Background: Plakoglobin (Jup) is involved in intercellular junctions. Mutation of Jup is associated with Naxos disease.
Results: Epidermis-restricted Jup knock-outs largely recapitulated human palmoplantar keratoderma. Ultrastructural analyses revealed the disruption of the assembly of desmosomes and adherens junctions in Jup mutant epidermis.
Conclusion: Jup is critical for maintaining normal epidermis.
Significance: Our findings provide important insights for the pathogenesis of palmoplantar keratoderma.
Keywords: Cell Death, Cell Junctions, Desmosome, Epidermis, Inflammation, Mouse Genetics
Abstract
Loss-of-function mutation of Jup has been associated with Naxos disease, which is characterized by arrhythmogenic cardiomyopathy and the cutaneous disorder palmoplantar keratoderma. Previously, we have shown that genetic ablation of Jup in cardiomyocytes in mice leads to arrhythmogenic cardiomyopathy similar to Naxos disease in humans. Currently, to determine the pathogenesis of Naxos disease-associated keratoderma, we generated Jup mutant mice by inactivating Jup restrictively in keratinocytes. Jup mutant mice largely recapitulated the clinical features of human palmoplantar keratoderma: overcornification and thickening of the epidermis. Jup mutant mice also suffered skin ulceration and inflammation. Cell apoptosis and proliferation were significantly elevated in Jup mutant epidermis. Ultrastructural analyses revealed the disruption of the assembly of desmosomes and adherens junctions in Jup mutant epidermis. We also demonstrated the compensational increase in β-catenin at Jup mutant cell-cell junctions without altering its signaling activities. Our findings provide important insights for understanding the pathogenesis of human palmoplantar keratoderma.
Introduction
The epidermis, a striated squamous epithelium, acts as the major barrier against pathogens or immunogens from the environment. This is achieved mainly through numerous intercellular junctions between keratinocytes, such as desmosomes, adherens junctions, and tight junctions. Among these junctions, desmosomes provide the primary resistance to the incessant mechanical stresses applied to the epidermis. Epidermal desmosomes bridge between two neighbor cells by homophilic or heterophilic binding of transmembranous desmosomal cadherins (desmoglein and desmocollin) (1). The cytoplasmic tail of desmosomal cadherins interacts with plakoglobin (Jup, also called γ-catenin), which is then associated with the intermediate filament network via desmoplakin. Among these desmosomal proteins, Jup is also a structural component for the adherens junction, in which it is interchangeable with its close homolog, the Armadillo family member β-catenin. Jup and β-catenin share the same degradation machinery and potentially same binding partners (T-cell factor/lymphoid-enhancing factor) in mediating canonical Wnt signaling (2). Thus, Jup has been hypothesized to inhibit canonical Wnt signaling by competing with β-catenin, although this assumption is highly controversial, particularly in terms of different temporal and spatial contexts. Loss-of-function mutation of Jup has been identified in patients with Naxos disease, an autosomal recessive disease characterized by cardiomyopathy and the skin disorder palmoplantar keratoderma (3). Palmoplantar keratoderma is characterized by thick hyperkeratosis over the palms and soles. Interestingly, the deregulation of Wnt signaling has been suggested to play a causal role in the pathogenesis of palmoplantar keratoderma (4), although this suggestion awaits further supporting evidence. In mice, global deletion of Jup results in heart malformations and blister formations on the epidermis (5). However, early embryonic lethality prevents further functional analyses of Jup deletion in postnatal epidermis pathogenesis.
In this study, to better address whether Jup inactivation is sufficient for the emergence of palmoplantar keratoderma and its potential underlying mechanisms, we generated an epidermal conditional knock-out mouse model of Jup (Jup mutant). These Jup mutants largely mimic the human palmoplantar keratoderma condition. We found that cell proliferation, apoptosis, and differentiation are markedly perturbed in Jup mutants, likely contributing to the development of keratoderma. Furthermore, we show that Jup mutant mice exhibited compromised immune defense, which is likely caused by the loose intercellular junctions between keratinocytes. Ultrastructural analyses revealed abnormal desmosomes and adherens junctions in Jup mutant mice. Finally, we demonstrate that there is a compensatory increase in β-catenin at cell-cell junctions, whereas Wnt/β-catenin signaling is unaffected by the loss of Jup in keratinocytes.
MATERIALS AND METHODS
Mice
Jupf/f mice were described previously (6). Cytokeratin 14 K14-Cre transgenic mice (7) were a gift from Dr. Jingwu Xie (Indiana University, Indianapolis, IN). fos-lacZ transgenic mice (8) were purchased from The Jackson Laboratory. All studies involving the use of mice were in compliance with the National Institutes of Health and were approved by the Indiana University School of Medicine Animal Care and Use Committee.
Quantitative RT-PCR
TRIzol reagent (Invitrogen) was used to extract total RNA from skin. SYBR Green quantitative RT-PCR analyses were performed using the Roche Applied Science LightCycler 480 system. Primers used for quantitative RT-PCR are listed in Table 1.
TABLE 1.
