Abstract
Background:
Frequency of FGFR2 amplification, its clinicopathological features, and the results of high-throughput screening assays in a large cohort of gastric clinical samples remain largely unclear.
Methods:
Drug sensitivity to a fibroblast growth factor receptor (FGFR) inhibitor was evaluated in vitro. The gene amplification of the FGFRs in formalin-fixed, paraffin-embedded (FFPE) gastric cancer tissues was determined by a real-time PCR-based copy number assay and fluorescence in situ hybridisation (FISH).
Results:
FGFR2 amplification confers hypersensitivity to FGFR inhibitor in gastric cancer cell lines. The copy number assay revealed that 4.1% (11 out of 267) of the gastric cancers harboured FGFR2 amplification. No amplification of the three other family members (FGFR1, 3 and 4) was detected. A FISH analysis was performed on 7 cases among 11 FGFR2-amplified cases and showed that 6 of these 7 cases were highly amplified, while the remaining 1 had a relatively low grade of amplification. Although the difference was not significant, patients with FGFR2 amplification tended to exhibit a shorter overall survival period.
Conclusion:
FGFR2 amplification was observed in 4.1% of gastric cancers and our established PCR-based copy number assay could be a powerful tool for detecting FGFR2 amplification using FFPE samples. Our results strongly encourage the development of FGFR-targeted therapy for gastric cancers with FGFR2 amplification.
Keywords: FGFR2 , gastric cancer, gene amplification
Intensive investigations of anticancer treatments for gastric cancer have been done over the past three decades; however, the prognosis for patients with unresectable advanced or recurrent gastric cancer remains poor (Bittoni et al, 2010; Fujii et al, 2010), and new therapeutic modalities are needed.
Fibroblast growth factors (FGFs) and their receptors are considered to be associated with multiple biological activities, including fundamental developmental pathways, cellular proliferation, differentiation, motility and transforming activities (Itoh et al, 1994; Moffa et al, 2004; Grose and Dickson, 2005). Fibroblast growth factor signalling is also involved in many physiological roles in the adult organism, such as the regulation of angiogenesis and wound repair, and FGF receptors (FGFRs) are expressed on many different cell types and regulate key cell behaviours of cancer cells (Turner and Grose, 2010). Emerging evidence has demonstrated that the deregulation of FGF signalling is frequently observed in various solid cancers and haematological malignancies (Beenken and Mohammadi, 2009). The most well-known association with FGFR mutations is the FGFR3 mutation observed in bladder cancer, in which somatic mutations in coding regions are observed in about 50% of all specimens (Cappellen et al, 1999; Turner and Grose, 2010). Other genetic alterations in FGFR3 include gene amplification in bladder cancer and translocation in myeloma (Turner and Grose, 2010). Similarly, the deregulation of FGF signalling has been reported in various malignancies. Glioblastoma exhibits FGFR1 kinase domain gain-of-function mutations, and FGFR1 is abnormally activated in malignant prostate cells. In 8p11 myeloproliferative syndrome, translocations fuse different proteins in frame with the FGFR1 kinase domain, causing the constitutive dimerisation of the kinase (Giri et al, 1999; Rand et al, 2005; Beenken and Mohammadi, 2009). The FGFR1 amplification has been reported in approximately 10% of breast cancers (Courjal et al, 1997) and oral squamous carcinomas, and has been also found at a low incidence in ovarian cancer, bladder cancer and rhabdomyosarcoma (Turner and Grose, 2010). FGFR2 mutations are observed in 12% of endometrial cancers but are reportedly rare in gastric cancers (Jang et al, 2001; Dutt et al, 2008). The K-sam gene was first identified and characterised as an amplified gene in the human gastric cancer cell line KATO-III (Hattori et al, 1990; Ueda et al, 1999), and its product was later found to be identical to the bacteria-expressed kinase, or keratinocyte growth factor receptor, and FGF receptor 2 (FGFR2). FGFR2 amplification has been found in diffuse-type gastric cancer-derived cell lines and the amplification was preferentially detected in diffuse-type gastric cancer. FGFR2 protein overexpression was detected using immunohistochemical staining in 20 of 38 advanced cases of diffuse-type gastric cancer (Hattori et al, 1996). FGFR2 protein expression was observed in 31% of the gastric carcinomas and was positively correlated with scirrhous cancer, a diffuse type, the invasion depth, the infiltration type and a poor prognosis (Toyokawa et al, 2009).
