Abstract
Human-caused environmental changes are creating regional combinations of environmental conditions that, within the next 50 to 100 years, may fall outside the envelope within which many of the terrestrial plants of a region evolved. These environmental modifications might become a greater cause of global species extinction than direct habitat destruction. The environmental constraints undergoing human modification include levels of soil nitrogen, phosphorus, calcium and pH, atmospheric CO2, herbivore, pathogen, and predator densities, disturbance regimes, and climate. Extinction would occur because the physiologies, morphologies, and life histories of plants limit each species to being a superior competitor for a particular combination of environmental constraints. Changes in these constraints would favor a few species that would competitively displace many other species from a region. In the long-term, the “weedy” taxa that became the dominants of the novel conditions imposed by global change should become the progenitors of a series of new species that are progressively less weedy and better adapted to the new conditions. The relative importance of evolutionary versus community ecology responses to global environmental change would depend on the extent of regional and local recruitment limitation, and on whether the suite of human-imposed constraints were novel just regionally or on continental or global scales.
The earth is undergoing rapid environmental changes because of human actions (1–6). Humans have greatly impacted the rates of supply of the major nutrients that constrain the productivity, composition, and diversity of terrestrial ecosystems. Specifically, the natural rates of nitrogen addition and phosphorus liberation to terrestrial ecosystems (1, 7, 8) have been doubled, and atmospheric CO2 concentrations have been increased to about 40% above preindustrial levels (9). Soil calcium levels are declining in some ecosystems because of increased rates of leaching caused by acidic deposition (10). Humans have relaxed biogeographic barriers to dispersal by accidentally or deliberately moving exotic species to new biogeographic realms (e.g., ref. 11). Through both active fire suppression and increased use of fire as a land clearing or management tool, humans have regionally changed fire frequency (12, 13), which is a major force structuring communities and ecosystems (14). Humans now appropriate more than a third of all terrestrial primary production (15), and, in doing so, have simplified or destroyed large portions of some types of ecosystems, leaving behind fragments that often lack herbivores or predators that provided important top-down constraints. Moreover, many human environmental impacts are projected to be two to three times stronger within 50 years (16). In total, humans may be imposing combinations of constraints that already do, or may soon, fall outside the ranges within which many species evolved.
Here we explore how and whether such changes could result in the loss of local diversity and accelerated extinction (3), and thus potentially decrease ecosystem functioning (e.g., refs. 17–19). The effects of environmental change on species composition, diversity, and ecosystem functioning are poorly understood. As a tool to explore this issue, we use theories that potentially can explain multispecies coexistence (20–29). These models are based on the interplay of environmental constraints and the trade-offs organisms face in dealing with these constraints. They can predict both the persistence of a large number of species (24–29) and the conditions that could lead to extinctions. Although mechanisms differ, all solutions to Hutchinson's (20) paradox of diversity have a similar structure (26, 28, 29). All mechanisms assume that two or more factors constrain fitness, and that intraspecific and interspecific trade-offs constrain each individual or species to having optimal performance at a particular value of these constraints. These processes provide a basis for interpreting the impacts of global human ecosystem domination on community composition, extinction, and speciation.
The physiology, morphology, and life history of a plant necessarily constrains it to survival in only a range of environmental conditions. In the classical literature, these conditions were called its fundamental niche. Each species is, at best, a superior competitor for a narrower range of conditions, classically called its realized niche (30–32). The attributes of sites and regions thus limit the types of species that can occur in them. These classical concepts of fundamental and realized niches underlie recent mechanistic approaches to competition, coexistence, and community structure (24, 25, 28, 33–36) and are a useful way to summarize natural history (e.g., refs. 37–39). Moreover, they suggest that human-caused environmental changes could create “vacant niches” (40)—i.e., evolutionarily novel suites of environmental conditions for which no species in a region are well adapted. In this paper, we use recent mechanistic theory to explore the potential impacts of human-driven environmental change on the composition and diversity of terrestrial plant communities, and on their patterns of speciation.
Environmental Constraints in Plant Communities
What are the major environmental variables that limit the abundance of terrestrial and aquatic plants, and which of these variables are being impacted significantly by human actions? In essence, plants may be limited by nutrients and other resources, by pathogens and herbivores, by disturbances, by dispersal abilities, and by the physical environment, including its climate. These constraints are elaborated below.
Resource Limitation.
Plants require N, P, K, Ca, Mg, S, trace metals, CO2, water, light, and other resources. Depending on the habitat and species, any one or several of these may be limiting. The most commonly limiting resources of terrestrial habitats are N, P, and water (24, 41–44). N limitation is common because the parent materials in which soils form contain almost no N. Rather, the chemically stable form of nitrogen is atmospheric N2, which is usable only by N-fixing plants via microbial symbionts. Non-N-fixing plants obtain N as nitrate, ammonium, or organic N. Some soils are either initially low in other mineral elements, especially phosphorus and calcium, or become low in these after millennia of leaching. The Park Grass plots of Rothamsted, England have joint limitation by N, P, K, and early spring rainfall (43, 44). The greatest changes in plant community biomass, composition, and diversity came from N addition in the grasslands of both Rothamsted and Cedar Creek, Minnesota (45–47). Water is a limiting factor in many terrestrial habitats, as can be the atmospheric concentration of CO2. Light may also be limiting, especially on productive soils in areas with low disturbance and low grazing rates.
Recruitment Limitation.
