Background: Nrf2 has been implicated in regulating immune cell signaling and function.
Results: Nrf2-deficient murine DCs exhibit enhanced maturation phenotype, increased ROS levels with dysregulation of antigen uptake capabilities, and altered intracellular signaling.
Conclusion: Nrf2 regulates DC intracellular redox and immune function.
Significance: Defining the role of Nrf2 in DC biology underpins development of potential Nrf2 targeted immunotherapeutics.
Keywords: Dendritic Cells, Immunology, Nrf2, Redox Regulation, Signaling
Abstract
Dendritic cells (DCs) are critical mediators of immunity and immune tolerance by orchestrating multiple aspects of T cell activation and function. Immature DCs (iDCs) expressing low levels of co-stimulatory receptors are highly efficient at antigen capture but are poor activators of T cells. Maturation of DCs is associated with increased expression of co-stimulatory molecules. Co-stimulatory receptor gene expression is regulated by intracellular redox, NF-κB, and MAPK pathways and by histone deacetylase (HDAC) activity. The transcription factor, Nrf2, is important for maintaining intracellular glutathione (GSH) levels and redox homeostasis and has been implicated in modulating DC co-stimulatory receptor expression. It is unclear whether Nrf2 mediates this effect by GSH-dependent mechanisms and whether it influences DC signaling pathways. Using bone marrow-derived iDCs from Nrf2+/+ and Nrf2−/− mice, we demonstrate that Nrf2−/− iDCs have lower basal GSH levels, enhanced co-stimulatory receptor expression, impaired phagocytic functions, and increased antigen-specific CD8 T cell stimulation capacity. Interestingly, lowering GSH levels in Nrf2+/+ iDCs did not recapitulate the Nrf2−/− iDC phenotype. Loss of Nrf2 resulted in elevated basal levels of reactive oxygen species but did not affect basal NF-κB activity or p38 MAPK phosphorylation. Using pharmacological inhibitors, we demonstrate that enhanced co-stimulatory receptor phenotype of Nrf2−/− iDC does not require ERK activity but is dependent on HDAC activity, indicating a potential interaction between Nrf2 function and HDAC. These results suggest that Nrf2 activity is required to counter rises in intracellular reactive oxygen species and to regulate pathways that control DC co-stimulatory receptor expression.
Introduction
Dendritic cells (DCs)4 are potent antigen-presenting cells that are responsible for orchestrating immune responses (1). DCs reside in the periphery in an immature antigen-capturing state, enabling them to survey the environment for the presence of soluble antigens, microbes, and dying cells (2, 3). Immature DCs (iDCs) have high phagocytic capabilities but are poorly immunogenic as they express low levels of MHC class II and the co-stimulatory receptors CD40, CD80, and CD86 (4). Immature DCs also play a critical role in maintaining peripheral T cell tolerance, which is dependent upon their low expression of co-stimulatory receptors (4). Maturation of DCs can be triggered by engagement of receptors such as Toll-like receptors, resulting in the up-regulation of co-stimulatory molecules and associated loss of phagocytic activity (1). Mature DCs expressing high levels of co-stimulatory receptors can activate CD8 T lymphocytes and generate effector cytotoxic T lymphocytes that possess antiviral and antitumor activity (5–7). DC maturation involves the activation of several intracellular signaling pathways including those of the nuclear factor-κB (NF-κB) and MAPK pathways (8–10). NF-κB belongs to the NF-κB/Rel family of transcription factors and has been implicated in DC development, maturation, and survival (9, 11). Similarly, the MAP kinases such as ERK1/2 and p38 MAPK have been implicated in regulating DC co-stimulatory receptor expression and inflammatory cytokine production (8, 12). Furthermore, epigenetic changes in DC gene expression can influence their maturation status and subsequent immune function (13, 14). Histone deacetylase (HDACs) enzymes (along with histone acetylases) are responsible for the epigenetic regulation of co-stimulatory receptor gene expression through deacetylation of histone residues (13–15). Evidence has shown that the intracellular redox status and reactive oxygen species (ROS) production have a significant impact on DC functions such as activation, maturation cytokine production, and immunosenescence (16–19). One of the key antioxidant molecules responsible for maintenance of DC intracellular redox homeostasis is glutathione (GSH) (16). Cellular GSH levels are mainly determined by the activity of a basic leucine zipper transcription factor, nuclear factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2), through transcribing genes involved in GSH biosynthesis such as the glutamate-cysteine ligase catalytic (GCLC) domain (17, 20). Nrf2 has been shown to influence DC redox homeostasis and impact on aspects of DC biology (17, 18, 21, 22). However, it is not clear whether the effects of Nrf2 deficiency in DC phenotype and function can be attributed to a potential lowering of GSH levels in these cells. Furthermore, the impact of altered phenotype of Nrf2−/− DCs on CD8 T cell stimulatory capacity has not been examined. Finally, the molecular basis for the altered co-stimulatory phenotype is unclear. In particular, the potential effect of loss of Nrf2 on ROS levels, signaling by the NF-κB and MAPK pathways, and involvement of HDACs in DCs has not yet been investigated extensively. In this study, bone marrow-derived Nrf2-deficient iDCs (Nrf2−/− iDCs) demonstrate lower basal levels of GSH and GSH-independent enhanced co-stimulatory receptor expression, impaired phagocytic function, and enhanced CD8 T cell stimulation capacity when compared with Nrf2+/+ iDCs. We also demonstrate increase in ROS levels and delayed LPS-induced NF-κB signaling events without changes in the basal NF-κB activity in Nrf2−/− iDCs. Furthermore, loss of Nrf2 affected p42/44 (ERK1/2) but not p38 signaling. Finally, our results provide evidence for HDAC-dependent higher co-stimulatory receptor expression in Nrf2−/− iDCs. Our findings highlight the potential role of Nrf2 in modulating intracellular ROS, MAPK, and NF-κB signaling and HDAC-dependent co-stimulatory gene expression in DCs.
EXPERIMENTAL PROCEDURES
Reagents
Fetal calf serum (FCS) and recombinant GM-CSF were purchased from Invitrogen (Paisley, UK) and PeproTech, respectively. All other reagents were of analytical grade and purchased from Sigma-Aldrich (Poole, UK) unless otherwise stated.
Mice
Nrf2+/+ and Nrf2−/− mice were purchased from Riken BRC (Ibaraki, Japan) and maintained at the Biomedical Services Unit, University of Liverpool (23). Mice transgenic for the H-2Db-restricted TCR-αβ transgene, F5, was a kind gift from Dr. James Matthews, Cardiff, Wales, UK. Protocols described herein were undertaken in accordance with criteria outlined in licenses granted under the Animals (Scientific Procedures) Act 1986 (PPL 40/2937 and PPL 40/2544).
Generation of Bone Marrow-derived DCs
Bone marrow-derived iDCs were generated according to published protocols (24).