On the other hand, along with another group, we previously reported that FGFR2 amplification confers hypersensitivity to FGFR inhibitor in gastric cancer cell lines both in vitro and in vivo (Nakamura et al, 2006; Takeda et al, 2007), strongly suggesting that FGFR2 amplification may be a promising molecular target for the treatment of FGFR2-amplified gastric cancer. However, very limited information on FGFR2 amplification is available regarding the frequency, the degree of the increase in the copy number, the histology and a high-throughput screening method in gastric cancer. In this report, we retrospectively studied these issues using formalin-fixed, paraffin-embedded (FFPE) samples in patients with gastric cancer who underwent surgery in an attempt to advance FGFR2-targeted therapy for gastric cancer.
Materials and methods
Cell culture
All of the gastric cancer cell lines used in this study were maintained in RPMI-1640 medium (Sigma, St Louis, MO, USA), except for IM95 (DMEM; Nissui Pharmaceutical, Tokyo, Japan), supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL, Grand Island, NY, USA), penicillin and streptomycin in a humidified atmosphere of 5% CO2 at 37 °C. IM95 and OCUM1 were obtained from the Japanese Collection of Research Bioresources (Osaka, Japan) and the others were provided from National Cancer Center Research Institute (Tokyo, Japan).
Patients
A total of 267 patients with histologically confirmed gastric cancer who had undergone surgery at the National Cancer Center Hospital between 1996 and 2006 were included in this study. All the patients in this series had an Eastern Cooperative Oncology Group performance status of 0 to 2 and had undergone surgery. Of these patients, one subject was excluded because an insufficient quantity of DNA was extracted from the patient's specimen. Thus, samples from the remaining 267 patients were analysed. This study was approved by the institutional review board of the National Cancer Center Hospital.
Isolation of genomic DNA
Genomic DNA samples were extracted from surgical specimens preserved as FFPE tissue using a QIAamp DNA Micro kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Macro-dissection of the FFPE samples was performed to select a cancer region, which was marked by a pathologist after deparaffinisation. The DNA concentration was determined using the NanoDrop2000 (Thermo Scientific, Waltham, MA, USA).
Real-time reverse-transcription PCR (RT–PCR)
cDNA was prepared from the total RNA of each cultured cell line using a GeneAmp RNA-PCR kit (Applied Biosystems, Foster City, CA, USA). Real-time RT–PCR amplification was carried out using a Thermal Cycler Dice (Takara, Otsu, Japan) in accordance with the manufacturer's instructions under the following conditions: 95 °C for 5 min, and 50 cycles of 95 °C for 10 s and 60 °C for 30 s. The primers used for the real-time RT–PCR were as follows: FGFR2, forward 5′-GATAAATACTTCCAATGCAGAAGTGCT-3′ and reverse 5′-TGCCCTATATAATTGGAGACCTTACA-3′ GAPDH, forward 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse 5′-ATGGTGGTGAAGACGCCAGT-3′. GAPDH was used to normalise the expression levels in the subsequent quantitative analyses.
Immunoblotting
A western blot analysis was performed as described previously (Matsumoto et al, 2009). The following antibodies were used: monoclonal FGFR2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), β-actin antibody and HRP-conjugated secondary antibody (Cell Signaling Technology, Beverly, MA, USA).
Cell growth inhibitory assay
To evaluate growth inhibition in the presence of various concentrations of PD173074 (Sigma), we used an MTT assay and a previously described method (Kaneda et al, 2010). Briefly, the cells were seeded at a density of 2 × 103 cells per well in 96-well plates. After 24 h, PD173074 was added and the incubation was further continued for 72 h at 37 °C. The assay was conducted in triplicate.
Copy number assay for four FGFR family genes
The copy numbers for FGFR 1–4 were determined using commercially available and pre-designed TaqMan Copy Number Assays according to the manufacturer's instructions (Applied Biosystems). The primer IDs used for FGFRs were as follows: FGFR1, Hs02862256_cn; FGFR2, HS05182482_cn (intron 14) and Hs05114211_cn (intron 12); FGFR3, Hs03518314_cn; and FGFR4, Hs01949336_cn. The TERT locus was used for the internal reference copy number. Human Genomic DNA (Takara) was used as a normal control. Real-time genomic PCR was performed in a total volume of 20 μl in each well, containing 10 μl of TaqMan genotyping master mix, 20 ng of genomic DNA and each primer. The PCR conditions were 95 °C for 10 min and 40 cycles of 95 °C for 15 s and 60 °C for 1 min; the resulting products were detected using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). Data were analysed using SDS 2.2 software and CopyCaller software (Applied Biosystems).
