstract
Humans affect biodiversity at the genetic, species, community, and ecosystem levels. This impact on genetic diversity is critical, because genetic diversity is the raw material of evolutionary change, including adaptation and speciation. Two forces affecting genetic variation are genetic drift (which decreases genetic variation within but increases genetic differentiation among local populations) and gene flow (which increases variation within but decreases differentiation among local populations). Humans activities often augment drift and diminish gene flow for many species, which reduces genetic variation in local populations and prevents the spread of adaptive complexes outside their population of origin, thereby disrupting adaptive processes both locally and globally within a species. These impacts are illustrated with collared lizards (Crotaphytus collaris) in the Missouri Ozarks. Forest fire suppression has reduced habitat and disrupted gene flow in this lizard, thereby altering the balance toward drift and away from gene flow. This balance can be restored by managed landscape burns. Some have argued that, although human-induced fragmentation disrupts adaptation, it will also ultimately produce new species through founder effects. However, population genetic theory and experiments predict that most fragmentation events caused by human activities will facilitate not speciation, but local extinction. Founder events have played an important role in the macroevolution of certain groups, but only when ecological opportunities are expanding rather than contracting. The general impact of human activities on genetic diversity disrupts or diminishes the capacity for adaptation, speciation, and macroevolutionary change. This impact will ultimately diminish biodiversity at all levels.
Biodiversity has been defined at several levels of biological organization, including genes, species, communities, and ecosystems (1). Human activities are causing massive impacts on biodiversity at all these levels, but the impacts are most apparent to the general public at the species level and above as people witness loss of habitat, species extinction, disrupted communities, and polluted or otherwise damaged ecosystems. The impact of human activities on genetic diversity within a species is the least apparent and hence is often ignored. Genetic diversity is at the lowest hierarchy in this biodiversity sequence, which enhances—not diminishes—its importance. Without genetic diversity, a population cannot evolve, and it cannot adapt to environmental change. Environmental change is now occurring on a global scale because of human activities, and many species will have to adapt to this change or experience an ever-increasing chance of extinction. Moreover, as is common with many hierarchical systems, genetic diversity has an impact on the higher levels of biodiversity. Species, in their most basic sense, are evolving lineages (2–4), and the maintenance of their capacity to evolve requires the existence of genetic diversity (5). In this manner, species diversity emerges from genetic diversity over evolutionary time. Species diversity is seen in diversity of habitat needs and responses to other species. These species attributes in turn are the basis for much community and ecosystem structure. Hence, the impact of genetic diversity percolates through all levels of biodiversity via the evolutionary process.
Human activities can and do have dramatic effects on the amount and distribution of genetic diversity within species. As a consequence, human activities are directly altering the dynamics of evolution itself with respect to the fundamental evolutionary processes of adaptation and speciation. In this paper, examples are given of how human activities can disrupt the evolutionary potential for both adaptation and speciation.
Factors Controlling the Amount and Distribution of Genetic Variation Within Species
Genetic diversity is ultimately created by the process of mutation, which creates allelic diversity (alternative forms of genes at the same locus). This diversity is lost during the evolutionary process; some of it is lost at random (genetic drift in the species as a whole) and some because of natural selection (elimination of deleterious alleles and fixation of favorable alleles). The amount of allelic diversity in a species represents a dynamic balance among mutation, drift, and selection.
Species exist in both space and time, and so does intraspecific genetic diversity. In some species, allelic diversity is widely distributed across a species' entire geographical range, and all local populations contain virtually the same alleles and at similar allele frequencies. At the other extreme are species in which local populations have little or no internal allelic diversity, but different local populations can be fixed for alternative alleles. The forces that partition and create genetic hierarchies within a species are collectively known as population structure and include such factors as system of mating, genetic drift, and gene flow. The partitioning of allelic diversity within and among local breeding populations is primarily because of the dynamic balance between local genetic drift (which causes the local breeding population to lose allelic diversity but causes an increase in genetic differentiation among local populations) versus gene flow (which brings new allelic diversity into the local population and reduces genetic differentiation among populations). The allelic diversity within a reproducing population is translated into genotypic diversity through the mechanisms of gamete formation and gamete union (system of mating). During gamete formation, alleles at different loci are put together into various combinations by the processes of recombination and assortment, which greatly augments the potential for genotypic diversity. The system of mating determines the extent to which diploid individuals will themselves carry allelic diversity (in the form of heterozygosity).
