Abstract
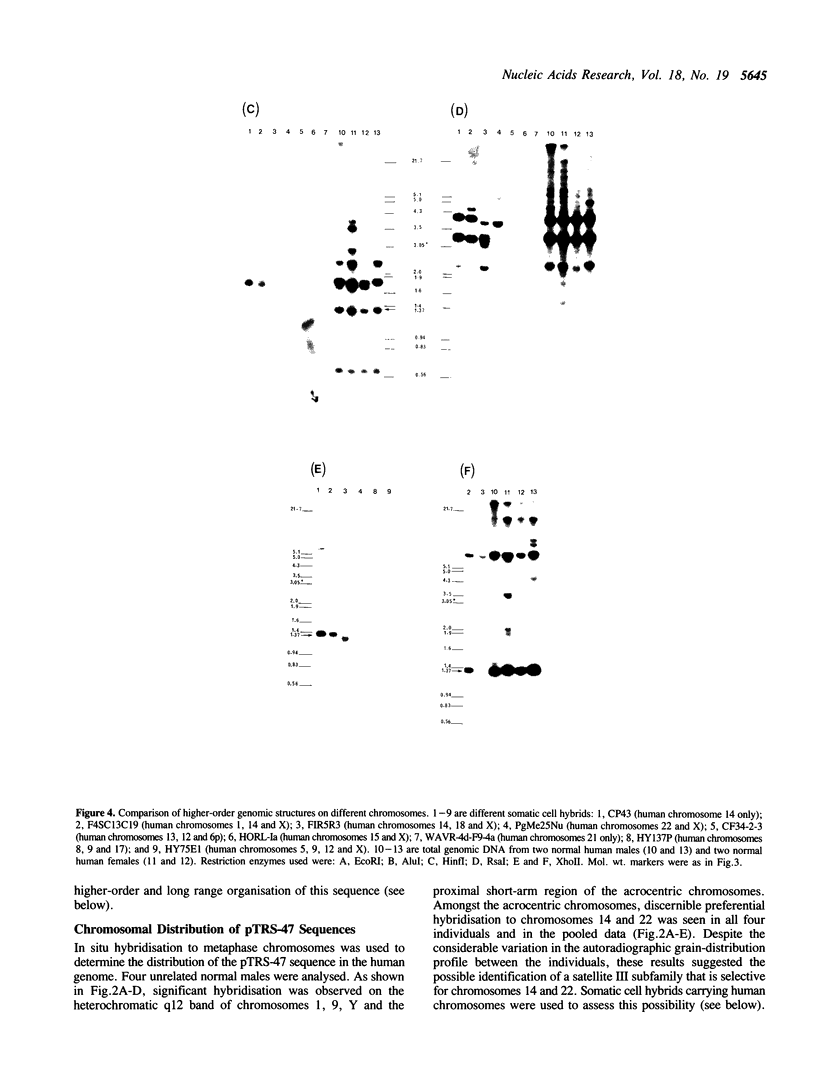
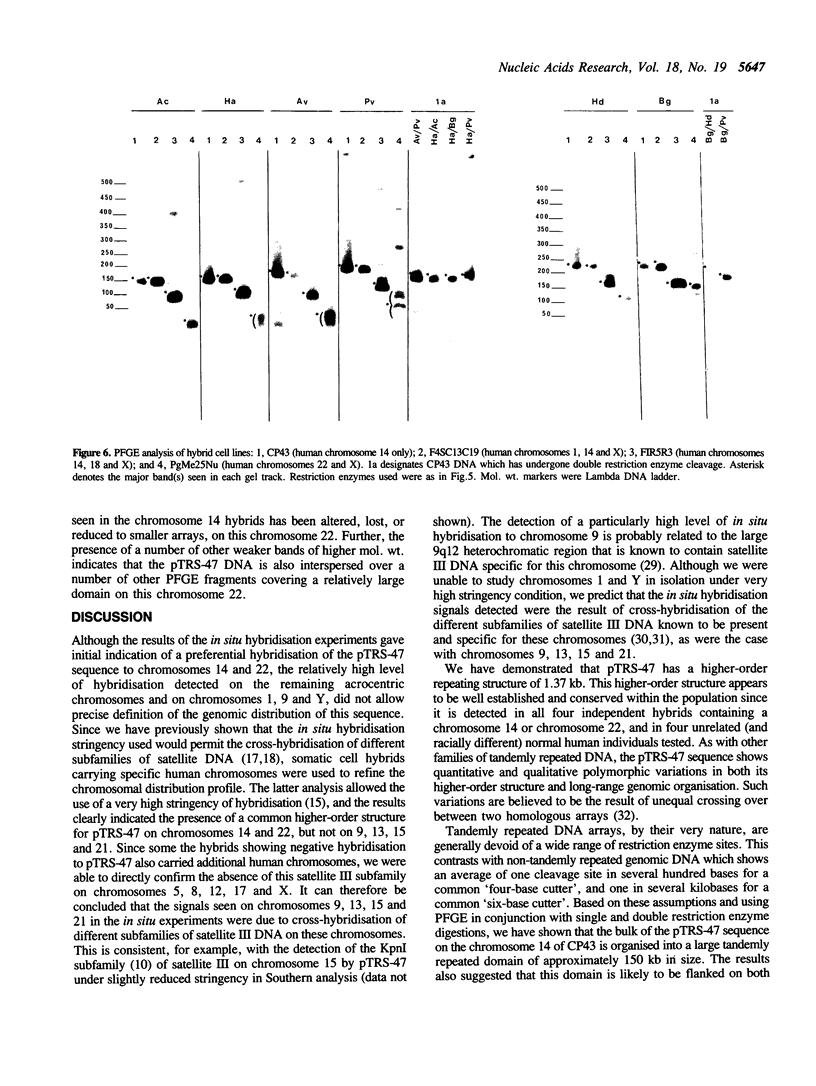
We describe a new subfamily of human satellite III DNA that is represented on two different acrocentric chromosomes. This DNA is composed of a tandemly repeated array of diverged 5-base-pair monomer units of the sequence GGAAT or GGAGT. These monomers are organised into a 1.37-kilobase higher-order structure that is itself tandemly reiterated. Using a panel of somatic cell hybrids containing specific human chromosomes, this higher-order structure is demonstrated on chromosomes 14 and 22, but not on the remaining acrocentric chromosomes. In situ hybridisation studies have localised the sequence to the proximal p-arm region of these chromosomes. Analysis by pulsed-field gel electrophoresis (PFGE) reveals that 70-110 copies of the higher-order structure are tandemly organised on a chromosome into a major domain which appears to be flanked on both sides by non-tandemly repeated genomic DNA. In addition, some of the satellite III sequences are interspersed over a number of other PFGE fragments. This study provides fundamental knowledge on the structure and evolution of the acrocentric chromosomes, and should extend our understanding of the complex process of interchromosomal interaction which may be responsible for Robertsonian translocation and meiotic nondisjunction involving these chromosomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agresti A., Rainaldi G., Lobbiani A., Magnani I., Di Lernia R., Meneveri R., Siccardi A. G., Ginelli E. Chromosomal location by in situ hybridization of the human Sau3A family of DNA repeats. Hum Genet. 1987 Apr;75(4):326–332. doi: 10.1007/BF00284102. [DOI] [PubMed] [Google Scholar]
- Arnheim N., Krystal M., Schmickel R., Wilson G., Ryder O., Zimmer E. Molecular evidence for genetic exchanges among ribosomal genes on nonhomologous chromosomes in man and apes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7323–7327. doi: 10.1073/pnas.77.12.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K. H., Brown R., Webb G., Craig I. W., Filby R. G. Genomic organization of human centromeric alpha satellite DNA: characterization of a chromosome 17 alpha satellite sequence. DNA. 1987 Aug;6(4):297–305. doi: 10.1089/dna.1987.6.297. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Earle E., Vissel B., Filby R. G. Identification of two distinct subfamilies of alpha satellite DNA that are highly specific for human chromosome 15. Genomics. 1990 Jun;7(2):143–151. doi: 10.1016/0888-7543(90)90534-2. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Vissel B., Brown R., Filby R. G., Earle E. Homologous alpha satellite sequences on human acrocentric chromosomes with selectivity for chromosomes 13, 14 and 21: implications for recombination between nonhomologues and Robertsonian translocations. Nucleic Acids Res. 1988 Feb 25;16(4):1273–1284. doi: 10.1093/nar/16.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K. H., Vissel B., Earle E. Evolution of alpha-satellite DNA on human acrocentric chromosomes. Genomics. 