Glutathione Assay
DCs were lysed in 10 mm HCl. 6.5% salicylic acid was added to cell lysates (1:4 v/v), and protein precipitates were removed by centrifugation. Cell supernatants were assayed for GSH according to Ref. 25 and normalized to protein content.
Cell Surface Receptor Expression
DCs were labeled with αCD11cTC (Invitrogen) and αCD86FITC, αCD40FITC, or αMHC IIPE (BD Biosciences) antibodies for 30 min on ice, washed, acquired on a BD FACSCanto II flow cytometer (BD Biosciences), and analyzed using the Cyflogic version 1.2.1 software (CyFlo Ltd.). Peripheral T cells from lymph nodes were labeled with αCD8TC (Invitrogen), αCD4PE, and αCD62LFITC (BD Biosciences) antibodies and analyzed as above.
DC Endocytosis Assay
DCs were incubated with 0.5 μg/ml DextranFITC (40,000 Mr) (Invitrogen) for 60 min at 37 or 4 °C. Cells were stained for surface expression of CD11c as described above and analyzed by flow cytometry.
DC Phagocytosis Assay
Apoptotic thymocytes were generated from thymi of C57BL/6 mice by treatment with 1 μm dexamethasone for 18 h. Apoptotic thymocytes were labeled with the intracellular fluorescent dye, carboxyfluorescein succinimidyl ester (CFSE), and co-cultured with plate-adherent DCs for 2 h at 37 or 4 °C. Cells were stained with αCD11cTC prior to analysis by flow cytometry. Jurkat T cells were fluorescently labeled with CFSE, and necrosis was induced by snap freezing in liquid nitrogen. Necrotic cells were co-cultured with DCs as above and analyzed by flow cytometry.
Intracellular IFN-γ Staining
F5 CD8 T cells were co-cultured for 72 h with Nrf2+/+ and Nrf2−/− iDCs prepulsed with 1 × 10−7 m NP68 peptide. T cells were harvested and co-cultured for 6 h with Nrf2+/+ mature DCs prepulsed with 1 × 10−7 m NP68. Brefeldin A was added 1 h after start of culture. Cells were stained with αCD8TC followed by fixing, permeabilization, and intracellular staining for interferon-γ (IFN-γ) according to the manufacturer's instructions (Cytofix/Cytoperm Plus; BD Biosciences) and analyzed by flow cytometry.
F5 CD8 T Cell Proliferation
F5 CD8 T cell proliferation was quantified as described in Ref. 26. Briefly, Nrf2+/+ and Nrf2−/− iDCs were pulsed with a dose range of antigenic peptides (NP68 or NP34), washed, and co-cultured with F5 T cells for 72 h. [3H]Thymidine was added for the last 16 h. Cells were harvested onto glass fiber filter mats and read on a scintillation counter (MicroBeta Trilux; PerkinElmer Life Sciences, Buckinghamshire, UK).
Measurement of ROS
Unstimulated or stimulated DCs were labeled with the fluorescent ROS indicator, dihydroethidium, according to Ref. 27 and analyzed by flow cytometry.
Gel Electrophoresis and Western Blot
Immature DCs treated with LPS over the indicated time points were lysed, and 20 μg of lysate protein/sample was resolved by SDS-PAGE, transferred to nitrocellulose membranes (GE Healthcare), blocked, and probed for the indicated proteins using the appropriate primary antibodies (phospho-p65, IκBα, phospho-ERK1/2, ERK1/2, phospho-p38, and p38, Cell Signaling Technology; and p65 and actin, Santa Cruz Biotechnologies) followed by horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich) and visualized using the ECL system (PerkinElmer Life Sciences).
Measurement of NF-κB Activity
NF-κB activity of iDC nuclear extracts was determined using the EZ-DetectTM NF-κB p65 transcription factor kit (Pierce Biotechnology) following the manufacturer's instructions. Results are expressed as relative luminescence units.
ELISA
Supernatants were recovered from iDC cultures and assayed for TNF-α using a mouse TNF-α Quantikine kit (R&D Systems) according to the manufacturer's protocol.
HDAC Activity Assay
HDAC activity of iDC nuclear extracts was determined using the HDAC assay kit (Millipore) following the manufacturer's instructions. Results are expressed as absorbance at 405 nm (A405).
Statistics
Data were analyzed using the unpaired t test, one-way analysis of variance, and the Mann-Whitney U test
RESULTS
Loss of Nrf2 Results in Altered Phenotype and Antigen Capture Function of DCs
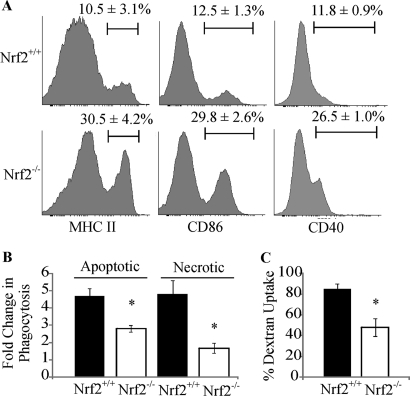
To evaluate the role of Nrf2 in regulating the phenotype and function of iDCs, we compared the maturation states of Nrf2−/− and Nrf2+/+ iDCs in addition to measuring endocytic and phagocytic function. Maturation is accompanied by an increase in the proportion of DCs that express higher levels of co-stimulatory molecules (1). Immature DCs are composed of subpopulations that express either high or low levels of co-stimulatory molecules (Fig. 1A). Flow cytometric analysis of iDCs demonstrate that the proportion of iDCs that express high levels of CD86, CD40, and MHC II was significantly increased in the Nrf2−/− iDCs in comparison with the Nrf2+/+ iDCs as shown in Fig. 1A (CD86, 29.75 versus 12.5%, p < 0.05; CD40, 26.5 versus 11.75%, p < 0.05; MHC II, 30.5 versus 10.5%, p < 0.05). DC maturation is accompanied by a diminished capacity for phagocytosis and endocytosis. As Nrf2−/− iDCs exhibited a more mature phenotype, we tested whether they also have impaired antigen capture functions. As shown in Fig. 1B, in comparison with Nrf2+/+ iDCs, Nrf2−/− iDCs had a significantly lower capacity to phagocytose apoptotic cells (4.7 versus 2.8-fold increase over baseline, p < 0.05) and necrotic cells (4.8- versus 1.7-fold increase over baseline, p < 0.05). Endocytic capacity of Nrf2−/− iDCs was also impaired when compared with the Nrf2+/+ iDCs (45.1 versus 88.1%, p < 0.05), as shown in Fig. 1C. These results indicate that loss of Nrf2 affects iDC maturation state and concomitant antigen capture functions.
FIGURE 1.