All sessile plants have the potential to have their abundance limited by dispersal (25, 48–51). This occurs because dispersal is a neighborhood process, and because interspecific interactions also occur locally. Such “contact” processes can cause plants to have spatially patchy distributions (52), and thus to be missing from suitable habitat because of recruitment limitation. A one-time addition of seed of plant species that occurred in a savanna, but were absent from the local sites, led to an 83% increase in local plant species diversity and to a 31% increase in total community plant abundance (53). Because the added species occurred nearby, but were absent locally, their ability to germinate, grow, survive, and reproduce after a one-time seed addition showed that their abundance was limited by recruitment. Long-term observations in a Panamanian rainforest (51) also demonstrated strong recruitment limitation, as have seed addition experiments in other habitats (54, 55). Other evidence of dispersal limitation and of the rate of movement of plant species comes from studies of secondary succession. For instance, 10 to 15 years are required for Schizachyrium scoparium, a prairie plant that is a strong nitrogen competitor, to disperse from margins into abandoned fields, and another 30 years are required for it to attain peak abundance (46). This 40-year time delay between creation of a site and dominance is reduced to 3 years simply by adding seed of little bluestem. Cornell and Lawton (56) found that local diversity was limited less by local interspecific interactions than by recruitment from regional pools. Davis (57, 58) followed the dynamics of North American forests after glacial recession, and observed time lags of thousands of years between a region having the appropriate climate for a tree species and the arrival of that species. Such time lags could greatly influence responses of plant communities to human-caused environmental changes (58). Habitat fragmentation would lengthen such time delays.
Predators and Pathogens.
Plant abundance in both terrestrial and aquatic ecosystems is also limited by the densities and species identities of pathogens and herbivores, which in turn can be limited both by their predators and by dispersal. Thus, top-down forces can greatly constrain both terrestrial and aquatic ecosystems.
Disturbance.
Physical disturbances also limit terrestrial plant communities and sessile (benthic) freshwater and marine plant communities. For many terrestrial ecosystems, fire frequency has been a major constraint, as have been such physical disturbances as wind storms, landslides, mudslides, avalanches, clearings caused by gophers or other fossorial animals, disturbances caused by hooves, wallows, etc.
Temperature/Climate.
The growth rates of terrestrial and aquatic plants are temperature-dependent, with species (and genotypes) having optimal growth and competitive ability at particular temperatures, and thus in particular climates. This is likely the greatest cause of the geographic separation of species along continental climatic gradients, such as north–south gradients and elevational gradients. In addition, the geographic ranges and abundance of many terrestrial plants are limited by temperature extremes, especially by tissue damage associated with freezing or subfreezing temperatures. In addition, within a region, differences in temperature-dependent growth could cause different plant species to be specialized on different portions of the growing season.
Temporal Variation.
Plants respond not just to the mean levels of limiting factors, but also to the extent and patterning of their temporal variation. Some species may be limited or inhibited by such temporal variation, whereas other species may have traits that allow them to exploit such temporal variation (21, 22). This means that temporal variation, itself, can function as an additional limiting factor.
In total, there are a large number of factors and processes that constrain abundance of plants in both terrestrial and aquatic habitats. All of these limiting factors have been implicated as potential determinants of the species composition and diversity of various plant communities. Various combinations of two or, at times, three of these limiting factors have been formally incorporated into theories that are potentially capable of explaining the diversity and composition of terrestrial and aquatic plant communities. Changes in any of these constraints could thus change the abundance of species and genotypes in a habitat.
Anthropogenic Global Change and Plant Constraints
Many of these constraints are undergoing large, rapid changes because of human actions. Recent human activities have more than doubled the preindustrial rate of supply of N to terrestrial ecosystems (7). Nitrogen had a preindustrial terrestrial cycle that involved the annual fixation of about 90 to 140 Tg (teragrams) of N/yr (1, 7), with an additional 10 Tg of N/yr provided by atmospheric N fixation via lightening. Industrial N fixation for fertilizer currently totals about 88 Tg/yr. About 20 Tg/yr of N is fixed during the combustion of fossil fuels, and about 40 Tg/yr of N is fixed by legume crops. In addition, land clearing, biomass burning, and other human activities mobilize and release about an additional 70 Tg of N/yr. The projected expansion of global population to about 9 billion people by year 2050 and shifts to diets higher in animal protein suggest that, by 2050, global food production will be double its current rate (19). If so, anthropogenic terrestrial N inputs in 2050 would be about three to four times the preindustrial rate (16, 19). Much of this N would enter rivers and be carried to near-shore marine ecosystems. N would also be deposited atmospherically on nonagricultural terrestrial ecosystems
Nitrate is readily leached from soil, carrying with it positively charged ions such as Ca. Atmospheric N deposition may be depleting Ca and other cations in hardwood forests of the eastern United States (10). This depletion of base cations could cause elements that had not been limiting in a region to become limiting. Plant species often have distributions constrained by soil pH and Ca.
Phosphorus is a commonly applied agricultural fertilizer, and current P application is a doubling of the natural global rate for terrestrial ecosystems (8). Projections to year 2050 are that agricultural P fertilization will more than double. Much of this P may enter aquatic ecosystems, which can be P-limited.
The accumulation of such greenhouse gases as CO2 and methane may lead to global climate change, with the greatest changes, especially warmer winter temperatures, forecast for temperate and polar ecosystems (e.g., ref. 2). Because climate change and its potential impacts on terrestrial ecosystems are widely studied, we will not review them here. Rather, we merely note that rainfall patterns, the frequency and severity of droughts, and other aspects of climatic mean and variance, which all constrain plant communities, are also forecast to change. In addition, CO2 is a plant nutrient, and elevated levels of CO2 represent atmospheric eutrophication with a limiting plant resource.
Fire frequency is a major variable controlling the species composition and diversity of forests and grasslands (e.g., ref. 14). In the United States, active fire suppression, habitat fragmentation, and other human activities have decreased by 10-fold the area burned each year, from about 22 × 106 ha/yr in 1930 to about 1.5 × 106 ha/yr since about 1960 (13). In contrast, fire frequency is greatly increasing in other habitats, especially tropical habitats, where fire is used as a land-clearing or land-management tool (59).
Modern transportation and commerce have immensely increased both accidental and deliberate introductions of species to novel biogeographic realms (11). About one quarter of the vascular plant species of California, for instance, are exotics. Exotic species are the second largest cause of native species of the United States being listed as endangered (60). Exotic species can impact the abundance of native species in a large number of ways, including via competitive suppression, via changes in disease incidence or some other trophic interaction, via inducing changes in the physical habitats, such as in fire frequency, and changes in nutrient cycles (61, 62). For instance, the invasion of the N-fixing Myrica fava into the Hawaiian Islands greatly increased local N fixation and thence soil N fertility. This increased soil fertility allowed other exotic species to increase in abundance once they were freed from N competition with native plants that where efficient N users (63).