Human-Induced Alterations in the Balance of Genetic Drift and Gene Flow
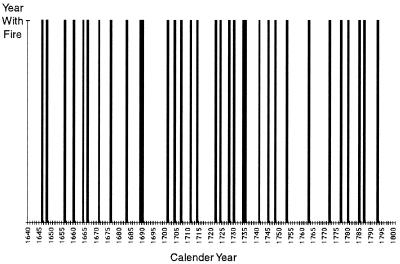
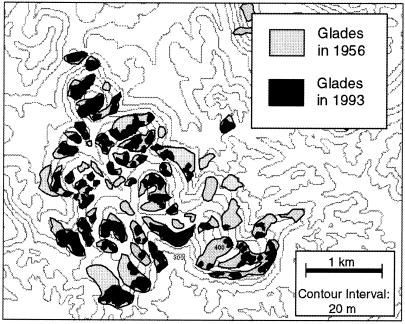
One of the primary impacts of many human activities is habitat fragmentation; that is, human use of the landscape creates habitat “islands”, and the species within them often have little or no genetic contact with conspecific populations inhabiting other such islands. As an example, consider the eastern collared lizard (Crotaphytus collaris collaris), a species restricted to glades in the portion of its range located in the Ozarks. Ozark glades are barren, rocky outcrops, usually with a southern or southwestern exposure on a ridge top that creates a desert or dry prairie-like microhabitat (6). Desert-adapted plants and animals (such as scorpions, tarantulas, cacti, and collared lizards) invaded the Ozarks during the Xerothermic maximum about 8,000 years ago (the period of maximum warmth in our current interglacial period) and were cut off from their southwestern ancestral range at the end of the Xerothermic about 4,000 years ago (7). After that time and until European settlement, the fragmented Ozark glades were mostly separated by savannas—open mixed woodland and grassland areas (8, 9). Ozark savannas were a fire-maintained community, and before European settlement, fires occurred frequently in the Ozarks (10). For example, one of our field sites is in the Stegall Mountain Natural Area. Fig. 1 presents the fire history of this mountain from 1640 to 1800 as reconstructed from fire scar data on tree stumps (11, 12). As can be seen, the average interval between fires of sufficient intensity to produce fire scars was about once every 5 years, and no decade in this period had no fires. However, with European settlement, clear cutting occurred throughout most of the Ozarks, often followed by cutting of second-growth forest as well. The present forest grew during a time in which fires were suppressed, particularly from about 1950 to the present. This new forest is an oak–hickory forest with a dense understory. Although savanna was the dominant community type in the Ozarks in the early 1800s, less than 100 acres of it survived this replacement by the dense oak–hickory forest (13). In addition to changing the nature of the forest that separates the glades, the suppression of fire also allowed the invasion of glades by fire-sensitive eastern red cedars (Juniperus virginiana), which in turn allowed successional invasion by other woody species. As a consequence, many glades have been reduced in size, and some have disappeared completely (14). This destruction and increased fragmentation of glade habitats can be documented on Stegall Mountain by comparing a series of aerial photos taken in 1956 (graciously provided by the Missouri Department of Conservation) to a glade map of the same area from 1993 (Fig. 2).
Figure 1.
The fire history of Stegall Mountain, as reconstructed from fire scars on stumps between 1640 and 1800.
Figure 2.
Glade habitat on Stegall Mountain in 1956 versus 1993 as inferred from aerial photos.
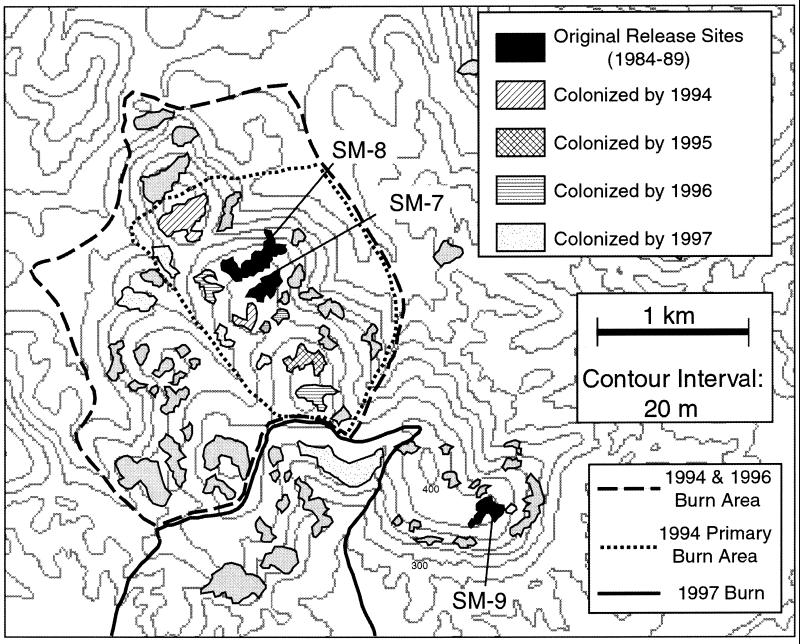
These environmental changes had a drastic impact on the population structure of the collared lizard, particularly in the northeastern part of the Ozarks where European settlement first occurred. On the basis of microsatellite loci, the present populations have extreme population subdivision (FST = 0.40, which measures the proportion of the genetic variation in the Total population that exists as differences between Subpopulations), with little genetic diversity within any single glade population but many fixed genetic differences among even nearby glade populations (15, 16). This pattern indicates a combination of small local population sizes and little to no gene flow. The small population sizes are expected from the reduction in glade numbers and sizes and are confirmed by direct observations. For example, at Sandy Ridge, a glade with one of the more abundant and reliable collared lizard populations in the eastern Ozarks, the adult population size fluctuated between 21 and 79 individuals in the period 1975–1985 (O. Sexton, personal communication). Given that eigenvalue effective size (which measures the rate of loss of genetic variation) tends to be weighted most heavily by the smaller population size values when size fluctuates with time, the size fluctuations observed by Sexton imply that the Sandy Ridge population should be losing its genetic variation at a high rate. Most other glade populations in the northeastern Ozarks are even smaller. Hence, unless counteracted by gene flow, glade populations should experience intense genetic drift and an attendant loss of local population genetic variation. The genetic evidence indicates there is little to no gene flow among glade populations under present conditions. For example, fixed differences exist between populations