Loss of Nrf2 leads to dysregulation in multiple DC functions. A, Nrf2+/+ and Nrf2−/− iDCs were labeled with antibodies against MHC II, CD86, and CD40 co-stimulatory receptors. Co-stimulatory receptor expression was determined by flow cytometry. The percentage of iDCs expressing high levels of co-stimulatory receptors is indicated above the marker. Representative histograms are presented with average percentage ± S.D. Statistical significance was tested by unpaired Student's t test (*, p < 0.05). Data are derived from four independent experiments. B, Nrf2+/+ and Nrf2−/− iDCs were co-cultured with CFSE-labeled apoptotic thymocytes or necrotic Jurkat cells at 37 °C for 2 h. DC phagocytic capacity was measured by flow cytometry as an increase in CFSE levels when compared with corresponding 4 °C baseline control samples. Data are expressed as average -fold changes ± S.E. Statistical significance was tested by Mann-Whitney U test (*, p < 0.05). Data are derived from five independent experiments. C, endocytic capacity was measured by incubating Nrf2+/+ or Nrf2−/− iDCs cells with DextranFITC for 60 min at 37 °C. DextranFITC uptake by iDCs was assessed by flow cytometry. Data are presented as average percentage of uptake ± S.D. Statistical significance was tested by unpaired Student's t test (*, p < 0.05). Data are derived from four independent experiments.
Dysregulation in Phenotype and Function of Nrf2−/− iDCs Is Not a Direct Consequence of Lower GSH Levels
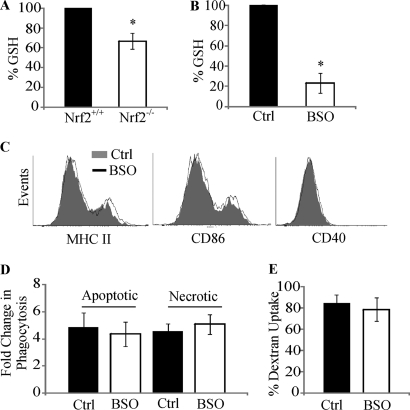
Nrf2 is a critical regulator of GSH biosynthesis (20), and loss of Nrf2 is hypothesized to result in lowered intracellular GSH in DCs. We therefore measured basal levels of GSH and show that Nrf2−/− iDCs have up to 33% reduction in GSH levels relative to Nrf2+/+ iDCs (p < 0.05) (Fig. 2A). To test whether the changes in phenotype and function of Nrf2−/− iDCs could be due to lowered GSH levels, buthionine sulfoximine (BSO), a potent inhibitor of the glutamate-cysteine ligase catalytic domain (an essential enzyme involved in GSH biosynthesis) (28), was used to reduce GSH levels and assess effects on phenotype and function in Nrf2+/+ iDCs. Fig. 2B demonstrates that up to 77% reduction in basal GSH levels can be achieved through treatment of Nrf2+/+ iDCs with BSO (p < 0.05). When the expression levels of MHC II, CD86, and CD40 were analyzed on BSO-treated iDCs, no detectable changes in surface expression of these receptors could be observed (Fig. 2C). Furthermore, lowering GSH levels did not affect either the phagocytic or the endocytic functions of iDCs (Fig. 2, D and E). These results suggest that although loss of Nrf2 results in reduced GSH levels, the changes in phenotype and function of Nrf2−/− iDCs cannot be attributed to the lowered basal GSH.
FIGURE 2.
Dysregulation in function of Nrf2−/− DC is not a consequence of lower GSH levels. A and B, GSH levels were measured in Nrf2+/+ and Nrf2−/− iDC (A) or Nrf2+/+ iDCs untreated (Control) or treated with BSO (100 μm) (B) for 24 h. Data are presented as average percentage (when compared with Nrf2+/+ or untreated iDCs) ± S.D. Statistical significance was tested by unpaired Student's t test (*, p < 0.05). Data are derived from five independent experiments. C, immature Nrf2+/+ DCs untreated or treated with BSO were labeled with antibodies against MHC II, CD86, and CD40 co-stimulatory receptors. Co-stimulatory receptor expression was determined by flow cytometry. Histogram overlays show fluorescence intensity of respective co-stimulatory receptors and are representative of four independent experiments. D, immature Nrf2+/+ DCs untreated or treated with BSO were co-cultured with CFSE-labeled apoptotic thymocytes or necrotic Jurkat cells at 37 °C for 2 h. DC phagocytic capacity was measured by flow cytometry as an increase in CFSE levels when compared with corresponding 4 °C baseline control samples. Data are presented as average -fold changes ± S.E. Data are derived from five independent experiments. E, endocytic capacity was measured by incubating Nrf2+/+ iDCs that were untreated or treated with BSO with DextranFITC for 60 min at 37 °C. DextranFITC uptake by DCs was assessed by flow cytometry. Data are derived from four independent experiments and presented as average percentage of uptake ± S.D.
Increased Co-stimulatory Receptor Expression of Nrf2−/− iDCs Is Associated with Enhanced Antigen-specific CD8 T Cell Stimulatory Capacity
Immature DCs expressing low levels of co-stimulatory molecules are unable to stimulate a fully competent antigen-specific CD8 T cell response (4). Given that Nrf2−/− iDCs have elevated expression of CD86, CD40, and MHC II, we postulated that these iDCs would be capable of inducing an antigen-specific CD8 T cell response.
