Background: Phospho-modulation of SNARE function has not yet been shown.
Results: Phospho-SNAP-25 was dephosphorylated by protein phosphatase-1, whose activity was regulated by PRIP, thus regulating exocytosis.
Conclusion: Protein phosphatase-1, whose activity is regulated by PRIP, is the major phosphatase responsible for the dephosphorylation of SNAP-25.
Significance: The results provide the first information regarding the phosphatases responsible for phospho-modulation of SNAP-25 and the regulation of exocytosis.
Keywords: Exocytosis, Phospholipase C, PP1, Protein Kinase A (PKA), Protein Kinase C (PKC), SNARE Proteins, cAMP-dependent Protein Kinase, Protein Phosphatase
Abstract
Exocytosis is one of the most fundamental cellular events. The basic mechanism of the final step, membrane fusion, is mediated by the formation of the SNARE complex, which is modulated by the phosphorylation of proteins controlled by the concerted actions of protein kinases and phosphatases. We have previously shown that a protein phosphatase-1 (PP1) anchoring protein, phospholipase C-related but catalytically inactive protein (PRIP), has an inhibitory role in regulated exocytosis. The current study investigated the involvement of PRIP in the phospho-dependent modulation of exocytosis. Dephosphorylation of synaptosome-associated protein of 25 kDa (SNAP-25) was mainly catalyzed by PP1, and the process was modulated by wild-type PRIP but not by the mutant (F97A) lacking PP1 binding ability in in vitro studies. We then examined the role of PRIP in phospho-dependent regulation of exocytosis in cell-based studies using pheochromocytoma cell line PC12 cells, which secrete noradrenalin. Exogenous expression of PRIP accelerated the dephosphorylation process of phosphorylated SNAP-25 after forskolin or phorbol ester treatment of the cells. The phospho-states of SNAP-25 were correlated with noradrenalin secretion, which was enhanced by forskolin or phorbol ester treatment and modulated by PRIP expression in PC12 cells. Both SNAP-25 and PP1 were co-precipitated in anti-PRIP immunocomplex isolated from PC12 cells expressing PRIP. Collectively, together with our previous observation regarding the roles of PRIP in PP1 regulation, these results suggest that PRIP is involved in the regulation of the phospho-states of SNAP-25 by modulating the activity of PP1, thus regulating exocytosis.
Introduction
Neurotransmitters, neuropeptides, and peptide hormones are released from secretory vesicles through regulated and constitutive secretory processes. Exocytosis is the final stage of the secretory pathway and involves the membrane fusion of secretory vesicles with the plasma membrane. Extensive studies on the molecular mechanism of exocytosis have revealed that the basic mechanism of membrane fusion uses the proteins termed soluble N-ethylmaleimide-sensitive factor (NSF)3 attachment protein receptors (SNAREs) (1–5). The SNARE complex consists of members of the synaptobrevin (also called vesicle- associated membrane protein (VAMP)) family on the vesicular membrane (v-SNARE) and syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) families on the target plasma membrane (t-SNARE). Many other proteins also have been identified to play important regulatory roles in exocyotosis, such as synaptotagmin, NSF, Munc18, Munc13, and CAPS (Ca2+-activated protein for secretion) (6–13). The interaction among these numerous proteins enables sophisticated regulation of exocytosis in accordance with changing physiological requirements.
Protein phosphorylation and dephosphorylation through activation of protein kinases and phosphatases also play an important role in the regulation of exocytosis. Recent studies have indeed demonstrated that the SNARE proteins, SNAP-25, syntaxin, and VAMP, can be phosphorylated by protein kinases, such as cAMP-dependent protein kinase (PKA) (14, 15), protein kinase C (PKC) (16, 17), and casein kinase (18, 19). The SNARE accessory proteins, Munc18, synaptotagmin, NSF, and synaptophysin, can also be phosphorylated by various kinases (20–22). Thus, it is apparent that the multiple steps involved in exocytosis can be modulated by phosphorylation, which should be reversibly controlled by the coordinated actions of both protein kinases and phosphatases; however, fewer studies regarding the phosphatases responsible for the phospho-regulation of exocytosis have been performed than those regarding kinases. Furthermore, the combination of specific substrate proteins implicated in exocytosis, specific kinase, and phosphatase and their regulation to modulate exocytosis are still unknown.
Phospholipase C-related but catalytically inactive protein (PRIP), composed of type 1 and type 2, was originally identified in this laboratory as a novel d-myo-inositol 1,4,5-trisphosphate (Ins(1,4,5)P3)-binding protein, whose name was derived from the lack of catalytic activity despite the similarity to phospholipase Cδ-1 (23–28). Further studies revealed that PRIP has a number of binding partners, including the catalytic subunit of protein phosphatase 1α (PP1α) and PP2A (29, 30) and the phosphorylated (active) form of Akt (31) in addition to Ins(1,4,5)P3 and phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) (32–36). Thus, PRIP is a unique molecule that associates with both multiple phosphatases (PP1 and PP2A) and a kinase (activated form of Akt), suggesting that PRIP participates in the phospho-regulation of cellular events, by recruiting these enzymes to where the event occurs if PRIP can approach. We have recently reported that exocytosis of various peptide hormones, such as gonadotropins and insulin, was up-regulated in PRIP-1 and/or PRIP-2 knock-out (KO) mice (37, 38), indicating that PRIP is likely to be involved in dense core vesicle exocytosis in a negative manner, independent of the type of vesicular contents. The molecular mechanisms underlying the inhibition of exocytosis by PRIP are currently being studied in the laboratory. This observation with PRIP-KO mice also indicates that PRIP could exist closer to where exocytosis occurs through a mechanism currently being studied. Then, in the present study, we investigated the possible involvement of PRIP in the phospho-regulation of exocytosis through modulation of the dynamics of protein phosphorylation. For this purpose, we employed rat pheochromocytoma cell line PC12 cells, which express no intrinsic PRIP-1 and -2, as a cell-based experimental model, because they have many characteristics of adrenal chromaffin cells, including the ability to secrete catecholamines (39, 40), and furthermore, we have recently optimized the experimental procedures to prepare permeabilized cells with a preserved mechanism for exocytosis for manipulation from outside of cells (41).
We found that dephosphorylation of SNAP-25 phosphorylated by PKA and PKC in vitro was mainly catalyzed by PP1, the process of which was modulated by wild-type PRIP-1 but not by mutant PRIP-1 (F97A) lacking the ability to bind to PP1. On the basis of cell-based experiments, an immunoprecipitation experiment revealed that PRIP-1 interacted with both PP1 and SNAP-25 using PC12 cells expressing PRIP-1. Phosphorylation of SNAP-25 in PC12 cells was induced by forskolin or phorbol ester to stimulate PKA or PKC, respectively, followed by gradual dephosphorylation after the removal of stimulants. Exogenous expression of PRIP accelerated this dephosphorylation process. Correlating with the phospho-state of SNAP-25, pretreatment of the cells with forskolin or phorbol ester enhanced NA secretion, and the enhancement was gradually diminished by removing stimulants. Exogenous expression of PRIP inhibited NA secretion, but similar forskolin or phorbol ester stimulation was observed with the accelerated recovery process. Together with our previous observation of the roles of PRIP in PP1 regulation, the results suggest that PRIP is involved in the phospho-state of SNAP-25 through modulating the activity of protein phosphatase, thus regulating exocytosis.
EXPERIMENTAL PROCEDURES
Materials
Calyculin A and okadaic acid were from Millipore (Billerica, MA). Phorbol 12-myristate 13-acetate (PMA) and PKI 6-22 amide, a PKA inhibitor, were from Sigma-Aldrich. Forskolin and Go6976, a PKC inhibitor, were from Merck. Antibodies used were as follows: anti-SNAP-25 antibody (Sigma-Aldrich), anti-GFP antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and anti-PP1 antibody (Millipore)
Mutagenesis
Residue Thr-94 or Phe-97 in PRIP-1 was mutated into Ala by the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The mutagenic primer sequences for Thr-94 or Phe-97 were as follows (5′ to 3′): GTGGTGGCAGAAAGAAAGCCGTGTCTTTCAGCAGC (forward) and GCTGCTGAAAGACACGGCTTTCTTTCTGCCACCAC (reverse) or GAAAACAGTGTCTGCCAGGCAGCATGCCATCGG (forward) and CCGATGGCATGCTGCTGGCAGACACTGTTTTC (reverse), respectively. The sequences of mutants were confirmed by DNA sequencing.
Cell Culture and Generation of PC12 Cells Stably Expressing GFP-PRIP-1
PC12 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% horse serum and 5% fetal bovine serum. The generation of PC12 clonal cells stably expressing GFP-PRIP-1 was performed as follows. PC12 cells were first transfected with phGFP105, PRIP-1 (wild type)/phGFP105 (34), PRIP-1 (F97A)/phGFP105, or PRIP-1 (T94A)/phGFP105 with Lipofectamine2000 (Invitrogen) according to the manufacturer's instructions. Transfected cells were maintained in growth medium containing 400 μg/ml G418 (Invitrogen) for about a month. The growing colonies were visually selected for GFP fluorescence and picked up.