1989 Aug;5(2):332–344. doi: 10.1016/0888-7543(89)90066-9. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Hindley J. Cloning of human satellite III DNA: different components are on different chromosomes. Nucleic Acids Res. 1979 Jul 25;6(10):3177–3197. doi: 10.1093/nar/6.10.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Devilee P., Cremer T., Slagboom P., Bakker E., Scholl H. P., Hager H. D., Stevenson A. F., Cornelisse C. J., Pearson P. L. Two subsets of human alphoid repetitive DNA show distinct preferential localization in the pericentric regions of chromosomes 13, 18, and 21. Cytogenet Cell Genet. 1986;41(4):193–201. doi: 10.1159/000132229. [DOI] [PubMed] [Google Scholar]
- Frommer M., Prosser J., Tkachuk D., Reisner A. H., Vincent P. C. Simple repeated sequences in human satellite DNA. Nucleic Acids Res. 1982 Jan 22;10(2):547–563. doi: 10.1093/nar/10.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts van Kessel A. H., den Boer W. C., van Agthoven A. J., Hagemeijer A. Decreased tumorigenicity of rodent cells after fusion with leukocytes from normal and leukemic donors. Somatic Cell Genet. 1981 Nov;7(6):645–656. doi: 10.1007/BF01538754. [DOI] [PubMed] [Google Scholar]
- Gosden J. R., Lawrie S. S., Gosden C. M. Satellite DNA sequences in the human acrocentric chromosomes: information from translocations and heteromorphisms. Am J Hum Genet. 1981 Mar;33(2):243–251. [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R., Spurr N. K., Goodfellow P. N., Solomon E., Carritt B., Bodmer W. F. Chromosomal localization of human cellular homologues of two viral oncogenes. Nature. 1982 Oct 21;299(5885):747–749. doi: 10.1038/299747a0. [DOI] [PubMed] [Google Scholar]
- Higgins M. J., Wang H. S., Shtromas I., Haliotis T., Roder J. C., Holden J. J., White B. N. Organization of a repetitive human 1.8 kb KpnI sequence localized in the heterochromatin of chromosome 15. Chromosoma. 1985;93(1):77–86. doi: 10.1007/BF01259449. [DOI] [PubMed] [Google Scholar]
- Hobart M. J., Rabbitts T. H., Goodfellow P. N., Solomon E., Chambers S., Spurr N., Povey S. Immunoglobulin heavy chain genes in humans are located on chromosome. Ann Hum Genet. 1981 Oct;45(Pt 4):331–335. doi: 10.1111/j.1469-1809.1981.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen A. L., Bostock C. J., Bak A. L. Homologous subfamilies of human alphoid repetitive DNA on different nucleolus organizing chromosomes. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1075–1079. doi: 10.1073/pnas.84.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen A. L., Kølvraa S., Jones C., Bak A. L. A subfamily of alphoid repetitive DNA shared by the NOR-bearing human chromosomes 14 and 22. Genomics. 1988 Aug;3(2):100–109. doi: 10.1016/0888-7543(88)90139-5. [DOI] [PubMed] [Google Scholar]