Fluorescence in situ hybridisation analysis
The fluorescence in situ hybridisation (FISH) method was previously descried (Motoi et al, 2010). Probes designed to detect the FGFR2 gene and the CEN10p on chromosome 10 were labelled with fluorescein isothiocyanate or Texas red and were designed to hybridise to the adjacent genomic sequence spanning approximately 0.33 and 0.64 Mb, respectively. The probes were generated from appropriate clones from a library of human genomic clones (GSP Laboratory, Kawasaki, Japan). Deparaffinised tissue sections were air dried and pre-treated with the GSP paraffin pre-treatment kit (GSP Laboratory). In all, 10 μl of fluorescent FISH probe was heated for 5 min at 73–75 °C in a waterbath for denaturation. The tissue sections were then placed in a denaturant solution (70% formamide/2 × saline sodium citrate (SSC) pH 7-8) in a 73–75 °C waterbath, denatured for 5 min, dehydrated in 70 and 100% ethanol for 1 min each at room temperature, and air-dried. Denatured probes were applied, and the specimens were covered with a coverglass and placed on a heated block at 45–50 °C. Then, the slides were sealed with rubber cement and placed in a pre-warmed humidified box overnight at 37 °C. Stringent washing was performed using 2 × SSC/0.3% NP-40 at room temperature and at 72 °C for 5 min and then with 2 × SSC at room temperature. The signals were observed using fluorescence microscopy, and the FISH signals were evaluated by independent observers (TM and AK). After screening all the complete sections, images of the tumour cells were captured and recorded and the signals for 20 random nuclei were counted for an area where individual cells were recognised on at least 10 representative images. The positive result of copy number gain is determined as follows (FGFR2/CEN10p⩾2.0).
Statistical analysis
The statistical analyses of the clinicopathological features were performed using the Student t-test and the χ2 test using PAWS Statistics 18 (SPSS Japan Inc., Tokyo, Japan). The overall survival (OS) curves were estimated using the Kaplan–Meier method.
Results
FGFR2 amplification confers hypersensitivity to FGFR inhibitor in gastric cancer cell lines
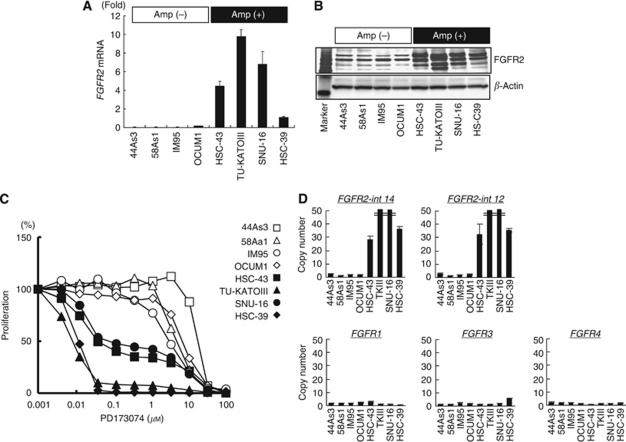
We examined the growth inhibitory effect of PD173074 (0.004–80 μM) on four FGFR2-amplified (HSC-43, TU-KATPIII, SNU-16 and HSC-39) and four non-amplified (44As3, 58As1, IM95 and OCUM1) gastric cancer cell lines. The FGFR2 amplification status of each cell line had already been examined using a CGH analysis (unpublished data). The mRNA and protein expressions of FGFR2 were overexpressed in the FGFR2-amplified cell lines (Figures 1A and B). A growth inhibitory assay showed that the IC50 values of the FGFR inhibitor PD173074 in FGFR2-amplified cells were 0.01–0.07 μM, whereas those in non-amplified cells were 2.6–13.2 μM, indicating that FGFR2 amplification conferred an approximately 100-fold hypersensitivity to FGFR inhibitor in gastric cancer cell lines (Figure 1C).
Figure 1.