Noradrenalin Secretion Assay
PC12 cells were seeded in a 12-well plate coated with 10 μg/ml poly-l-lysine (3 × 105 cells). Twenty-four h later, cells were labeled with 0.5 μCi of [3H]noradrenalin (NA) (GE Healthcare) per well in 1 ml of complete culture medium containing serum in the presence of 0.5 mm ascorbic acid for 12–16 h. The medium was replaced with fresh complete medium, and the cells were further incubated for 1–2 h to remove unincorporated [3H]NA. For the intact cell assay, cells were washed with physiological salt saline (PSS) containing 145 mm NaCl, 5.6 mm KCl, 2.2 mm CaCl2, 0.5 mm MgCl2, 5.6 mm d-glucose, and 15 mm Hepes, pH 7.4, and the secretion of [3H]NA was triggered with high K+/PSS (81 mm NaCl, 70 mm KCl). At the end of the secretion assay, the medium was transferred into a scintillation vial, and cells were collected to assess the remaining radioactivity in 0.3 ml of 0.5% Triton X-100, enabling calculation of the ratio of secretion relative to the total radioactivity. The radioactivity of [3H]NA remaining in cells and secreted into the medium was measured by a liquid scintillation counter. For the permeabilized cell assay, cells were permeabilized by 10 μm digitonin in 0.3 ml of KGlu buffer (120 mm potassium glutamate, 20 mm potassium acetate, 2 mm EGTA, 0.1% bovine serum albumin, and 20 mm HEPES, pH 7.2) containing 2 mm MgATP. [3H]NA secretion was triggered by adding 2 mm CaCl2 to give a free Ca2+ concentration of 1 μm. [3H]NA counting was performed as described for intact cells. By digitonin permeabilization, [3H]NA radioactivity present in intact cells decreased about 10%, whereas about half of cellular protein with a molecular size of 25 kDa as assessed by the amount of GFP was lost, but exocytosis regulated by free Ca2+ concentration was observed normally (see Ref. 41).
Expression and Purification of Recombinant Proteins
For purification of recombinant full-length SNAP-25 and a variety of mutants (described below) conjugated with glutathione S-transferase (GST), the SNAP-25/pGEX-2T construct was transformed into Escherichia coli BL-21 (DE3). Bacterial cells were grown to 0.25 of absorbance at 600 nm at 37 °C and then with 0.1 μm isopropyl β-d-1-thiogalactopyranoside at 16 °C for an additional 12–14 h. Bacterial lysate was prepared by sonication in a lysis buffer containing 50 mm Tris-HCl (pH 8.0), 300 mm NaCl, 1 mm EDTA, 1 mm dithiothreitol, and protease inhibitor mixture containing 5 μg/ml pepstatin A, 10 μm leupeptin, 1.7 μg/ml aprotinin, and 50 μm 4-amidinophenylmethanesulfonyl fluoride hydrochloride, followed by rotation after the addition of 1% Triton X-100 for 20 min. Purification was achieved using glutathione-Sepharose 4B beads (GE Healthcare). After extensive washing with lysis buffer without protease inhibitor mixture, the proteins were eluted with 20 mm reduced glutathione in a lysis buffer containing 1 mm dithiothreitol. Purity was checked by Coomassie Brilliant Blue staining after SDS-PAGE. His-tagged PRIP-1, wild type and F97A mutant, were purified from insect cells using the baculovirus expression system as described previously (34). The recombinant molecules of interest were dialyzed against the solution for more than 6 h, followed by centrifugation at 100,000 × g for 30 min before use.
In Vitro Phosphorylation and Dephosphorylation of SNAP-25
GST-tagged SNAP-25 immobilized on glutathione-Sepharose 4B beads was phosphorylated in a solution (50 mm Tris-HCl, pH 7.5, 10 mm MgCl2) containing 10 μm ATP and 0.5 μCi of [γ-32P]ATP (PerkinElmer Life Sciences) and 0.4 μg of the catalytic subunit of PKA (Promega, Madison, WI) or 0.2 μg of PKC (Millipore) at 30 °C for 30 min. For the dephosphorylation assay, beads were then washed with dephosphorylation buffer (for PP1, 50 mm Tris-HCl at pH 7.5, 1 mm MnCl2, 150 mm NaCl, 2 mm EGTA and 1 mm dithiothreitol; for PP2A, 50 mm Tris-HCl at pH 8.5, 20 mm MgCl2, and 1 mm dithiothreitol; for PP2B, 50 mm Tris-HCl at pH 7.0, 1 mm NiCl2, and 10 μg/ml calmodulin). The resulting beads were incubated in 60 μl of dephosphorylation buffer containing 0.5 or 1 unit of PP1 (Sigma-Aldrich) or PP2A or PP2B (Promega) at 30 °C for 10 min. The liberated 32P was counted by a scintillation counter, and the dephosphorylation of GST-SNAP-25 was also visualized by SDS-PAGE and subsequent autoradiography.
Labeling of PC12 Cells and Immunoprecipitation for Phosphorylation Assay
PC12 cells (1 × 106 cells) were seeded in a 6-well plate coated with 10 μg/ml poly-l-lysine. After culture for 48 h, PC12 cells were starved for 2 h in phosphate-free DMEM containing 2% dialyzed fetal bovine serum. Cells were labeled with 0.3 mCi of [32P]orthophosphate (PerkinElmer Life Sciences) for 4 h in 1 ml of phosphate-free DMEM, followed by extensive washing with phosphate-free DMEM to remove unincorporated radioactivity. After treating the cells with the substance of interest, cells were lysed for 1 h in ice-cold lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 10% SDS, 1 mm dithiothreitol) containing phosphatase inhibitors (50 mm NaF, 10 mm Na4P2O7, 20 mm β-glycerophosphate, and 1 mm Na3VO4) and protease inhibitors (5 μg/ml pepstatin A, 10 μm leupeptin, 1.7 μg/ml aprotinin, and 50 μm 4-amidinophenylmethanesulfonyl fluoride hydrochloride). The cell lysate was then mixed with a specific antibody against SNAP-25 (Sigma-Aldrich) at 4 °C overnight, followed by the addition of 15 μl of protein G-Sepharose beads for an additional 1 h at 4 °C. The beads were precipitated by centrifugation and washed three times with lysis buffer. After the final wash, the beads were resuspended in 50 μl of sample buffer for SDS-PAGE. The proteins in the precipitated immunocomplex were separated by SDS-PAGE, and the radiolabeled molecules were visualized by autoradiography.
Immunoprecipitation and Western Blotting
Cell lysates were prepared from PC12 cells using ice-cold lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA, 1% Triton X-100, and 1 mm dithiothreitol) containing phosphatase inhibitors and protease inhibitors as described above. The cell lysates were subjected to immunoprecipitation using the indicated antibody and the Pierce Crosslink Immunoprecipitation Kit or Exacta Cruz (Santa Cruz Biotechnology, Inc.) according to the manufacturer's instructions. The protein samples were then separated by SDS-PAGE, followed by transfer to polyvinylidene fluoride membranes (Millipore). After blocking, the membrane was blotted with the appropriate antibody and horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (GE Healthcare), followed by the detection of chemiluminescent signals using an LAS-3000 mini gel documentation system (Fujifilm, Tokyo, Japan). Digital images were analyzed with NIH Image software to measure the density of each band.
RESULTS
Regulation of Exocytosis by Protein Phosphorylation
We previously found that the absence of ATP after permeabilization of PC12 cells diminished Ca2+-triggered exocytosis (41). In agreement with many reports regarding the requirement of PtdIns(4,5)P2 to maintain the priming state for exocytosis (42, 43), this diminishment is assumed to be caused by the conversion of membrane PtdIns(4,5)P2 to phosphatidylinositol phosphate and then phosphatidylinositol in the absence of ATP. We assumed that protein phosphorylation involved in exocytosis is also implicated in its regulation.
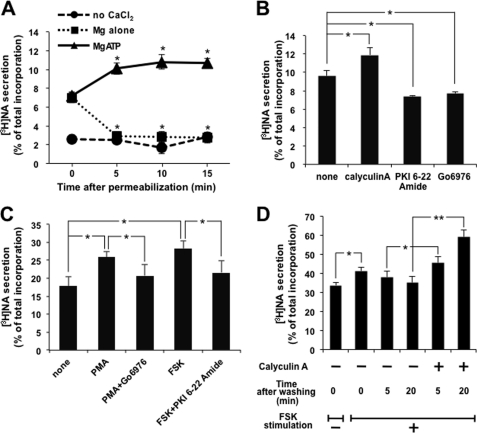
We here confirmed the previous observation; PC12 cells labeled with [3H]NA were permeabilized with 10 μm digitonin and left at room temperature for 5, 10, and 15 min in the presence or absence of ATP, and then NA secretion was triggered by the addition of 2 mm CaCl2 to adjust the free Ca2+ concentration to 1 μm. [3H]NA released from permeabilized cells for 2 min was assayed (Fig. 1A). Ca2+-triggered NA secretion was decreased to the unstimulated level for the first 5 min and was stable for up to 15 min in the absence of ATP, while the presence of ATP somehow increased. While permeabilized cells were left at room temperature in the presence of ATP, a protein phosphatase inhibitor, calyculin A, at 100 nm was included for 5 min, followed by an assay of Ca2+-triggered [3H]NA secretion for 2 min. As shown in Fig. 1B, [3H]NA secretion was increased by about 25%. On the other hand, the addition of a protein kinase inhibitor (e.g. PKA inhibitor (PKI 6-22 amide at 100 nm) or PKC inhibitor (Go6976 at 1 μm)) inhibited NA secretion by about 25% (Fig. 1B), indicating that protein phosphorylation might be involved; however, it is still possible that the amount of PtdIns(4,5)P2 is modified by treatment with these inhibitors. We then quantified PtdIns(4,5)P2 in PC12 cells to examine whether calyculin A treatment increases PtdIns(4,5)P2. PtdIns(4,5)P2 was not increased by calyculin A, although permeabilization decreased PtdIns(4,5)P2, and incubation in the presence of ATP increased PtdIns(4,5)P2 (see supplemental Fig. 1).