separated by as little as 50 m of intervening forest (16). This lack of gene flow has also been confirmed by field experimentation. In 1983, in cooperation with the Missouri Department of Conservation (MDOC), we began a translocation program for collared lizards (17). The initial releases of translocated animals were made on glades at the Peck Ranch, a 23,000-acre wildlife area owned and managed by the MDOC that contains the Stegall Mountain Natural Area. This area had been ecologically devastated, first by extensive clear-cutting of its primary pine and oak woodlands in the 1800s, followed by clear-cutting of the secondary oak–hickory forest in the early 1900s and then by the raising of hogs and cattle on an open range. Protection of this area began in 1953, shortly after its purchase by the MDOC, and that protection included effective suppression of forest fires that unintentionally resulted in extensive destruction and reduction of glade habitat (Fig. 2). Nothing is definitively known about collared lizard populations before 1980, but in that year, an extensive survey revealed that no collared lizard populations could be found on the Peck Ranch. Three populations were translocated onto glades on Stegall Mountain (Fig. 3), one each in 1984 (glade SM-7), 1987 (glade SM-8), and 1989 (glade SM-9). All three translocations were successful in the sense that the lizards were able to live and reproduce on these three glades. However, there was no gene flow or dispersal among these glades. Glades SM-7 and SM-8 are separated by only 50 m of intervening forest (Fig. 3), but despite annual observation trips, no animals were observed to have dispersed between them (on the basis of mark/recapture studies by using toe clipping to mark individuals) while the forest fire suppression policy was still in effect (up to and including 1993). Moreover, several empty glades existed in this area, the closest being only 60 m from glade SM-7. Several of these nearby glades were regularly monitored but were never colonized in the period 1984–1993. These monitoring studies support the inference from genetic data of little to no gene flow among the fragmented populations. Overall, this combination of low population sizes and no gene flow explains the high FST values observed in the northeastern Ozarks.
Figure 3.
Map of glades and burn areas on Stegall Mountain. The glades with areas filled in black were the sites of release of translocated collared lizard populations in 1984 (SM-7), 1987 (SM-8), and 1989 (SM-9). Different patterns within glades indicate the first year in which collared lizards were observed in that glade for the period 1984–1997.
The genetic data also suggest that the lack of gene flow is a relatively recent phenomenon. First, within the northeastern Ozarks, there is no correlation between geographical distance with either pairwise FST values or their variances (15). This is the pattern one expects when a relatively genetically homogeneous ancestral population is suddenly fragmented into many small isolated units (15). The validity of this explanation can be tested directly by altering the fire regime once again. A Biodiversity Task Force assembled by the MDOC and the U.S. Forest Service made several management recommendations, including the use of managed forest fires on a landscape level (13). An initial fire management area was designated on Stegall Mountain (Fig. 3), although the first burn, in April 1994, was primarily confined to the northwestern portion of the designated burn area (Fig. 3). A subsequent burn in 1996 included the entire initial fire management area. In 1997, a second segment to the south of the 1994/1996 management area was burned (Fig. 3). As of 1999, all of the area shown in Fig. 3 and even beyond has been included in the fire management program, for a total of about 5,000 acres. After 1997, the situation with the lizards has become more complex and will require genetic testing in addition to mark/recapture data to sort out the dispersal among glades. These surveys are in progress, so for now we will confine our analysis to the initial response to the new forest fire management policy up to and including 1997.
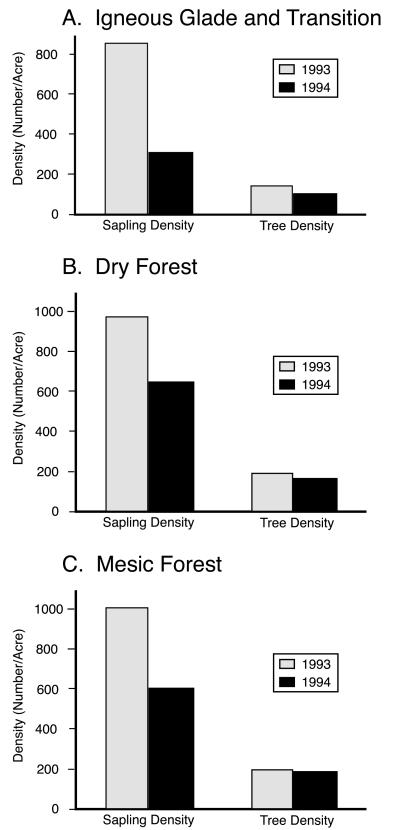
These burns had a dramatic effect biologically. Three transects through the burned area set up and monitored by the MDOC revealed a marked change in woody vegetation (Fig. 4, on the basis of data kindly provided by T. Nigh and K. Kramer of the MDOC). The fire helped keep the glades clear of encroaching trees and saplings (Fig. 4 Top) and greatly altered the structure of the intervening forest. There was little impact on the canopy trees, but the understory was significantly thinned of saplings (Fig. 4 B and C) and became more open and dominated by grasses and herbaceous plants rather than woody species. The burns also had a dramatic effect on the population structure of the collared lizards.