Human actions have also fragmented habitats via conversion of native ecosystems to agricultural lands, urban or suburban lands, roads, power line rights-of-way, etc. Fragmentation is likely to escalate as population and per capita incomes increase globally. Habitat destruction can cause immediate extinction of those species that lived only in areas destroyed, and delayed extinction of poorly dispersing, perhaps competitively superior, species of extant ecosystems (64).
Finally, humans have decreased the geographic ranges and abundance of top predators, especially large carnivores. Decreased abundance of predators have had impacts in both aquatic and terrestrial habitats that have cascaded down the food chain (e.g., refs. 65 and 66), increasing abundance of some herbivores, decreasing abundance of their preferred plant species, and freeing herbivore-resistant species from competitive pressure.
In total, human actions are modifying many environmental constraints that, in combination with intraspecific and interspecific trade-off, led to the evolution of extant plant species and thus influenced the composition, diversity, and functioning of terrestrial and aquatic plant communities. If current trends continue, within 50 to 100 years the suites of factors constraining the structure of many plant communities may fall outside the envelope of values that existed both before the industrial revolution and when many of the plant species evolved.
Ecological Responses to Environmental Change
How would such changes in environmental constraints impact plant communities? Although there would be a continuum of responses, it is instructive to consider two ends of this spectrum: the more immediate, or “ecological” responses, and the more long-term, or “evolutionary” responses, especially patterns of speciation. Clearly, both ecological and evolutionary responses happen simultaneously. We separate them because the evolutionary response in which we are most interested is speciation, which is much slower than changes in species abundance. Ecological responses would depend on the constraints and trade-offs that had structured a given community and on how these had changed. Let us consider a case in which the composition and diversity of a plant community are determined by competition for nitrogen and light (e.g., ref. 28) and by dispersal limitation (25, 49), and explore the impacts of elevated N deposition. The qualitative changes that would occur in this plant community in response to elevated N deposition are the same as those that would occur in response to changes in any other environmental constraint.
Concepts and Theory.
Assuming similar underlying physiologies, each plant species can be represented by the proportion of its biomass that is in either roots (for uptake of nitrogen), stem (which determines plant height and thus light capture), seed (which determines dispersal ability), or leaves (light capture via photosynthesis). For a given spatially homogeneous habitat—a site with a uniform soil of a given fertility (measured by the annual in-site mineralization rate of nitrogen)—and for a given physiology, there would be one pattern of biomass in root, stem, seed, and leaf that led to maximal competitive ability (28). On a low N soil, such as nutrient-maintained (rather than grazing-maintained) grasslands, the best competitor would have high root biomass, enough leaf biomass to provide photosynthate to meet the needs of roots, little biomass in stem (because light is not limiting), and little biomass in seed or rhizome. It would, in essence, be a short species that is an excellent N competitor but a poor disperser, perhaps much like the bunchgrass S. scoparium (little bluestem) of prairie grasslands on sandy soils in the United States, which are ecosystems that have historically experienced frequent burns. Plants with long-lived tissues, such as eracoids, might fill this role in less frequently burned habitats, because greater tissue longevity decreases plant N requirements (67).
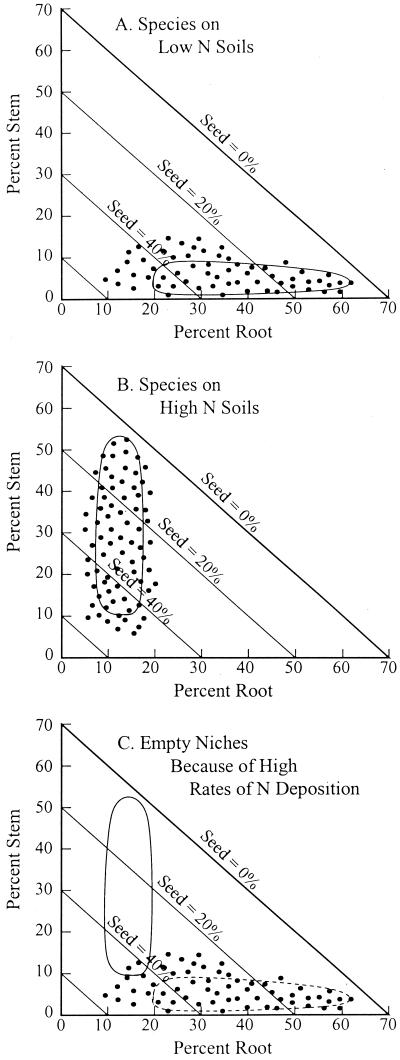
Even if soils were spatially homogeneous, theory predicts that many other plant species could coexist with the best N competitor if they had appropriate trade-off between their competitive ability for N and their dispersal ability (23, 25, 27). Although there is an analytical limit to similarity for this mechanism of coexistence (25), there is no simple limit to the number of species that can stably coexist via this metacommunity process. This is the predominant mechanism of coexistence illustrated in Fig. 1A. It allows numerous species, each represented by a dot, to coexist with the major axis of differentiation being between root biomass (i.e., competitive ability for soil N) and seed biomass (i.e., dispersal ability). This defines the region of trait space in which species can coexist (28), which has a highly elongated shape (closed curve in Fig. 1A). This region of multispecies coexistence spans species with seed biomass from a few percent (the best competitor for N, which is more than 60% root) to more than 40% (the poorest competitor, but the best disperser). The region of coexistence includes species with different stem biomasses because of assumed spatial heterogeneity in the N content of soils. On more N rich soils, species with greater stem biomass are favored over those with more root biomass, because greater stem biomass allows better access to light. This, though, is a minor axis of coexistence compared with the seed–root trade-off for low N habitats.