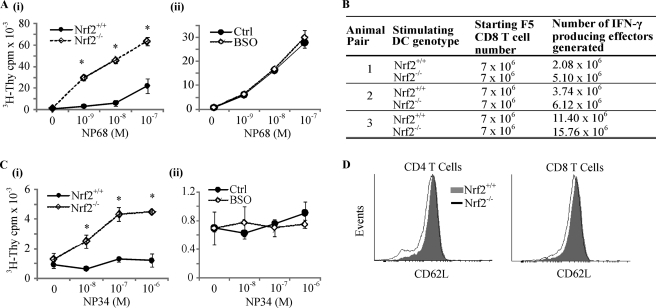
To address this, we utilized a TCR transgenic mouse model, F5, wherein the CD8 T cells express a T cell receptor (F5 TCR) that recognizes an antigenic peptide, NP68, when presented by DCs (29). The functional consequence of changes in DC co-stimulatory receptor expression can be assessed by the ability of NP68-bearing DCs to stimulate F5 CD8 T cell proliferation. Nrf2+/+ iDCs pulsed with increasing doses of NP68 did not induce specific F5 CD8 T cell proliferation until 10 nm NP68 (Fig. 3A, panel i). A higher dose of NP68 (100 nm) induced greater proliferation. In contrast, Nrf2−/− iDCs were able to induce significant F5 CD8 T cell proliferation even at 1 nm NP68 (Fig. 3A, panel i) and a 3-to-10-fold higher degree of T cell proliferation when compared with Nrf2+/+ iDCs at all NP68 doses (Fig. 3A, panel i, p < 0.05). As with the phenotype and antigen capture functions, lowering of GSH did not increase the ability of Nrf2+/+ iDCs to stimulate antigen-specific CD8 T cells (Fig. 3A, panel ii). Following primary antigen-specific activation, CD8 T cells develop into IFN-γ-producing effectors (30). Our results indicate that Nrf2−/− iDCs can generate between 1.4- and 2.4-fold more effectors from naive F5 CD8 T cells when compared with Nrf2+/+ iDCs (Fig. 3B). These results highlight the association between increased co-stimulatory marker expression of Nrf2−/− iDCs and the ability to induce enhanced CD8 T cell proliferation and acquisition of effector function. The induction of CD8 T cell tolerance by iDCs is largely due to the presentation of self-peptides (31). An altered peptide ligand, termed NP34, binds to the F5 TCR when presented by iDCs, does not activate naive F5 CD8 T cells, and simulates, to a degree, the interaction between self-peptides with TCRs on naive T cells (32, 33). NP34-pulsed Nrf2+/+ iDCs did not induce significant F5 CD8 T cell proliferation even at doses as high as 1 μm (Fig. 3C, panel i). In contrast, NP34 presentation by Nrf2−/− iDCs resulted in a 2-fold increase in proliferation of F5 CD8 T cells at 10 nm NP34 and above when compared with unpulsed iDCs (Fig. 3C, panel i, p < 0.05 for all NP34 doses). As with T cell responses with NP68, depletion of GSH from Nrf2+/+ iDCs does not recapitulate the T cell responses observed with NP34-pulsed Nrf2−/− iDCs (Fig. 3C, panel ii). The ability to provoke T cell responses by self-peptide bearing Nrf2−/− iDCs could lead to inappropriate T cell activation in vivo. To investigate this possibility, we compared the levels of CD62L, a marker of T cell activation in the lymph node T cells from Nrf2+/+ and Nrf2−/− mice. T cell activation results in the down-regulation of CD62L (34). Our results demonstrate that T cells from Nrf2−/− mice manifest signs of low-level T cell activation with a small but consistent reduction in CD62L expression when compared with their Nrf2+/+ counterpart (Fig. 3D).
FIGURE 3.
Increased T cell stimulatory capacity of Nrf2−/− iDCs. A, Nrf2+/+ and Nrf2−/− iDCs (panel i) and untreated (Control) or BSO (100 μm for 24 h) (panel ii)-treated Nrf2 +/+ iDCs were pulsed with increasing concentrations of NP68 antigenic peptide, and DCs were then co-cultured with F5 CD8 T cells for 72 h. [3H]Thymidine (3H-Thy) was added for the last 16 h. Proliferation of T cells was determined by scintillation counting of incorporated [3H]thymidine. Data are presented as average [3H]thymidine scintillation counts ± S.D. Statistical significance was tested by one-way analysis of variance (*, p < 0.05). Data are derived from four independent experiments. B, naive F5 CD8 T cells were co-cultured with Nrf2+/+ or Nrf2−/− iDCs as described under “Experimental Procedures.” Total numbers of IFN-γ-producing effector T cells were quantified by intracellular cytokine staining. C, Nrf2+/+ and Nrf2−/− iDCs (panel i) and untreated or BSO-treated Nrf2 +/+ iDCs (panel ii) were pulsed with increasing concentrations of NP34 partial agonist. DC-induced T cell proliferation was determined as in panel A. D, CD62L expression on CD4 and CD8 T cells from the lymph nodes of Nrf2+/+ and Nrf2−/− mice was determined by flow cytometry. Histogram overlays are representative of three independent experiments.
Loss of Nrf2 Results in Increased ROS Levels in Immature DCs
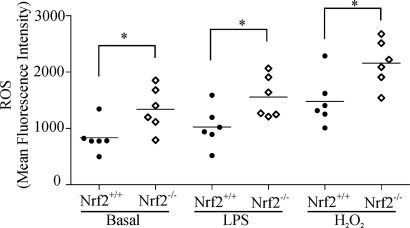
Elevations in DC ROS levels have been shown to enhance co-stimulatory receptor expression and maturation (19). Nrf2 signaling increases transcription of antioxidant genes that counter elevations in intracellular ROS (20). We therefore examined ROS levels in Nrf2−/− and Nrf2+/+ iDCs basally and in response to the oxidative stress inducer hydrogen peroxide (H2O2) and the Toll-like receptor agonist LPS using the fluorescent ROS indicator, dihydroethidium. As demonstrated in Fig. 4, Nrf2−/− iDCs had significantly higher basal ROS levels in comparison with Nrf2+/+ iDCs (mean fluorescence intensity 1341 versus 833.5, p < 0.05). Furthermore, Nrf2−/− iDC ROS level were significantly increased in comparison with their wild type counterpart following treatment with H2O2 (mean fluorescence intensity 2159 versus 1482, p < 0.05) and LPS (mean fluorescence intensity 1557 versus 1026, p < 0.05). Taken together, these results suggest that loss of Nrf2 impairs the capacity of iDCs to maintain redox homeostasis.
FIGURE 4.
Loss of Nrf2 elevates intracellular ROS levels. Nrf2+/+ and Nrf2−/− iDCs were untreated (basal) or treated with LPS (1 μg/ml) or H2O2 (1 mm) for 10 min at 37 °C. Cells were then incubated with the ROS indicator dihydroethidium before analysis by flow cytometry. Data points represent mean fluorescence intensity of six separate measurements from two independent experiments. The horizontal line represents the mean. Statistical significance was tested by unpaired Student's t test (*, p < 0.05).
Elevated ROS Levels in Nrf2-deficient DCs Do Not Affect Basal Nuclear NF-κB Activity
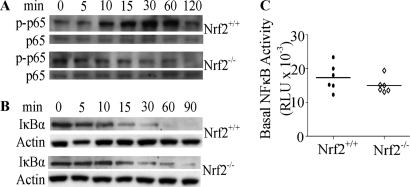
Elevated ROS levels in DCs have been associated with alterations in NF-κB signaling (35). We examined basal and LPS-induced phosphorylation of p65 and degradation of IκBα to investigate the effect of Nrf2 deficiency on NF-κB signaling pathway. These events are essential upstream events required for NF-κB activation (36). Although there were no basal differences in phosphorylated p65, LPS-induced phosphorylation of p65 was impeded in the Nrf2−/− iDCs when compared with the Nrf2+/+ iDCs (Fig. 5A). In Nrf2+/+ iDCs, an increase in p65 phosphorylation can be detected at 10 min, peaking at 30 min, followed by a decrease to basal levels at 120 min after stimulation (Fig. 5A). In contrast, no increases in p65 phosphorylation can be detected in Nrf2−/− iDCs (Fig. 5A) at any of the time points. Similarly, as can be seen in Fig. 5B, the basal (at 0 min) levels of IκBα were comparable in both cell types. However, upon stimulation with LPS, IκBα degradation can be seen as early as 10 min, progressing to total loss of IκBα at 60 min in Nrf2+/+ iDCs, whereas in Nrf2−/− iDCs, IκBα degradation was delayed in onset (at 15 min) and incomplete in extent with residual IκBα being detected at 90 min after stimulation (Fig. 5B). We examined the basal nuclear NF-κB (p65) activity and demonstrate that there are no significant basal differences in NF-κB activity between Nrf2−/− and Nrf2+/+ iDCs (Fig. 5C). Taken together, these results suggest that although an increase in ROS levels in Nrf2-deficient iDCs may affect upstream elements of the NF-κB pathway (phosphorylation of p65 and IκBα degradation), it however did not affect basal NF-κB activity.