Figure 4.
Summary of three vegetation transects taken in 1993 (before the burn) and in 1994 (after the burn) in the fire management area on Stegall Mountain by the MDOC. The transects are subdivided into three major habitat types: (A) glades and the transitional areas between the glades and the dry forest; (B) dry forest; and (C) mesic forest.
The 1994 and 1996 burn areas include two glades on which collared lizards were translocated in 1984 and 1987 (SM-7 and SM-8, Fig. 3). A third glade, SM-9, was the site of a collared lizard release in 1989 but was outside the areas burned by 1997 (Fig. 3). Before 1994, a total of 63 lizards were marked on these 3 glades, and 9 were recaptured, all on the glade of original capture. Between 1994 and 1997, an additional 65 animals were marked in the burn area and 39 outside the burn area. Of 18 recaptures in the burn area, 9 represent dispersal events. Of 34 recaptures in the nonburned area, all 34 were on glade SM-9, the glade of initial capture. These data can be used to test the null hypothesis that burning does not affect dispersal, both temporally (before and after April 1994 in the burn area, Table 1) and spatially (after April 1994 in the burned versus unburned areas, Table 2). Table 1 reveals a highly significant (P = 0.012) change in dispersal rates before and after the 1994 burn within the initial fire management area. Table 2 reveals a highly significant (P < 0.001) difference in dispersal in the period 1994–1997 in the burned versus unburned portions of Stegall Mountain. Hence, interglade dispersal went from being nondetectable to being common in the areas that were burned.
Table 1.
Recapture data on marked collared lizards in the areas burned between 1994 and 1997 on Stegall Mountain before (1984–1993) and after the burns (1994–1997)
| Time period | Recaptured in glade other than glade of original capture | Recaptured in glade of original capture |
|---|---|---|
| 1984–1993 | 0 | 9 |
| 1994–1994 | 9 | 9 |
A two-tailed Fisher's Exact Test is used to test the null hypothesis of no temporal effects. Two-tailed Fisher's Exact Test: P = 0.012.
Table 2.
Recapture data on marked collared lizards in the burned versus unburned areas between 1994 and 1997 on Stegall Mountain
| Area | Recaptured in glade other than glade of original capture | Recaptured in glade of original capture |
|---|---|---|
| Unburned | 0 | 34 |
| Burned | 9 | 9 |
A two-tailed Fisher's Exact Test is used to test the null hypothesis of no spatial effects. Two-tailed Fisher's Exact Test: P < 0.001.
The burns also had a significant impact on colonization rate. Before the 1994 burn, no glades had been colonized in the 10 summers that lizards were present in the area eventually burned. Between 1994 and 1997, 13 glades were colonized (Fig. 4), indicating a dramatic increase in colonization rate after the initiation of burning. Moreover, the colonization within burned areas has continued on Stegall Mountain, and 32 glades have been colonized between 1994 and 1999 versus no glades colonized between 1984 and 1993. For a spatial contrast, one glade was colonized in the unburned portion of Stegall between 1994 and 1997, whereas 12 were colonized in the burn area (Fig. 3). The one glade that was colonized in the unburned area actually shows the importance of clearing the understory for dispersal in this species. As can be seen from Fig. 2, glade SM-9 and the glade colonized in 1997 in the unburned area were originally part of a single large glade. In anticipation of extending the burn area to include this southwestern part of the main ridge of Stegall Mountain, during the winter of 1996/1997, MDOC workers cut with chain saws much of the woody vegetation that separated these two fragments of what was formerly a single glade. Although the population on glade SM-9 was very dense, no lizards colonized this nearby fragment between 1989 and 1996; they did so only after the clearing by chain saws. This 1997 colonization event indicates that the lizards' dispersal behavior is cued not by burning per se but rather by having an open understory. Therefore, the ability of collared lizards to disperse among and colonize glades depends strongly on the intervening forest structure, particularly the understory. These managed fires have established a gene flow and colonization regime today that is consistent with the inferred ancestral population structure before European settlement (15). Thus, human activities have had and continue to have a dramatic effect on gene flow in these lizard populations.
