Background: PSGL-1 amino acid sequence that binds ERMs may be involved in signaling and rolling.
Results: Deletion or mutation of PSGL-1 ERM-binding sequence severely reduced leukocyte capture by selectins and abrogated PSGL-1 signaling through ERK but did not affect Syk activation.
Conclusion: ERM-binding sequence regulates ERK activation and leukocyte recruitment by selectins.
Significance: Investigating leukocyte activation and recruitment is critical in understanding their trafficking.
Keywords: Adhesion, Cell Signaling, ERK, Glycoprotein, Leukocyte
Abstract
P-selectin glycoprotein ligand-1 (PSGL-1) mediates the capture (tethering) of free-flowing leukocytes and subsequent rolling on selectins. PSGL-1 interactions with endothelial selectins activate Src kinases and spleen tyrosine kinase (Syk), leading to αLβ2 integrin-dependent leukocyte slow rolling, which promotes leukocyte recruitment into tissues. In addition, but through a distinct pathway, PSGL-1 engagement activates ERK. Because ezrin, radixin and moesin proteins (ERMs) link PSGL-1 to actin cytoskeleton and because they serve as adaptor molecules between PSGL-1 and Syk, we examined the role of PSGL-1 ERM-binding sequence (EBS) on cell capture, rolling, and signaling through Syk and MAPK pathways. We carried out mutational analysis and observed that deletion of EBS severely reduced 32D leukocyte tethering and rolling on L-, P-, and E-selectin and slightly increased rolling velocity. Alanine substitution of Arg-337 and Lys-338 showed that these residues play a key role in supporting leukocyte tethering and rolling on selectins. Importantly, EBS deletion or Arg-337 and Lys-338 mutations abrogated PSGL-1-induced ERK activation, whereas they did not prevent Syk phosphorylation or E-selectin-induced leukocyte slow rolling. These studies demonstrate that PSGL-1 EBS plays a critical role in recruiting leukocytes on selectins and in activating the MAPK pathway, whereas it is dispensable to phosphorylate Syk and to lead to αLβ2-dependent leukocyte slow rolling.
Introduction
Leukocyte migration into inflammatory lesions is tightly regulated by adhesion receptors and mediators of inflammation. Selectins play a critical role in initiating the leukocyte adhesion cascade by mediating leukocyte tethering and rolling along inflamed vascular endothelium or on adherent platelets/leukocytes (1). P-selectin glycoprotein ligand 1 (PSGL-1) is expressed by most leukocytes and functions as a common ligand for the three selectins (2). Early in inflammation, PSGL-1 interacts with P-selectin on activated platelets/endothelium (3) and with l-selectin on adherent leukocytes (4, 5), leading to the capture of free-flowing leukocytes. PSGL-1 also cooperates with CD44 and E-selectin ligand-1 (ESL-1) to support leukocyte rolling on endothelial E-selectin (6, 7). During leukocyte rolling on P- and/or E-selectin, PSGL-1 engagement induces intracellular signals that activate αLβ2leukocyte integrin, which then adopts an extended conformation, allowing slow rolling (8–11). In mice, slow rolling is dependent on the phosphorylation of Src kinases Fgr, Hck, and Lyn, which activate the ITAM2-containing adaptors DAP12 and FcRγ (9, 12). During rolling, PSGL-1 engagement by selectins induces the recruitment of the spleen tyrosine kinase (Syk) into lipid rafts (13). Syk associates with activated DAP12 and FcRγ (9, 12), leading to the successive phosphorylation of Bruton's tyrosine kinase (Btk), a member of the Tec kinases, phospholipase Cγ2, and p38 MAPK (10, 12, 14). Finally, p38 MAPK activates CalDAG-GEFI and Rap1 (Ras-proximate-1), which primes αLβ2 leukocyte integrin for slow rolling on intercellular adhesion molecule-1 (ICAM-1) (14).
The role of Src family kinases (SFKs) DAP12/FcRγ in Syk activation has been well established (9, 12), but PSGL-1 amino acid residues involved in this reaction as well as in ERK activation (15) have not been identified. Biochemical studies previously showed that the adaptor proteins ezrin, radixin, and moesin (ERMs) can bind to PSGL-1 cytoplasmic domain and to Syk through their ITAM-like motif (16, 17). Tyrosine phosphorylation of Syk and SRE-dependent transcriptional activity was observed following PSGL-1 engagement (16). However, whether PSGL-1 ERM-binding sequence (EBS) controls Syk activation has not yet been examined. In addition, it is not known whether EBS is specifically involved in controlling leukocyte tethering and rolling. ERMs link the actin cytoskeleton to several membrane glycoproteins, including CD43, CD44, and PSGL-1 (17–20), and contribute to maintain the structure of microvilli (21). Earlier studies showed that pharmacologic inhibition of actin polymerization in cells expressing PSGL-1 inhibits rolling on P-selectin (18). These data indicate that PSGL-1 anchorage to cytoskeleton is essential for leukocyte recruitment on P-selectin (18). Because the stability of leukocyte rolling is dependent on the formation of bonds to ligand(s) and on the ability of microvilli to deform and generate tethers (22–25), we hypothesized that altered interactions of PSGL-1 EBS with ERMs and actin may affect leukocyte recruitment and stability of cell displacements.
PSGL-1 amino acids that interact with ERMs have been revealed by crystal structure analysis and biochemical studies, which showed the involvement of three highly evolutionarily conserved amino acids: Ser-336, Arg-337, and Lys-338 (17, 26, 27). In preliminary experiments performed with CHO cells, we observed that alanine substitution of these residues strongly impaired cell recruitment on P-selectin. More detailed studies were then performed with 32D murine leukocytes to examine the involvement of the EBS in leukocyte tethering, rolling, and signaling. Deletion or mutation of EBS strongly reduced leukocyte capture and recruitment on selectins. Syk phosphorylation and αLβ2-dependent leukocyte slow rolling on E-selectin plus ICAM-1 have not been affected either by deletion of EBS or by alanine substitution of Ser-336, Arg-337, and Lys-338, indicating that they are not required to activate Syk. Because PSGL-1 engagement also activates MAPK pathway (15), we examined whether ERK activation was affected by EBS mutations. Indeed, ERK phosphorylation was abolished, whereas Syk activation was not affected, revealing a crucial role for PSGL-1 EBS in activating the MAPK pathway.