FIGURE 1.
Protein phosphorylation is involved in the regulation of exocytosis. A, PC12 cells labeled with [3H]NA were permeabilized with 10 μm digitonin and then maintained at room temperature for the period indicated in the presence or absence of 2 mm MgATP. [3H]NA secretion was triggered by adding 2 mm CaCl2 to give a free Ca2+ concentration of 1 μm for 2 min. NA release is indicated as the percentage of released [3H]NA relative to the total [3H]NA incorporated into the cells. B, after digitonin permeabilization, [3H]NA-labeled PC12 cells were incubated at room temperature for 5 min in the presence of 2 mm MgATP, along with either calyculin A (100 nm), PKI 6-22 amide (100 nm), or Go6976 (1 μm). [3H]NA secretion by 1 μm Ca2+ for 2 min was assayed. C, PC12 cells labeled with [3H]NA were treated with PMA (1 μm) or forskolin (FSK) (50 μm) with or without each inhibitor for 30 or 5 min, respectively, followed by the assay of [3H]NA secretion by high K+ solution (70 mm KCl) for 2 min. D, PC12 cells labeled with [3H]NA were treated with 50 μm FSK for 5 min. After removal of the stimulus, cells were left for 5 or 20 min in the presence or absence of 100 nm calyculin A, followed by the assay of [3H]NA secretion by 10 μm ionomycin for 5 min. All of the results are the means ± S.E. (error bars) of five independent experiments. Student's t tests were used, and significance is represented by * or ** for p < 0.05 or p < 0.01, respectively. The same statistical methods were applied to the following results.
The effects of inhibitors of protein kinases and phosphatase were also examined with intact PC12 cells. [3H]NA secretion from PC12 cells was triggered by high K+ solution, causing depolarization of the plasma membrane in the presence of extracellular Ca2+. Forskolin (50 μm) or PMA (1 μm) was added before high K+ stimulation to activate adenylate cyclase, resulting in PKA activation or PKC directly, respectively, and both enhanced secretion by up to about 50%, which was inhibited to almost the basal level by the respective protein kinase inhibitors, PKI 6-22 amide or Go6976, indicating that protein phosphorylation mediated by kinases is important for enhancement (Fig. 1C). Calyculin A has been reported to inhibit the calcium channel independent of the inhibition of protein phosphatases (44). To bypass the calcium channel, ionomycin instead of high K+ was used to trigger exocytosis. As shown in Fig. 1D, ionomycin-induced [3H]NA secretion was enhanced by forskolin by about 25% and declined slowly after removal of forskolin. The presence of calyculin A after removal of forskolin augmented the secretion. These results clearly indicate that protein phosphorylation is involved in the regulation of exocytosis in a positive manner.
Dephosphorylation of SNAP-25
Many proteins important for exocytosis can be phosphorylated by various protein kinases. One of the most investigated SNARE proteins is SNAP-25, the phosphorylation of which has been previously reported to be catalyzed by both PKA and PKC to enhance exocytosis (15, 16, 45, 46). The finding that PC12 cells up-regulate [3H]NA secretion by both activation of PKA and PKC led us to focus on the phospho-modulation of exocytosis via SNAP-25.
First, it was examined whether GST-fused SNAP-25 was phosphorylated by the catalytic subunit of PKA using [γ-32P]ATP. The mixture was separated by SDS-PAGE, followed by autoradiography to monitor 32P incorporation. The presence of PKA elicited robust 32P incorporation into GST-SNAP25, whereas no radioactivity was incorporated in its absence (Fig. 2A). Because GST itself was not phosphorylated (results not shown), the phosphorylation observed is attributed to that of SNAP-25, consistent with the previous reports (14, 15).
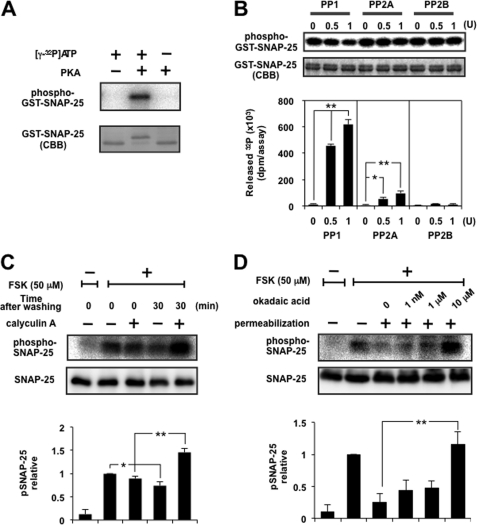
FIGURE 2.
Dephosphorylation of SNAP-25 was mainly catalyzed by PP1. A, GST-tagged SNAP-25 was phosphorylated with [γ-32P]ATP using the catalytic subunit of PKA. The mixture was separated by SDS-PAGE, followed by CBB staining and autoradiography. Note that phosphorylated SNAP-25 shifted up. B, GST-tagged SNAP-25 immobilized on glutathione beads was phosphorylated as described above, followed by dephosphorylation by PP1, PP2A, or PP2B in an appropriate buffer solution. The radioactivity of released 32P was counted using a liquid scintillation counter, and beads were analyzed by SDS-PAGE for CBB staining and autoradiography. The top and bottom panels show a typical autoradiogram and CBB staining of GST-SNAP-25 after treatment, respectively. The graph shows a summary of the released radioactivity of five separate experiments. Doublet bands of SNAP-25 seen by CBB staining showed little difference after treatment with PP, indicating that liberation of radioactivity is more sensitive, and the portion of SNAP-25 dephosphorylated appears to be small. Each enzyme was supplied by the respective manufacturer, designating the activity as a unit, but the assay condition (buffer composition, time, temperature, etc.) was different from that used in the present study, so we re-evaluated each activity using 100 μm p-nitrophenyl phosphate as a substrate under exactly the same assay conditions as described here. One unit of PP1, PP2A, and PP2B by the manufacturer was about 0.3, 0.7, and 0.2 unit, respectively. Re-evaluated activity of each enzyme was used here. C, PC12 cells labeled with [32P]orthophosphate were stimulated with 50 μm FSK for 5 min. After removal of the stimulus, cells were left in the presence or absence of 100 nm calyculin A for 30 min. Cellular extracts were subjected to immunoprecipitation by anti-SNAP-25 antibody overnight at 4 °C, and the immunocomplexes were examined by SDS-PAGE followed by autoradiography and Western blotting using anti-SNAP-25 antibody. The panels show a typical autoradiogram and immunoblot, and the graph shows the summary of five separate experiments. Doublet bands of SNAP-25 depending on the phospho-state seen by CBB staining in vitro (see A and B) were not observed in that immunoprecipitated by anti-SNAP-25 antibody from living cells, for unknown reasons. D, PC12 cells labeled with [32P]orthophosphate were incubated with 50 μm FSK for 5 min, followed by digitonin permeabilization and subsequent incubation with okadaic acid at the concentrations indicated for another 10 min. The cell lysates were subjected to immunoprecipitation as described above. The panels show a typical autoradiogram and immunoblot, and the results are expressed as the mean ± S.E. (error bars) of five separate experiments.
Although there are multiple reports about the phosphorylation of SNAP-25 and its functional modulation of exocytosis (14–16), the dephosphorylation process has not yet been investigated. We therefore investigated which protein phosphatase is responsible for the dephosphorylation of PKA-phosphorylated SNAP-25. Purified recombinant GST-fused SNAP-25 immobilized on glutathione-Sepharose 4B beads was mixed together with the catalytic subunit of PKA and [γ-32P]ATP for phosphorylation. After washing extensively, the catalytic subunit of PP1, PP2A, or PP2B was added to the mixture. Phosphatase activity catalyzing dephosphorylation was measured by the radioactivity of 32P released from the beads and furthermore by analyzing the beads by SDS-PAGE and autoradiography. PP1 caused the release of 32P from GST-SNAP-25 in a dose-dependent manner, and PP2A also catalyzed the release but to a lesser extent, whereas PP2B showed no activity (Fig. 2B), indicating that SNAP-25 phosphorylated by PKA was mainly dephosphorylated by PP1 and to a lesser extent by PP2A in vitro.
To examine whether phosphorylated SNAP-25 could be a target of PP1 and PP2A in intact PC12 cells, we used okadaic acid as well as calyculin A as the specific inhibitor of PP2A and PP1. PC12 cells metabolically labeled with [32P]orthophosphate were stimulated with forskolin to activate PKA for 5 min, followed by immunoprecipitation of SNAP-25. Immunoprecipitates contained several phospho-bands, but stimulation by forskolin resulted in the marked incorporation of 32P into a major band with a molecular mass of 25 kDa (Fig. 2C), which was not observed in the immunoprecipitates using control IgG antibody (data not shown), indicating that the phospho-band is SNAP-25. The phosphorylation level was decreased 30 min after the removal of forskolin, but the process appeared very slow, which was probably attributable to overstimulation with forskolin for continued activation of PKA by persistent cAMP. This decrease was abolished by the addition of calyculin A (100 nm), a relatively nonspecific inhibitor of PP1 and PP2A. The same experiments were performed using okadaic acid, a specific inhibitor of PP2A at nanomolar concentrations but an inhibitor of PP1 as well as PP2A at over 10 μm (47). In this case, cells were permeabilized in order to observe the concentration-dependent specific inhibition by okadaic acid without the obstacle of cell membranes. As shown in Fig. 2D, the inclusion of okadaic acid at 1 nm or 1 μm, a concentration at which PP2A is selectively inhibited, slightly blocked dephosphorylation but with no statistical significance. Okadaic acid increased to 10 μm, a concentration sufficient to block both PP1 and PP2A, recovered the dephosphorylation. These results clearly indicate that dephosphorylation of intrinsic SNAP-25 phosphorylated by PKA in PC12 cells was mainly catalyzed by PP1 and also by PP2A to a lesser extent, consistent with the results obtained from the in vitro assay. It was noted that permeabilized cells showed a more profound decrease of the phosphorylation of SNAP-25 than intact cells, probably because cAMP was washed away along with PKA by permeabilization.