FGFR2 amplification in gastric cancer cell lines. (A) The mRNA expression levels of FGFR2 were determined using real-time RT–PCR for eight gastric cancer cell lines. FGFR2 mRNA: normalised mRNA expression levels (FGFR2/GAPDH × 103). (B) Western blot analysis for FGFR2 expression. β-Actin was used as an internal control. Marker, molecular marker. (C) Growth inhibition assay for the FGFR inhibitor PD173074, evaluated at the indicated concentrations using an MTT assay. (D) Evaluation of DNA copy number assay using gastric cancer cell lines. A TaqMan copy number assay was performed to determine the copy number using specific primers for the genomic loci of the FGFR1–4 genes against DNA samples. Amp, gene amplification. FGFR2-int-14 and FGFR2-int-12, different primers for intron 14 or intron 12 of FGFR2.
FGFR2 amplification in clinical gastric cancer cell lines and surgical specimens
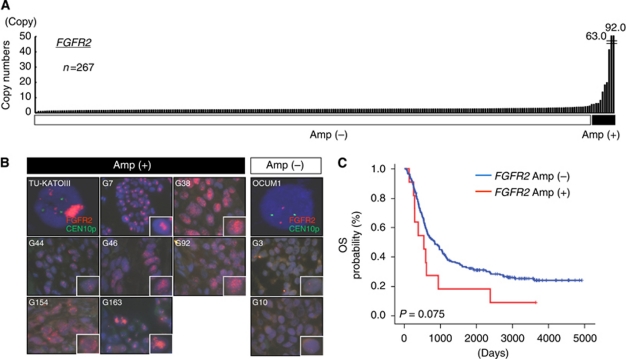
To develop a high-throughput method for detecting FGFR2 gene amplification in a clinical setting, we verified a real-time PCR-based detection method, the TaqMan Copy Number Assay. The FGFR2 copy number was 1.4–2.7 copies in the four non-amplified cell lines; however, the numbers in the four FGFR2-amplified cell lines were 28.2, 231.7, 88.2 and 36.3 copies, respectively (Figure 1D). In addition, another primer in intron 12 of FGFR2 produced a very similar result (R=0.99, Figure 1D). Collectively, these results suggested that a DNA copy number assay for FGFR2 was a sensitive and reproducible method. We also examined the copy numbers of FGFR1, FGFR3 and FGFR4, but no obvious gene amplification was observed in all of the eight cell lines (Figure 1D). Next, FGFR2 amplification was evaluated using the copy number assay in 267 FFPE samples of primary gastric cancer specimens. FGFR2 amplification of more than 5 copies was observed in 11 cases (92.0, 63.0, 41.4, 19.9, 18.4, 13.7, 8.3, 6.2, 6.2, 5.7 and 5.6 copies), with a frequency of 4.1% (Figure 2A). The mean copy number in the non-amplified cases was 2.4±0.6 copies. Meanwhile, no obvious gene amplification of FGFR1, FGFR3 or FGFR4 was observed (data not shown).
Figure 2.
(A) Amplification of FGFRs in surgical specimens of gastric cancer. A TaqMan copy number assay for FGFR2 was performed using DNA samples obtained from 267 FFPE samples. Human normal genomic DNA was used as a normal control. FGFR2 amplification over 5 copies was observed in 11 cases (92.0, 63.0, 41.4, 19.9, 18.4, 13.7, 8.3, 6.2, 6.2, 5.7 and 5.6 copies). (B) Fluorescence in situ hybridisation analysis of FGFR2-amplified KATO-III cells, non-amplified OCUM1 cells and nine surgical specimens of gastric cancer. Green, signal of CEN10P locus; Red, signal of FGFR2 locus; G3∼G92, sample numbers; Amp, gene amplification. High-power images are presented for a single cancer cell. (C) Overall survival in FGFR2-amplified gastric cancer. Kaplan–Meier curves for OS according to the FGFR2 amplification status.
FISH analysis for FGFR2 amplification
We used a FISH analysis to examine FGFR2 amplification in the same samples to verify the results of the above PCR-based DNA copy number assay. Highly amplified TU-KATOIII cells showed numerous and large clustered signals, whereas non-amplified OCUM1 cells contained two normally paired signals (Figure 2B). A FISH analysis was performed on seven cases among 11 FGFR2-amplified cases and two non-amplified cases. The FISH analysis revealed that FGFR2 was highly amplified in six of the seven FGFR2-amplified clinical samples (four showed multiple scattered signals and two showed large clustered signals), while the remaining sample exhibited a relatively low grade of amplification (FGFR2/CEN10p=2.2, Figure 2B). The FGFR2 signals in the G3 and G10 samples, which were determined not to be amplified based on the results of the DNA copy number assay, were not increased. These results clearly demonstrated the presence of FGFR2-amplified gastric cancers among clinical samples.