EXPERIMENTAL PROCEDURES
Antibodies, Chimeric Selectins, and Reagents
mAbs CSLEX-1 (anti-sialyl Lewis x (sLex), ATCC HB-10135), anti-PSGL-1 mAbs PS5 and PS4, LAM1-3, and WAPS12.2 were purified from hybridoma culture medium (28). Isotypic mouse IgG1 control mAb, goat anti-mouse Ig-PE F(ab′)2, FITC-labeled rabbit anti-human IgM, and goat anti-mouse Ig were purchased from Dako. KPL1 was from BD Pharmingen. Antibodies reacting with Syk were from Santa Cruz Biotechnology, Inc. and Upstate; antibodies against phospho-Syk, ERK, and phospho-ERK were from Cell Signaling. l-Selectin/IgM heavy chain (l-selectin/μ) and P- and E-selectin/μ chimeras were produced in CHOdhFr− cells and used for immunostaining or cell rolling assays as described (2, 28–30). Recombinant human P-selectin and recombinant mouse ICAM-1/Fc chimera were purchased from R&D Systems. PD98059, methyl-β-cyclodextrin (mβCD), and peroxidase-conjugated cholera toxin B subunit were from Sigma-Aldrich, and PP2 and PP3 were from Calbiochem.
cDNA Constructs and Transfectants
PSGL-1 S336A/R337A/K338A (AAA) and PSGL-1 R337A/K338A (SAA) mutant cDNAs (Fig. 1A) were obtained using the QuikChange site-directed mutagenesis kit (Stratagene), according to the manufacturer's instructions. Sequences of the forward and reverse primers are listed in Table 1. Each primer pair was annealed at 55 °C. PSGL-1 deletion mutant devoid of EBS (ΔEBS) cDNA was obtained using overlapping primers devoid of the EBS. We amplified the region upstream to EBS with P1 fw and ΔEBS rev and the region downstream with ΔEBS fw and P1 rev (annealing temperature of 54 °C for 10 cycles, followed by 20 cycles at 60 °C). Both PCR products were then used as template for 40 PCR cycles using P1 fw and P1 rev primers to amplify ΔEBS coding sequence. All constructs were verified by DNA sequencing.
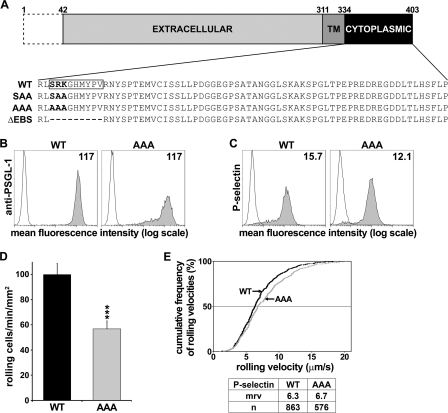
FIGURE 1.
SRK mutations inhibit CHO-PSGL-1 transfectant rolling on P-selectin but do not affect P-selectin/μ chimera binding and cell rolling velocity. A, schematic representation of WT and mutant PSGL-1 used in this study. The dashed box represents the signal peptide and propeptide. TM, transmembrane domain. The EBS is boxed on WT PSGL1. Residues are numbered according to the PSGL-1 amino acid sequence reported by Sako et al. (42). B and C, flow cytometric histograms of CHO transfectants showing matched expression levels of WT PSGL-1 and AAA mutant detected by anti-PSGL-1 mAb PS5 (B) and P-selectin/μ chimera binding (gray histograms) (C). White histograms indicate immunostaining with isotype-matched control mAb (B) or complete inhibition of staining with 10 mm EDTA (C). Mean fluorescence intensity is indicated in each histogram. D, CHO cells expressing matched levels of WT PSGL-1 or AAA mutant were perfused at 1.5 dynes/cm2 on recombinant P-selectin. Cell recruitment was assessed by video microscopy, and results represent the mean ± S.E. (error bars) of 11 experiments. E, cumulative rolling velocity of CHO transfectants on P-selectin. The number of analyzed rolling cells (n) and the mrv are indicated in the bottom table (***, p < 0.001 compared with WT).
TABLE 1.
Sequences of primers used to generate PSGL-1 cytoplasmic mutants
| Primer | Sequence |
|---|---|
| SAA fw | 5′-CGGTCCGCCTCTCCGCCGCGGGCCACATGTACC-3′ |
| SAA rev | 5′-GGTACATGTGGCCCGCGGCGGAGAGGCGGACCG-3′ |
| AAA fw | 5′-CGGTCCGCCTCGCCGCCGCGGGCCACATGTACC-3′ |
| AAA rev | 5′-GGTACATGTGGCCCGCGGCGGCGAGGCGGACCG-3′ |
| P1 fw | 5′-AGCGGATCCCCACCATGCCTCTGCAACTCC-3′ |
| P1 rev | 5′-GCGCTCGAGCTAAGGGAGGAAGCTGTGC-3′ |
| ΔEBS fw | 5′-GCGGTCCGCCTCCGTAATTACTCCCCCACC-3′ |
| ΔEBS rev | 5′-GGAGTAATTACGGAGGCGGACCGCCAGCAC-3′ |
WT and mutant forms of human PSGL-1 cDNA sequences were inserted into pcDNA3 vector (Invitrogen) and stably expressed in CHO cells (ATCC catalog no. CRL 9096) coexpressing core-2 β1,6-N-acetylglucosaminyltransferase and fucosyltransferase-VII cDNA sequences (28, 31). 32D leukocytes (DSMZ ACC 411) were nucleofected (Nucleofector, Lonza AG) with WT or mutant PSGL-1 cDNAs. Stable transfectants were selected by limiting dilution for matched PSGL-1, sLex, and Syk expression (28) as assessed by flow cytometry. In order to fuse GFP to the C terminus of Syk protein, human Syk cDNA sequence was cloned into pmaxFP-Green-N vector (Lonza AG) in which the neomycin resistance had been replaced by the blasticidin resistance from pcDNA6/TR (Invitrogen). This construct was stably nucleofected in 32D cells expressing human WT or mutant PSGL-1.
Cell Rolling Assays
Adhesion assays were performed under flow conditions, as described (28). Briefly, cells were perfused under constant shear stress in a parallel plate flow chamber (GlycoTech Corp., Rockville, MD) mounted on a glass coverslip coated with optimal concentration of selectins (28, 30). Cell displacements were recorded by video microscopy and analyzed using a digital image analysis system (Mikado software (GPL SA, Martigny, Switzerland) or Imaris (Bitplane Scientific Software)) (28–30). Cell displacements shown in Fig. 4A were measured every 40 ms for at least 2 s (28, 30). Analyses of tethering were performed on subsaturating concentrations of selectins (32). Cells were perfused at 1.5 dynes/cm2 on L- or P-selectin and 1.0 dyne/cm2 on E-selectin, and transient tethering was defined as cells that rolled between 0.2 and 1.0 s at velocities of <200 μm/s on l-selectin or <60 μm/s on P-selectin or E-selectin/μ chimera. Adhesion assays comparing leukocyte rolling velocities on E-selectin and E-selectin plus ICAM-1 were performed at 1.0 dyne/cm2. Transfectants were perfused into the flow chamber on glass coverslips coated with E-selectin/μ (0.4 μg/ml) or E-selectin/μ plus ICAM-1/Ig (3.0 μg/ml).