Primers used for quantitative RT-PCR analyses
| Forward (5′ → 3′) | Reverse (5′ → 3′) | |
|---|---|---|
| TNFα | TCTTCTCATTCCTGCTTGTGG | GGTCTGGGCCATAGAACTGA |
| IL-1β | TGTAATGAAAGACGGCACACC | TCTTCTTTGGGTATTGCTTGG |
| IL-6 | GCTACCAAACTGGATATAATCAGGA | CCAGGTAGCTATGGTACTCCAGAA |
| Cyclin D1 | GAGATTGTGCCATCCATGC | CTCCTCTTCGCACTTCTGCT |
| c-myc | CCTAGTGCTGCATGAGGAGA | TCTTCCTCATCTTCTTGCTCTTC |
| c-fos | CAGCCTTTCCTACTACCATTCC | ACAGATCTGCGCAAAAGTCC |
| c-jun | CCAGAAGATGGTGTGGTGTTT | CTGACCCTCTCCCCTTGC |
| GAPDH | TCCTGGTATGACAATGAATACGGC | TCTTGCTCAGTGTCCTTGCTGG |
Immunoblot Analyses
Skin samples were lysed with radioimmune precipitation assay buffer, and proteins were subsequently separated on 4–12% BisTris2 gels, transferred, and immunoblotted. The following antibodies were used: polyclonal antibody (pAb) against Jup and pan-cadherin and monoclonal antibody (mAb) against α-catenin (Santa Cruz Biotechnology); pAb against β-catenin and β-catenin phosphorylated at Ser-33, Ser-37, and Thr-41 (Cell Signaling Technology); pAb against keratin 5, involucrin, and filaggrin and mAb against keratin 10 (Abcam); pAb against keratin 14 (Covance); mAb against desmogleins 1 and 2 (PROGEN); mAb against Gr-1 (Pharmingen); mAb against E-cadherin (BD Transduction Laboratories); and pAb against ZO-1 (zona occludens-1) (Invitrogen).
Immunofluorescence Staining
Fluorophore-conjugated wheat germ agglutinin and Alexa Fluor-labeled secondary antibodies were from Molecular Probes. Nuclei were stained with DAPI. Photomicrographs were acquired with either a Leica microscope or an Olympus FV1000 confocal microscope using a single exposure time for the same set of sections in each staining.
BrdU Staining and Terminal Deoxynucleotidyltransferase-mediated dUTP Nick End Labeling (TUNEL) Assay
BrdU and TUNEL assays were performed as described previously (9). Briefly, mice were intraperitoneally injected with BrdU labeling reagent (30 mg/kg of body weight) and killed 3–4 h later. The skin was harvested and fixed in 4% paraformaldehyde in PBS overnight. A BrdU labeling and alkaline phosphatase detection kit (Roche Applied Science) was used to detect proliferating cells on paraffin sections from different samples. TUNEL assays were performed on paraffin slides using an ApopTag kit (Chemicon). Eight photographs on average were randomly taken for each sample. The percentage of positively stained cells was quantified using ImageJ software.
Transmission Electron Microscopy
Transmission electron microscopy was performed as described previously (9). Skin samples were fixed in modified Karnovsky's fixative (2% paraformaldehyde and 2% glutaraldehyde in 0.1 m phosphate buffer) at 4 °C overnight.
Statistical Analyses
Results are presented as the mean ± S.E. for parametric data. Student's t test was performed for comparison between two groups. A p value of <0.05 was considered significant.
RESULTS
Keratinocyte-restricted Loss of Jup Results in Keratoderma
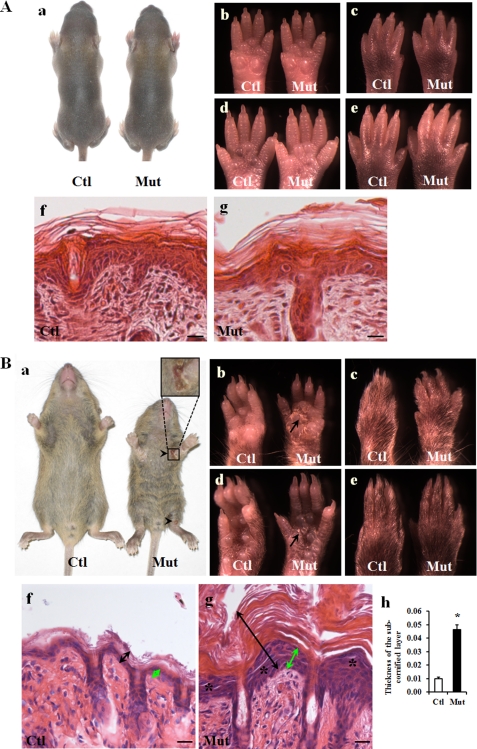
The cross of Jupf/f and Jupf/+/K14-Cre mice was expected to give rise to 25% Jupf/f/K14-Cre (designated Jup mutant) offspring. We designated mice with genotypes of Jupf/+, Jupf/f, and Jupf/+/K14-Cre as controls in this study because we did not observe skin abnormalities in any of these mice. Jup mutant mice appeared normal by the first postnatal week compared with littermate controls (Fig. 1A, panels a–g). By 2–3 weeks, however, Jup mutant mice were significantly smaller than Jupf/f littermate controls and exhibited ulcerations in the skin near joint areas (Fig. 1B, panel a). All Jup mutant mice died before weaning. Jup mutant skin was markedly stiffer than Jupf/f littermate control skin. Severe keratoderma was apparent in Jup mutant mice through hyperkeratosis in the palms and soles (Fig. 1B, panels b and d). Histology further revealed a marked thickening of Jup mutant epidermal layers, including the shedding of the cornified layer and the underlying subcornified layer (granular layer and basal layer), compared with littermate control epidermis (Fig. 1B, panels g and h). In addition, detachment in the granular layer was apparent in Jup mutant epidermis. No overt abnormality was found in Jup mutant hair follicles.