Phosphorylation of SNAP-25 by PKC and dephosphorylation by a catalytic subunit of PP1, PP2A, or PP2B were also performed in vitro, yielding results that were basically similar to those with PKA except that PP2A appeared more effective. Furthermore, cell-based experiments using 32P-labeled cells stimulated with PMA in place of forskolin in the presence of okadaic acid provided basically similar results, with the only difference being that okadaic acid at 1 μm was as effective as at 10 μm to block (see supplemental Fig. 2), indicating that SNAP-25 phosphorylated by PKC is dephosphorylated mainly by PP1 and by PP2A more effectively.
There are 10 serines and 7 threonines in SNAP-25, of which Ser-28, Thr-29, Thr-46, Thr-138, and Ser-187 are present in the central region to form a SNARE complex with syntaxin and VAMP (2), which have been reported to be phosphorylated by PKA or PKC (15, 16, 45, 46). Therefore, we investigated possible amino acid residues phosphorylated by PKA using recombinant SNAP-25 (either WT or mutants to alanine) and [γ-32P]ATP and 32P-labeled COS7 cells transfected with genes for either WT or mutant SNAP-25 and thus found that Ser-28, Thr-29, Thr-138, and Ser-187 but not Thr-46 were phosphorylated (see supplemental Fig. 3, A and B for in vitro and cellular experiments, respectively). We performed further cellular experiments; FLAG-tagged SNAP-25 (either WT or mutants shown in supplemental Fig. 3C) was transfected into COS7 cells metabolically labeled with [32P]orthophosphate in advance, followed by stimulation with forskolin, immunoprecipitation by anti-FLAG antibody, and autoradiography. As shown in supplemental Fig. 3C, the density at the phospho-band corresponding to FLAG-SNAP-25 was increased by stimulation of COS7 cells expressing WT SNAP-25 with forskolin, which was reduced when cells expressing mutant SNAP-25 were used, roughly depending on the number of mutations, indicating that Ser-28, Thr-29, Thr-138, and Ser-187 are equally phosphorylated in living cells. It was also noted that radioactivity corresponding to one or two amino acid residues was found in FLAG-SNAP-25 in cells with no stimulation, and no extra residue in addition to Ser-28, Thr-29, Thr-138, and Ser-187 appeared to be phosphorylated because the radioactivity found in the mutations of these residues was much the same as that in WT before stimulation. The similar in vitro and cellular experiments were performed using PKC and PMA, respectively. As shown in supplemental Fig. 3D, PKC appeared to phosphorylate Ser-187 most preferentially and Thr-138 over Ser-28 and Thr-29 using recombinant SNAP-25. On the contrary, PMA stimulation appeared to phosphorylate all four residues in intact cells, as shown in supplemental Fig. 3E. This difference would be caused by either the difference of substrate property in intact cells from that of recombinant molecule or the activation of the PKA system triggered by PMA stimulation through an unidentified mechanisms in intact cells.
We then investigated whether PP1 and PP2A differentially catalyze dephosphorylation using recombinant GST-SNAP-25 in vitro. SNAP-25 of either WT or mutants phosphorylated by PKA was analyzed for dephosphorylation by PP1 and PP2A (supplemental Fig. 4). Noteworthy findings were as follows: (i) dephosphorylation of WT by PP1 was almost double that of the mutant S28A/T29A, indicating that Ser-28/Thr-29 and Thr-138/Ser-187 are equally dephosphorylated by PP1; (ii) dephosphorylation of WT and the mutant S28A/T29A by PP2A were much the same, indicating that PP2A fails to dephosphorylate at Ser-28 and Thr-29; and (iii) the mutant S28A/T29A/T138A/S187A was still dephosphorylated by PP1 and PP2A, indicating that unidentified residues corresponding to one or two residues are targets of PP1 and PP2A.
Regulation of Dephosphorylation of SNAP-25 by PRIP-1
We have previously shown that PRIP is a negative modulator of PP1 (29). PRIP binds to PP1 to inhibit the phosphatase activity. When PRIP-1 itself is phosphorylated by PKA at residue Thr-94, PP1 could no longer associate with PRIP to be an active form. Because PP1 catalyzes the dephosphorylation of SNAP-25 phosphorylated by PKA and PKC, PRIP must also be involved in the regulation of the phospho-state of SNAP-25 through regulating the activity of PP1.
GST-fused SNAP-25 immobilized on glutathione-Sepharose 4B beads was phosphorylated by PKA and [γ-32P]ATP, which was subjected to dephosphorylation by PP1 (Fig. 3). PP1 caused the release of 32P from phosphorylated SNAP-25, which was inhibited by the addition of purified recombinant PRIP-1 but not by the mutant PRIP-1, whose residue Phe-97 was replaced with Ala, lacking PP1 binding ability (29), as shown in Fig. 3A. Dephosphorylation of SNAP-25 catalyzed by PP1 was not inhibited by previously phosphorylated PRIP-1 (Fig. 3B). The results indicate that PRIP could be involved in the modulation of the phospho-state of SNAP-25 through regulating the activity of PP1.
FIGURE 3.
Dephosphorylation of SNAP-25 is modulated by PRIP-1 in vitro. GST-tagged SNAP-25 immobilized on glutathione beads was phosphorylated as described and then dephosphorylated by 1 unit of PP1. A, recombinant PRIP-1 (WT) or the mutant F97A (1 nmol) was included in the reaction mixture. The panels show an autoradiogram and CBB staining of GST-SNAP-25 after treatment with PP1 alone or along with PRIP-1. Results are expressed as the mean ± S.E. of five separate experiments. CBB staining of SNAP-25 yielded double bands; upper and lower bands represent phosphorylated and unphosphorylated, respectively (see Fig. 2A). B, PRIP-1 was phosphorylated in advance by non-radioactive ATP plus the catalytic subunit of PKA (pPRIP), followed by a dephosphorylation assay of SNAP-25. In this experiment, radioactivity to phosphorylate SNAP-25 was reduced. Results are expressed as the mean ± S.E. (error bars) of four separate experiments.
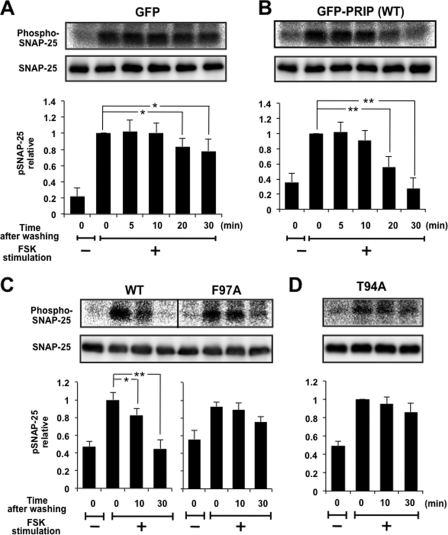
We then examined the roles of PRIP in regulating the phospho-state of SNAP-25 in PC12 cells. Because there is no intrinsic PRIP in PC12 cells, as assessed by Western blotting, and the transfection efficiency of the PRIP gene was relatively low, we have generated PC12 cells stably expressing either GFP or GFP-PRIP-1 to investigate the role of PRIP in intact PC12 cells. We first examined the dephosphorylation of SNAP-25 in PC12 cells expressing either GFP or GFP-PRIP-1. PC12 cells labeled with [32P]orthophosphate were treated with 50 μm forskolin for 5 min to activate PKA, followed by maintenance of the cells without forskolin at room temperature for 5, 10, 20, and 30 min. Endogenous SNAP-25 was immunoprecipitated using anti-SNAP-25 antibody, followed by SDS-PAGE and autoradiography. The experiments were similar to those shown in Fig. 2C. Forskolin treatment caused robust 32P incorporation into SNAP-25, slowly decreasing for up to 30 min after the removal of forskolin (Fig. 4A). However, in PRIP-1-expressing cells, although forskolin treatment led to a similar increase of the phosphorylation of SNAP-25, phosphorylation began decreasing 10 min after removal of forskolin and decreased rapidly and intensively in the following 20 min. The phosphorylation reached the basal level after 30 min (Fig. 4B). In contrast, PC12 cells expressing mutant PRIP-1 (F97A), which lacks PP1 binding ability, behaved similarly to the control cells (Fig. 4C). Furthermore, PC12 cells expressing mutant PRIP-1 (T94A), which is not phosphorylated and therefore keeps PP1 sequestered and inactivated, were also examined. As shown in Fig. 4D, the phospho-pattern of SNAP-25 was similar to that in cells expressing GFP alone or mutant PRIP (F97A). These results indicate that the dephosphorylation process of SNAP-25 catalyzed mainly by PP1 was accelerated by the presence of PRIP-1 in PC12 cells, and PP1 binding ability is required for PRIP to execute the role in regulating the dephosphorylation of SNAP-25.
FIGURE 4.