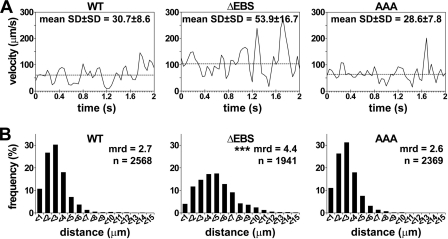
FIGURE 4.
ERMs stabilize PSGL-1-dependent rolling on L-selectin. A, illustration of 1 of 20 representative 32D cells expressing WT PSGL-1 or ΔEBS or AAA mutants tracked each 40 ms for 2 s; mrv is indicated by a dashed line. B, distribution of distances traveled by 32D transfectants during successive 40 ms time lapses (mrd, mean rolling distance; ***, p < 0.001 compared with WT).
Cell Activation and Western Blot
32D cells (1 × 106) stably expressing matched densities of WT or mutant PSGL-1 were incubated for various time periods at 37 °C with anti-PSGL-1 mAb PS4 and goat anti-mouse antibody (10 μg/ml). When indicated, cells were treated with MAP kinase kinase (MEK) inhibitor PD98059 (40 μm) for 15 min at 37 °C. Cells were then lysed in ice-cold 20 mm Tris-HCl buffer, pH 7.5, containing 1% Triton and protease/phosphatase inhibitors. After centrifugation, supernatants were boiled in buffer containing 5% β-2-mercaptoethanol, subjected to 8.5% SDS-PAGE, and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked for 1 h at room temperature in 10 mm TBST buffer, pH 7.4, containing 5% nonfat dry milk, washed, and incubated with the indicated primary antibodies overnight at 4 °C. Antibody binding was revealed with horseradish peroxidase (HRP)-conjugated secondary antibody and enhanced chemiluminescence (ECL Plus, Amersham Biosciences). Densitometric analyses were performed using an Amersham Biosciences Image Scanner with LabScan ImageQuant TL software. Phospho-ERK or phospho-Syk expression ratios were calculated as activated samples relative to unactivated samples after normalization to respective ERK or Syk proteins.
Syk Immunoprecipitation
32D cells (5 × 106) were activated by PSGL-1 cross-linking, lysed, and centrifuged as described above. Supernatants were incubated for 3 h with 1 μg of anti-Syk antibody and 7 μl of protein G-agarose (GE Healthcare) under constant rotation at 4 °C. Beads were then washed and analyzed by SDS-PAGE and Western blotting with anti-phospho-Syk antibody followed by HRP-labeled secondary antibody and reblotted with anti-Syk antibody. When indicated, leukocytes were incubated with the SFK inhibitor PP2 (30 μm) or its inactive analog PP3 (30 μm) for 15 min at 37 °C before PSGL-1 cross-linking. Lipid rafts were disrupted by cell exposure to 12 mm mβCD for 30 min at 37 °C.
Lipid Raft Isolation and Cholera Toxin Assay
Detergent-resistant membrane fractions were isolated as described (13). CHO cells (14 × 106) were lysed in 25 mm Tris-HCl, pH 7.6, containing 0.5% Brij 58 and centrifuged for 18 h at 2 × 105 × g at 4 °C on discontinuous sucrose gradients. Fractions were collected and analyzed by immunoblotting. HRP-conjugated cholera toxin binding to lipid raft fractions was detected by chemiluminescence (13).
Statistical Analysis
Differences between groups were disclosed using Mann-Whitney test or Kruskal-Wallis nonparametric analysis of variance followed by Dunn's multiple comparisons test. p values of <0.05 were considered significant.
RESULTS
Previous biochemical studies performed with fusion proteins of various mutant forms of PSGL-1 cytoplasmic domain (17) and crystal structure analysis (26) indicated that the evolutionarily conserved (27) residues Ser-336, Arg-337, and Lys-338 are involved in ERM protein binding. To further investigate the role of the EBS in cell rolling on selectins, we performed preliminary experiments with CHO cells expressing a mutant form of PSGL-1, in which Ser-336, Arg-337, and Lys-338 (SRK) were substituted with alanines (PSGL-1 S336A/R337A/K338A; AAA mutant) (Fig. 1A). We compared the ability of CHO cells expressing WT PSGL-1 or AAA mutant to bind to and roll on P-selectin.
Additional experiments were then performed with 32D leukocytes expressing 1) ΔEBS PSGL-1, devoid of Ser-336 to Val-344 residues forming the EBS or 2) AAA mutant or SAA mutant, the latter exhibiting alanine substitution of Arg-337 and Lys-338 (Figs. 1A and 2). We confirmed with 32D transfectants the involvement of EBS in leukocyte recruitment on P-selectin and extended our observations to L- and E-selectin. Using this leukocyte cell line, which was more appropriate than CHO cells for signal transduction studies, we examined whether EBS deletion or mutations 1) decrease leukocyte tethering and rolling on L-, P-, and E-selectin, 2) reduce rolling stability on L-selectin, 3) prevent slow rolling on E-selectin plus ICAM-1, or 4) impair PSGL-1-induced Syk or ERK activation.
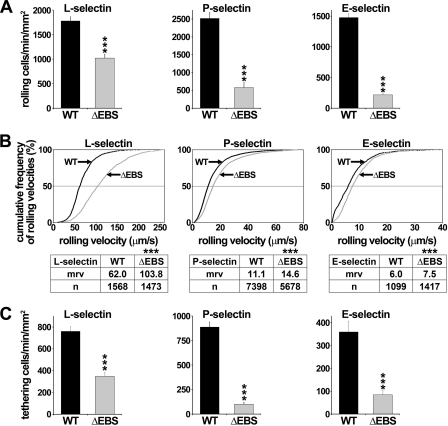
FIGURE 2.
EBS deletion or mutations do not impair L- or P-selectin binding to 32D leukocytes. Flow cytometric histograms showing that 32D transfectants express matched levels of PSGL-1 (A) and are bound by l-selectin/μ (B) and P-selectin/μ chimera (C) similarly (gray histograms). Immunostaining with isotype-matched control mAb or inhibition of selectin binding with 10 mm EDTA is indicated by white histograms. The figure illustrates one of three representative experiments. Mean fluorescence intensity is indicated in each histogram.
Mutation of SRK Amino Acid Residues of EBS Does Not Affect PSGL-1 Binding to P-selectin/μ Chimera
As a prerequisite for flow adhesion assays, we analyzed P-selectin/μ chimera binding to CHO cells expressing WT PSGL-1 or AAA mutant to ensure that EBS mutations did not affect selectin binding to PSGL-1 N terminus (31). Flow cytometric analysis showed that equivalent levels of P-selectin bound to WT PSGL-1 or AAA mutant (Fig. 1C), which is consistent with unmodified P-selectin binding activity.