Clinicopathological features of FGFR2-amplified gastric cancer
We evaluated the clinicopathological features including age, sex, histology and pathological stage according to the FGFR2 amplification status. Patients age with FGFR2 amplification were significantly higher than the others, but sex and pathological stage were not associated with FGFR2 amplification in this study (Table 1). Among the patients with FGFR2 amplification, the histologies of two cases were intestinal-type gastric cancer and one was unclassified type, while the others were diffuse-type (Table 2). The tumours were located in either the upper or lower stomach. These results are summarised in Table 2. Finally, we examined the prognostic impact of FGFR2 amplification on OS after surgery. FGFR2 amplification tended to be associated with a poorer outcome, compared with non-amplified cases, but no significant difference was observed in the current study (log-rank test, P=0.075; Figure 2C).
Table 1. Frequency of FGFR2 amplification in gastric cancers and its association with clinical and pathologic factors.
|
FGFR2 (+)
|
FGFR2 (−)
|
||||
|---|---|---|---|---|---|
| n=11 | % | n=256 | % | P-value | |
| Age | |||||
| Range | 55–91 | 31–88 | 0.047 | ||
| Median | 67 | 63 | |||
| Gender | |||||
| Male | 11 | 100 | 173 | 68 | 0.052 |
| Female | 0 | 0 | 83 | 32 | |
| Pstage | |||||
| I | 0 | 0 | 25 | 10 | 0.16a |
| II | 0 | 0 | 32 | 13 | |
| III | 3 | 27 | 73 | 29 | |
| IV | 8 | 73 | 125 | 49 | |
| Unknown | 0 | 0 | 1 | 0 | |
| Histology | |||||
| Tub1 | 0 | 0 | 41 | 16 | 0.55b |
| Tub2 | 2 | 18 | 51 | 20 | |
| Pap | 1 | 9 | 5 | 2 | |
| Muc | 2 | 18 | 8 | 3 | |
| Sig | 1 | 9 | 15 | 6 | |
| Por1 | 0 | 0 | 28 | 11 | |
| Por2 | 5 | 45 | 108 | 42 | |
Abbreviations: Amp=gene amplification; FGFR=fibroblast growth factor receptor; Muc=mucinous adenocarcinoma; Pap=papillary adenocarcinoma; Por=poorly differentiated adenocarcinoma; pStage=pathological stage; Sig=signet ring-cell carcinoma; Tub=tubular adenocarcinoma.
Comparison between pStage I+II and III+IV.
Comparison between intestinal (Tub1, Tub2 and Pap) and others. P-values were calculated using the t-test for age and the χ2 test for the other variables.
Table 2. Summary of FGFR2-amplified gastric cancers.
| No. | Age | Sex | Location | Size of lesion (cm) | Macroscopic type a | Lauren's classification | Histology | pStage | OS (days) | FGFR2 (CN) | FISH (type, copies) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G7 | 55 | M | Lower | 8.5 × 8 | 3 | Diffuse | Muc>Por2, Sig | IV | 612 | 41.4 | LC, +++ |
| G38 | 70 | M | Upper | 8.5 × 8 | 1 + IIc | Intestinal | Pap>Tub1, Tub2, Por2 | IIIa | 591 | 92.0 | MS, +++ |
| G44 | 70 | M | Lower | 9.5 × 8 | 3 | Diffuse | Por2>Pap, Tub1, Muc | IIIa | 938 | 5.6 | Low, 2.2b |
| G46 | 75 | M | Middle | 10 × 6 | 4 | Intestinal | Tub2>Por2 | IV | 2380 | 13.7 | MS, +++ |
| G92 | 75 | M | Middle | 6.5 × 5.5 | 3 | Diffuse | Por2>Tub2 | IV | 280 | 19.9 | MS, +++ |
| G154 | 59 | M | Middle | 14 × 12 | 4 | Diffuse | Por2 | IV | 132 | 5.7 | MS, +++ |
| G163 | 64 | M | Lower | 15 × 10 | 3 | Diffuse | Muc>Sig>Tub2 | IV | 540 | 6.2 | LC, +++ |
| G203 | 64 | M | Lower | 10.5 × 6.5 | 4 | Diffuse | Sig>Por2>Muc | IV | 283 | 8.3 | ND |
| G271 | 91 | M | Upper | 7 × 6.5 | 2 | Intestinal | Tub2>Por1 | IV | 383 | 63.0 | ND |
| G299 | 65 | M | Middle | 20 × 20 | 4 | Diffuse | Por2>Sig | IV | 256 | 6.2 | ND |
| G329 | 67 | M | Middle | 6.5 × 6 | 3 | Diffuse | Por2>Sig | IIIa | 3642+ | 18.4 | ND |
Abbreviations: CN=copy number of FGFR2 determined using a copy number assay; Diffuse=diffuse-type gastric cancer; FISH=fluorescence in situ hybridisation; FGFR2=fibroblast growth factor receptor 2; Intestinal=intestinal-type gastric cancer; Location=tumor location in stomach; LC=large clustered signals; Low=low copy number gain; M=male; MS=multiple scattered signals; ND, not determined; No.=sample numbers; OS=overall survival; pStage=pathological stage; +++=numerous FGFR2 signals; +=patients. alive.