Dephosphorylation of SNAP-25 was modulated in PC12 cells expressing PRIP-1. PC12 cells expressing GFP (A), GFP-PRIP-1 (WT) (B), GFP-RPIP-1 (F97A) (C), or GFP-PRIP-1 (T94A) (D) were labeled with [32P]orthophosphate, followed by stimulation with 50 μm FSK for 5 min. After removal of the stimulus, cells were left for the time period indicated. Cell extracts were subjected to immunoprecipitation by anti-SNAP-25 antibody overnight at 4 °C, and the precipitates were examined by SDS-PAGE for autoradiography and Western blotting using anti-SNAP-25 antibody. The panels show a typical autoradiogram and immunoblot. Results are expressed as the mean ± S.E. (error bars) of five (A–C) or four (D) separate experiments.
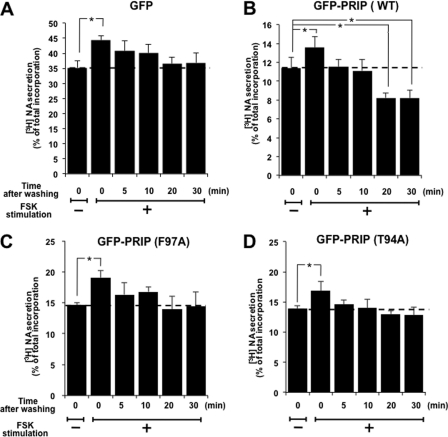
Based on the previous report that the phosphorylation of SNAP-25 by PKA or PKC enhances exocytosis, acceleration of the dephosphorylation of SNAP-25 by PRIP should decrease noradrenalin secretion from PC12 cells. To assess this point, we examined high K+-induced [3H]NA secretion from PC12 cells, which were treated in a manner similar to that described in the legend to Fig. 4. As shown in Fig. 5A, in control PC12 cells, high K+ solution triggered about 35% [3H]NA secretion for 2 min, which was enhanced by about 25% by forskolin stimulation. NA secretion was then recovered gradually after removal of forskolin to the basal level after 30 min. In contrast, in PC12 cells expressing PRIP-1, high K+ solution triggered only about 9% of the total [3H]NA secretion, the degree of which roughly correlated with the amount of expressed PRIP-1 in a reverse manner; the presence of PRIP itself inhibited the secretion as described in the Introduction,4 although forskolin stimulation similarly increased the secretion by about 20%. The recovery process of NA secretion after washing out of forskolin occurred rapidly (Fig. 5B); recovery was completed as early as 5 min after removal of the stimulus, and NA secretion decreased further below the basal level after 30 min. In PC12 cells with the mutant PRIP-1 (F97A), the recovery process appeared to be moderate between the control and WT (Fig. 5C). PC12 cells expressing mutant PRIP-1 (T94A) were also examined for [3H]NA secretion, showing that the recovery process following forskolin stimulation was similar to that seen in cells expressing mutant PRIP-1 (F97A) (Fig. 5D). Together with the result shown in Fig. 1D, these results indicate that PRIP-1 is involved in the regulation of NA secretion through modulating the dephosphorylation of some proteins important for exocytosis. This correlated with the regulation of the phospho-state of SNAP-25 by PRIP-1 through PP1 binding. [3H]NA secretion was also assayed in cells stimulated with PMA (supplemental Fig. 5). PMA stimulation increased [3H]NA secretion by about 40%, as shown in Fig. 1C, and the recovery process after washing out PMA was as slow as that of forskolin stimulation. Furthermore, cells expressing PRIP-1 (WT) accelerated the recovery, indicating that PKC phosphorylates Thr-94 to liberate PP1, similarly to PKA.
FIGURE 5.
[3H]NA secretion was modulated by PRIP-1. PC12 cells expressing GFP (A), GFP-PRIP-1 (WT) (B), GFP-RPIP-1 (F97A) (C), or GFP-PRIP-1 (T94A) (D) were labeled with [3H]NA, followed by stimulation with 50 μm FSK for 5 min. After removal of the stimulus, cells were left at room temperature for 5, 10, 20, or 30 min, followed by [3H]NA secretion assay with high K+ solution for 5 min. All the data are the means ± S.E. (error bars) of five (A–C) or three (D) experiments.
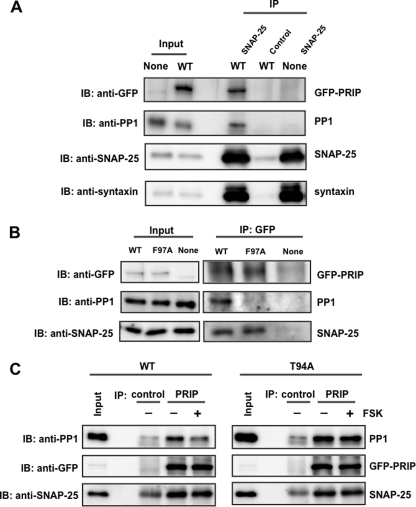
Complex Formation of PRIP-1 with PP1 and SNAP-25 in PC12 Cells
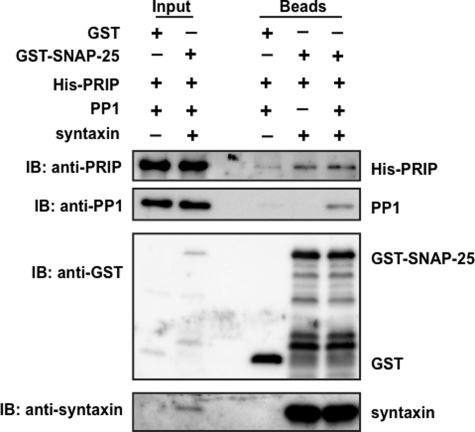
SNAP-25 is mainly localized at the plasma membrane, whereas PP1 exists throughout the cytosol in the cells; therefore, PP1 needs to be recruited to the site where SNAP-25 is localized in order to function in exocytosis. Thus, we hypothesized that PRIP may help PP1 to be recruited to the site where SNAP-25 is localized and exocytosis takes place in addition to the regulation of PP1 activity. To examine this possibility, we performed a co-immunoprecipitation assay using PC12 cells expressing PRIP-1. Endogenous SNAP-25 was immunoprecipitated from the cell lysate of PC12 cells expressing PRIP-1 using a specific antibody against SNAP-25, followed by immunoblotting using the antibody of interest. Anti-SNAP-25 antibody co-immunoprecipitated syntaxin in addition to SNAP-25 in control PC12 cells. PRIP-1 along with PP1 was co-immunoprecipitated when GFP-PRIP-1 was expressed, by anti-SNAP-25 antibody but not by control IgG, indicating the complex formation of SNAP-25 with PRIP-1, PP1, and syntaxin (Fig. 6A). For quantitative analysis of the interaction, the band density in the precipitates compared with that in the input sample was analyzed; about 3% of SNAP-25 present in cells was immunoprecipitated. Taking this value into account, a similar calculation based on band densities by each antibody led to the finding that about 6.7% and 6.7% of GFP-PRIP-1 and PP1 were co-precipitated with SNAP-25, respectively. Reciprocally, RPIP-1 was immunoprecipitated using anti-GFP antibody, and the immunoprecipitates were blotted using anti-SNAP-25 and anti-PP1 antibodies. SNAP-25 was co-precipitated with PRIP-1 in cells expressing WT and mutant F97A; however, PP1 was only co-precipitated with WT of PRIP-1, not with F97A (Fig. 6B). A similar calculation to that described above was also performed; about 6.8 and 10.4% of cellular PP1 and SNAP-25 were immunoprecipitated with GFP-PRIP-1, based on the immunoprecipitation efficiency of GFP-PRIP-1 (3.8%). PC12 cells expressing either WT or the T94A mutant of PRIP-1 were first treated with forskolin for 5 min to induce the phosphorylation of PRIP-1, probably along with SNAP-25, followed by immunoprecipitation by anti-PRIP-1 antibody. The immunoprecipitates were then analyzed for the presence of PP1 and SNAP-25. As shown in Fig. 6C, forskolin treatment reduced the amount of PP1 immunoprecipitated with PRIP-1 from PC12 cells expressing WT, but not cells expressing T94A of PRIP-1, despite a similar amount of SNAP-25. The same assays were performed using PMA (supplemental Fig. 6). Similar reduction of the interaction of PRIP-1 with PP1 was observed when cells expressing PRIP-1 were stimulated with PMA. These results support our assumption that PRIP-1 recruits PP1 to SNAP-25 by the complex formation, probably along with syntaxin. To corroborate these interactions in a quantitative manner, the in vitro pulldown assay was performed using recombinant molecules, including PRIP-1, PP1, syntaxin, and SNAP-25. GST-SNAP-25 at 10 pmol was incubated with the same amount of His-PRIP-1, PP1, and syntaxin and then with an excess amount of glutathione beads, followed by extensive washing, SDS-PAGE, and immunoblotting with each antibody, indicating the complex formation of these four molecules (Fig. 7). Band densities, by taking the amount applied into each lane of SDS-gel account, revealed that about 4–5% of SNAP-25 was precipitated with an equal amount of syntaxin, along with 0.2–0.3% of PRIP-1 and PP1. The values indicate that the complexes between SNAP-25 and syntaxin and between PRIP and PP1 are formed in a 1:1 ratio, and about 5–6% of SNAP-25·syntaxin forms a complex with PRIP-1·PP1.
FIGURE 6.