SRK Mutations Impair CHO Cell Recruitment on P-selectin without Affecting Cell Rolling Velocity
CHO cells expressing matched surface levels of WT PSGL-1 or AAA mutant (Fig. 1B) were compared side-by-side in rolling assays to assess cell recruitment and rolling velocities under shear stress conditions that mimic leukocyte-endothelial interactions in postcapillary venules. CHO cells expressing WT PSGL-1 exhibited efficient tethering and rolling between 1.0 and 2.0 dynes/cm2. The specificity of PSGL-1 interactions with P-selectin was demonstrated by the abrogation of cell recruitment in the presence of blocking mAbs KPL1 or WAPS12.2, directed against PSGL-1 or P-selectin, respectively. Mock-transfected cells did not interact with P-selectin, and selectin-mediated rolling was abolished by EDTA (data not shown).
Ala substitution of Ser-336, Arg-337, and Lys-338 residues in AAA mutant inhibited CHO cell recruitment on P-selectin by 45% (Fig. 1D, p < 0.001). However, the median rolling velocity (mrv) was not affected (6.3 versus 6.7 μm/s for WT PSGL-1 versus AAA mutant, respectively; Fig. 1E). The lack of significant effect of SRK mutations on rolling velocity is consistent with the hypothesis that velocity is mainly determined by receptor off-rate (33, 34), which here depends on the interactions of PSGL-1 extracellular domain with P-selectin. Cell capture and subsequent cell recruitment on selectins is mainly dependent on microvillar distribution of the receptor (34, 35) or on its ability to bind ERMs and actin (35). Because PSGL-1 positioning on leukocyte microvilli is not dependent on its cytoplasmic domain (11), we performed supplementary experiments with 32D leukocytes to determine whether deletion or mutations in the EBS interfere with leukocyte tethering and rolling on L-, P-, or E-selectin.
EBS Deletion Impairs Leukocyte Capture and Subsequent Rolling on Selectins
To further examine EBS function, ΔEBS cDNA was stably expressed in 32D leukocytes. Transfectants with matched expression of WT PSGL-1 or ΔEBS mutant (Figs. 1A and 2A) bound equivalent levels of L- or P-selectin/μ chimera (Fig. 2, B and C). Compared with WT PSGL1 transfectants, the recruitment of leukocytes expressing ΔEBS mutant was reduced by 43% on L-selectin, 77% on P-selectin, and 85% on E-selectin (Fig. 3A; p < 0.001), and the mrv increased by 1.7-fold on L-selectin and by 1.3-fold on P- and E-selectin (Fig. 3B; p < 0.001).
FIGURE 3.
EBS deletion impairs 32D leukocyte rolling on selectins and increases rolling velocity. Transfectants (0.5 × 106/ml) were perfused at 1.5 dynes/cm2 on L- or P-selectin/μ chimera and at 1.0 dyn/cm2 on E-selectin/μ chimera. A, leukocyte recruitment; B, cumulative rolling velocity. The number of analyzed rolling cells (n) and mrv are indicated in the tables. C, tethering of transfectants expressing WT PSGL-1 or ΔEBS mutant. Results represent the mean ± S.E. (error bars) of at least three independent experiments. ***, p < 0.001 compared with WT.
Because a decrease in leukocyte capture (tethering) by selectins results in reduced cell recruitment, we analyzed the tethering efficiency of 32D leukocytes expressing WT or ΔEBS PSGL-1. EBS deletion decreased tethering efficiency by 54% on L-selectin, 89% on P-selectin, and 77% on E-selectin (Fig. 3C; p < 0.001), consistent with the hypothesis that PSGL-1 interactions with ERMs and actin play a key role in leukocyte capture by selectins.
EBS Deletion Impairs Leukocyte Rolling Stability
Because EBS deletion increased leukocyte rolling velocity on l-selectin (Fig. 3B), we analyzed cell displacements on l-selectin to determine whether it also affected rolling stability. Leukocytes were tracked within successive video frames (40 ms) for 2 s (Fig. 4A); peaks correspond to acceleration, and valleys correspond to deceleration (28). Dashed lines indicate mrv, which was included within percentiles 40 and 60 of the velocity curves illustrated on Fig. 3B. The stability of rolling velocity is indicated by the mean S.D. ± S.D. of tracked cell velocities (28). Cell displacements are the most stable, with low peaks and shallow valleys, for WT transfectants, which displayed a mean stability of rolling velocity on L-selectin of 30.7 ± 8.6 (Fig. 4A, left). By contrast, cells expressing ΔEBS mutant rolled faster and significantly less stably (mean S.D. ± S.D., 53.9 ± 16.7; p < 0.001; Fig. 4A, middle).
The distribution of distances traveled by all examined 32D leukocytes illustrates how unstable displacements are correlated with longer rolling distances (Fig. 4B). Indeed, 86% of WT PSGL-1 cells traveled less than 4 μm on L-selectin within 40-ms periods, whereas only 48% of cells expressing ΔEBS mutant did. These results indicated that PSGL-1 EBS contributes to stabilize leukocyte rolling.
SRK or RK Mutations Impair Leukocyte Recruitment on Selectins
Additional flow adhesion assays were performed to determine whether SRK mutations 1) affect 32D leukocyte recruitment and 2) mimic the effects of EBS deletion. The recruitment of transfectants expressing AAA or SAA mutants was strongly impaired on L-selectin (AAA or SAA: 29 or 40% inhibition, respectively; p < 0.001, AAA or SAA compared with WT), P-selectin (58 or 47% inhibition; p < 0.001), and E-selectin (77 or 58% inhibition; p < 0.001). A key role was observed for Arg-337 and Lys-338, whose substitution by Ala inhibited cell recruitment on L-, P-, and E-selectin to a similar extent as SRK substitution (Fig. 5A). However, compared with transfectants expressing ΔEBS PSGL-1, the recruitment on L-, P-, or E-selectin of AAA or SAA transfectants was less affected (p < 0.01), suggesting that SRK or RK mutations are not as efficient as EBS deletion at inhibiting selectin-dependent rolling.
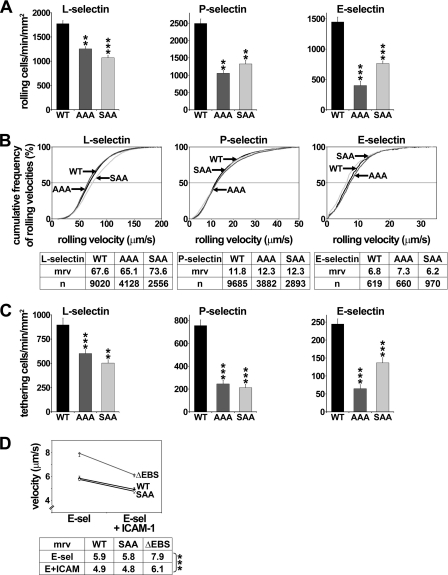
FIGURE 5.