FIGURE 1.
Epidermal phenotypes of Jup mutant mice. A, normal epidermis of 1-week-old Jup mutant mice. Panel a, general morphology of a Jup mutant (Mut) mouse and a control (Ctl) mouse. Panels b–e, normal appearance of the Jup mutant palm and planta. No apparent plaques are visible. Panels f and g, normal histology of control and Jup mutant epidermis, respectively. Scale bars = 20 μm. B, Jup mutant mice develop an abnormal epidermal phenotype at ∼2 weeks after birth. Panel a, general morphology of a Jup mutant mouse and a control mouse (16 days old). The Jup mutant is smaller than its control littermate, and the hair on the Jup mutant abdomen is rough. Ulcerations (arrowheads) are seen near joint areas. The Jup mutant palm exhibits severe hyperkeratosis with plaques (panel b, arrow), whereas keratosis is mild in the Jup mutant planta (panel d). No apparent plaques are visible in either the Jup mutant opisthenar (panel c) or instep (panel e). Jup mutant epidermis (panel g, double-headed black arrows) is markedly thicker than control epidermis (panel f), with a thick, shedding cornified layer and thickened subcornified layer (double-headed green arrows). Detachment of granular layer (asterisks) is apparent in Jup mutant epidermis. Scale bars = 30 μm. Quantitation of the thickness of the subcornified layer is shown in panel h. *, significantly different from the control (p < 0.01; n = 4 (control) and n = 3 (mutant)).
Inflammation in Jup Mutant Skin
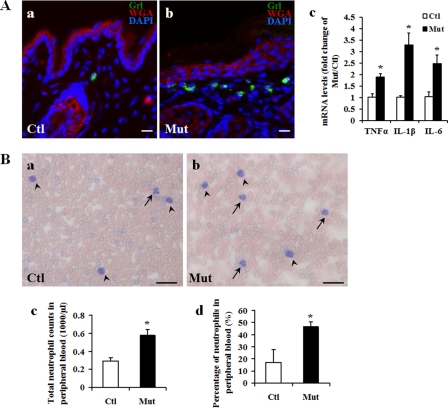
Given the ulcerations seen in Jup mutant skin, we suspected that these mice suffered from inflammation. Indeed, we found excessive neutrophil infiltration in Jup mutant derma (Fig. 2A). Consistently, inflammatory cytokines, including TNFα, IL-1β, and IL-6, were significantly elevated in Jup mutant skin (Fig. 2A). Furthermore, both the total number and the percentage of peripheral blood neutrophils were significantly higher in Jup mutants compared with controls (Fig. 2B). Collectively, these data indicate enhanced inflammation in Jup mutant mice.
FIGURE 2.
Inflammation in Jup mutant skin. A, neutrophil infiltration in Jup mutant (Mut) derma (16 days old). Note that rare resident neutrophils (Gr-l-positive cells) can be found in the distal control (Ctl) derma. Scale bars = 20 μm. WGA, wheat germ agglutinin. B, increase in peripheral blood neutrophils in Jup mutants. Representative graphs of Giemsa staining on a peripheral blood smear from Jup mutant and control mice (16 days old) are shown in panels a and b, respectively. Arrows and arrowheads indicate neutrophils and lymphocytes, respectively. Scale bars = 40 μm. HEMAVET analyses in panels c and d show increased counts and percentage of peripheral blood neutrophils in Jup mutants (n = 7) compared with controls (n = 10). *, p < 0.001.