Interaction of PRIP-1, SNAP-25, and PP1 in PC12 cells. A, PC12 cells expressing GFP alone (None) or GFP-PRIP-1 (WT) were lysed, and SNAP-25 was immunoprecipitated (IP) by anti-SNAP25 antibody. Immunoprecipitate was separated by SDS-PAGE and analyzed by Western blotting (IB) using anti-GFP, anti-PP1, anti-SNAP25, and anti-syntaxin antibodies. Control, immunoprecipitation by control IgG instead of anti-SNAP-25 antibody. Similar results were seen in three other independent experiments. 0.1 or 30% of the total amounts of cell lysates or immunoprecipitates, respectively, was applied to SDS-PAGE, the values of which were taken into account for the calculation (see “Results”). B, PC12 cells expressing GFP (None), GFP-PRIP-1 (WT), or GFP-PRIP-1 (F97A) were lysed and subjected to immunoprecipitation with anti-GFP antibody using the Pierce Crosslink Immunoprecipitation Kit. The eluted samples were separated by SDS-PAGE and analyzed by Western blotting using anti-GFP, anti-SNAP25, and anti-PP1 antibody. Similar results were seen in three other independent experiments. C, PC12 cells expressing GFP-PRIP-1 (WT) or GFP-PRIP-1 (T94A) were first stimulated to phosphorylate PRIP-1 itself with FSK (50 μm) for 5 min, followed by immunoprecipitation by anti-PRIP-1 antibody (PRIP). The immunoprecipitates were immunoblotted by anti-PP1 and anti-SNAP-25 antibodies. Control, immunoprecipitation by control IgG instead of anti-PRIP-1. The band seen in the blot by anti-SNAP-25 in the control would be a light chain of IgG because a band with a similar density was observed even in the absence of cell lysates. Similar results were seen in two other independent experiments.
FIGURE 7.
In vitro complex formation of SNAP-25 with PRIP, PP1 and syntaxin. An equal amount of each molecule was assayed for the complex formation. GST alone or GST-SNAP-25 along with His-PRIP-1, PP1, and syntaxin at the same amount (10 pmol) was incubated at 4 °C for 2 h, followed by the addition of excess capacity of glutathione beads, precipitation by centrifugation, separation by SDS-PAGE, and immunoblotting (IB) with each antibody. A typical blot is shown, and two other blots yielded similar results. Bands seen between GST-SNAP-25 and GST by immunoblotting with anti-GST antibody would be degradative products of GST-SNAP-25. 0.1 or 30% of the recombinant molecules used or beads after experiments, respectively, were applied to SDS-PAGE, the values of which were taken into account for the calculation (see “Results”).
PP2A was also immunoprecipitated (data not shown), indicating that this phosphatase might also be involved in the phospho-modulation of SNAP-25 and thus the regulation of exocytosis to a lesser extent. Our extensive binding studies suggest that complex formation of PRIP-1 with SNAP-25 appears to be direct in addition to the indirect interaction through syntaxin that directly binds PRIP-1.5
DISCUSSION
SNARE and synaptotagmin are believed to be “minimal membrane fusion machinery” in Ca2+-regulated exocytosis (1–5). In addition, numerous proteins are assumed to participate in the process in a modulatory manner; modulation is important for homeostatic adjustment according to physiological requirements (6–13). Of the proteins of the basic fusion machinery, SNAP-25 has been well investigated (14–16, 48, 49) because protein phosphorylation is one of the issues to be studied as a modulatory mechanism. SNAP-25 has multiple phosphorylation sites, including Ser-28, Thr-29, Thr-138, and Ser-187 phosphorylated by PKA and PKC (see Refs 50 and 51 and this work). It has been reported that phosphorylation of SNAP-25 by PKC prevents inappropriate SNARE association (16), and another report showed that PKA controls the size of the readily releasable pool of vesicles by phosphorylating SNAP-25 (15), both resulting in increased secretion. We here confirmed the phosphorylation of SNAP-25 by PKA and PKC, causing increased secretion, indicating that phosphorylation of SNAP-25 is implicated in the modulation of exocytosis, although the exact molecular mechanisms underlying the increased exocytosis are still unknown. Phosphorylation of proteins must be generally regulated by the balance between kinases and phosphatases; however, few studies regarding phosphatases, compared with kinases, have been performed so far to our knowledge; the type of protein phosphatases responding to dephosphorylation is unknown. Our mutagenesis studies of SNAP-25 using in vitro recombinant molecules and cells expressing SNAP-25 could be summarized as follows: (i) PKA seems to phosphorylate SNAP-25 equally at residues Ser-28, Thr-29, Thr-138, and Ser-187, whereas PKC also phosphorylates these four residues but with preference for Thr-138 and Ser-187 over Ser-28 and Thr-29; (ii) PP1 is the major phosphatase responsible for dephosphorylation of SNAP-25 by both PKA and PKC; and (iii) PP1 seems to target residues Ser-28, Thr-29, Thr-138, and Ser-187 equally, whereas PP2A prefers Thr-138 and Ser-187. The observation that PP2A, which fails to dephosphorylate at Ser-28 and Thr-29, was more effective when SNAP-25 was phosphorylated by PKC supports this summary. The up-regulation of [3H]NA secretion in cells stimulated with forskolin and PMA and the recovery process after stimulation behaved similarly, suggesting that the phosphorylation of SNAP-25 at Thr-138 and/or Ser-187 is more likely to be responsible for the phospho-regulation of exocytosis.
Furthermore, the mechanisms for phosphatase to target SNAP-25 are not known to ensure dynamic phospho-dependent modulation of its function by antagonizing the function of protein kinases. We isolated PRIP as a novel inositol 1,4,5-trisphosphate/PtdIns(4,5)P2-binding protein (23, 32, 34, 35) and later identified it also as a protein phosphatase (PP1 and PP2A)-anchoring protein (29, 30). Furthermore, very recently we found that PRIP associates with Akt when it is phosphorylated to be active (31), indicating that PRIP is a unique molecule participating in phosphorylation events from both sides. PRIP gene-deficient mice exhibited up-regulation of exocytosis, including gonadotropins and insulin (37, 38). During the course of extensive experiments to elucidate the mechanisms by which PRIP inhibits exocytosis, we learned that PRIP might also be implicated in the modulation of exocytosis through phospho-regulation, in addition to its fundamental inhibitory roles in exocytosis. Our unpublished observation shows that active Akt failed to phosphorylate SNAP-25 in an in vitro experiment,6 so here we did not assume the involvement of PRIP in the phosphorylation process but first elucidated which phosphatase is responsible for the dephosphorylation of SNAP-25 and further examined the possible involvement of PRIP in the phospho-regulation of exocytosis as PP1 and PP2A-anchoring proteins.
In this study, we showed that SNAP-25 phosphorylated by PKA and PKC was dephosphorylated by PP1 most efficiently and by PP2A to a lesser extent but not by PP2B in vitro. Cellular experiments in combination with inhibitors of phosphatases also support the results obtained by in vitro experiments. It was reported that inhibition of the Ca2+/calmodulin-dependent protein phosphatase, calcineurin (PP2B), leads to increased neurotransmitter release (48, 49, 52), and the releasable vesicle pools in chromaffin cells are regulated by PKA and calcineurin, being correlated with the phospho-state of SNAP-25 (15). Although these studies showed that a balance between PKA and calcineurin activity might control exocytosis, there is no direct evidence that dephosphorylation of SNAP-25 is mediated by calcineurin. We demonstrate here for the first time that SNAP-25 is the substrate of PP1 and PP2A, thus providing an important physiological perspective for understanding the phospho-dependent modulation of SNARE protein-mediated exocytosis.
The mechanisms by which protein phosphatases are targeted to SNAP-25 to ensure dynamic phospho-dependent modulation of SNARE protein function remain to be addressed. Because PP1 and PP2A to a lesser extent appear to be responsible for dephosphorylation, PRIP could be a promising molecule because PRIP associates with both phosphatases, as described above. Furthermore, we have previously shown that PRIP plays an important role in controlling GABAA receptor activity via regulation of the phospho-states of the β subunit of the receptor (53). In this case, PRIP was found to interact with GABAA receptor β subunit, which was also a substrate of PP1. Indeed, PRIP-1, SNAP-25, syntaxin, and PP1 all existed in the immunocomplex precipitated from PC12 cell lysate, supporting the notion that PRIP promotes PP1 to be closer to the sites where SNAP-25 acts as SNARE. We found that PRIP-1 expression in PC12 cells accelerated the dephosphorylation process of SNAP-25. This acceleration could be attributed to the higher activity of PP1 targeting the SNAP-25 location by PRIP. This was further supported by the lack of effect of the mutants of PRIP-1, F97A and T94A. These results suggest that PRIP plays a role as a scaffold to facilitate the targeting of phosphatases to SNAP-25 in order to ensure dynamic phospho-dependent modulation of SNARE protein function. The phospho-level of SNAP-25 was roughly but not precisely correlated with the up-regulation of NA secretion in PC12 cells. PRIP binds to membrane PtdIns(4,5)P2 through its pleckstrin homology domain (32–35). It is also reported that Ca2+-triggered dense core vesicle exocytosis takes place at microdomains where PtdIns(4,5)P2 are abundant (54–56); therefore, the pleckstrin homology domain might be responsible for PRIP recruiting protein phosphatases, PP1 and PP2A, to the locations where exocytosis occurs. To prove this notion, we examined PC12 cells stably expressing the mutant PRIP-1, which fails to associate with PtdIns(4,5)P2/Ins(1,4,5)P3 in the dephosphorylation process after forskolin stimulation; however, these cells showed the results between the control PC12 cells and PC12 cells expressing PRIP-1 but still similar to the control. We have found that the direct interaction of PRIP-1 with t-SNARE proteins, including syntaxin and SNAP-25, is mediated by the C2 domain of PRIP-15; therefore, multiple devices appear to be available for PRIP to approach the location where exocytosis occurs.