SRK or RK mutations inhibit leukocyte recruitment and tethering on L-, P-, and E-selectin but do not affect slow rolling on E-selectin plus ICAM-1. A, 32D cell recruitment; B, cumulative rolling velocity; C, tethering on L-, P-, and E-selectin. Transfectants expressing matched levels of WT PSGL-1 or SAA or AAA mutants (0.5–1 × 106 cells/ml) were perfused under constant shear stress on selectins. Results represent the mean ± S.E. (error bars) of 3–9 independent experiments (**, p < 0.01; ***, p < 0.001, compared with WT PSGL-1). D, mrv of 32D leukocytes expressing WT PSGL-1 or ΔEBS or SAA mutants on E-selectin or E-selectin plus ICAM-1 (***, p < 0.001, compared with mrv on E-selectin alone). Results represent the mean ± S.E. of at least three independent experiments.
As observed in preliminary experiments performed with CHO cells on P-selectin, neither SRK nor RK mutations significantly affected leukocyte rolling velocity of 32D cells on the three selectins (Fig. 5B). Finally, in contrast with results obtained with ΔEBS mutant, SRK mutations did not affect leukocyte rolling stability (mean S.D. ± S.D. of rolling velocities; WT versus AAA, 30.7 ± 8.6 versus 28.6 ± 7.8; Fig. 4A), indicating that additional residues within the EBS may play a role in stabilizing leukocyte rolling. Moreover, rolling distances of AAA transfectants were not significantly different from those traveled by WT PSGL-1 cells (Fig. 4B, right).
Further analyses were performed to determine whether SRK or RK mutations impair leukocyte capture by L-, P-, or E-selectin. Compared with 32D cells expressing WT PSGL-1, the tethering efficiency of transfectants expressing AAA or SAA mutant was diminished by 33 or 44% on L-selectin (p < 0.001), 68 or 72% on P-selectin (p < 0.001), and 72 or 47% on E-selectin, respectively (p < 0.001) (Fig. 5C). All together, these data indicate that SRK residues of the EBS play a key role in supporting leukocyte capture by all three selectins and that the decrease in the recruitment of SAA or AAA transfectants results mainly from diminished tethering efficiency.
EBS Deletion or Mutations Do Not Impair Integrin-dependent Slow Rolling
Several studies showed that leukocyte rolling on E-selectin induces αLβ2 integrin-mediated slow rolling on ICAM-1, which is dependent on SFK and Syk activation (9, 10, 12, 14). Because ERMs can act as adaptor molecules between Syk and PSGL-1 (16), we examined whether EBS deletion or mutations prevent E-selectin-induced slow rolling on ICAM-1. Flow adhesion assays performed with 32D leukocytes expressing WT PSGL-1 or ΔEBS or SAA mutants revealed a significant decrease in rolling velocities of all transfectants on E-selectin plus ICAM-1, compared with E-selectin alone (p < 0.001; Fig. 5D). As expected (9, 12), pretreatment of transfectants with SFK inhibitor PP2 prevented αLβ2 integrin-dependent slow rolling, whereas its inactive analog PP3 or MEK inhibitor PD98059 did not (data not shown). These results indicate that ERM protein binding to PSGL-1 is not required to activate αLβ2 integrin and induce slow rolling on ICAM-1.
PSGL-1 EBS Is Involved in ERK Phosphorylation but Is Dispensable for Syk Activation
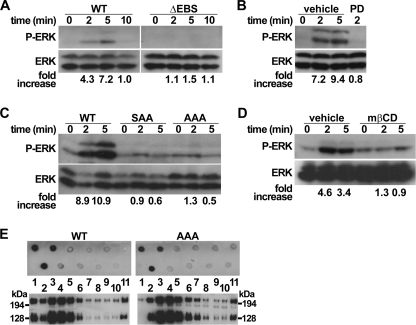
Several studies indicated that PSGL-1 engagement by mAbs or by P- or E-selectin induces ERK phosphorylation (15), SFK, and Syk activation (9, 10, 12). Because PSGL-1 amino acid residues involved in ERK activation are not known, we examined if the EBS is implicated. Experiments were performed with 32D leukocytes stably expressing matched levels of WT or mutant PSGL-1. Cross-linking of WT PSGL-1 with mAb induced a rapid and transient activation of ERK, which peaked between 2 and 5 min, followed by its dephosphorylation after 10 min (Fig. 6A). The dependence of ERK activation on MEK activity was confirmed by its abrogation after pretreatment with MEK inhibitor PD98059 (Fig. 6B). Moreover, crucial involvement of the EBS in MAPK pathway activation was indicated by the absence of ERK phosphorylation in 32D leukocytes expressing ΔEBS mutant (Fig. 6A). A key role was disclosed for Arg-337 and Lys-338 because alanine substitution of these residues was sufficient to abrogate ERK phosphorylation induced by PSGL-1 engagement (Fig. 6C). These results reveal for the first time the role of PSGL-1 EBS in ERK activation. In addition, to determine whether leukocyte interactions with selectins are dependent on ERK activity, we prevented its activation by pharmacologic inhibition of MEK. Neither leukocyte recruitment nor leukocyte velocity was impaired by 32D leukocyte pretreatment with PD98059, suggesting that ERK does not regulate leukocyte rolling (data not shown).
FIGURE 6.
PSGL-1 signaling on ERK is dependent on the EBS and on lipid raft integrity. A–C, 32D leukocytes expressing WT PSGL-1 or ΔEBM, SAA, or AAA mutants were incubated with anti-PSGL-1 mAb at 37 °C for the indicated times and then lysed. Equivalent amounts of lysates were analyzed by Western blotting with antibody directed against phospho-ERK (P-ERK) followed by ERK antibody. When indicated, transfectants expressing WT PSGL-1 were pretreated with vehicle or MEK inhibitor PD98059. D, THP1 cells exposed to mβCD or vehicle were incubated and immunoblotted as indicated in A–C. E, detergent-insoluble fractions of CHO cells expressing WT or AAA PSGL-1 were isolated by centrifugation on a sucrose gradient and analyzed by immunoblotting with anti-PSGL-1 PS5 mAb (bottom panels). Phospho-ERK or phospho-Syk expression ratios were calculated as described under “Experimental Procedures.” Lipid raft-containing fractions were identified with HRP-labeled cholera toxin (top panels). Data are representative of at least three independent experiments.