Altered Epidermal Differentiation in Jup Mutant Mice
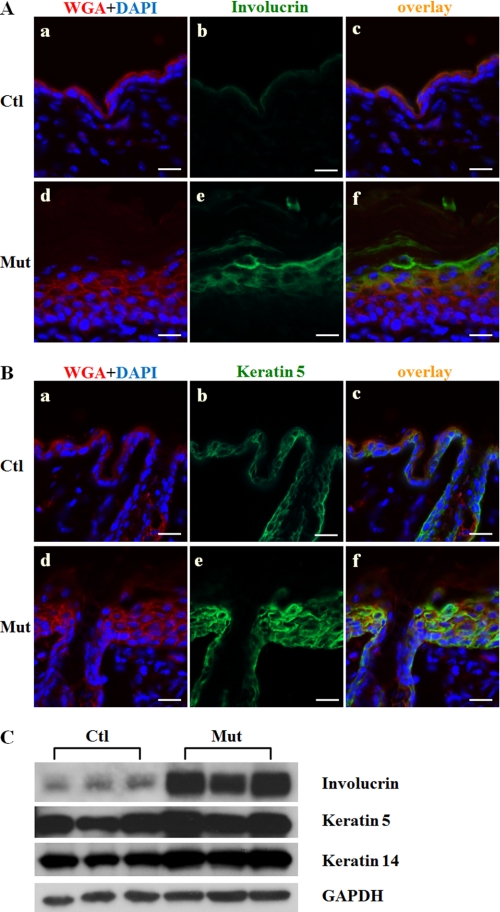
Because Jup mutant epidermal layers were markedly thickened, we next wanted to understand whether or not and how epidermal differentiation was affected. Immunostaining of involucrin, filaggrin, and keratin 10 (suprabasal layer markers) was considerably expanded in Jup mutant epidermis compared with control epidermis (Fig. 3A and supplemental Fig. 1). The staining of keratin 5 (a basal layer marker) was greatly extended to most of the epidermis with the exception of the cornified layer, which consists of only dead cells without nuclei (Fig. 3B). Western blot findings showed increased expression of involucrin and keratin 5 (Fig. 3C). These data indicate that epidermal differentiation is remarkably affected by the loss of Jup in keratinocytes.
FIGURE 3.
Altered epidermal differentiation of Jup mutant mice (16 days old). A, markedly thickened Jup mutant (Mut) suprabasal layer (panel e) compared with the control (Ctl; panel b) as revealed by involucrin staining. Scale bars = 30 μm. B, significantly expanded basal layer in Jup mutant epidermis (panel e) compared with the control (panel b) as shown by keratin 5 staining. Scale bars = 30 μm. WGA, wheat germ agglutinin. C, Western blot analyses of involucrin, keratin 5, and keratin 14. GAPDH served as a total protein loading control.
Increased Cell Apoptosis and Proliferation in Jup Mutant Epidermis
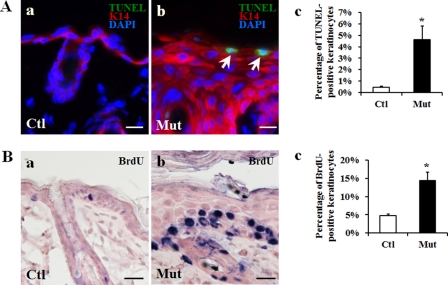
Given the development of keratoderma and perturbed epidermal differentiation seen in Jup mutant epidermis, we asked whether cell apoptosis or proliferation was affected in Jup mutants. First, we found that cell apoptosis was significantly enhanced in Jup mutant epidermis as revealed by TUNEL staining (Fig. 4A). Notably, the apoptotic cells were located close to the outermost granular layer. Cell proliferation was significantly increased in Jup mutant epidermis as assessed by BrdU assay (Fig. 4B). Proliferating keratinocytes were confined to the basal layer.
FIGURE 4.
Enhanced cell apoptosis and proliferation in Jup mutant epidermis (16 days old). A, TUNEL staining shows increased cell apoptosis in the Jup mutant (Mut) suprabasal layer (panel b) compared with controls (Ctl; panel a). White arrows indicate apoptotic cells. Scale bars = 20 μm. K14, keratin 14. Quantification is shown in panel c. *, p < 0.001. B, increased cell proliferation in the Jup mutant basal layer (panel b) compared with controls (panel a). The positive BrdU staining is shown as dark blue nuclear staining. Scale bars = 30 μm. Quantification is shown in panel c. *, p < 0.001.
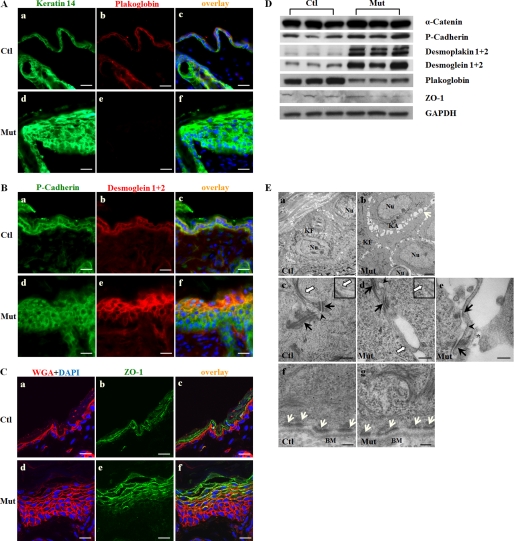
Jup is required for proper cell-cell junctions in the epidermis: Jup is a structural component for both desmosomes and adherens junctions. Although Jup is interchangeable with β-catenin in adherens junctions, only Jup is found in desmosomes under physiological conditions (10, 11). To understand how desmosomes and adherens junctions are affected by Jup deficiency, we first used immunofluorescence staining to demonstrate the specific loss of Jup expression in Jupf/f/K14-Cre keratinocytes, confirming the successful deletion of Jup in keratinocytes (Fig. 5A). Next, we assessed the expression of cadherin (specific for adherens junctions), ZO-1 (specific for tight junctions), and desmoglein (specific for desmosomes). The immunostaining intensity of P-cadherin and, more specifically, E-cadherin was similar in both Jup mutant and control keratinocytes, although the staining was more expanded in Jup mutant epidermis (Fig. 5B and supplemental Fig. 2). In contrast, the staining intensity of desmoglein was much stronger in Jup mutant keratinocytes compared with control keratinocytes (Fig. 5B), in addition to its wider expression in Jup mutant epidermis, as seen similarly in the cadherin staining pattern of Jup mutant epidermis. ZO-1 staining was also extended in Jup mutant epidermis, whereas the staining intensity was similar compared with control epidermis (Fig. 5C). Interestingly, the wide-spreading