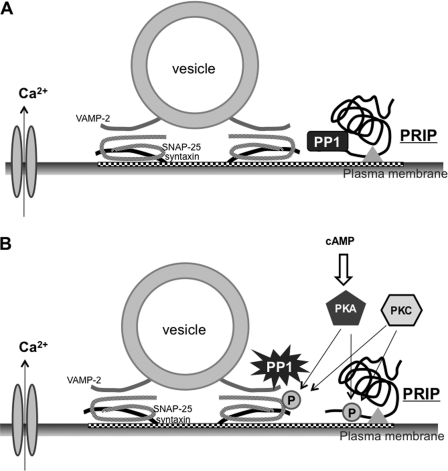
Collecting the data presented in this study, a model explaining that PRIP modulates the phospho-states of SNAP-25 and exocytosis is shown in Fig. 8. PRIP recruits PP1 and PP2A to the site where t-SNARE proteins exist but inhibits PP1 activity, probably with the aid of the pleckstrin homology and C2 domains (Fig. 8A). When the intracellular cAMP level is elevated or PKC is activated by cellular stimulation, PKA and PKC phosphorylate both SNAP-25 and PRIP. Following the phosphorylation of PRIP, PP1 is released to be active near SNAP-25; thus, PP1 can dephosphorylate SNAP-25 effectively to abolish the effect (Fig. 8B).
FIGURE 8.
Schematic representation of the role of PRIP in phospho-dependent regulation of SNAP-25. A, under basal conditions, PRIP facilitates the targeting of PP1 to the site where SNAP-25 exists. PP1 bound by PRIP is inactive. B, PKA or PKC triggers the phosphorylation of SNAP-25 and PRIP, thus modulating exocytosis. Phosphorylated PRIP releases PP1 to be activated for dephosphorylation to cease phospho-modulation by SNAP-25. The triangle indicates the devices available for PRIP to approach the site where exocytosis occurs (see “Discussion”).
The current study showed the possible involvement of PRIP in PKA- and PKC-dependent phospho-modulation of regulatory exocytosis by regulating the location and activities of protein phosphatases, PP1 and PP2A, using PC12 cells expressing PRIP-1. To our knowledge, there have been few studies regarding the dephosphorylation (OFF) process of SNARE proteins for exocytosis compared with those regarding the phosphorylation (ON) process. For the first time, we here elucidated that PP1 is a major phosphatase responsible for the OFF process, which is regulated by PRIP. Further studies are clearly required using cells intrinsically expressing PRIP for a more physiological point of view. Other issues to be addressed are the role of PP2A binding of PRIP and the regulation of catalytic activity in phospho-dependent modulation of exocytosis, although the participation of PP2A appears to be reduced. Furthermore, other proteins of exocytosis, including syntaxin as a substrate, have been reported to participate in the phospho-dependent regulation of exocytosis (14, 19). Whether PRIP modulates the phospho-states of these proteins should also be investigated to better understand the mechanism of the OFF process in the phospho-modulation of exocytosis.
Supplementary Material
Acknowledgment
We thank the Research Support Center, Graduate School of Medical Sciences, Kyushu University, for technical support.
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Science, Sports, and Technology of Japan (to J. G., H. T., and M. H.), the Shimabara Foundation (to H. T.), and the Japan China Medical Association (to J. G.).

This article contains supplemental Figs. 1–6.
H. Takeuchi, J. Gao, Z. Zhang, and M. Hirata, manuscript in preparation.
Z. Zhang, H. Takeuchi, J. Gao, and M. Hirata, manuscript in preparation.
J. Gao, H. Takeuchi, and M. Hirata, unpublished observation.
- NSF
- N-ethylmaleimide-sensitive factor
- CBB
- Coomassie Brilliant Blue
- FSK
- forskolin
- Ins(1,4,5)P3
- d-myo-inositol 1,4,5-trisphosphate
- NA
- noradrenalin
- PMA
- phorbol 12-myristate 13-acetate
- PP
- protein phosphatase
- PRIP
- phospholipase C-related but catalytically inactive protein
- PtdIns(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- SNAP-25
- synaptosome-associated protein of 25 kDa
- SNARE
- soluble NSF-attachment protein receptors
- VAMP
- vesicle-associated membrane protein.
REFERENCES
- 1. Banerjee A., Barry V. A., DasGupta B. R., Martin T. F. (1996) N-Ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J. Biol. Chem. 271, 20223–20226 [DOI] [PubMed] [Google Scholar]
- 2. Sutton R. B., Fasshauer D., Jahn R., Brunger A. T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347–353 [DOI] [PubMed] [Google Scholar]
- 3. Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Söllner T. H., Rothman J. E. (1998) SNAREpins. Minimal machinery for membrane fusion. Cell 92, 759–772 [DOI] [PubMed] [Google Scholar]
- 4. Jahn R., Lang T., Südhof T. C. (2003) Membrane fusion. Cell 112, 519–533 [DOI] [PubMed] [Google Scholar]
- 5. Söllner T. H. (2003) Regulated exocytosis and SNARE function (Review). Mol. Membr. Biol. 20, 209–220 [DOI] [PubMed] [Google Scholar]
- 6. Tolar L. A., Pallanck L. (1998) NSF function in neurotransmitter release involves rearrangement of the SNARE complex downstream of synaptic vesicle docking. J. Neurosci. 18, 10250–10256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu T., Ashery U., Burgoyne R. D., Neher E. (1999) Early requirement for α-SNAP and NSF in the secretory cascade in chromaffin cells. EMBO J. 18, 3293–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dulubova I., Sugita S., Hill S., Hosaka M., Fernandez I., Südhof T. C., Rizo J. (1999) A conformational switch in syntaxin during exocytosis. Role of Munc18. EMBO J. 18, 4372–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Südhof T. C., Rothman J. E. (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiavo G., Stenbeck G., Rothman J. E., Söllner T. H. (1997) Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc. Natl. Acad. Sci. U.S.A. 94, 997–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chapman E. R., Hanson P. I., An S., Jahn R. (1995) Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J. Biol. Chem. 270, 23667–23671 [DOI] [PubMed] [Google Scholar]
- 12. Walent J. H., Porter B. W., Martin T. F. (1992) A novel 145-kDa brain cytosolic protein reconstitutes Ca2+-regulated secretion in permeable neuroendocrine cells. Cell 70, 765–775 [DOI] [PubMed] [Google Scholar]
- 13. Grishanin R. N., Kowalchyk J. A., Klenchin V. A., Ann K., Earles C. A., Chapman E. R., Gerona R. R., Martin T. F. (2004) CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2-binding protein. Neuron 43, 551–562 [DOI] [PubMed] [Google Scholar]
- 14. Risinger C., Bennett M. K. (1999) Differential phosphorylation of syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) isoforms. J. Neurochem. 72, 614–624 [DOI] [PubMed] [Google Scholar]
- 15. Nagy G., Reim K., Matti U., Brose N., Binz T., Rettig J., Neher E., Sørensen J. B. (2004) Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron 41, 417–429 [DOI] [PubMed] [Google Scholar]
- 16. Nagy G., Matti U., Nehring R. B., Binz T., Rettig J., Neher E., Sorensen J. B. (2002) Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser-187 potentiates vesicle recruitment. J. Neurosci. 22, 9278–9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shimazaki Y., Nishiki T., Omori A., Sekiguchi M., Kamata Y., Kozaki S., Takahashi M. (1996) Phosphorylation of 25-kDa synaptosome-associated protein. Possible involvement in protein kinase C-mediated regulation of neurotransmitter release. J. Biol. Chem. 271, 14548–14553 [DOI] [PubMed] [Google Scholar]
- 18. Foletti D. L., Lin R., Finley M. A., Scheller R. H. (2000) Phosphorylated syntaxin 1 is localized to discrete domains along a subset of axons. J. Neurosci. 20, 4535–4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dubois T., Kerai P., Learmonth M., Cronshaw A., Aitken A. (2002) Identification of syntaxin-1A sites of phosphorylation by casein kinase I and casein kinase II. Eur. J. Biochem. 269, 909–914 [DOI] [PubMed] [Google Scholar]
- 20. Fujita Y., Sasaki T., Fukui K., Kotani H., Kimura T., Hata Y., Südhof T. C., Scheller R. H., Takai Y. (1996) Phosphorylation of Munc-18/n-Sec1/rbSec1 by protein kinase C. Its implication in regulating the interaction of Munc-18/n-Sec1/rbSec1 with syntaxin. J. Biol. Chem. 271, 7265–7268 [DOI] [PubMed] [Google Scholar]
- 21. Davletov B., Sontag J. M., Hata Y., Petrenko A. G., Fykse E. M., Jahn R., Südhof T. C. (1993) Phosphorylation of synaptotagmin I by casein kinase II. J. Biol. Chem. 268, 6816–6822 [PubMed] [Google Scholar]
- 22. Matveeva E. A., Whiteheart S. W., Vanaman T. C., Slevin J. T. (2001) Phosphorylation of the N-ethylmaleimide-sensitive factor is associated with depolarization-dependent neurotransmitter release from synaptosomes. J. Biol. Chem. 276, 12174–12181 [DOI] [PubMed] [Google Scholar]
- 23. Kanematsu T., Takeya H., Watanabe Y., Ozaki S., Yoshida M., Koga T., Iwanaga S., Hirata M. (1992) Putative inositol 1,4,5-trisphosphate binding proteins in rat brain cytosol. J. Biol. Chem. 267, 6518–6525 [PubMed] [Google Scholar]