Macroscopic type, classification is based on the definitions of the Japanese Research Society for Gastric Cancer.
Ratio of FGFR2/CEN10p.
Discussion
To date, several studies have reported on the protein expression of FGFR2 and clinicopathological analyses using immunohistochemistry, with 20 of 49 (41%) and 42 of 134 (31%) gastric cancers expressing FGFR2 protein when evaluated using positive or negative staining (Hattori et al, 1996; Toyokawa et al, 2009). Regarding genomic alteration, the frequency of FGFR2 amplification has been reported to be 3 out of 19 (16%, among diffuse-type gastric cancers) detected using comparative genomic hybridisation (CGH), 3 out of 57 (5%) detected using Southern blot analysis, and 2 out of 30 (7%) detected using CGH (Tsujimoto et al, 1997; Peng et al, 2003; Kim et al, 2010). These results suggest that the frequency of FGFR2 amplification is around 5%, which is lower than the positive staining results obtained using immunohistochemistry. However, the frequency of amplification has not been determined in a large cohort. Our results indicated that the frequency of FGFR2 amplification was 4.1% (11 out of 267), consistent with these previous reports on genomic alterations. To select a sub-population of gastric cancers sensitive to FGFR inhibitors in the future, gene amplification may be a more suitable biomarker than positive staining using immunohistochemistry based on the results of preclinical studies (Figure 1, Takeda et al, 2007).
In six cases, the copy number of FGFR2 was larger than 10 copies and numerous signals were observed by the FISH analysis (Figure 2B), indicating that these gastric cancer cells harboured high levels of amplification, similar to the results obtained using gastric cancer cell lines. Preclinical studies suggest that these cases may be likely to respond to FGFR inhibitors. In the remaining case, FGFR2 amplification was relatively low (4∼8 copies, G44). Such cases with low levels of FGFR2 amplification may require further investigation regarding their sensitivity to FGFR inhibitors in the future. Meanwhile, we used a copy number assay to detect gene amplification in FFPE samples. Although DNA extracted from FFPE samples was considered to be of low quality with a DNA degradation in general, a copy number assay was capable of detecting and screening amplification in the FFPE samples, which had been stored for as long as 10 years. The results were consistent with the results of FISH studies in several cell lines, with seven positive cases and two negative cases. Our findings suggest that a copy number assay is a powerful tool for detecting and screening gene amplification using FFPE samples.
Recently, trastuzumab in combination with chemotherapy has been regarded as a new standard option for patients with HER2-positive advanced gastric or gastro-oesophageal junction cancer (Bang et al, 2010). Therefore, the evaluation of both the HER2 and FGFR2 status before anti-cancer treatment may be needed in gastric cancer patients in the near future. Many small molecules of VEGFR2 tyrosine kinase inhibitors, categorised as anti-angiogenic agents, are now under clinical evaluation, and some of them, including sorafenib for hepatocellular carcinoma and sunitinib for renal cell carcinoma, are being clinically used as standard treatment options (Ellis and Hicklin, 2008). These compounds are also known to have a potential kinase inhibitory effect on FGFRs (Takeda et al, 2007; Turner et al, 2010), indicating that the development of these multi-kinase inhibitors may be a promising approach to the treatment of FGFR2-amplified gastric cancer. In addition to small molecular FGFR tyrosine kinase inhibitors, anti-FGFR antibodies, such as IMC-A1, PRO-001a and R3Mab, also offer promise as molecular-based drugs (Turner and Grose, 2010). We plan to conduct a prospective study in a cohort of Japanese patients with FGFR2-amplified gastric cancers.