Figure 1.
(A) Plant species can be represented by the proportion of biomass in leaves, roots, stems, and seeds (28). In low nutrient habitats, superior competitors have high biomass in root, low biomass in stem and seed, and moderate biomass in leaves. Such superior competitors stably coexist with species that are progressively poorer competitors, but better dispersers (25). (B) In a fertile habitat, plant height and thus stem biomass is a determinant of competitive ability for light. (C) A nutrient-poor region, experiencing high rates of nutrient deposition. The region of coexistence includes only a few of the species originally present in the nutrient-poor region. These species would be competitively dominant and displace all of the other species, but be subject to invasion by species in the vacant region enclosed by the solid curve. Because Percent Root + Percent Stem + Percent Seed + Percent Leaf = 100%, Percent Leaf is about 30% for all cases shown.
A comparable pattern occurs for habitats with soils that have high N content (Fig. 1B). The elongated region of coexistence shown again represents coexistence mainly via a competition–colonization trade-off, but in this case the trade-off is between stem allocation (for light capture during competition for light) and seed allocation (dispersal ability that depends on the number and size of seed). Soils of intermediate fertility would favor species intermediate between the extremes shown in Fig. 1 A and B.
About a third of the globe has sandy soils with low N content. What would happen if a region with such soils were to receive projected increased rates of atmospheric N deposition? If all possible species were present throughout the region (i.e., if the whole triangular trait space of Fig. 1 were reasonably well covered with species), there would be a transition, as N accumulated, from a suite of species like those of Fig. 1A to a suite like that of Fig. 1B. However, given that the region receiving elevated N inputs started with low-N soil, the species of Fig. 1B, which occur on N-rich soils, would not be present. Rather, the responses observed would come from those species that happened to be present in the region—those shown in Fig. 1A.
The long-term response of this low-N habitat to greatly elevated N deposition should be dominance by superior light competitors, which have greater stem biomass. However, only two of the original species of the originally low N region would fall within the new trait space favored by N addition (Fig. 1C). These are both weedy species—i.e., species with high seed biomass compared with those that would be expected to be the competitive dominants of the elevated-N habitat. These species are favored initially because, of all of the species present in the original low-N habitat, they have relatively high stem biomass. Under conditions of elevated N, these two species would be expected to increase greatly in abundance where present and to rapidly spread to suitable sites because of their high seed biomass. Some of the other original species of the low-N community might coexist with them, if these additional species had the appropriate trade-off between their competitive ability for light and their dispersal ability. However, most species would be competitively displaced. Thus, a striking feature of Fig. 1C is that the vast majority of the species of the originally species-rich flora of this originally low N region would be competitively displaced by the new dominants. Thus, greatly elevated N deposition should lead to great local extinction.
A second striking feature is the extent to which there are “vacant niches” caused by environmental change—i.e., there are almost no species present in the regional flora that have traits that would normally be favored in such habitats. This is shown by the large empty area within the solid closed curve of Fig. 1C. Any species with traits that fell in this empty area should be able to invade into the region. In total, because of N deposition, the majority of the species that had been the dominants of a region when it was a low N habitat would be competitively displaced by a few formerly rare species, creating an ecosystem highly susceptible to invasion and species turnover until a community like that of Fig. 1B had developed.
Results of Experimental N Additions.
Just such changes in plant diversity and composition are seen when one or a few such factors have been experimentally manipulated for extended periods of time. For instance, fertilization of the Park Grass plots with 4.8 g⋅m−2 of N, as ammonium sulfate, led to dominance by the grass Agrostis (84% of community biomass compared with an average abundance in unfertilized control plots of 12%) and to the loss of 14 of the 19 plant species found, on average, in unfertilized control plots (44, 68). The addition of 14.4 g⋅m−2 of N as ammonium sulfate together with P, K, Mg, and other nutrients led to extreme dominance by Holcus lanatus (Yorkshire fog, a grass), which had an average abundance of 96% in the two replicate high-N plots, compared with an average abundance in the three unfertilized and unlimed control plots of 2%. Both of the high-N plots contained only two plant species, whereas the controls averaged 19 plant species. Experimental N addition in a set of 207 grassland plots in Minnesota showed similarly strong loss of grassland species diversity and similar shifts in species composition at high rates of N addition (28, 69). Moreover, similar shifts in plant community diversity and composition have been reported for ecosystems experiencing high rates of atmospheric N deposition because of nearby intensive agriculture (70, 71). For instance, the heathlands of The Netherlands are an ecosystem type that had dominated sandy soils for millennia. Agricultural intensification in The Netherlands in the 1960s and later was associated with high rates of N fertilization. Much of this N was first captured by crops, then entered cattle as feed, and later was volatilized as ammonia from their wastes. This led to about an order of magnitude increase in the rate of atmospheric N deposition, which contributed to the conversion of species-rich heathlands first into low-diversity stands of a weedy grass (Molinia) and then into shrubby forest (71).
A Generalization of Constraint Surfaces.
These losses of diversity and shifts in species composition have, at their core, a conceptually simple basis (24, 44). The plant species that coexist in the unfertilized control plots do so for a variety of reasons, including interspecific trade-off in their ability to compete for limiting resources (e.g., ref. 24), or trade-off between competitive ability versus local dispersal ability (e.g., refs. 23, 25, and 27), or a trade-off between competitive ability versus resistance to herbivory or disease (e.g., refs. 24 and 72). If plant species coexist in the Park Grass plots because of competition for soil nutrients and light in a spatially heterogeneous environment (24), competitive abilities can be summarized by the relative shapes and positions of the resource-dependent growth isoclines of the species (24). Addition of N pushes this system toward an edge for which all plant species are limited by the same resource, light, and a single species is the superior competitor (24, 44). Moreover, the resource requirements of the Rothamsted species also depend on soil pH (24). The average soil pH of the unmanipulated Rothamsted soils was 5.3, whereas soil pH fell to 4.1 in the plot receiving 4.8 g⋅m−2 of N, and to 3.7 in the plots receiving 14.4 g⋅m−2 of N (68). In essence, the addition of the major limiting soil resource, N, and the associated shift to much more acidic soils, favored the plant species that could live in and were superior competitors for the novel conditions of high N, high plant biomass, low light penetration to the soil, and low soil pH.