staining of ZO-1 suggests the disturbed cell differentiation in some skin lesions or diseases (12, 13). Thus, this extended ZO-1 staining seen in Jup mutant keratinocytes further reinforced the finding of disturbed epidermal differentiation in Jup mutants. The results from Western blot analyses of these proteins along with other junctional proteins (α-catenin, specific for adherens junctions; and desmoplakins 1 and 2, specific for desmosomes) were consistent with the results from immunostaining (Fig. 5D). To more directly assess the impact of Jup deficiency on cell-cell junctions, ultrastructural analyses by transmission electron microscopy were performed. Wide segregating spaces were seen between adjacent Jup mutant keratinocytes (Fig. 5E), which was consistent with the histological observation (Fig. 1), indicating cell-cell detachment. Keratin aggregates were often seen in the perinuclear space in Jup mutant keratinocytes, but not in control keratinocytes (Fig. 5E). Furthermore, atypical desmosomes (the outer plaque of Jup mutant desmosomes connected with very few keratin plexuses) were seen in Jup mutant keratinocytes (Fig. 5E). Adherens junctions in Jup mutant keratinocytes displayed abnormally, with wide intercellular spaces. Tight junctions appeared normal in Jup mutant epidermis, although abnormal intracellular cell body dissociation was evident in Jup mutant keratinocytes (Fig. 5E). These intracellular breaks, together with the intercellular gaps in Jup mutant epidermis, greatly compromised the competence of the epidermis as a biological barrier. In contrast, hemidesmosomes, in which Jup is known not to be a structural component (14), were still intact in Jup mutant keratinocytes (Fig. 5E). Collectively, these data suggest that the loss of Jup in keratinocytes affects desmosome assembly and compromises the overall integrity of cell-cell junctions, leading to cell-cell detachment.
FIGURE 5.
Assessment of cell-cell junction in Jup mutant epidermis. A, plakoglobin staining signal was lost in Jup mutant (Mut) keratinocytes (panel e) as opposed to membranous localization in control (Ctl) cells (panel b), whereas keratin 14 staining intensity was markedly increased in Jup mutant cells (panel d) compared with control cells (panel a). Panels c and f, overlay images. Scale bars = 30 μm. B, P-cadherin staining was similar between Jup mutant (panel d) and control (panel a) epidermis. Staining of desmogleins 1 and 2 was greatly increased in Jup mutant epidermis (panel e) compared with the control (panel b). Scale bars = 30 μm. C, the staining intensity of ZO-1 was similar between Jup mutant (panel e) and control (panel b) epidermis, although the signal was much expanded in Jup mutant epidermis. WGA, wheat germ agglutinin. Scale bars = 15 μm. D, Western blot analyses of cell junction proteins. E, ultrastructural analyses of Jup mutant epidermis. Loose cell-cell contacts between Jup mutant keratinocytes (panel b) are shown by segregated spaces (white arrows). Keratin fibers (KF) are at cell peripheries in both control (panel a) and Jup mutant (panel b) keratinocytes. Keratin aggregates (KA) are seen in the perinuclear (Nu) region of Jup mutant keratinocytes (panel b), but not in control cells (panel a). Desmosomes (black arrows) are anchored to the keratin plexus in control keratinocytes (panel c), whereas significantly fewer keratins are at Jup mutant desmosomes (panels d and e). The intercellular spaces of adherens junctions (arrowheads) are wider in Jup mutant keratinocytes (panels d and e) than in control keratinocytes (panel c). Tight junctions (white arrows) appear normal in Jup mutant keratinocytes (panel d). Tight junctions are also presented at higher magnification in the insets in panels c and d. Note that the partial cell membrane and associated cytoplasm (asterisk) are disintegrated from the Jup mutant keratinocyte cell body (panel e). In contrast, hemidesmosomes (white arrows) are intact in Jup mutant keratinocytes (panel g). BM, basal membranes. Scale bars = 2 μm (panels a and b), 250 nm (panels c–e), and 125 nm (panels f and g).