There have also been dramatic increases in population sizes after fire management. The founder population on Stegall Mountain consisted of 28 adult animals (10 on two glades and 8 on the third). Low recapture rates preclude a meaningful estimate of population size before the burns, but no obvious large increase in population size occurred at either of the glades in the area burned in 1994 and 1996, whereas the 1989 release population appeared to have achieved the greatest local population size, with 14 animals caught in 1993 on glade SM-9 in contrast to 4 apiece on glades SM-7 and SM-8 in 1993. In 1999, 233 individual animals were captured on Stegall Mountain, consisting of 107 adults and 126 hatchlings. This number of captures indicates at least a nearly 10-fold increase in population size from the original release population of 28 individuals, and the high percentage of hatchlings indicates a rapidly expanding population. Most of this increase in population size is attributable to the postburn colonization of 32 new glades. Moreover, the fires are increasing both the area and the quality of existing glades (Fig. 4 Top) and are allowing the colonization of small glades. For example, two of the glades colonized in 1996 lie well below the size range of 42 glades with natural populations of collared lizards in the northeastern Ozarks that we have surveyed since 1981. It is doubtful whether these small glades could maintain a viable population of collared lizards in isolation, but they are sufficiently large to provide good foraging territories for about two to five lizards. Now that lizards can disperse and are dispersing among glades, these small habitat islands are available for exploitation and help augment the total lizard population size. Interestingly, one of these smaller glades subsequently became unoccupied again, only to be recolonized later. Hence, metapopulation dynamics, defined by local extinctions and recolonizations, has now become established after the burns. Thus, the restoration of forest fires in this area has dramatically altered the balance between drift and gene flow in a manner that should maintain much higher levels of genetic diversity at the local glade population level and at the total Stegall Mountain population level (because of less overall genetic drift caused by dramatic increase in the total population size). Although the lizards still live in fragmented glade habitats, the extreme fragmentation induced by fire suppression has been replaced by frequent and effective gene flow among glade populations.
Disrupting the Evolutionary Potential for Adaptation
The balance between drift and gene flow and its impact on genetic variation in the local population's gene pool is important for three reasons: (i) the possibility that genetic uniformity makes populations more likely to experience high infection rates and rapid spreads of pathogens; (ii) the possibility that loss of local genetic diversity will reduce a population's ability to respond to environmental change through the process of adaptation; and (iii) the possibility that local adaptations will be unable to spread throughout the species from their local population of origin. Caro and Laurenson (18) questioned the importance of genetic variation with respect to increased risk to short-term extinction. (Note: Caro and Laurenson incorrectly state that it is loss of heterozygosity that may reduce a population's adaptive flexibility, but adaptive flexibility is bestowed by having genetic diversity in the gene pool—which may or not be in the form of heterozygosity at the diploid level.) Concerning point i, Caro and Laurenson (18) argue that the cases of increased epidemiological impact of pathogens in natural populations that are low in genetic variation are not definite proofs of the importance of genetic variation, although they are consistent with this conclusion (19, 20). However, the agricultural literature clearly shows the dangers of genetic monocultures with respect to pathogen epidemiology (21, 22). Given the consistency of the natural examples with agricultural work, it would be unwise to dismiss this role of genetic variation in mediating the intensity and ecological consequences of host/pathogen interactions. Greater epidemiological impact of pathogens can make it more likely that small local populations will go extinct—the ultimate disruption in evolutionary potential.
The lack of genetic diversity in local populations can disrupt adaptive evolution long before extinction. The need for genetic diversity as a prerequisite for adaptive evolution is well established theoretically and experimentally (e.g., ref. 23). There are also abundant natural examples of organisms using their genetic diversity to adapt to environmental, including human-induced, change (21). The importance of genetic diversity as a necessary component of adaptive evolution cannot be doubted, but adaptive flexibility is realized only over evolutionary time. Hence, the criterion of short-term extinction risk (18) is inherently an inappropriate criterion for assessing the importance of genetic diversity on adaptive flexibility. Moreover, the adaptive flexibility associated with high genetic diversity is typically interwoven with ecological conditions that also diminish extinction risk. For example, the collared lizards on Stegall Mountain now have the capacity to maintain high levels of genetic diversity available for local adaptation because of the larger population sizes and large amounts of gene flow that unite many glades into a single effective breeding population. The increased gene flow is caused by the lizards' ability to disperse through recently burned forests, which, as we have already noted, also allows colonization of unoccupied glades (another buffer against local extinction), including glades too small to support an isolated viable population (allowing increases in total population size, another powerful buffer against extinction). In general, the factors that promote increased genetic diversity for local populations also provide an ecological buffer against local extinction.
One area of potential confusion about the need for gene flow in facilitating local adaptive flexibility is Wright's shifting balance theory (24). Wright argued that restricted gene flow resulting in population subdivision creates the optimal conditions for adaptive breakthroughs. However, it would be a mistake to interpret the shifting balance theory as implying that human-induced fragmentation facilitates adaptation. Wright's shifting balance process requires gene flow to be restricted but not eliminated. With complete isolation of small local populations, the shifting balance process grinds to a halt for lack of variation within local populations. Moreover, recent theoretical and experimental work indicates that the shifting balance process works at higher levels of gene flow than Wright had first envisioned (25–29), and that it works with metapopulation structures with local extinction coupled with recolonization (30–32), as is now occurring with the collared lizards on Stegall Mountain. Thus, when gene flow is reduced to extremely low levels, as had occurred in the lizards during the period of forest fire suppression, even shifting balance ceases to contribute to adaptive change. Complete or nearly complete fragmentation therefore disrupts the process of local adaptation even under shifting balance.