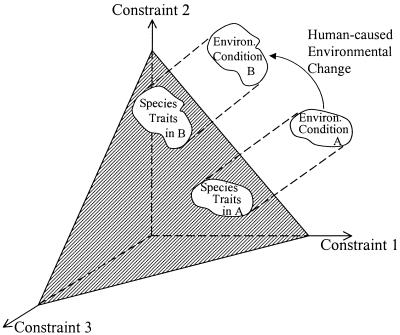
Comparable patterns of dominance by a few formerly rare species, of competitive displacement of most existing species by these newly dominant species, and of high susceptibility to invasion by exotic species would be expected to occur for each of the types of human-caused changes in environmental constraints summarized above. In essence, a given habitat has various factors that constrain the fitness of the organisms that live there, and there is a trade-off surface that defines the potential responses (both within and among species) to these constraints (Fig. 2). Ecological processes, such as interspecific competition, map these environmental conditions onto the constraint surface and thus show the region of traits within which species must fall to persist in a region that has a given suite of environmental conditions (Fig. 2). Changes in any environmental conditions that limit organismal fitness, such as decreased fire frequency, increased N deposition, elevated CO2, increased leaching loss of Ca and P, decreased herbivory, etc., would move the region of coexistence, as illustrated in Fig. 2.
Figure 2.
The qualitative mapping of environmental conditions onto the traits of competitively superior species. The set of values of Constraints 1, 2, and 3 for Environmental Condition A, map into species traits on the trade-off surface, indicated by the shaded plane. Human-caused environmental change moves environmental conditions from Region A to Region B, causing a corresponding shift in the traits of the competitively dominant species.
The High Dimensionality of Environmental Change.
The greater the dimensionality of a habitat is (i.e., the greater its number of constraints), the more its diversity and composition would be impacted by a given amount of environmental change in each variable. As reviewed above, human actions are changing many environmental constraints simultaneously, including N, P, Ca, CO2, pH, fire frequency, trophic structure, and climate. The high dimensionality of these changes may lead to much greater impacts on plant communities than anticipated from a consideration of only one or a few of these factors.
A simple example illustrates this. Consider a habitat in which there are three constraints, factors 1, 2, and 3. The low and high values of these factors might map into a cubic trait space for competitive coexistence. If the values of factor 1 were shifted up by 50%, but nothing else changed, the old trait space and the new trait space would share 50% of their volume, indicating that this change would eliminate about half of the original species and create vacant niches that could be colonized by a comparable number of species, should they exist regionally. If both factor 1 and 2 were increased 50%, the new trait space would overlap with only 25% of the old (i.e., 1/2 × 1/2 = 1/4). If each of the three factors were shifted by 1/2, new trait space would overlap with only 1/8 of the original. In this case, 7/8 of the original species would be driven locally extinct. Comparably, if each of three variables were to be shifted by 2/3, the resultant trait space would overlap only 1/27 of its original volume, and 26/27 of the original species would be lost, on average.
A more formal, although still highly abstracted, treatment of this matter can be provided by a simple extension of Hutchinson's (30) abstraction of the niche as a hypervolume. Suppose species abundance is limited by multiple environmental factors defining orthogonal niche axes and forming a niche space whose boundaries are determined by the largest and smallest possible values of the environmental factors. Suppose that physiological and morphological trade-offs, as well as adaptation to past interspecific interactions, imply some optimal point in the niche space at which the species performs best, and away from which performance drops off. In two dimensions, for example, the axes might be soil pH and temperature, and performance might drop off as in a bivariate normal surface whose peak is at the optimal point (19). In a discrete approximation, the bivariate normal surface becomes a circle within which the species can survive, outside of which it cannot. In multiple dimensions, the circle becomes a hypersphere.
In this abstract view of the niche, prevailing environmental conditions are points in the niche space, and if the species can survive in the prevailing environment, those points fall within the species' niche hypersphere. Anthropogenic actions that change environmental conditions move those points to new locations in the niche space. What is the chance that the moved points will fall within the hypersphere of the species?
With random and independent changes, that chance can be calculated simply by dividing the volume of the species' niche hypersphere by that of the entire niche space. Assuming the species niche is smaller than the entire niche space, then using formulae for the volumes of n-dimensional hyperspheres and hypercubes, that chance can be shown to be always less than
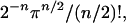 |
where n is the number of environmental conditions changed, and where the factorial is computed via the gamma function when n is odd. Under these assumptions, if two environmental conditions were changed (n = 2), at most about 80% of the species on average would survive, but if eight conditions were changed randomly at once, at most about 1% of the species on average would survive. This multiplicative effect of changes in limiting factors means that several small changes can have as great an impact as one larger change, and that various combinations of small and large environmental changes can, in combination, have an immense impact. Thus, the ecological impacts of human-caused environmental change should depend on the dimensionality of the suite of factors that constrain species abundance, and, in a multiplicative manner, on the magnitudes of changes in all these factors.
In the short-term, such shifts in environmental constraints would eliminate many species and favor once-rare species. The longer-term dynamics of these terrestrial plant communities would depend on the dispersal rates of species both within a region and from other regions, if any, that formerly had characteristics similar to those that occur in the human-impacted region. They also would depend on the evolutionary responses of the species that remain in these habitats.