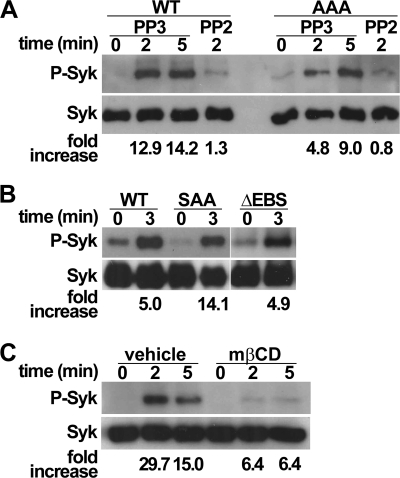
Previous reports indicated that interaction of ERMs with Syk mediates signaling by PSGL-1 (16). However, in vivo studies performed later with gene-deficient mice demonstrated that Syk activation was dependent on PSGL-1 signaling through SFKs and ITAM adaptors DAP12 and FcRγ (9, 12, 14). Thus, whether ERMs play a role in activating Syk has remained controversial for several years (36). The potential role of EBS in Syk activation was examined using ΔEBS, AAA, or SAA transfectants. Cross-linking of WT or AAA PSGL-1 induced a rapid phosphorylation of Syk, which was, as expected (9, 12, 14), abrogated by leukocyte pretreatment with the SFK inhibitor PP2 but not by its inactive control PP3 (Fig. 7A). Although ERK phosphorylation was abolished in leukocytes expressing ΔEBS, AAA, or SAA mutants, Syk activation was not affected (Fig. 7, A and B), indicating that it is not dependent on ERM binding to PSGL-1.
FIGURE 7.
PSGL-1 binding to ERMs is not required to activate Syk. A and B, 32D leukocytes expressing WT PSGL-1, ΔEBS, SAA, or AAA mutants were incubated with anti-PSGL-1 mAb at 37 °C for the indicated times and then lysed. Equivalent amounts of lysates were immunoblotted with antibody directed against phospho-Syk (P-Syk) and then Syk. When indicated, leukocytes were incubated with the SFK inhibitor PP2 or its inactive analog PP3 before PSGL-1 cross-linking. Data are representative of 3–6 independent experiments. C, THP1 cells exposed to mβCD or vehicle were incubated and immunoblotted as indicated in A and B. Data are representative of at least three independent experiments.
PSGL-1-induced Activation of ERK and Syk Is Dependent on Lipid Rafts
Lipid raft disruption, induced by the exposure of THP1 cells to mβCD (13), abrogated ERK phosphorylation (Fig. 6D) and, as expected (11, 12), Syk activation (Fig. 7C). These observations reveal an essential role for lipid rafts in activating MAPK pathways. To examine whether the EBS is involved in partitioning PSGL-1 into lipid rafts, CHO cells expressing WT PSGL-1 or AAA mutant were treated with Brij 58 and fractionated on sucrose gradients (13). As expected (12), both WT PSGL-1 and AAA mutant were detected in lipid rafts containing fractions (Fig. 6E), indicating that ERM binding to PSGL-1 is dispensable for its localization in lipid rafts.
DISCUSSION
In this study, we examined the contribution of PSGL-1 EBS in 1) recruiting leukocytes on L-, P-, and E-selectin and 2) activating the MAPK pathway. Because earlier studies showed that ERMs serve as adaptors between the cytoplasmic tail of PSGL-1 and Syk (16), additional experiments were performed to clarify (36) whether PSGL-1 binding to ERMs is required to activate Syk. Our results show that the EBS strongly contributes to capture and recruit leukocytes on selectins. In addition, we demonstrate that it plays a crucial role in activating ERK upon PSGL-1 engagement, whereas it is dispensable for Syk activation.
Flow adhesion assays show that EBS is required to efficiently capture leukocytes (Figs. 3C and 5C) and to stabilize cell displacements (Fig. 4A). These observations reveal that EBS binding to ERMs and actin cytoskeleton (37) plays a key role in supporting leukocyte capture by selectins.
Previous studies performed with transfectants expressing chimeric l-selectin/CD44 molecules demonstrated that leukocyte tethering on endothelium (34) or PSGL-1 (35) is dependent on the distribution of L-selectin on leukocyte microvilli (34, 35) and on the binding of L-selectin cytoplasmic domain to ERMs and actin cytoskeleton (35). By contrast, leukocyte rolling velocity was not affected by the topographic distribution of L-selectin (34, 35). Most probably, the impairment of leukocyte tethering observed in our study (Figs. 3 and 5) does not result from an alteration in the positioning of mutant PSGL-1 on microvilli because deletion of the whole cytoplasmic tail does not impair its distribution at leukocyte cell surface (11). Thus, the impaired capture of leukocytes by selectins is probably due to defective binding of cytoplasmic tail to ERMs and actin.
Deletion of the EBS decreased the stability of leukocyte displacements on L-selectin (Fig. 4A). These results are in agreement with biophysical studies showing that loss of PSGL-1 anchorage to cytoskeleton decreases the ability of leukocytes to elongate microvilli and form tethers (25, 38). They are also consistent with the concept that the stability of cell rolling is dependent on the ability of microvilli to deform (25). Thus, the EBS plays a crucial role in stabilizing cell rolling velocity. Impairment of this process does not allow the formation of prolonged adhesive bonds at high physiological shear stresses, which are required to stabilize cell displacements and allow optimal leukocyte capture by selectins (23, 24). These properties are central features of immune defense because maintaining rolling velocity more or less constant improves immune surveillance by enabling uniform leukocyte exposure to proinflammatory molecules along vessels.
L-selectin and PSGL-1 share similarities in their interactions with ERMs. Two basic residues of L-selectin cytoplasmic tail, Arg-357 and Lys-362, bind to ERMs and regulate L-selectin shedding and leukocyte tethering on PSGL-1 (32). Substitution of these residues with alanines strongly reduces leukocyte tethering efficiency and recruitment on PSGL-1 (32), suggesting that they may be necessary for microvillar positioning of L-selectin (32). However, more recent data showed that they are not sufficient (35) and that the transmembrane domain is also required to allow L-selectin sorting to microvilli (35). As observed for L-selectin, alanine substitution of Arg-337 and Lys-338 on PSGL-1 also strongly reduced tethering efficiency and leukocyte recruitment on selectins (Fig. 5, A and C). Thus, for both L-selectin and PSGL-1, two basic residues play a key role in ERM binding and leukocyte tethering on PSGL-1 or selectins.
PSGL-1 engagement simultaneously induces ERK (15) and Syk activation (9, 12). ERK phosphorylation results from Ras activation, which may successively activate Raf, MEK, and ERK (15). Syk activation is dependent on signaling through SFKs and leads to αLβ2 integrin activation and slow rolling on ICAM-1 (9, 12). ERK and Syk activation are transient and occur within the first minutes of PSGL-1 engagement. The abrogation of ERK phosphorylation in leukocytes exhibiting deletion of EBS or alanine substitution of Arg-337 and Lys-338 indicates that ERMs may contribute to activate the MAPK pathway. How they are involved in generating signals that activate ERK following PSGL-1 engagement remains to be elucidated, and whether ezrin and moesin play redundant or specific roles is not yet known either.
We previously reported that PSGL-1 is expressed in signaling platforms called lipid rafts (13). Data presented here further show that their integrity is required to activate the MAPK pathway (Fig. 6D) and, as previously reported (11), Syk. In addition, SRK mutation did not affect PSGL-1 distribution in lipid rafts (Fig. 6E). Although it is well established that PSGL-1 signaling through SFKs and Syk activates αLβ2 integrin and slows down rolling on ICAM-1 (Fig. 5D) (9, 10, 12, 14), little is known about the role of ERK activation induced by PSGL-1 engagement. The importance of the MAPK pathway in regulating cell proliferation and differentiation may suggest that PSGL-1 signaling through ERK may also play a role in these processes, which is consistent with recent observations showing that bone marrow microenvironment interactions with PSGL-1 regulate multiple myeloma cell proliferation (39).