FIGURE 5.
Loss of Nrf2 perturbs NF-κB signaling in iDCs. Nrf2+/+ and Nrf2−/− iDCs were treated with LPS (1 μg/ml) for the indicated times, and whole cell lysates were subjected to SDS-PAGE. A and B, the levels of phosphorylation of p65 (p-p65) (A) and of IκBα levels (B) were assessed by Western blotting. Data are representative of three independent experiments. C, NF-κB activity in the nuclear lysates of Nrf2+/+ and Nrf2−/− iDCs was measured and presented as dot plots with each dot representing mean relative luminescence units (RLU) (from triplicates). Data are from six independent experiments. The horizontal lines indicate means for each group.
Elevated ROS Levels in Nrf2-deficient DCs Do Not Affect MAPK-mediated Co-stimulatory Receptor Expression
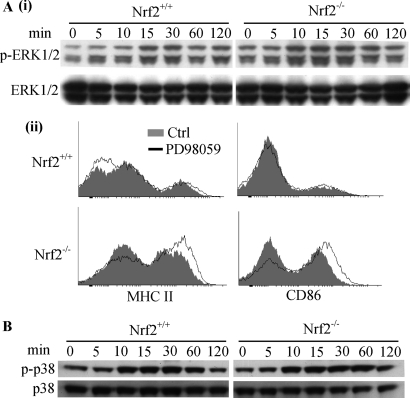
Alterations in ROS levels in DCs are associated with changes in ERK1/2 and p38 MAPK signaling pathways (8). Activation of MAPK pathways by stimuli such as LPS results in phosphorylation of ERK1/2 and p38 MAPK (37). The level of phosphorylated ERK1/2 was slightly higher in Nrf-2−/− iDCs in comparison with Nrf2+/+ iDCs both basally and upon LPS stimulation (Fig. 6A, panel i). Furthermore, Nrf2−/− iDCs exhibited enhanced phosphorylation kinetics of ERK1/2 in response to LPS in comparison with their wild type counterpart. The onset of LPS-induced phosphorylation of ERK1/2 was observed at 10 min in Nrf2−/− when compared with 15 min for Nrf2+/+ iDCs. To investigate whether this change in basal ERK1/2 phosphorylation contributes to the enhanced co-stimulatory phenotype in Nrf2−/− iDCs, we treated Nrf2−/− and Nrf2+/+ iDCs with the ERK inhibitor, PD98059, and assessed its effect on iDC co-stimulatory expression. ERK inhibition did not reverse the enhanced expression of MHC II and CD86 in Nrf2−/− iDCs but rather caused a slight increase (Fig. 6A, panel ii) as reported previously for human DCs (8). This suggests that enhanced co-stimulatory receptor expression in Nrf2−/− iDCs is not dependent on ERK1/2 activity. We next assessed the levels of phospho-p38 in Nrf2−/− and Nrf2+/+ iDC lysates. There were no detectable differences in phospho-p38 between the two cell types (basal or LPS-induced), indicating that p38 MAPK pathway may not be affected by the loss of Nrf2 (Fig. 6B).
FIGURE 6.
Effects of Nrf2 loss on MAPK signaling in DCs. Nrf2+/+ and Nrf2−/− iDCs were treated with LPS (1 μg/ml) for the indicated time points, and whole cell lysates were subjected to SDS-PAGE. A, panel i, phosphorylation of ERK1/2 (p-ERK1/2) assessed by Western blotting. B, phosphorylation of p38 MAPK (p-p38) assessed by Western blotting. Data are representative of three independent experiments. A, panel ii, Nrf2+/+ and Nrf2−/− iDCs were untreated or treated with 50 μm PD98059 (MEK1 inhibitor/ERK inhibitor). Co-stimulatory receptor expression was determined by flow cytometry. Data are representative of three independent experiments.
Enhanced Co-stimulatory Receptor Expression of Nrf2−/− iDCs Is Dependent on Histone Deacetylase Activity
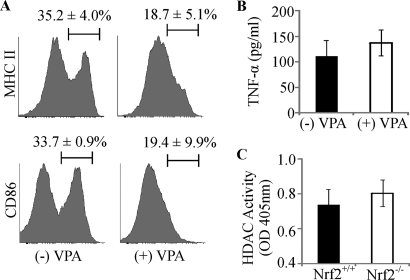
HDAC is involved in regulating DC differentiation, maturation, cytokine production, and immune function (13, 14). To investigate whether enhanced co-stimulatory receptor expression of Nrf2−/− iDCs was dependent on HDAC activity, valproic acid (VPA), an HDAC inhibitor (38), was used. VPA at a concentration of 100 μg/ml reduces iDC HDAC activity by ∼50% (data not shown). As shown in Fig. 7A, VPA caused a reduction in MHC II and CD86 co-stimulatory expression levels in the Nrf2−/− iDCs (MHC II, 35.2 versus 18.7%, p < 0.05; CD86, 33.7 versus 19.4% p < 0.05). In addition to altered co-stimulatory receptor expression, it has been previously shown that secretion of TNF-α is also dysregulated in Nrf2−/− iDCs (21). We therefore were interested in whether TNF-α secretion is subject to modulation by HDAC inhibition in Nrf2−/− iDCs. Untreated Nrf2−/− iDCs secrete measurable quantities of TNF-α. However, inhibition of HDAC with VPA did not significantly alter TNF-α secretion (Fig. 7B). To examine whether the increased co-stimulatory receptor expression is accompanied by changes in HDAC activity, we measured HDAC activity in the nuclear lysates of Nrf2+/+ and Nrf2−/− iDCs. Although there is a marginal increase in HDAC activity in the Nrf2−/− iDCs when compared with Nrf2+/+ iDCs (0.80 ± 0.08 versus 0.74 ± 0.09 respectively), this was not statistically significant (p = 0.08) (Fig. 7C). Despite the absence of a marked increase in the HDAC activity in the Nrf2−/− iDCs, our results suggest that the enhanced co-stimulatory expression of Nrf2−/− iDCs is nevertheless dependent on HDAC activity.