Increase in β-Catenin at Cell-Cell Junction without Affecting Wnt/β-Catenin Signaling in Jup Mutant Epidermis
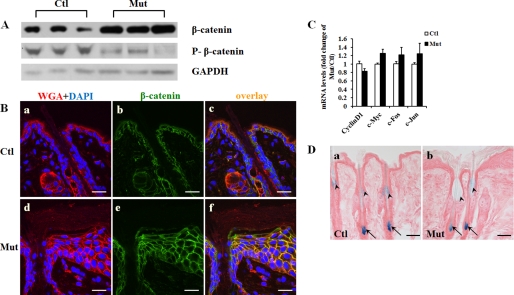
Jup has been postulated to have an inhibitory function in Wnt/β-catenin signaling possibly by competing with β-catenin for the same binding partners (2). However, this inhibitory function of Jup in Wnt signaling was still controversial, as shown by existing studies (15, 16). Moreover, the potential influence on Wnt signaling by Jup was considered to be pathogenic (17). Nonetheless, it would be important to determine whether β-catenin and its signaling activities are affected by the loss of Jup in keratinocytes. Interestingly, Western blot analyses showed that the total level of β-catenin was overtly increased in Jup mutant skin compared with control skin (Fig. 6A). A major regulation of the β-catenin level in the cytoplasm is through protein phosphorylation and consequent ubiquitination (18). Phosphorylation of β-catenin at Ser-33, Ser-37, and Thr-41 by glycogen synthase kinase 3β is a primary control for destabilization of β-catenin (19). Using a specific antibody against β-catenin phosphorylated at Ser-33, Ser-37, and Thr-41, we found that phosphorylated β-catenin was markedly reduced in Jup mutant skin compared with control skin. This reduction in phosphorylated β-catenin very likely accounts for the increase in total β-catenin. The stabilized β-catenin can either localize to the cell membrane to become a cell-cell junctional constituent or transport into nuclei to behave as a transcriptional activator (20). Immunofluorescence staining revealed that the increase was at the cell membrane, with no detectable signal in the nucleus or cytosol (Fig. 6B), thus not suggesting transcriptional activity of β-catenin. To further examine whether the canonical Wnt signaling is altered in Jup mutant skin, we analyzed the expression levels of several well known β-catenin transcriptional targets, including cyclin D1, c-Myc, c-Fos, and c-Jun (21), and found that they were similar between Jup mutant and control skin (Fig. 6C). To further examine canonical Wnt signaling activities in vivo, a specific Wnt/β-catenin signaling reporter mouse line, fos-lacZ transgenic mouse (8), was crossed with Jup mutant mice. There was no obvious positive X-gal staining signal observed in Jup mutant and control epidermis (Fig. 6D). In contrast, X-gal staining signals were readily detected in the sebaceous gland and hair roots in both Jup mutant and control hair follicles (Fig. 6D). These observations were consistent with previous findings (8). The fact that no noticeable difference in staining intensity was found between Jup mutants and controls indicates that β-catenin signaling activities are not significantly affected in Jup mutant keratinocytes.
FIGURE 6.
Increase in β-catenin at cell membrane in Jup mutant epidermis. A, Western blot of total β-catenin and β-catenin phosphorylated at Ser-33, Ser-37, and Thr-41. B, representative confocal photographs of β-catenin in 16-day-old Jup mutant (Mut) and control (Ctl) epidermis. The expression of β-catenin at the membrane of Jup mutant keratinocytes was markedly increased compared with the control keratinocyte membrane. The signal was undetectable in the nuclei or cytosol in either Jup mutant or control keratinocytes. Scale bars = 20 μm. WGA, wheat germ agglutinin. C, assessment of Wnt/β-catenin signaling targets by quantitative RT-PCR. There was no statistical difference between controls (n = 3) and Jup mutants (n = 3). D, representative pictures of X-gal staining of Jupf/f/fos-lacZ epidermis (panel a) and Jupf/f/K14-Cre/fos-lacZ skin (panel b). There was no positive signal in the epidermis of either controls (panel a) or Jup mutants (panel b). X-Gal staining signals were readily detected in the sebaceous gland region (arrowheads) and hair roots (arrows) in both Jup mutant and control hair follicles. No overt difference in staining intensity was found between Jup mutants and controls. Scale bars = 50 μm.
DISCUSSION
Keratoderma, a thickening of the stratum corneum of the skin, can be acquired or genetically inherited (22). A homozygous loss-of-function mutation of Jup (Jup2157del2) has been documented in patients with Naxos disease, which manifests cardiomyopathy and palmoplantar keratoderma (3). More recently, several other genetic mutations of Jup have been identified in patients with keratoderma or epidermolysis bullosa (23–25). These Jup mutations lead to either the production of truncated JUP protein or the complete loss of JUP protein. However, the potential underlying mechanisms are not well understood. Herein, we demonstrated that keratinocyte-restricted ablation of Jup in mice results in palmoplantar keratoderma.
In this study, we have shown that Jup is critical for the cell-cell junctions among keratinocytes. Loss of Jup in keratinocytes results in inappropriate desmosome assembly and severe cell-cell detachment. The findings of abnormal formation of desmosomes in Jup mutant epidermis are consistent with an early report of global Jup knock-out mouse embryos, although the cell-cell detachment phenotype seemed to be much more severe in Jup mutant pups (5). The discrepancy is most likely ascribed to the difference in the timing of Jup inactivation and the mechanical tension from the surrounding environment: rough cage beddings versus amniotic fluid in the uterus. Interestingly, the similar cell-cell dissociation phenotype was also evident in the skin of patients with a homozygous nonsense JUP mutation (1615C → T) (24), which further supports the pivotal role of Jup as a cell-cell junction constituent.