Evolutionary Responses to Global Change
What might the long-term outcome be of evolution under novel environmental conditions? For one possibility, let us consider again, but on an evolutionary time scale, the effects on a low-N terrestrial plant community of a large increase in the regional rate of N deposition. This could cause light and dispersal ability to become major limiting factors, as illustrated in Fig. 1C. As already discussed, the immediate effect of a high rate of N deposition would be dominance by a few formerly rare, fast-growing, rapidly dispersing plant species. These species would rapidly spread and overtop low-N-adapted species and thus out-compete them for light. However, a large portion of the viable trait space of this community would be empty, as in Fig. 1C. Assuming that N deposition is occurring on a geographically large region, or that habitat fragmentation or other dispersal barriers prevent colonization by suitable superior light competitors, or that the region has experienced other environmental changes (e.g., Ca leaching, soil acidification, invasion by pathogens) that make it inhospitable for otherwise suitable superior light competitors, its longer-term dynamics would be driven as much, or more, by internal evolutionary processes than by colonization.
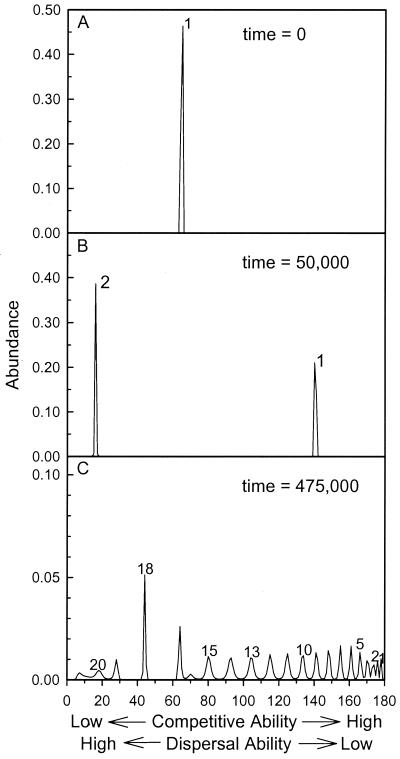
The evolutionary dynamics of such systems have been explored for situations in which it is assumed that there is a strict trade-off between competitive ability and dispersal ability (36, 73, 74). Let us ask what might happen to a weedy plant species that was the initial dominant of a formerly N-poor habitat that experienced elevated N deposition, as shown in Fig. 1C. Numerical solutions to a partial differential equation model (36) show that, within the initially dominant weedy species (species 1 of Fig. 3A), those individuals that are better light competitors have greater fitness than those that are better dispersers. This causes the weedy species to evolve into a progressively better light competitor (acquiring such traits as a larger proportion of biomass in stem, greater height, and larger seed), but to produce fewer seeds and/or allocate less to vegetative spread. Thus, species 1 evolves to the right in Fig. 3A. As species 1 evolves into a better local competitor (and thus a poorer disperser), it occupies fewer sites in the spatial habitat. After this has progressed sufficiently far, an interesting phenomenon occurs. Individuals at the far end of the range of phenotypes, which are good dispersers but poor light competitors, are also favored (species 2 of Fig. 3B). These individuals are poor light competitors, and thus do not competitively inhibit species 1. However, they are good dispersers, which allows them to live in the sites not occupied by species 1.
Figure 3.
Numerical solutions of evolutionary change in a weedy species growing in a spatially implicit habitat in which fitness is limited both by dispersal ability and by competitive ability, based on a model of phenotypic diffusion (36). (A) Given this trade-off, an initially weedy species, species 1, undergoes evolutionary change, with its peak shown moving to the right. (B) After 50,000 years, species 1 has evolved into a much better competitor, but a much poorer disperser than it originally was, and a new species, species 2, has appeared. Species 2 is a superior disperser, but an inferior competitor. It survives in vacant sites in this spatial habitat. (C) Species 1 and 2 each evolve toward being superior competitors. After some time a third species appears that is a poor competitor, but excellent disperser. This third species evolves into a superior competitor and a fourth species appears, etc. Shown here is the result after 475,000 years, at which time 21 peaks of abundance appear, each peak representing a different phenotype, thus corresponding with different species.
In essence, there is a bimodal selective pressure created by competition in a spatial habitat and by an analytical limit to similarity for coexistence of organisms with traits at different points on the trade-off curve (36). This leads to two peaks on the trade-off curve, each peak corresponding to an incipient species (Fig. 3B). Such peaks appear even when all phenotypes are initially rare, and result from the interplay of selection, mutation/recombination, and the competitive limit to similarity. Within each of these peaks, those individuals that are superior light competitors but inferior dispersers are favored, causing the peaks to move to the right in Fig. 3B. Once the second peak, incipient species 2, moves sufficiently far to the right, a third peak appears. It also evolves toward the right, and a fourth peak appears, etc. In numerical solutions of the underlying reaction-diffusion model, after a 475,000 year period, a single weedy species had speciated into 21 species (Fig. 3C) that spanned the empty niche space of Fig. 1C. Such speciation processes would occur within each of the original weedy species, and eventually would yield a local flora as species-rich as occurred before N deposition.
In total, this process suggests that the imposition of novel environmental constraints would lead to the eventual diversification of the flora of a region, with the new flora filling in the empty niches created by novel human-caused environmental conditions. The process by which this is predicted to occur is one in which the ancestral progenitors of this new flora are small, fast-growing, weedy species. Interestingly, this is just what has been suggested to have occurred during the evolution of the angiosperms, during diversification in corals, and during the diversification of terrestrial mammals.
Conclusions
Anthropogenic changes in environmental limiting factors are likely to cause significant loss of plant diversity, leaving many niches empty and creating plant communities dominated by weedier species (poor competitors but good dispersers). The extent of this effect will depend both on the number of constraints that are changed (i.e., dimensionality) and on the magnitude of such changes. Because the impact of multidimensional environmental changes are expected to be multiplicative, a series of relatively small changes may be as important as a single major change. The vacant niches of a region experiencing a major change in an environmental constraint, such as a high rate of N deposition (Fig. 1C), indicate several things about such habitats. First, species that have traits that fall within the newly created vacant niches should be able to invade into, spread through, and persist if propagules are regionally available. Secondly, any heritable variation within existing species that allowed individuals to fill the vacant niches would be favored. For instance, following N deposition, there would be especially strong selection favoring those individuals with greater competitive ability for light, even if this cost dispersal ability. Until the available genetic variation for such traits was consumed, such evolution would be rapid. However, it seems unlikely that such species could rapidly evolve to be equivalent to the species of habitats that had a long evolutionary history of nitrogen rich soils. As such, these newer systems might long be susceptible to invasion by such species, with such invasion often leading to the displacement of the species that were evolving in situ.