FIGURE 7.
Histone deacetylase function is required for co-stimulatory receptor expression in Nrf2−/− iDCs. A, Nrf2−/− iDCs were treated with the HDAC inhibitor, valproic acid (100 μg/ml) (+VPA), for 72 h and labeled with antibodies against MHC II and CD86. The percentage of DCs expressing high levels of co-stimulatory receptors is indicated above the marker. Representative histograms are presented with average percentage ± S.D. Data were derived from four independent experiments. B, Nrf2−/− iDCs were untreated or treated with VPA for 24 h, supernatants were collected, and levels of TNF-α were measured. Data are presented as average pg/ml ± S.D. and derived from three independent experiments. C, HDAC activity was measured in nuclear lysates from Nrf2+/+ and Nrf2−/− iDCs. Data are presented as average absorbance at 405 nm ± S.D. and derived from three independent experiments. OD, optical density.
DISCUSSION
Our present study investigated the role of Nrf2 in DC immune function and intracellular signaling pathways. Studies have shown that the co-stimulatory molecules CD86 and MHC II are elevated in Nrf2−/− iDCs, and our results support these findings (17, 21). We also found a marked increase in CD40 expression in the Nrf2−/− iDCs, which has not been documented previously. The difference in findings between the two studies could be due to some differences in DC culture methods. Previous studies using Nrf2-deficient mice have shown lowered GSH levels in a variety of cell types, but GSH levels in Nrf2−/− DCs have not been measured (20, 39, 40). Our findings demonstrate that Nrf2−/− iDCs have lowered GSH levels, but this is not sufficient to cause the altered phenotype in Nrf2−/− iDCs. This is consistent with other studies that found that the enhanced co-stimulatory expression Nrf2−/− iDCs could not be reversed by repleting GSH using the GSH precursor N-acetyl cysteine (21). A major function of iDCs is to take up antigens via phagocytic and endocytic processes, critical for initiation of T cell immune response and for induction of T cell tolerance (1–3). As DCs mature, they lose their endocytic and phagocytic capacity, which correlates closely with higher expression of co-stimulatory molecules. Both endocytic and phagocytic functions were impaired in Nrf2−/− iDCs. Defects in endocytic/phagocytic function of DCs are associated with systemic lupus erythematosus and Gaucher disease (41, 42). It is intriguing that aged Nrf2−/− female mice develop systemic lupus erythematosus-like disease. It would therefore be useful to examine Nrf2 function in DCs from systemic lupus erythematosus patients to clarify a role for Nrf2 in DC phagocytosis and pathogenesis of systemic lupus erythematosus.
Generation of functional cytotoxic T lymphocytes from naive CD8 T cells requires co-stimulatory and T cell receptor signals (5–7). CD8 T cells cannot be fully stimulated by immature DCs that normally express low amounts of co-stimulatory molecules (4). The implications of the enhanced co-stimulatory receptor expression by Nrf2−/− iDCs on antigen-specific T cell outcomes have not been explored. In this study, we have made a clear demonstration that Nrf2−/− iDCs presenting the antigenic peptides can induce substantial CD8 T cell proliferation in the absence of any further maturation or co-stimulatory signaling. Additionally, we showed that the CD8 T cells activated by the Nrf2−/− iDCs develop effector functions. Under normal conditions, presentation of self-peptides to T cells by iDCs that express low levels of co-stimulatory molecules leads to the induction of T cell tolerance (31). The potential ability of Nrf2−/− iDCs to stimulate enhanced CD8 T cell activation suggests that these DCs may not be tolerogenic. To test this possibility, we used a partial agonist as a surrogate self-peptide to assess the ability of Nrf2−/− iDCs to stimulate CD8 T cells and discovered that these iDCs can induce CD8 T cell activation. Furthermore, we found lowered CD62L expression in the Nrf2−/− peripheral lymph node T cells, indicating that they have been undergoing low-level activation, presumably through interaction with DCs in the lymph nodes. It has been shown that DCs bearing self-peptides can deliver low-level activatory signals to T cells through the TCR (43). These low-level signals are important for T cell survival in the host animal (44). It is possible that the elevated co-stimulatory receptor expression by the Nrf2−/− iDCs augments these low-level TCR signals and causes a slight but significant activation of interacting T cells in vivo. These findings raise the possibility that Nrf2 could play an important role in the maintenance of peripheral T cell tolerance by iDCs.
Changes in ROS levels in DCs have been implicated in altering DC co-stimulatory expression, maturation, and subsequent immune response elicited (17, 19). Our results suggest that the enhanced maturation phenotype of Nrf2−/− iDCs is associated with increased basal ROS levels. In support of our finding, increases in basal H2O2 levels have been observed in Nrf2-deficient DCs (17). It would be interesting to test whether antioxidants could dampen ROS levels and reverse changes in co-stimulatory phenotype and function of Nrf2−/− iDCs.
NF-κB is involved in DC development, survival, and maturation (9, 11). NF-κB is activated by a variety of stimuli such as LPS and oxidative stress (36). Upon activation, upstream IκB kinase (IKK) complexes are activated, which in turn phosphorylate IκBα, resulting in its ubiquitination and subsequent proteasomal degradation. This facilitates NF-κB translocation into the nucleus, where it can transcribe genes involved in the immune response (36). ROS levels have been shown to both induce and repress NF-κB signaling depending on the cell type (45, 46). Interestingly, our results revealed delayed kinetics of LPS-induced phosphorylation of p65 and IκBα degradation in Nrf2−/− DCs. Elevated ROS levels in Nrf2−/− iDCs could induce modifications in the cysteine residues in upstream kinases, namely IKKβ, resulting in its inactivation and inability to phosphorylate phospho-65 and IκBα (47). However, we did not find any difference in basal NF-κB activation. This is consistent with previous studies in Nrf2-deficient mice whereby no differences in basal NF-κB activation in lung lysates were observed between Nrf2−/− and Nrf2+/+ mice (22, 39). However, it appears that changes to upstream elements of the NF-κB pathway in Nrf2−/− iDCs do not translate to alterations in nuclear NF-κB. Increased intracellular ROS and other stimuli such as LPS also induce activation of MAPKs, resulting in increased DC co-stimulatory expression, cytokine production, and capacity to stimulate T cells in secondary lymphoid organs (12). Although inhibitor studies have implicated ERK and p38 MAPK in CD86 and HLA-DR expression in human DCs under conditions where changes in ROS have been induced by chemical sensitizers, our results show that ERK and p38 MAPK are not responsible for the altered phenotype in the Nrf2-deficient DCs (8). The effect of chronic elevated basal levels of ROS in the intracellular environment of Nrf2-deficient DCs could be different from transient ROS elevations induced by chemical sensitizers.