Previous studies showed that ERMs may serve as adaptor molecules between Syk and PSGL-1 (16). To elucidate the role of ERMs in PSGL-1-mediated activation of Syk, we examined whether ERM binding to PSGL-1 is required to activate Syk. Data presented here show that despite deletion or mutation of the EBS, Syk is rapidly phosphorylated following PSGL-1 engagement. As expected (9, 12), this reaction is prevented by leukocyte exposure to SFK inhibitor PP2 (Fig. 7A). In addition, deletion of the EBS did not prevent slow rolling on E-selectin plus ICAM-1 (Fig. 5D). Taken together, these data demonstrate that ERM binding to PSGL-1 is not necessary for Syk activation or slow rolling.
Our results indicate that distinct amino acid sequences are involved in PSGL-1-induced activation of SFKs and the MAPK pathway. Little is known about the mechanisms that initiate the activation of SFKs following PSGL-1 engagement (9, 12, 40). SFKs are in a dynamic equilibrium between inactive and active conformation (41). The access to their catalytic site is prevented by the phosphorylation of a C-terminal tyrosine residue that interacts with the Src homology 2 domain of the involved kinase. The recovery of a full kinase activity requires dephosphorylation of the C-terminal tyrosine by a phosphatase. Further investigations are needed to examine whether phosphatases are activated following PSGL-1 engagement and if they activate SFKs. They may provide important insight into the molecular mechanisms that initiate slow rolling on ICAM-1 and may reveal novel target(s) for anti-inflammatory therapy.
Acknowledgment
We thank Dr. A. Ciuffi for critical reading of the manuscript.
This work was supported by Swiss National Foundation for Scientific Research Grant 310030_127368 (to O. S.).
- ITAM
- immunoreceptor tyrosine-based activation sequence
- mrv
- median rolling velocity
- ERM
- ezrin-radixin-moesin
- EBS
- PSGL-1 ERM-binding sequence
- Syk
- spleen tyrosine kinase
- ICAM-1
- intercellular adhesion molecule-1
- SFK
- Src family kinase
- SAA
- PSGL-1 R337A/K338A
- AAA
- PSGL-1 S336A/R337A/K338A
- ΔEBS
- PSGL-1 mutant exhibiting deletion of amino acid residues Ser-336 to Val-344.
REFERENCES
- 1. Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation. The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 2. Spertini O., Cordey A. S., Monai N., Giuffrè L., Schapira M. (1996) P-selectin glycoprotein ligand 1 is a ligand for L-selectin on neutrophils, monocytes, and CD34+ hematopoietic progenitor cells. J. Cell Biol. 135, 523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ley K., Bullard D. C., Arbonés M. L., Bosse R., Vestweber D., Tedder T. F., Beaudet A. L. (1995) Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 181, 669–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walcheck B., Moore K. L., McEver R. P., Kishimoto T. K. (1996) Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation of P-selectin in vitro. J. Clin. Invest. 98, 1081–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sperandio M., Forlow S. B., Thatte J., Ellies L. G., Marth J. D., Ley K. (2001) Differential requirements for core2 glucosaminyltransferase for endothelial L-selectin ligand function in vivo. J. Immunol. 167, 2268–2274 [DOI] [PubMed] [Google Scholar]
- 6. Hidalgo A., Peired A. J., Wild M. K., Vestweber D., Frenette P. S. (2007) Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity 26, 477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zarbock A., Ley K., McEver R. P., Hidalgo A. (2011) Leukocyte ligands for endothelial selectins. Specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118, 6743–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang H. B., Wang J. T., Zhang L., Geng Z. H., Xu W. L., Xu T., Huo Y., Zhu X., Plow E. F., Chen M., Geng J. G. (2007) P-selectin primes leukocyte integrin activation during inflammation. Nat. Immunol. 8, 882–892 [DOI] [PubMed] [Google Scholar]
- 9. Zarbock A., Abram C. L., Hundt M., Altman A., Lowell C. A., Ley K. (2008) PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR γ to induce slow leukocyte rolling. J. Exp. Med. 205, 2339–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mueller H., Stadtmann A., Van Aken H., Hirsch E., Wang D., Ley K., Zarbock A. (2010) Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) γ2 and PI3Kγ pathways. Blood 115, 3118–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miner J. J., Xia L., Yago T., Kappelmayer J., Liu Z., Klopocki A. G., Shao B., McDaniel J. M., Setiadi H., Schmidtke D. W., McEver R. P. (2008) Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood 112, 2035–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yago T., Shao B., Miner J. J., Yao L., Klopocki A. G., Maeda K., Coggeshall K. M., McEver R. P. (2010) E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin αLβ2-mediated slow leukocyte rolling. Blood 116, 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abbal C., Lambelet M., Bertaggia D., Gerbex C., Martinez M., Arcaro A., Schapira M., Spertini O. (2006) Lipid raft adhesion receptors and Syk regulate selectin-dependent rolling under flow conditions. Blood 108, 3352–3359 [DOI] [PubMed] [Google Scholar]
- 14. Stadtmann A., Brinkhaus L., Mueller H., Rossaint J., Bolomini-Vittori M., Bergmeier W., Van Aken H., Wagner D. D., Laudanna C., Ley K., Zarbock A. (2011) Rap1a activation by CalDAG-GEFI and p38 MAPK is involved in E-selectin-dependent slow leukocyte rolling. Eur. J. Immunol. 41, 2074–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hidari K. I., Weyrich A. S., Zimmerman G. A., McEver R. P. (1997) Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J. Biol. Chem. 272, 28750–28756 [DOI] [PubMed] [Google Scholar]
- 16. Urzainqui A., Serrador J. M., Viedma F., Yáñez-Mó M., Rodríguez A., Corbí A. L., Alonso-Lebrero J. L., Luque A., Deckert M., Vázquez J., Sánchez-Madrid F. (2002) ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity 17, 401–412 [DOI] [PubMed] [Google Scholar]
- 17. Serrador J. M., Urzainqui A., Alonso-Lebrero J. L., Cabrero J. R., Montoya M. C., Vicente-Manzanares M., Yáñez-Mó M., Sánchez-Madrid F. (2002) A juxtamembrane amino acid sequence of P-selectin glycoprotein ligand-1 is involved in moesin binding and ezrin/radixin/moesin-directed targeting at the trailing edge of migrating lymphocytes. Eur. J. Immunol. 32, 1560–1566 [DOI] [PubMed] [Google Scholar]