In conclusion, we found that FGFR2 amplification was observed in gastric cancer at a frequency of about 4.1%, and a copy number assay was a powerful tool for screening for FGFR2 amplifications using FFPE samples. Our results warrant strong consideration of the development of FGFR inhibitors for the treatment of gastric cancers with FGFR2 amplification.
Acknowledgments
We thank Miss Tomoko Kitayama and Miss Hideko Morita for their technical assistance. This study was supported by the Third-Term Comprehensive 10-Year Strategy for Cancer Control and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
References
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK (2010) Trastuzumab in combination with chemotherapy vs chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742): 687–697 [DOI] [PubMed] [Google Scholar]
- Beenken A, Mohammadi M (2009) The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8(3): 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittoni A, Maccaroni E, Scartozzi M, Berardi R, Cascinu S (2010) Chemotherapy for locally advanced and metastatic gastric cancer: state of the art and future perspectives. Eur Rev Med Pharmacol Sci 14(4): 309–314 [PubMed] [Google Scholar]
- Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, Chopin D, Thiery JP, Radvanyi F (1999) Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nature Genet 23: 18–20 [DOI] [PubMed] [Google Scholar]
- Courjal F, Cuny M, Simony-Lafontaine J, Louason G, Speiser P, Zeillinger R, Rodriguez C, Theillet C (1997) Mapping of DNA amplifications at 15 chromosomal localizations in 1875 breast tumors: definition of phenotypic groups. Cancer Res 57: 4360–4367 [PubMed] [Google Scholar]
- Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C, Nicoletti R, Winckler W, Grewal R, Hanna M, Wyhs N, Ziaugra L, Richter DJ, Trovik J, Engelsen IB, Stefansson IM, Fennell T, Cibulskis K, Zody MC, Akslen LA, Gabriel S, Wong KK, Sellers WR, Meyerson M, Greulich H (2008) Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci USA 105: 8713–8717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LM, Hicklin DJ (2008) VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8(8): 579–591 [DOI] [PubMed] [Google Scholar]
- Fujii M, Kochi M, Takayama T (2010) Recent advances in chemotherapy for advanced gastric cancer in Japan. Surg Today 40(4): 295–300 [DOI] [PubMed] [Google Scholar]
- Giri D, Ropiquet F, Ittmann M (1999) Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res 5: 1063–1071 [PubMed] [Google Scholar]
- Grose R, Dickson C (2005) Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev 16: 179–186 [DOI] [PubMed] [Google Scholar]
- Hattori Y, Itoh H, Uchino S, Hosokawa K, Ochiai A, Ino Y, Ishii H, Sakamoto H, Yamaguchi N, Yanagihara K, Hirohashi S, Sugimura T, Terada M (1996) Immunohistochemical detection of K-sam protein in stomach cancer. Clin Cancer Res 2: 1373–1381 [PubMed] [Google Scholar]
- Hattori Y, Odagiri H, Nakatani H, Miyagawa K, Naito K, Sakamoto H, Katoh O, Yoshida T, Sugimura T, Terada M (1990) K-sam, an amplified gene in stomach cancer, is a member of the heparin-binding growth factor receptor genes. Proc Natl Acad Sci USA 87: 5983–5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Hattori Y, Sakamoto H, Ishii H, Kishi T, Sasaki H, Yoshida T, Koono M, Sugimura T, Terada M (1994) Preferential alternative splicing in cancer generates a K-sam messenger RNA with higher transforming activity. Cancer Res 54: 3237–3241 [PubMed] [Google Scholar]
- Jang JH, Shin KH, Park JG (2001) Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res 61: 3541–3543 [PubMed] [Google Scholar]