Wright's shifting balance theory also emphasizes another important role for gene flow: the spread of an adaptive trait from its local population of origin to the remainder of the species (26, 27, 29, 33–35). This spread is called phase III of shifting balance and illustrates the importance of gene flow not only in local adaptation but also in global adaptation. As habitat fragmentation increases and severs gene flow, the spread of adaptive traits throughout a species becomes increasingly difficult, thereby disrupting global adaptation at the same time that local adaptive flexibility is diminished.
Disrupting the Evolutionary Potential for Speciation
It can be argued that although fragmentation disrupts adaptation, it may partially compensate in promoting biodiversity by facilitating the evolutionary process of speciation (ref. 1, p. 75). This idea is based on the idea of founder-induced speciation (36–40). Of these models, the theory of genetic transilience is not just a theory of how founder events can induce speciation but rather primarily of why the vast majority of founder events do not induce speciation (40). Very restrictive conditions must hold before a founder event is likely to trigger speciation (40), conditions of: innate properties (e.g., genomic recombination size, system of mating), historical properties (e.g., the nature of the ancestral population structure, founder numbers, the manner in which the founders were sampled), and ecological factors (the requirement for a rapid increase in population size shortly after the founder event). Recently there have been empirical tests of genetic transilience (41, 42), and the results have supported the predictions of genetic transilience theory, in its predictions both in factors favoring founder-induced speciation and those preventing such speciation (43, 44).
When conditions are favorable for genetic transilience, they can lead not only to explosive speciation rates but also to major adaptive breakthroughs and innovations and to the evolution of higher taxa. For example, the Hawaiian Drosophila have the right combination of innate and historical properties in an appropriate ecological context for genetic transilience (38). The Hawaiian Drosophila not only represent the most speciose group of Drosophila; they also display an extraordinary range of morphological, developmental, and ecological diversity for the genus as a whole and have led to the creation of new genera (45). The ecological context in this case consisted of the regular creation of new volcanic islands to serve as sites of colonization from the older islands. This ecological context creates a situation in which rare interisland founder events to newer islands should lead to explosive population growth after the founder event because of open ecological niches. Such rapid population growth shortly after the founder event is a critical and essential element to speciation via genetic transilience (40).
The requirement of rapid population growth immediately after the founder event means that founder events are likely to induce speciation only in environmental contexts of open or expanding ecological opportunities. However, the founder events induced by human fragmentation are often characterized by diminished, not enhanced, ecological opportunity. Consequently, we expect most human-induced fragmentation events to reduce genetic diversity and increase local extinction with no compensating facilitation of speciation. We know of no compelling examples, in either nature or the laboratory, of speciation via founder events without the flush phase of rapid population growth after the founder event.
Studies of the eastern collared lizard illustrate a fate of rapid local extinction after founder events induced by fragmentation. As noted above in our work on Stegall Mountain, under a fire regime, collared lizards successfully exploit small glade habitats as feeding and breeding territories. Once isolated (as they were when fires were suppressed), these small glade populations must inevitably go extinct. Since 1981, we have surveyed 130 glades in the northeastern Ozarks that had open areas that were as large as or larger than other nearby glades that had a population of collared lizards. Of these larger glades, collared lizard populations were still on 42 of them, indicating that 68% of these glades have experienced local extinction with no subsequent recolonization under the extreme fragmentation induced by fire suppression. This calculation assumes that all 130 glades had collared lizards before fire suppression occurred. In light of the fact that all these glades are close to a currently inhabited glade, this seems to be a reasonable assumption, given the results obtained at the Peck Ranch that lizards readily disperse to nearby glades when frequent fires occur. Indeed, we feel that this percentage is undoubtedly an underestimate of local extinction on larger glades, because we primarily surveyed areas with prior reports that collared lizards were present.
This local extinction process was directly observed for one glade, Victoria Glade. Because of its proximity to St. Louis, this glade has been included in a large number of scientific studies and is a common destination of field trips sponsored by Washington University and other local universities. As a consequence, there is excellent documentation of the plants and animals on this glade since the early 1950s. In the 1950s, this glade had a healthy population of collared lizards, but because of a lack of fire, eastern red cedars began to encroach on the glade, thereby destroying the open microhabitat essential for collared lizards. By 1962, the lizards had become extinct (O. Sexton, personal communication). In the 1980s, this glade was purchased in part by the MDOC and in part by The Nature Conservancy, both agencies initiating a management regime of clearing and burning (only the glade proper was initially burned and not the surrounding oak–hickory forest). By 1990, the glade had been returned to excellent condition (as judged by the plant community), but no collared lizards had recolonized the glade despite the existence of nearby natural populations on private property. Hence, fragmentation of the collared lizards in the eastern Ozarks has resulted in much local extinction without compensatory recolonization events. (This glade was subsequently recolonized by collared lizards, but only after fire management included the surrounding forest.) This situation resulted in an “extinction ratchet” (16, 46), in which each local extinction brings the total population closer and closer to global extinction. An extinction ratchet, not speciation, is the primary impact of human-induced fragmentation.