- 18. Snapp K. R., Heitzig C. E., Kansas G. S. (2002) Attachment of the PSGL-1 cytoplasmic domain to the actin cytoskeleton is essential for leukocyte rolling on P-selectin. Blood 99, 4494–4502 [DOI] [PubMed] [Google Scholar]
- 19. Serrador J. M., Nieto M., Alonso-Lebrero J. L., del Pozo M. A., Calvo J., Furthmayr H., Schwartz-Albiez R., Lozano F., González-Amaro R., Sánchez-Mateos P., Sánchez-Madrid F. (1998) CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood 91, 4632–4644 [PubMed] [Google Scholar]
- 20. Gal I., Lesley J., Ko W., Gonda A., Stoop R., Hyman R., Mikecz K. (2003) Role of the extracellular and cytoplasmic domains of CD44 in the rolling interaction of lymphoid cells with hyaluronan under physiologic flow. J. Biol. Chem. 278, 11150–11158 [DOI] [PubMed] [Google Scholar]
- 21. Fehon R. G., McClatchey A. I., Bretscher A. (2010) Organizing the cell cortex. The role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11, 276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen S., Springer T. A. (1999) An automatic braking system that stabilizes leukocyte rolling by an increase in selectin bond number with shear. J. Cell Biol. 144, 185–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shao J. Y., Ting-Beall H. P., Hochmuth R. M. (1998) Static and dynamic lengths of neutrophil microvilli. Proc. Natl. Acad. Sci. U.S.A. 95, 6797–6802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park E. Y., Smith M. J., Stropp E. S., Snapp K. R., DiVietro J. A., Walker W. F., Schmidtke D. W., Diamond S. L., Lawrence M. B. (2002) Comparison of PSGL-1 microbead and neutrophil rolling. Microvillus elongation stabilizes P-selectin bond clusters. Biophys. J. 82, 1835–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pospieszalska M. K., Ley K. (2009) Dynamics of microvillus extension and tether formation in rolling leukocytes. Cell Mol. Bioeng. 2, 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takai Y., Kitano K., Terawaki S., Maesaki R., Hakoshima T. (2007) Structural basis of PSGL-1 binding to ERM proteins. Genes Cells 12, 1329–1338 [DOI] [PubMed] [Google Scholar]
- 27. Baïsse B., Galisson F., Giraud S., Schapira M., Spertini O. (2007) Evolutionary conservation of P-selectin glycoprotein ligand-1 primary structure and function. BMC Evol. Biol. 7, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tauxe C., Xie X., Joffraud M., Martinez M., Schapira M., Spertini O. (2008) P-selectin glycoprotein ligand-1 decameric repeats regulate selectin-dependent rolling under flow conditions. J. Biol. Chem. 283, 28536–28545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie X., Rivier A. S., Zakrzewicz A., Bernimoulin M., Zeng X. L., Wessel H. P., Schapira M., Spertini O. (2000) Inhibition of selectin-mediated cell adhesion and prevention of acute inflammation by nonanticoagulant sulfated saccharides. Studies with carboxyl-reduced and sulfated heparin and with trestatin A sulfate. J. Biol. Chem. 275, 34818–34825 [DOI] [PubMed] [Google Scholar]
- 30. Martinez M., Joffraud M., Giraud S., Baïsse B., Bernimoulin M. P., Schapira M., Spertini O. (2005) Regulation of PSGL-1 interactions with L-selectin, P-selectin, and E-selectin. Role of human fucosyltransferase-IV and -VII. J. Biol. Chem. 280, 5378–5390 [DOI] [PubMed] [Google Scholar]
- 31. Bernimoulin M. P., Zeng X. L., Abbal C., Giraud S., Martinez M., Michielin O., Schapira M., Spertini O. (2003) Molecular basis of leukocyte rolling on PSGL-1. Predominant role of core-2 O-glycans and of tyrosine sulfate residue 51. J. Biol. Chem. 278, 37–47 [DOI] [PubMed] [Google Scholar]
- 32. Ivetic A., Florey O., Deka J., Haskard D. O., Ager A., Ridley A. J. (2004) Mutagenesis of the ezrin-radixin-moesin binding domain of L-selectin tail affects shedding, microvillar positioning, and leukocyte tethering. J. Biol. Chem. 279, 33263–33272 [DOI] [PubMed] [Google Scholar]
- 33. Krasik E. F., Hammer D. A. (2004) A semianalytic model of leukocyte rolling. Biophys. J. 87, 2919–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stein J. V., Cheng G., Stockton B. M., Fors B. P., Butcher E. C., von Andrian U. H. (1999) L-selectin-mediated leukocyte adhesion in vivo. Microvillous distribution determines tethering efficiency but not rolling velocity. J. Exp. Med. 189, 37–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buscher K., Riese S. B., Shakibaei M., Reich C., Dernedde J., Tauber R., Ley K. (2010) The transmembrane domains of L-selectin and CD44 regulate receptor cell surface positioning and leukocyte adhesion under flow. J. Biol. Chem. 285, 13490–13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mócsai A., Ruland J., Tybulewicz V. L. (2010) The SYK tyrosine kinase. A crucial player in diverse biological functions. Nat. Rev. Immunol. 10, 387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alonso-Lebrero J. L., Serrador J. M., Domínguez-Jiménez C., Barreiro O., Luque A., del Pozo M. A., Snapp K., Kansas G., Schwartz-Albiez R., Furthmayr H., Lozano F., Sánchez-Madrid F. (2000) Polarization and interaction of adhesion molecules P-selectin glycoprotein ligand 1 and intercellular adhesion molecule 3 with moesin and ezrin in myeloid cells. Blood 95, 2413–2419 [PubMed] [Google Scholar]
- 38. Python J. L., Wilson K. O., Snook J. H., Guo B., Guilford W. H. (2010) The viscoelastic properties of microvilli are dependent upon the cell surface molecule. Biochem. Biophys. Res. Commun. 397, 621–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azab A. K., Quang P., Azab F., Pitsillides C., Thompson B., Chonghaile T., Patton J. T., Maiso P., Monrose V., Sacco A., Ngo H. T., Flores L. M., Lin C. P., Magnani J. L., Kung A. L., Letai A., Carrasco R., Roccaro A. M., Ghobrial I. M. (2012) P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood 119, 1468–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zarbock A., Ley K. (2011) Protein-tyrosine kinases in neutrophil activation and recruitment. Arch. Biochem. Biophys. 510, 112–119 [DOI] [PubMed] [Google Scholar]
- 41. Hermiston M. L., Zikherman J., Zhu J. W. (2009) CD45, CD148, and Lyp/Pep. Critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol. Rev. 228, 288–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sako D., Chang X. J., Barone K. M., Vachino G., White H. M., Shaw G., Veldman G. M., Bean K. M., Ahern T. J., Furie B. (1993) Expression cloning of a functional glycoprotein ligand for P-selectin. Cell 75, 1179–1186 [DOI] [PubMed] [Google Scholar]