- Kaneda H, Arao T, Tanaka K, Tamura D, Aomatsu K, Kudo K, Sakai K, De Velasco MA, Matsumoto K, Fujita Y, Yamada Y, Tsurutani J, Okamoto I, Nakagawa K, Nishio K (2010) FOXQ1 is overexpressed in colorectal cancer and enhances tumorigenicity and tumor growth. Cancer Res 70(5): 2053–2063 [DOI] [PubMed] [Google Scholar]
- Kim HK, Choi IJ, Kim CG, Kim HS, Oshima A, Yamada Y, Arao T, Nishio K, Michalowski A, Green JE (2010) Three-gene predictor of clinical outcome for gastric cancer patients treated with chemotherapy. Pharmacogenomics J; e-pub ahead of print 21 December 2010 [DOI] [PMC free article] [PubMed]
- Matsumoto K, Arao T, Tanaka K, Kaneda H, Kudo K, Fujita Y, Tamura D, Aomatsu K, Tamura T, Yamada Y, Saijo N, Nishio K (2009) mTOR signal and hypoxia-inducible factor-1 alpha regulate CD133 expression in cancer cells. Cancer Res 69(18): 7160–7164 [DOI] [PubMed] [Google Scholar]
- Moffa AB, Tannheimer SL, Ethier SP (2004) Transforming potential of alternatively spliced variants of fibroblast growth factor receptor 2 in human mammary epithelial cells. Mol Cancer Res 2: 643–652 [PubMed] [Google Scholar]
- Motoi T, Kumagai A, Tsuji K, Imamura T, Fukusato T (2010) Diagnostic utility of dual-color break-apart chromogenic in situ hybridization for the detection of rearranged SS18 in formalin-fixed, paraffin-embedded synovial sarcoma. Hum Pathol 41(10): 1397–1404 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yashiro M, Matsuoka T, Tendo M, Shimizu T, Miwa A, Hirakawa K (2006) A novel molecular targeting compound as K-samII/FGF-R2 phosphorylation inhibitor, Ki23057, for Scirrhous gastric cancer. Gastroenterology 131(5): 1530–1541 [DOI] [PubMed] [Google Scholar]
- Peng DF, Sugihara H, Mukaisho K, Tsubosa Y, Hattori T (2003) Alterations of chromosomal copy number during progression of diffuse-type gastric carcinomas: metaphase- and array-based comparative genomic hybridization analyses of multiple samples from individual tumours. J Pathol 201(3): 439–450 [DOI] [PubMed] [Google Scholar]
- Rand V, Huang J, Stockwell T, Ferriera S, Buzko O, Levy S, Busam D, Li K, Edwards JB, Eberhart C, Murphy KM, Tsiamouri A, Beeson K, Simpson AJ, Venter JC, Riggins GJ, Strausberg RL (2005) Sequence survey of receptor tyrosine kinases reveals mutations in glioblastomas. Proc Natl Acad Sci USA 102: 14344–14349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Arao T, Yokote H, Komatsu T, Yanagihara K, Sasaki H, Yamada Y, Tamura T, Fukuoka K, Kimura H, Saijo N, Nishio K (2007) AZD2171 shows potent antitumor activity against gastric cancer over-expressing fibroblast growth factor receptor 2/keratinocyte growth factor receptor. Clin Cancer Res 13(10): 3051–3057 [DOI] [PubMed] [Google Scholar]
- Toyokawa T, Yashiro M, Hirakawa K (2009) Co-expression of keratinocyte growth factor and K-sam is an independent prognostic factor in gastric carcinoma. Oncol Rep 21(4): 875–880 [DOI] [PubMed] [Google Scholar]
- Tsujimoto H, Sugihara H, Hagiwara A, Hattori T (1997) Amplification of growth factor receptor genes and DNA ploidy pattern in the progression of gastric cancer. Virchows Arch 431(6): 383–389 [DOI] [PubMed] [Google Scholar]
- Turner N, Grose R (2010) Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10(2): 116–129 [DOI] [PubMed] [Google Scholar]
- Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, Natrajan R, Marchio C, Iorns E, Mackay A, Gillett C, Grigoriadis A, Tutt A, Reis-Filho JS, Ashworth A (2010) FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res 70(5): 2085–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Sasaki H, Kuwahara Y, Nezu M, Shibuya T, Sakamoto H, Ishii H, Yanagihara K, Mafune K, Makuuchi M, Terada M (1999) Deletion of the carboxyl-terminal exons of K-sam/FGFR2 by short homology-mediated recombination, generating preferential expression of specific messenger RNAs. Cancer Res 59(24): 6080–6086 [PubMed] [Google Scholar]