Clearly, all of the ideas we have discussed are speculative extensions of a few simple models of community structure and assembly. Such models merit further testing and deeper exploration of their ecological and evolutionary implications.
Acknowledgments
We thank Nancy Larson for her skilled assistance, the colloquium participants for their comments on our presentation, Richard McGehee and Chris Klausmeier for their comments, and National Science Foundation/Division of Environmental Biology Grant 9411972, and The Andrew Mellon Foundation for support.
Footnotes
This paper was presented at the National Academy of Sciences colloquium, “The Future of Evolution,” held March 16–20, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Vitousek P M. Ecology. 1994;75:1861–1876. [Google Scholar]
- 2.Houghton J T, Meiro Filho L G, Callandar B A, Harris N, Kattenberg A, Maskell K. Climate Change 1995: The Science of Climate Change. Cambridge, U.K.: Cambridge Univ. Press; 1996. [Google Scholar]
- 3.Pimm S L, Russell G J, Gittleman J L, Brooks T M. Science. 1995;269:347–350. doi: 10.1126/science.269.5222.347. [DOI] [PubMed] [Google Scholar]
- 4.Vitousek P M, Mooney H A, Lubchenco J, Melillo J M. Science. 1997;277:494–499. [Google Scholar]
- 5.Matson P A, Parton W J, Power A G, Swift M J. Science. 1997;277:504–509. doi: 10.1126/science.277.5325.504. [DOI] [PubMed] [Google Scholar]
- 6.Tilman D. Proc Natl Acad Sci USA. 1999;96:5995–6000. doi: 10.1073/pnas.96.11.5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitousek P M, Aber J D, Howarth R W, Likens G E, Matson P A, Schindler D W, Schlesinger W H, Tilman D. Ecol Appl. 1997;7:737–750. [Google Scholar]
- 8.Carpenter S R, Caraco N F, Correll D L, Howarth R W, Sharpley A N, Smith V H. Ecol Appl. 1998;8:559–568. [Google Scholar]
- 9.Schlesinger W H. Biogeochemistry: An Analysis of Global Change. San Diego: Academic; 1997. [Google Scholar]
- 10.Likens G E, Driscoll C T, Buso D C, Siccama T G, Johnson C E, Lovett G M, Fahey T J, Reiners W A, Ryan D F, Martin C W, et al. Biogeochemistry. 1998;41:89–173. [Google Scholar]
- 11.Lonsdale W M. Ecology. 1999;80:1522–1536. [Google Scholar]
- 12.Bird M I, Cali J A. Nature (London) 1998;394:767–769. [Google Scholar]
- 13.Tilman D, Reich P, Phillips H, Menton M, Patel A, Vos E, Peterson D, Knops J. Ecology. 2000;81:2680–2685. [Google Scholar]
- 14.Clark J S. Ecol Monogr. 1990;60:135–159. [Google Scholar]
- 15.Vitousek P M, Ehrlich P R, Ehrlich A H, Matson P A. Bioscience. 1986;36:368–373. [Google Scholar]
- 16.Tilman, D., Fargione, J., Wolff, B., D'Antonio, C., Dobson, A., Howarth, R., Schindler, D., Schlesinger, W., Simberloff, D. & Swackhamer, D. (2001) Science292, in press. [DOI] [PubMed]
- 17.Chapin F S, III, Zavaleta E S, Eviner V T, Naylor R L, Vitousek P M, Reynolds H L, Hooper D U, Lavorel S, Sala O E, Hobbie S E, et al. Nature (London) 2000;405:234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- 18.Tilman D, Downing J A. Nature (London) 1994;367:363–365. [Google Scholar]
- 19.Tilman D. Ecology. 1999;80:1455–1474. [Google Scholar]
- 20.Hutchinson G E. Am Nat. 1961;95:137–147. [Google Scholar]
- 21.Armstrong R A, McGehee R. Am Nat. 1980;115:151–170. [Google Scholar]
- 22.Levins R. Am Nat. 1979;114:765–783. [Google Scholar]
- 23.Horn H S, MacArthur R H. Ecology. 1972;53:749–752. [Google Scholar]
- 24.Tilman D. Resource Competition and Community Structure. Monographs in Population Biology. Princeton: Princeton Univ. Press; 1982. [PubMed] [Google Scholar]
- 25.Tilman D. Ecology. 1994;75:2–16. [Google Scholar]
- 26.Tilman D, Pacala S. In: Species Diversity in Ecological Communities. Ricklefs R E, Schluter D, editors. Chicago: Univ. of Chicago Press; 1993. pp. 13–25. [Google Scholar]
- 27.Hastings A. Theor Popul Biol. 1980;18:363–373. doi: 10.1016/0040-5809(80)90015-5. [DOI] [PubMed] [Google Scholar]
- 28.Tilman D. Plant Strategies and the Dynamics and Structure of Plant Communities. Princeton: Princeton Univ. Press; 1988. [Google Scholar]
- 29.Chesson P, Huntly N. Am Nat. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson G E. Quant Biol. 1957;22:415–427. [Google Scholar]
- 31.MacArthur R H. In: Population Biology and Evolution. Lewontin R C, editor. Syracuse, NY: Syracuse Univ. Press; 1968. pp. 159–176. [Google Scholar]
- 32.Whittaker R H, Levin S A, editors. Niche: Theory and Application, Benchmark Papers in Ecology. Stroudsburg, PA: Halsted Press; 1975. [Google Scholar]
- 33.Maguire B. Am Nat. 1973;107:213–246. [Google Scholar]