- Krystal M., D'Eustachio P., Ruddle F. H., Arnheim N. Human nucleolus organizers on nonhomologous chromosomes can share the same ribosomal gene variants. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5744–5748. doi: 10.1073/pnas.78.9.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnit D. M., Neve R. L., Morton C. C., Bruns G. A., Ma N. S., Cox D. R., Klinger H. P. Recent evolution of DNA sequence homology in the pericentromeric regions of human acrocentric chromosomes. Cytogenet Cell Genet. 1984;38(2):99–105. doi: 10.1159/000132039. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Kao F. T., Law M. L., Woo S. L. Assignment of the alpha 1-antitrypsin gene and a sequence-related gene to human chromosome 14 by molecular hybridization. Am J Hum Genet. 1983 May;35(3):385–392. [PMC free article] [PubMed] [Google Scholar]
- McDermid H. E., Duncan A. M., Higgins M. J., Hamerton J. L., Rector E., Brasch K. R., White B. N. Isolation and characterization of an alpha-satellite repeated sequence from human chromosome 22. Chromosoma. 1986;94(3):228–234. doi: 10.1007/BF00288497. [DOI] [PubMed] [Google Scholar]
- Meneveri R., Agresti A., Della Valle G., Talarico D., Siccardi A. G., Ginelli E. Identification of a human clustered G + C-rich DNA family of repeats (Sau3A family). J Mol Biol. 1985 Dec 5;186(3):483–489. doi: 10.1016/0022-2836(85)90123-8. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shulkin J. D., Sparkes M. C., Moedjono S. Assignment of PGM3 to the long arm of human chromosome 6. Studies using Chinese hamster X human cell hybrids containing a human 6/15 translocation. Cytogenet Cell Genet. 1980;28(1-2):116–120. doi: 10.1159/000131519. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Albright K. L., Bartholdi M. F., Cram L. S., Deaven L. L., Hildebrand C. E., Joste N. E., Longmire J. L., Meyne J., Schwarzacher-Robinson T. Human chromosome-specific repetitive DNA sequences: novel markers for genetic analysis. Chromosoma. 1987;95(6):375–386. doi: 10.1007/BF00333988. [DOI] [PubMed] [Google Scholar]
- Prosser J., Frommer M., Paul C., Vincent P. C. Sequence relationships of three human satellite DNAs. J Mol Biol. 1986 Jan 20;187(2):145–155. doi: 10.1016/0022-2836(86)90224-x. [DOI] [PubMed] [Google Scholar]
- Slate D. L., Shulman L., Lawrence J. B., Revel M., Ruddle F. H. Presence of human chromosome 21 alone is sufficient for hybrid cell sensitivity to human interferon. J Virol. 1978 Jan;25(1):319–325. doi: 10.1128/jvi.25.1.319-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Mitchell A. R., Willard H. F. Organization and genomic distribution of "82H" alpha satellite DNA. Evidence for a low-copy or single-copy alphoid domain located on human chromosome 14. Hum Genet. 1988 Jan;78(1):27–32. doi: 10.1007/BF00291229. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Human beta satellite DNA: genomic organization and sequence definition of a class of highly repetitive tandem DNA. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6250–6254. doi: 10.1073/pnas.86.16.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Sutherland J., Sylvester J. E., Willard H. F., Bodrug S., Dubé I., Duff C., Kean V., Ray P. N., Schmickel R. D. Human ribosomal RNA genes: orientation of the tandem array and conservation of the 5' end. Science. 1988 Jan 1;239(4835):64–68. doi: 10.1126/science.3336775. [DOI] [PubMed] [Google Scholar]