The skin inflammation present in Jup mutant mice seems contradictory given that Jup mutant epidermis was remarkably thickened. However, because Jup mutant cell junctions were severely compromised and keratinocytes detached, the skin's function as a defending barrier against innate intruding pathogens is expected to be severely compromised. Indeed, the complication of skin infections was also reported in palmoplantar keratoderma patients (26). All Jup mutant mice died before weaning. The following facts likely contributed to the lethality of Jup mutants. First, Jup mutant skin was thick and stiff, which greatly affected body movement, e.g. crawling and suckling. Second, Jup mutant skin had ulceration and inflammation accompanied by systemic inflammation as evidenced by the increase in neutrophils in the peripheral blood. The persistent and severe inflammation undoubtedly lessened their survival chance.
The relationship between Jup and β-catenin has been controversial (2). Our data demonstrate that β-catenin was increased at the plasma membrane to compensate for the loss of Jup at cell-cell junctions. Our results are consistent with some early findings of the ectopic localization of β-catenin in Jup-deficient desmosomes (10, 27). Nonetheless, β-catenin appears to incompetently substitute for Jup at cell junctions because Jup mutant cell junctions are loose; consequently, cells detach and undergo apoptosis. As a response, Jup mutant epidermis accelerates cell proliferation and differentiation in an attempt to further compensate for the loss of upper layer keratinocytes. Future work will be to analyze the underlying molecular signaling mechanism for the compensatory increase in keratinocyte proliferation associated with Jup deficiency. Interestingly, the increase in β-catenin in Jup mutant epidermis is specific to cell junctions without affecting Wnt/β-catenin signaling activities, which was clearly evidenced by the lack of nuclear localization of β-catenin, the unaffected levels of its transcriptional targets, and undisturbed T-cell factor/lymphoid-enhancing factor signaling reporter activities. This finding is consistent with our previous observation in cardiomyocyte-restricted Jup knock-out mutant mice: a compensational increase in β-catenin at junctions with no effect on its signaling activities (28). Thus, our findings do not suggest that the disturbance of Wnt/β-catenin signaling activities contributes to the pathogenesis of Jup-related keratoderma.
In summary, Jup mutant mice recapitulate the pathophysiological features of human palmoplantar keratoderma. We found that Jup is critical for maintaining the integrity of intercellular junctions and cell differentiation and proliferation. Additionally, we demonstrated the increase in β-catenin at cell junctions in compensation for the loss of Jup without affecting its signaling activities. Our current findings have provided important insights for understanding the pathogenesis of keratoderma.
Supplementary Material
Acknowledgments
We thank Dr. Jingwu Xie for generously providing K14-Cre mice and Dr. Mark H. Kaplan (Indiana University, Indianapolis) for anti-involucrin and anti-filaggrin antibodies. We thank Dr. Mark Soonpaa (Indiana University, Indianapolis) for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL81092, HL70259, and HL85098 (all to W. S.). This work was also supported by the Riley Children's Foundation (to W. S.).

This article contains supplemental Figs. 1 and 2.
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- pAb
- polyclonal antibody
- mAb
- monoclonal antibody
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
REFERENCES
- 1. Jamora C., Fuchs E. (2002) Intercellular adhesion, signaling, and the cytoskeleton. Nat. Cell Biol. 4, E101–E108 [DOI] [PubMed] [Google Scholar]
- 2. Zhurinsky J., Shtutman M., Ben-Ze'ev A. (2000) Plakoglobin and β-catenin: protein interactions, regulation, and biological roles. J. Cell Sci. 113, 3127–3139 [DOI] [PubMed] [Google Scholar]
- 3. McKoy G., Protonotarios N., Crosby A., Tsatsopoulou A., Anastasakis A., Coonar A., Norman M., Baboonian C., Jeffery S., McKenna W. J. (2000) Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 355, 2119–2124 [DOI] [PubMed] [Google Scholar]
- 4. Van Steensel M. A. (2010) Recessive palmoplantar keratodermas: a tale of wings, hands, hair. and cancer. G. Ital. Dermatol. Venereol. 145, 763–770 [PubMed] [Google Scholar]
- 5. Bierkamp C., Mclaughlin K. J., Schwarz H., Huber O., Kemler R. (1996) Embryonic heart and skin defects in mice lacking plakoglobin. Dev. Biol. 180, 780–785 [DOI] [PubMed] [Google Scholar]
- 6. Li D., Liu Y., Maruyama M., Zhu W., Chen H., Zhang W., Reuter S., Lin S. F., Haneline L. S., Field L. J., Chen P. S., Shou W. (2011) Restrictive loss of plakoglobin in cardiomyocytes leads to arrhythmogenic cardiomyopathy. Hum. Mol. Genet. 20, 4582–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jonkers J., Meuwissen R., van der Gulden H., Peterse H., van der Valk M., Berns A. (2001) Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat. Genet. 29, 418–425 [DOI] [PubMed] [Google Scholar]