- 34.Van den Bergh J P, Braakhekke W G. In: Structure and Functioning of Plant Populations. Freysen A H J, Woldendorp J W, editors. Amsterdam: North Holland Publishing Co.; 1978. pp. 125–138. [Google Scholar]
- 35.Comins H N, Noble I R. Am Nat. 1985;126:706–723. [Google Scholar]
- 36.Lehman C, Tilman D. In: Spatial Ecology: The Role of Space in Population Dynamics and Interspecific Interactions. Tilman D, Kareiva P, editors. Princeton: Princeton Univ. Press; 1997. pp. 185–203. [Google Scholar]
- 37.Cody M L. In: Community Ecology. Diamond J, Case T, editors. New York: Harper and Row; 1986. pp. 381–405. [Google Scholar]
- 38.Grubb P J. Biol Rev. 1977;52:107–145. [Google Scholar]
- 39.Chesson P. Trends Ecol Evol. 1991;6:26–28. doi: 10.1016/0169-5347(91)90144-M. [DOI] [PubMed] [Google Scholar]
- 40.Lawton J H. In: Ecological Communities: Conceptual Issues and the Evidence. Strong D R, Simberloff D, Abele L G, Thistle A B, editors. Princeton: Princeton Univ. Press; 1984. pp. 67–100. [Google Scholar]
- 41.Vitousek P. Am Nat. 1982;119:553–572. [Google Scholar]
- 42.Vitousek P M. Ecology. 1984;65:285–298. [Google Scholar]
- 43.Thurston J M, Williams E D, Johnston A E. Annales Agronomiques. 1976;27:1043–1082. [Google Scholar]
- 44.Tilman D, Dodd M E, Silvertown J, Poulton P R, Johnston A E, Crawley M J. In: Long-term Experiments in Agricultural and Ecological Sciences. Leigh R A, Johnston A E, editors. Wallingford, Oxon, U.K.: CAB International; 1994. pp. 287–303. [Google Scholar]
- 45.Tilman D. Ecol Monogr. 1987;57:189–214. [Google Scholar]
- 46.Tilman D. Oikos. 1990;58:3–15. [Google Scholar]
- 47.Wedin D A, Tilman D. Science. 1996;274:1720–1723. doi: 10.1126/science.274.5293.1720. [DOI] [PubMed] [Google Scholar]
- 48.Platt W, Weis I. Am Nat. 1977;111:479–513. [Google Scholar]
- 49.Hurtt G C, Pacala S W. J Theor Biol. 1995;176:1–12. [Google Scholar]
- 50.Chesson P. Aust J Ecol. 1998;23:234–240. [Google Scholar]
- 51.Hubbell S P, Foster R B, O'Brien S T, Harms K E, Condit R, Wechsler B, Wright S J, Loo de Lao S. Science. 1999;283:554–557. doi: 10.1126/science.283.5401.554. [DOI] [PubMed] [Google Scholar]
- 52.Durrett R, Levin V. Theor Popul Biol. 1994;46:363–394. [Google Scholar]
- 53.Tilman D. Ecology. 1997;78:81–92. [Google Scholar]
- 54.Cavers P B, Harper J L. J Ecol. 1967;55:59–71. [Google Scholar]
- 55.Robinson G R, Quinn J F, Stanton M L. Ecology. 1995;76:786–794. [Google Scholar]
- 56.Cornell H V, Lawton J H. J Anim Ecol. 1992;61:1–12. [Google Scholar]
- 57.Davis M B. In: Forest Succession. West D C, Shugart H H, Botkin D B, editors. New York: Springer; 1981. pp. 132–153. [Google Scholar]
- 58.Davis M B. In: Community Ecology. Diamond J, Case T, editors. New York: Harper and Row; 1986. pp. 269–284. [Google Scholar]
- 59.Goldammer J G. In: Global Biomass Burning: Atmospheric, Climatic, and Biospheric Implications. Levine J S, editor. Cambridge, MA: MIT Press; 1991. pp. 83–91. [Google Scholar]
- 60.Wilcove D S, Rothstein D, Dubow J, Phillips A, Losos E. Bioscience. 1998;48:607–615. [Google Scholar]
- 61.Walker L R, Smith S D. In: Assessment and Management of Plant Invasions. Luken J O, Thieret J W, editors. New York: Springer; 1997. pp. 69–86. [Google Scholar]
- 62.Mack M C, D'Antonio C M. Trends Ecol Evol. 1998;13:195–198. doi: 10.1016/S0169-5347(97)01286-X. [DOI] [PubMed] [Google Scholar]
- 63.D'Antonio C M, Vitousek P M. Annu Rev Ecol Syst. 1992;23:63–87. [Google Scholar]
- 64.Tilman D, May R M, Lehman C L, Nowak M A. Nature (London) 1994;371:65–66. [Google Scholar]
- 65.Ellenberg H. Vegetation Ecology of Central Europe. 4th Ed. Cambridge, U.K.: Cambridge Univ. Press; 1988. [Google Scholar]
- 66.Carpenter S R, Kitchell J F. The Trophic Cascade in Lakes. Cambridge, U.K.: Cambridge Univ. Press; 1993. [Google Scholar]
- 67.Chapin F S., III Annu Rev Ecol Syst. 1980;11:233–260. [Google Scholar]
- 68.Williams E D. Botanical composition of the Park Grass plots at Rothamsted 1856–1976. Harpenden, Herts, England: Rothamsted Experimental Station; 1978. [Google Scholar]
- 69.Tilman D. Ecology. 1996;77:350–363. [Google Scholar]
- 70.Berendse F, Aerts R. Acta Ecologica. 1984;5:3–14. [Google Scholar]
- 71.Aerts R, Berendse F. Vegetatio. 1988;76:63–69. [Google Scholar]
- 72.Levin B R, Stewart F M, Chao L. Am Nat. 1977;111:3–24. [Google Scholar]
- 73.Kinzig A P, Levin S A, Dushoff J, Pacala S. Am Nat. 1999;153:371–383. doi: 10.1086/303182. [DOI] [PubMed] [Google Scholar]
- 74.Lehman C. Ph.D. Thesis. St. Paul: Univ. of Minnesota; 2000. [Google Scholar]