HDAC has been associated with modulating DC differentiation, co-stimulatory expression, cytokine production, and immunogenicity (13). Our finding that the Nrf2-deficient co-stimulatory phenotype can be reversed by HDAC inhibition places HDACs as potential targets of Nrf2 function. Although the HDAC activity in Nrf2−/− DCs tended to be higher than in the wild type DCs, this difference was not statistically significant. However, it is possible that such small differences could lead to significant biological outcomes. In particular, a marginally higher HDAC function can lead to cumulative effects over time and may drive epigenetic changes, thus altering co-stimulatory molecule expression on DCs. Interestingly, dysregulation of HDAC functions has been associated with diseases such as cancer, chronic obstructive pulmonary disease, and asthma in which the pathology is associated with redox disequilibrium, implicating the link between redox status and HDAC (38, 48). Further investigation into the molecular pathways that lead from Nrf2 activity to ROS through to HDAC is merited. The availability of pharmaceuticals that target HDACs for therapeutic indications opens up the possibility of using such therapeutic strategies to modulate DC functions (14, 49, 50) in diseases that are associated with altered Nrf2 function.
This work was supported by the Wellcome trust and grants from the University Of Liverpool (to H. X. A. Y.) and the Biotechnology and Biological Sciences Research Council-Integrative Mammalian Biology (BBSRC-IMB) award (to J. M. H.), as part of the Drug Safety Centre supported by the Medical Research Council (grant number G0700654).
- DC
- dendritic cell
- iDC
- immature dendritic cell
- Nrf2
- NF-E2 p45-related factor-2
- NF-E2
- nuclear factor-erythroid 2
- HDAC
- histone deacetylase
- ROS
- reactive oxygen species
- NP68
- nucleoprotein 68
- NP34
- nucleoprotein 34
- CFSE
- carboxyfluorescein succinimidyl ester
- VPA
- valproic acid
- BSO
- buthionine sulfoximine
- TCR
- T cell receptor.
REFERENCES
- 1. Steinman R. M. (2003) Some interfaces of dendritic cell biology. Apmis 111, 675–697 [DOI] [PubMed] [Google Scholar]
- 2. Inaba K., Inaba M., Naito M., Steinman R. M. (1993) Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J. Exp. Med. 178, 479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albert M. L., Pearce S. F., Francisco L. M., Sauter B., Roy P., Silverstein R. L., Bhardwaj N. (1998) Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188, 1359–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bachmann M. F., Speiser D. E., Mak T. W., Ohashi P. S. (1999) Absence of co-stimulation and not the intensity of TCR signaling is critical for the induction of T cell unresponsiveness in vivo. Eur. J. Immunol. 29, 2156–2166 [DOI] [PubMed] [Google Scholar]
- 5. Chai J. G., Vendetti S., Bartok I., Schoendorf D., Takacs K., Elliott J., Lechler R., Dyson J. (1999) Critical role of co-stimulation in the activation of naive antigen-specific TCR transgenic CD8+ T cells in vitro. J. Immunol. 163, 1298–1305 [PubMed] [Google Scholar]
- 6. Kemball C. C., Lee E. D., Szomolanyi-Tsuda E., Pearson T. C., Larsen C. P., Lukacher A. E. (2006) Co-stimulation requirements for antiviral CD8+ T cells differ for acute and persistent phases of polyoma virus infection. J. Immunol. 176, 1814–1824 [DOI] [PubMed] [Google Scholar]
- 7. Celluzzi C. M., Falo L. D., Jr. (1998) Physical interaction between dendritic cells and tumor cells results in an immunogen that induces protective and therapeutic tumor rejection. J. Immunol. 160, 3081–3085 [PubMed] [Google Scholar]
- 8. Aiba S., Manome H., Nakagawa S., Mollah Z. U., Mizuashi M., Ohtani T., Yoshino Y., Tagami H. (2003) p38 mitogen-activated protein kinase and extracellular signal-regulated kinases play distinct roles in the activation of dendritic cells by two representative haptens, NiCl2 and 2,4-dinitrochlorobenzene. J. Invest. Dermatol. 120, 390–399 [DOI] [PubMed] [Google Scholar]
- 9. Ouaaz F., Arron J., Zheng Y., Choi Y., Beg A. A. (2002) Dendritic cell development and survival require distinct NF-κB subunits. Immunity 16, 257–270 [DOI] [PubMed] [Google Scholar]
- 10. Kim H. S., Kim J. Y., Ryu H. S., Park H. G., Kim Y. O., Kang J. S., Kim H. M., Hong J. T., Kim Y., Han S. B. (2010) Induction of dendritic cell maturation by β-glucan isolated from Sparassis crispa. Int. Immunopharmacol. 10, 1284–1294 [DOI] [PubMed] [Google Scholar]
- 11. van de Laar L., van den Bosch A., van der Kooij S. W., Janssen H. L., Coffer P. J., van Kooten C., Woltman A. M. (2010) A nonredundant role for canonical NF-κB in human myeloid dendritic cell development and function. J. Immunol. 185, 7252–7261 [DOI] [PubMed] [Google Scholar]
- 12. Nakahara T., Moroi Y., Uchi H., Furue M. (2006) Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J. Dermatol. Sci. 42, 1–11 [DOI] [PubMed] [Google Scholar]
- 13. Nencioni A., Beck J., Werth D., Grünebach F., Patrone F., Ballestrero A., Brossart P. (2007) Histone deacetylase inhibitors affect dendritic cell differentiation and immunogenicity. Clin. Cancer Res. 13, 3933–3941 [DOI] [PubMed] [Google Scholar]
- 14. Reddy P., Sun Y., Toubai T., Duran-Struuck R., Clouthier S. G., Weisiger E., Maeda Y., Tawara I., Krijanovski O., Gatza E., Liu C., Malter C., Mascagni P., Dinarello C. A., Ferrara J. L. (2008) Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J. Clin. Invest. 118, 2562–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dashwood R. H., Ho E. (2007) Dietary histone deacetylase inhibitors: from cells to mice to man. Semin. Cancer Biol. 17, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim H. J., Barajas B., Chan R. C., Nel A. E. (2007) Glutathione depletion inhibits dendritic cell maturation and delayed-type hypersensitivity: implications for systemic disease and immunosenescence. J. Allergy Clin. Immunol. 119, 1225–1233 [DOI] [PubMed] [Google Scholar]
- 17. Williams M. A., Rangasamy T., Bauer S. M., Killedar S., Karp M., Kensler T. W., Yamamoto M., Breysse P., Biswal S., Georas S. N. (2008) Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J. Immunol. 181, 4545–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim H. J., Barajas B., Wang M., Nel A. E. (2008) Nrf2 activation by sulforaphane restores the age-related decrease of TH1 immunity: role of dendritic cells. J. Allergy Clin. Immunol. 121, 1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kantengwa S., Jornot L., Devenoges C., Nicod L. P. (2003) Superoxide anions induce the maturation of human dendritic cells. Am. J. Respir. Crit. Care Med. 167, 431–437 [DOI] [PubMed] [Google Scholar]