- 24. Kanematsu T., Misumi Y., Watanabe Y., Ozaki S., Koga T., Iwanaga S., Ikehara Y., Hirata M. (1996) A new inositol 1,4,5-trisphosphate binding protein similar to phospholipase C-δ 1. Biochem. J. 313, 319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanematsu T., Yoshimura K., Hidaka K., Takeuchi H., Katan M., Hirata M. (2000) Domain organization of p130, PLC-related catalytically inactive protein, and structural basis for the lack of enzyme activity. Eur. J. Biochem. 267, 2731–2737 [DOI] [PubMed] [Google Scholar]
- 26. Essen L. O., Perisic O., Cheung R., Katan M., Williams R. L. (1996) Crystal structure of a mammalian phosphoinositide-specific phospholipase C δ. Nature 380, 595–602 [DOI] [PubMed] [Google Scholar]
- 27. Yoshida M., Kanematsu T., Watanabe Y., Koga T., Ozaki S., Iwanaga S., Hirata M. (1994) d-myo-Inositol 1,4,5-trisphosphate-binding proteins in rat brain membranes. J. Biochem. 115, 973–980 [DOI] [PubMed] [Google Scholar]
- 28. Uji A., Matsuda M., Kukita T., Maeda K., Kanematsu T., Hirata M. (2002) Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2. Comparison with PRIP-1. Life Sci. 72, 443–453 [DOI] [PubMed] [Google Scholar]
- 29. Yoshimura K., Takeuchi H., Sato O., Hidaka K., Doira N., Terunuma M., Harada K., Ogawa Y., Ito Y., Kanematsu T., Hirata M. (2001) Interaction of p130 with, and consequent inhibition of, the catalytic subunit of protein phosphatase 1α. J. Biol. Chem. 276, 17908–17913 [DOI] [PubMed] [Google Scholar]
- 30. Kanematsu T., Yasunaga A., Mizoguchi Y., Kuratani A., Kittler J. T., Jovanovic J. N., Takenaka K., Nakayama K. I., Fukami K., Takenawa T., Moss S. J., Nabekura J., Hirata M. (2006) Modulation of GABAA receptor phosphorylation and membrane trafficking by phospholipase C-related inactive protein/protein phosphatase 1 and 2A signaling complex underlying brain-derived neurotrophic factor-dependent regulation of GABAergic inhibition. J. Biol. Chem. 281, 22180–22189 [DOI] [PubMed] [Google Scholar]
- 31. Fujii M., Kanematsu T., Ishibashi H., Fukami K., Takenawa T., Nakayama K. I., Moss S. J., Nabekura J., Hirata M. (2010) Phospholipase C-related but catalytically inactive protein is required for insulin-induced cell surface expression of γ-aminobutyric acid type A receptors. J. Biol. Chem. 285, 4837–4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takeuchi H., Kanematsu T., Misumi Y., Yaakob H. B., Yagisawa H., Ikehara Y., Watanabe Y., Tan Z., Shears S. B., Hirata M. (1996) Localization of a high-affinity inositol 1,4,5-trisphosphate/inositol 1,4,5,6-tetrakisphosphate binding domain to the pleckstrin homology module of a new 130-kDa protein. Characterization of the determinants of structural specificity. Biochem. J. 318, 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takeuchi H., Kanematsu T., Misumi Y., Sakane F., Konishi H., Kikkawa U., Watanabe Y., Katan M., Hirata M. (1997) Distinct specificity in the binding of inositol phosphates by pleckstrin homology domains of pleckstrin, RAC-protein kinase, diacylglycerol kinase, and a new 130-kDa protein. Biochim. Biophys. Acta 1359, 275–285 [DOI] [PubMed] [Google Scholar]
- 34. Takeuchi H., Oike M., Paterson H. F., Allen V., Kanematsu T., Ito Y., Erneux C., Katan M., Hirata M. (2000) Inhibition of Ca2+ signaling by p130, a phospholipase-C-related catalytically inactive protein. Critical role of the p130 pleckstrin homology domain. Biochem. J. 349, 357–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao J., Takeuchi H., Zhang Z., Fujii M., Kanematsu T., Hirata M. (2009) Binding of phospholipase C-related but catalytically inactive protein to phosphatidylinositol 4,5-bisphosphate via the PH domain. Cell. Signal. 21, 1180–1186 [DOI] [PubMed] [Google Scholar]
- 36. Kanematsu T., Jang I. S., Yamaguchi T., Nagahama H., Yoshimura K., Hidaka K., Matsuda M., Takeuchi H., Misumi Y., Nakayama K., Yamamoto T., Akaike N., Hirata M., Nakayama K. (2002) Role of the PLC-related, catalytically inactive protein p130 in GABAA receptor function. EMBO J. 21, 1004–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matsuda M., Tsutsumi K., Kanematsu T., Fukami K., Terada Y., Takenawa T., Nakayama K. I., Hirata M. (2009) Involvement of phospholipase C-related inactive protein in the mouse reproductive system through the regulation of gonadotropin levels. Biol. Reprod. 81, 681–689 [DOI] [PubMed] [Google Scholar]
- 38. Doira N., Kanematsu T., Matsuda M., Takeuchi H., Nakano H., Ito Y., Nakayama K., Nakayama K. I., Hirata M. (2001) Hyperinsulinemia in PRIP-1 gene-deleted mice. Biomed. Res. 22, 157–165 [Google Scholar]
- 39. Oho C., Seino S., Takahashi M. (1995) Expression and complex formation of soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptors in clonal rat endocrine cells. Neurosci. Lett. 186, 208–210 [DOI] [PubMed] [Google Scholar]
- 40. De Camilli P. (1991) Co-secretion of multiple signal molecules from endocrine cells via distinct exocytotic pathways. Trends Pharmacol. Sci. 12, 446–448 [DOI] [PubMed] [Google Scholar]
- 41. Gao J., Takeuchi H., Umebayashi H., Zhang Z., Matsuda M., Hirata M. (2009) Assay of dense-core vesicle exocytosis using permeabilized PC12 cells. Adv. Enzyme Regul. 50, 237–246 [DOI] [PubMed] [Google Scholar]
- 42. Hay J. C., Fisette P. L., Jenkins G. H., Fukami K., Takenawa T., Anderson R. A., Martin T. F. (1995) ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature 374, 173–177 [DOI] [PubMed] [Google Scholar]
- 43. Eberhard D. A., Cooper C. L., Low M. G., Holz R. W. (1990) Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem. J. 268, 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gutierrez L. M., Viniegra S., Quintanar J. L., Reig J. A., Sala F. (1994) Calyculin A blocks bovine chromaffin cell calcium channels independently of phosphatase inhibition. Neurosci. Lett. 178, 55–58 [DOI] [PubMed] [Google Scholar]
- 45. Shu Y., Liu X., Yang Y., Takahashi M., Gillis K. D. (2008) Phosphorylation of SNAP-25 at Ser-187 mediates enhancement of exocytosis by a phorbol ester in INS-1 cells. J. Neurosci. 28, 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iwasaki S., Kataoka M., Sekiguchi M., Shimazaki Y., Sato K., Takahashi M. (2000) Two distinct mechanisms underlie the stimulation of neurotransmitter release by phorbol esters in clonal rat pheochromocytoma PC12 cells. J. Biochem. 128, 407–414 [DOI] [PubMed] [Google Scholar]
- 47. Favre B., Turowski P., Hemmings B. A. (1997) Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J. Biol. Chem. 272, 13856–13863 [DOI] [PubMed] [Google Scholar]
- 48. Victor R. G., Thomas G. D., Marban E., O'Rourke B. (1995) Presynaptic modulation of cortical synaptic activity by calcineurin. Proc. Natl. Acad. Sci. U.S.A. 92, 6269–6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gromada J., Høy M., Buschard K., Salehi A., Rorsman P. (2001) Somatostatin inhibits exocytosis in rat pancreatic α-cells by Gi2-dependent activation of calcineurin and depriming of secretory granules. J. Physiol. 535, 519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hepp R., Cabaniols J. P., Roche P. A. (2002) Differential phosphorylation of SNAP-25 in vivo by protein kinase C and protein kinase A. FEBS Lett. 532, 52–56 [DOI] [PubMed] [Google Scholar]
- 51. Genoud S., Pralong W., Riederer B. M., Eder L., Catsicas S., Muller D. (1999) Activity-dependent phosphorylation of SNAP-25 in hippocampal organotypic cultures. J. Neurochem. 72, 1699–1706 [DOI] [PubMed] [Google Scholar]
- 52. Nichols R. A., Suplick G. R., Brown J. M. (1994) Calcineurin-mediated protein dephosphorylation in brain nerve terminals regulates the release of glutamate. J. Biol. Chem. 269, 23817–23823 [PubMed] [Google Scholar]
- 53. Terunuma M., Jang I. S., Ha S. H., Kittler J. T., Kanematsu T., Jovanovic J. N., Nakayama K. I., Akaike N., Ryu S. H., Moss S. J., Hirata M. (2004) GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J. Neurosci. 24, 7074–7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Holz R. W., Hlubek M. D., Sorensen S. D., Fisher S. K., Balla T., Ozaki S., Prestwich G. D., Stuenkel E. L., Bittner M. A. (2000) A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J. Biol. Chem. 275, 17878–17885 [DOI] [PubMed] [Google Scholar]
- 55. Aoyagi K., Sugaya T., Umeda M., Yamamoto S., Terakawa S., Takahashi M. (2005) The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J. Biol. Chem. 280, 17346–17352 [DOI] [PubMed] [Google Scholar]
- 56. James D. J., Khodthong C., Kowalchyk J. A., Martin T. F. (2008) Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J. Cell Biol. 182, 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.