- 8. DasGupta R., Fuchs E. (1999) Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 [DOI] [PubMed] [Google Scholar]
- 9. Li D., Hallett M. A., Zhu W., Rubart M., Liu Y., Yang Z., Chen H., Haneline L. S., Chan R. J., Schwartz R. J., Field L. J., Atkinson S. J., Shou W. (2011) Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development 138, 303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Acehan D., Petzold C., Gumper I., Sabatini D. D., Müller E. J., Cowin P., Stokes D. L. (2008) Plakoglobin is required for effective intermediate filament anchorage to desmosomes. J. Invest. Dermatol. 128, 2665–2675 [DOI] [PubMed] [Google Scholar]
- 11. Fukunaga Y., Liu H., Shimizu M., Komiya S., Kawasuji M., Nagafuchi A. (2005) Defining the roles of β-catenin and plakoglobin in cell-cell adhesion: isolation of β-catenin/plakoglobin-deficient F9 cells. Cell Struct. Funct. 30, 25–34 [DOI] [PubMed] [Google Scholar]
- 12. Pummi K., Malminen M., Aho H., Karvonen S. L., Peltonen J., Peltonen S. (2001) Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J. Invest. Dermatol. 117, 1050–1058 [DOI] [PubMed] [Google Scholar]
- 13. Malminen M., Koivukangas V., Peltonen J., Karvonen S. L., Oikarinen A., Peltonen S. (2003) Immunohistological distribution of the tight junction components ZO-1 and occludin in regenerating human epidermis. Br. J. Dermatol. 149, 255–260 [DOI] [PubMed] [Google Scholar]
- 14. Tsuruta D., Hashimoto T., Hamill K. J., Jones J. C. (2011) Hemidesmosomes and focal contact proteins: functions and cross-talk in keratinocytes, bullous diseases, and wound healing. J. Dermatol. Sci. 62, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klymkowsky M. W., Williams B. O., Barish G. D., Varmus H. E., Vourgourakis Y. E. (1999) Membrane-anchored plakoglobins have multiple mechanisms of action in Wnt signaling. Mol. Biol. Cell 10, 3151–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimizu M., Fukunaga Y., Ikenouchi J., Nagafuchi A. (2008) Defining the roles of β-catenin and plakoglobin in LEF/T-cell factor-dependent transcription using β-catenin/plakoglobin-null F9 cells. Mol. Cell. Biol. 28, 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia-Gras E., Lombardi R., Giocondo M. J., Willerson J. T., Schneider M. D., Khoury D. S., Marian A. J. (2006) Suppression of canonical Wnt/β-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Invest. 116, 2012–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 19. Yost C., Torres M., Miller J. R., Huang E., Kimelman D., Moon R. T. (1996) The axis-inducing activity, stability, and subcellular distribution of β-catenin are regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 10, 1443–1454 [DOI] [PubMed] [Google Scholar]
- 20. Haq S., Michael A., Andreucci M., Bhattacharya K., Dotto P., Walters B., Woodgett J., Kilter H., Force T. (2003) Stabilization of β-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc. Natl. Acad. Sci. U.S.A. 100, 4610–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Itin P. H., Fistarol S. K. (2005) Palmoplantar keratodermas. Clin. Dermatol. 23, 15–22 [DOI] [PubMed] [Google Scholar]
- 23. Cabral R. M., Liu L., Hogan C., Dopping-Hepenstal P. J., Winik B. C., Asial R. A., Dobson R., Mein C. A., Baselaga P. A., Mellerio J. E., Nanda A., Boente Mdel C., Kelsell D. P., McGrath J. A., South A. P. (2010) Homozygous mutations in the 5′ region of the JUP gene result in cutaneous disease but normal heart development in children. J. Invest. Dermat. 130, 1543–1550 [DOI] [PubMed] [Google Scholar]
- 24. Pigors M., Kiritsi D., Krümpelmann S., Wagner N., He Y., Podda M., Kohlhase J., Hausser I., Bruckner-Tuderman L., Has C. (2011) Lack of plakoglobin leads to lethal congenital epidermolysis bullosa: a novel clinico-genetic entity. Hum. Mol. Genet. 20, 1811–1819 [DOI] [PubMed] [Google Scholar]
- 25. Erken H., Yariz K. O., Duman D., Kaya C. T., Sayin T., Heper A. O., Tekin M. (2011) Cardiomyopathy with alopecia and palmoplantar keratoderma (CAPK) is caused by a JUP mutation. Br. J. Dermatol. 165, 917–921 [DOI] [PubMed] [Google Scholar]
- 26. Gamborg Nielsen P. (1984) Psoriasis and hereditary palmoplantar keratoderma complicated with a dermatophyte infection. Case report. Dermatologica 168, 293–295 [DOI] [PubMed] [Google Scholar]
- 27. Bierkamp C., Schwarz H., Huber O., Kemler R. (1999) Desmosomal localization of β-catenin in the skin of plakoglobin-null mutant mice. Development 126, 371–381 [DOI] [PubMed] [Google Scholar]
- 28. Li D., Liu Y., Maruyama M., Zhu W., Chen H., Zhang W., Reuter S., Lin S. F., Haneline L. S., Field L. J., Chen P. S., Shou W. (2011) Restrictive loss of plakoglobin in cardiomyocytes leads to arrhythmogenic cardiomyopathy. Hum. Mol. Genet. 20, 4582–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.