How to Prevent the Disruption of Evolutionary Processes
Under extreme fragmentation, adaptive potential is lost as the genetic diversity within local populations is eroded by genetic drift and lack of gene flow. The lack of gene flow also prevents the spread of adaptive genetic complexes. Speciation is unlikely in these fragmented isolates; rather, an extinction ratchet is created by the fragmentation. The rate at which this extinction ratchet operates is primarily a function of local, not global, population size. Similarly, the rate of erosion of genetic diversity within the isolates also depends on their local effective sizes. The dominance of local factors makes the erosion of genetic diversity and the extinction ratchet virtually unmanageable, as separate management efforts would be needed for each isolate. The only practical manner of dealing with the erosion of genetic diversity and the extinction ratchet is to reestablish landscape-level population dynamics. That is, we need to end the isolation, both genetic and ecological, of fragmented local populations.
This can be done. The Biodiversity Task Force for the State of Missouri (13) recommended that the goal of conservation policy should not be to preserve a list of species or communities that were present at some reference time; rather, we should be preserving the processes that underlie a dynamic biodiversity at all levels. The experiences at Stegall Mountain demonstrate that a reversal of fragmentation is possible when management focus shifts from lists of items to be preserved to fundamental evolutionary and ecological processes, and from local isolates to the landscape in which the isolates are imbedded. This landscape focus does not mean that all efforts focused on local isolates or specific species and communities must cease; these efforts often continue to be needed. Rather, if we truly want to avoid disrupting the evolutionary process and want to ensure healthy biodiversity at all levels, from the genetic up, we must add landscape-level process-oriented considerations to our conservation efforts.
Acknowledgments
We thank the many people who helped in capturing collared lizards for this work, including Delbert Hutchison and family, Eric Routman, Christopher Phillips, L. Susan Pletscher, Ted Townsend, Jon Hess, and Aaron Hames. We also thank Melissa Kramer and Jon Hess for their excellent comments on an earlier version of this paper. This work was supported by National Science Foundation (NSF) Grant DEB-9610219 and Research Experiences for Undergraduates Program supplements to A.R.T. Graduate research fellowship support was provided for R.R. by an NSF Graduate Fellowship and for J.B. and J.S. by Howard Hughes Institute Graduate Fellowships. We also greatly appreciate the cooperation and logistical support we have received from the MDOC, the Department of Natural Resources of the State of Missouri, and The Nature Conservancy of Missouri.
Abbreviation
- MDOC
Missouri Department of Conservation
Footnotes
This paper was presented at the National Academy of Sciences colloquium, “The Future of Evolution,” held March 16–20, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Meffe G K, Carroll C R Contributors. Principles of Conservation Biology. Sunderland, MA: Sinauer; 1997. [Google Scholar]
- 2.Templeton A R. In: Speciation and Its Consequences. Otte D, Endler J A, editors. Sunderland, MA: Sinauer; 1989. pp. 3–27. [Google Scholar]
- 3.Templeton A R. In: Endless Forms: Species and Speciation. Howard D J, Berlocher S H, editors. Oxford: Oxford Univ. Press; 1998. pp. 32–43. [Google Scholar]
- 4.Templeton A R. In: Evolutionary Theory and Processes: Modern Perspectives, Papers in Honour of Eviatar Nevo. Wasser S P, editor. Dordrecht, The Netherlands: Kluwer; 1999. pp. 171–192. [Google Scholar]
- 5.Templeton A R. Annu Rev Ecol Syst. 1981;12:23–48. [Google Scholar]
- 6.Nelson P, Ladd D. Missouriensis. 1981;3:5–9. [Google Scholar]
- 7.Mondy D. Master's thesis. Edwardsville, IL: Southern Illinois University; 1970. [Google Scholar]
- 8.Anderson R C, Bowles M L. In: Savannas, Barrens, and Rock Outcrop Plant Communities of North America. Anderson R C, Fralish J S, Baskin J M, editors. Cambridge: Cambridge Univ. Press; 1999. pp. 155–170. [Google Scholar]
- 9.Heikens A L. In: Savannas, Barrens, and Rock Outcrop Plant Communities of North America. Anderson R C, Fralish J S, Baskin J M, editors. Cambridge: Cambridge Univ. Press; 1999. pp. 220–230. [Google Scholar]
- 10.Ladd D. Proceedings of the Oak Woods Management Workshop. Charleston, IL: Eastern Illinois University; 1991. pp. 67–80. [Google Scholar]