- 20. Reddy N. M., Kleeberger S. R., Yamamoto M., Kensler T. W., Scollick C., Biswal S., Reddy S. P. (2007) Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol. Genomics 32, 74–81 [DOI] [PubMed] [Google Scholar]
- 21. Rangasamy T., Williams M. A., Bauer S., Trush M. A., Emo J., Georas S. N., Biswal S. (2010) Nuclear erythroid 2 p45-related factor-2 inhibits the maturation of murine dendritic cells by ragweed extract. Am. J. Respir. Cell Mol. Biol. 43, 276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rangasamy T., Guo J., Mitzner W. A., Roman J., Singh A., Fryer A. D., Yamamoto M., Kensler T. W., Tuder R. M., Georas S. N., Biswal S. (2005) Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 202, 47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313–322 [DOI] [PubMed] [Google Scholar]
- 24. Lutz M. B., Kukutsch N., Ogilvie A. L., Rössner S., Koch F., Romani N., Schuler G. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 [DOI] [PubMed] [Google Scholar]
- 25. Vandeputte C., Guizon I., Genestie-Denis I., Vannier B., Lorenzon G. (1994) A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: performance study of a new miniaturized protocol. Cell Biol. Toxicol 10, 415–421 [DOI] [PubMed] [Google Scholar]
- 26. Johnson K. G., LeRoy F. G., Borysiewicz L. K., Matthews R. J. (1999) TCR signaling thresholds regulating T cell development and activation are dependent upon SHP-1. J. Immunol. 162, 3802–3813 [PubMed] [Google Scholar]
- 27. Bindokas V. P., Jordán J., Lee C. C., Miller R. J. (1996) Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci. 16, 1324–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Griffith O. W., Meister A. (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J. Biol. Chem. 254, 7558–7560 [PubMed] [Google Scholar]
- 29. Mamalaki C., Norton T., Tanaka Y., Townsend A. R., Chandler P., Simpson E., Kioussis D. (1992) Thymic depletion and peripheral activation of class I major histocompatibility complex-restricted T cells by soluble peptide in T cell receptor transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 89, 11342–11346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamann D., Baars P. A., Rep M. H., Hooibrink B., Kerkhof-Garde S. R., Klein M. R., van Lier R. A. (1997) Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186, 1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurts C., Kosaka H., Carbone F. R., Miller J. F., Heath W. R. (1997) Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 186, 239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams O., Tanaka Y., Bix M., Murdjeva M., Littman D. R., Kioussis D. (1996) Inhibition of thymocyte negative selection by T cell receptor antagonist peptides. Eur. J. Immunol. 26, 532–538 [DOI] [PubMed] [Google Scholar]
- 33. Evavold B. D., Sloan-Lancaster J., Allen P. M. (1993) Tickling the TCR: selective T cell functions stimulated by altered peptide ligands. Immunol. Today 14, 602–609 [DOI] [PubMed] [Google Scholar]
- 34. Rigby S., Dailey M. O. (2000) Traffic of L-selectin-negative T cells to sites of inflammation. Eur. J. Immunol. 30, 98–107 [DOI] [PubMed] [Google Scholar]
- 35. Sheng K. C., Pietersz G. A., Tang C. K., Ramsland P. A., Apostolopoulos V. (2010) Reactive oxygen species level defines two functionally distinctive stages of inflammatory dendritic cell development from mouse bone marrow. J. Immunol. 184, 2863–2872 [DOI] [PubMed] [Google Scholar]
- 36. Shih V. F.., Tsui R., Caldwell A., Hoffmann A. (2011) A single NF-κB system for both canonical and non-canonical signaling. Cell Res 21, 86–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCubrey J. A., Lahair M. M., Franklin R. A. (2006) Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 8, 1775–1789 [DOI] [PubMed] [Google Scholar]
- 38. Minucci S., Pelicci P. G. (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer 6, 38–51 [DOI] [PubMed] [Google Scholar]
- 39. Thimmulappa R. K., Lee H., Rangasamy T., Reddy S. P., Yamamoto M., Kensler T. W., Biswal S. (2006) Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 116, 984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan K., Han X. D., Kan Y. W. (2001) An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc. Natl. Acad. Sci. U.S.A. 98, 4611–4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Monrad S. U., Rea K., Thacker S., Kaplan M.. J. (2008) Myeloid dendritic cells display down-regulation of C-type lectin receptors and aberrant lectin uptake in systemic lupus erythematosus. Arthritis Res Ther. 10, R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Micheva I., Marinakis T., Repa C., Kouraklis-Symeonidis A., Vlacha V., Anagnostopoulos N., Zoumbos N., Symeonidis A. (2006) Dendritic cells in patients with type I Gaucher disease are decreased in number but functionally normal. Blood Cells Mol. Dis. 36, 298–307 [DOI] [PubMed] [Google Scholar]
- 43. Delon J., Bercovici N., Raposo G., Liblau R., Trautmann A. (1998) Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J. Exp. Med. 188, 1473–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goldrath A. W., Bevan M. J. (1999) Selecting and maintaining a diverse T cell repertoire. Nature 402, 255–262 [DOI] [PubMed] [Google Scholar]
- 45. Morgan M. J., Liu Z. G. (2011) Cross-talk of reactive oxygen species and NF-κB signaling. Cell Res. 21, 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zmijewski J. W., Zhao X., Xu Z., Abraham E. (2007) Exposure to hydrogen peroxide diminishes NF-κB activation, IκB-α degradation, and proteasome activity in neutrophils. Am. J. Physiol. Cell Physiol. 293, C255–C266 [DOI] [PubMed] [Google Scholar]
- 47. Reynaert N. L., van der Vliet A., Guala A. S., McGovern T., Hristova M., Pantano C., Heintz N. H., Heim J., Ho Y. S., Matthews D. E., Wouters E. F., Janssen-Heininger Y. M. (2006) Dynamic redox control of NF-κB through glutaredoxin-regulated S-glutathionylation of inhibitory κB kinase β. Proc. Natl. Acad. Sci. U.S.A. 103, 13086–13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chung K. F., Marwick J. A. (2010) Molecular mechanisms of oxidative stress in airways and lungs with reference to asthma and chronic obstructive pulmonary disease. Ann. N. Y. Acad. Sci. 1203, 85–91 [DOI] [PubMed] [Google Scholar]
- 49. Faraco G., Cavone L., Chiarugi A. (2011) The therapeutic potential of HDAC inhibitors in the treatment of multiple sclerosis. Mol. Med. 17, 442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vojinovic J., Damjanov N. (2011) HDAC inhibition in rheumatoid arthritis and juvenile idiopathic arthritis. Mol. Med. 17, 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]