- 11.Batek M J, Rebertus A J, Schroeder W A, Haithcoat T L, Compas E, Guyette R P. J Biogeogr. 1999;26:397–412. [Google Scholar]
- 12.Guyette R, McGinnes E A., Jr Trans Missouri Acad Sci. 1987;16:85–93. [Google Scholar]
- 13.Nigh T A, Pflieger W L, Redfearn P L, Jr, Schroeder W A, Templeton A R, Thompson F R., III . The Biodiversity of Missouri. Jefferson City, MO: Missouri Department of Conservation; 1992. [Google Scholar]
- 14.Gerber A S, Templeton A R. Oecologica. 1996;105:343–350. doi: 10.1007/BF00328737. [DOI] [PubMed] [Google Scholar]
- 15.Hutchison D W, Templeton A R. Evolution (Lawrence, Kans) 1999;53:1898–1914. doi: 10.1111/j.1558-5646.1999.tb04571.x. [DOI] [PubMed] [Google Scholar]
- 16.Templeton A R, Shaw K, Routman E, Davis S K. Ann Missouri Bot Gard. 1990;77:13–27. [Google Scholar]
- 17.Templeton A R. In: Biodiversity in Managed Landscapes: Theory and Practice. Szaro R C, Johnston D W, editors. Oxford: Oxford Univ. Press; 1996. pp. 315–325. [Google Scholar]
- 18.Caro T M, Laurenson M K. Science. 1994;263:485–486. doi: 10.1126/science.8290956. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien S J, Roelke M E, Marker L, Newman A, Winkler C A, Meltzer D, Colly L, Evermann J F, Bush M, Wildt D E. Science. 1985;227:1428–1434. doi: 10.1126/science.2983425. [DOI] [PubMed] [Google Scholar]
- 20.Moritz C, McCallum H, Donnellan S, Roberts J D. Proc R Soc London B. 1991;244:145–149. [Google Scholar]
- 21.Bishop J A, Cook L M. Genetic Consequences of Man Made Change. London: Academic; 1981. [Google Scholar]
- 22.Oldfield M L. The Value of Conserving Genetic Resources. National Park Service, Washington, DC: U.S. Department of the Interior; 1984. [Google Scholar]
- 23.Carson H L. Evolution (Lawrence, Kans) 1961;15:496–509. [Google Scholar]
- 24.Wright S. Proc Sixth Int Cong Genet. 1932;1:356–366. [Google Scholar]
- 25.Bergman A, Goldstein D B, Holsinger K E, Feldman M W. Genet Res. 1995;66:85–92. doi: 10.1017/s0016672300034418. [DOI] [PubMed] [Google Scholar]
- 26.Kawata M. Evol Ecol Res. 1999;1:663–680. [Google Scholar]
- 27.Peck S L, Ellner S P, Gould F. Evolution (Lawrence, Kans) 1998;52:1834–1839. doi: 10.1111/j.1558-5646.1998.tb02260.x. [DOI] [PubMed] [Google Scholar]
- 28.Wade M J, Goodnight C J. Science. 1991;253:1015–1018. doi: 10.1126/science.1887214. [DOI] [PubMed] [Google Scholar]
- 29.Wade M J, Griesemer J R. Am Nat. 1998;151:135–147. doi: 10.1086/286107. [DOI] [PubMed] [Google Scholar]
- 30.McCauley D E. Oxford Surv Evol Biol. 1993;9:109–134. [Google Scholar]
- 31.Wade M J, Goodnight C J. Evolution (Lawrence, Kans) 1998;52:1537–1553. doi: 10.1111/j.1558-5646.1998.tb02235.x. [DOI] [PubMed] [Google Scholar]
- 32.Wallace B. Genetica. 1992;87:119–125. [Google Scholar]
- 33.Gavrilets S. Evolution (Lawrence, Kans) 1996;50:1034–1041. doi: 10.1111/j.1558-5646.1996.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 34.Moore F, Tonsor S J. Evolution (Lawrence, Kans) 1994;48:69–80. doi: 10.1111/j.1558-5646.1994.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 35.Rogers A. Am Nat. 1990;135:398–413. [Google Scholar]
- 36.Mayr E. In: Mechanisms of Speciation. Barigozzi C, editor. New York: Liss; 1982. pp. 1–19. [Google Scholar]
- 37.Mayr E. In: Evolution as a Process. Huxley J, Hardy A C, Ford E B, editors. Princeton, NJ: Princeton Univ. Press; 1954. pp. 157–180. [Google Scholar]
- 38.Carson H L, Templeton A R. Annu Rev Ecol Syst. 1984;15:97–131. [Google Scholar]
- 39.Carson H L. In: Population Biology and Evolution. Lewontin R C, editor. Syracuse, NY: Syracuse Univ. Press; 1968. pp. 123–137. [Google Scholar]
- 40.Templeton A R. Genetics. 1980;94:1011–1038. doi: 10.1093/genetics/94.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galiana A, Moya A, Ayala F J. Evolution (Lawrence, Kans) 1993;47:432–444. doi: 10.1111/j.1558-5646.1993.tb02104.x. [DOI] [PubMed] [Google Scholar]
- 42.Rundle H D, Mooers A O, Whitlock M C. Evolution (Lawrence, Kans) 1998;52:1850–1855. doi: 10.1111/j.1558-5646.1998.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 43.Templeton A R. Evolution (Lawrence, Kans) 1996;50:909–915. doi: 10.1111/j.1558-5646.1996.tb03899.x. [DOI] [PubMed] [Google Scholar]
- 44.Templeton A R. Evolution (Lawrence, Kans) 1999;53:1628–1632. doi: 10.1111/j.1558-5646.1999.tb05429.x. [DOI] [PubMed] [Google Scholar]
- 45.Templeton A R. In: Evolutionary Processes and Theory. Karlin S, Nevo E, editors. New York: Academic; 1986. pp. 497–512. [Google Scholar]
- 46.Templeton A R. Nat Areas J. 1982;2:35–38. [Google Scholar]