Background: Apoptotic cell recognition triggers profound immunosuppressive responses; relevant recognition determinants are uncharacterized.
Results: Surface exposure of glycolytic enzymes is a common early apoptotic event.
Conclusion: Externalized glycolytic enzyme molecules are novel apoptotic biomarkers and candidate immunomodulatory/recognition determinants.
Significance: Apoptotic glycolytic enzyme externalization explicates plasminogen binding to mammalian cells and potential mechanisms of immune privilege by commensal bacteria and pathogens.
Keywords: Apoptosis, Cell Surface, Glycolysis, Immunology, Proteomics
Abstract
The intriguing cell biology of apoptotic cell death results in the externalization of numerous autoantigens on the apoptotic cell surface, including protein determinants for specific recognition, linked to immune responses. Apoptotic cells are recognized by phagocytes and trigger an active immunosuppressive response (“innate apoptotic immunity” (IAI)) even in the absence of engulfment. IAI is responsible for the lack of inflammation associated normally with the clearance of apoptotic cells; its failure also has been linked to inflammatory and autoimmune pathology, including systemic lupus erythematosus and rheumatic diseases. Apoptotic recognition determinants underlying IAI have yet to be identified definitively; we argue that these molecules are surface-exposed (during apoptotic cell death), ubiquitously expressed, protease-sensitive, evolutionarily conserved, and resident normally in viable cells (SUPER). Using independent and unbiased quantitative proteomic approaches to characterize apoptotic cell surface proteins and identify candidate SUPER determinants, we made the surprising discovery that components of the glycolytic pathway are enriched on the apoptotic cell surface. Our data demonstrate that glycolytic enzyme externalization is a common and early aspect of cell death in different cell types triggered to die with distinct suicidal stimuli. Exposed glycolytic enzyme molecules meet the criteria for IAI-associated SUPER determinants. In addition, our characterization of the apoptosis-specific externalization of glycolytic enzyme molecules may provide insight into the significance of previously reported cases of plasminogen binding to α-enolase on mammalian cells, as well as mechanisms by which commensal bacteria and pathogens maintain immune privilege.
Introduction
Apoptosis is the primary mechanism by which cells die physiologically and is ongoing throughout life in multicellular organisms. A surprising array of cellular components comprising autoantigens are exposed on the surface of apoptotic cells (1). Apoptotic cells are cleared rapidly in vivo; that this phagocytic clearance occurs in the absence of inflammation has long been recognized (2). The clearance of apoptotic cells is a key homeostatic process and represents a final step of the physiological cell death program (3, 4). The failure to promptly clear apoptotic cells even has been linked to chronic inflammation and autoimmunity characteristic of systemic lupus erythematosus, rheumatoid arthritis, and other pathologies, including atherosclerosis (5–8). Independent of phagocytosis, specific apoptotic recognition elicits a profound repertoire of affirmative signaling and effector responses in macrophages and neighboring cells associated generally with the suppression of inflammation and immune responsiveness; we have termed this “innate apoptotic immunity” (4, 9, 10). It may be that the autoimmune pathologies observed in association with the persistence of apoptotic corpses are consequences of deficits in recognition-specific non-phagocytic responses (11).
The anti-inflammatory effects elicited upon the specific recognition of apoptotic cells (12) result primarily from the triggering of transcriptional responses (especially the repression of inflammatory cytokine gene expression) in cells (both professional and non-professional phagocytes) that interact with them (10, 13). Subsequent responses, including the production of anti-inflammatory cytokines (e.g. TGF-β and IL-10), extend and may enhance the anti-inflammatory state (14). Although numerous molecules have been implicated in the process of apoptotic cell clearance (15), the critical determinants involved in the recognition of apoptotic cells and in the triggering of functional responses to them remain undefined. Our studies have demonstrated that these determinants are evolutionarily conserved and become membrane-exposed during the process of apoptotic cell death without a requirement for ensuing new gene expression (10, 13). Here, we add to this characterization and show that they are protease-sensitive. We note that determinants for apoptotic immune recognition and for the phagocytosis of apoptotic cells may not be identical; for example, phosphatidylserine has been implicated functionally in engulfment (16) and not in innate apoptotic recognition (12, 13).
In an effort to understand the molecular basis for innate immune responses to apoptotic cells, we have taken a comprehensive approach toward the identification of the determinants of apoptotic recognition. We have employed two distinct proteomic approaches based on two-dimensional electrophoretic separations and on isobaric tagging for relative and absolute quantification (iTRAQ),3 and we have exploited apoptotic membrane vesicles as an enriched source of apoptotic recognition determinants. From our analyses, we identified a large number of over- and underrepresented proteins in apoptotic vesicles. We categorized the identified molecules according to previously assigned molecular functions. Notably, these independent approaches both led to the novel observation that numerous components of the glycolytic pathway are enriched on the apoptotic cell surface. Through cytofluorometric analyses, we have confirmed the apoptosis-associated surface exposure of glycolytic enzymes. Moreover, we have extended these findings to reveal that externalization of glycolytic enzymes is a common attribute of apoptotic cell death, occurring independently of the particular suicidal stimulus and in a variety of cells of different tissue types and species of origin.
Although we have not completed our evaluation of all externalized glycolytic enzyme molecules as determinants of innate apoptotic responses, it is clear that surface-exposed glycolytic enzyme molecules represent novel, early, and unambiguous markers (biomarkers) of the apoptotic cell death process. Surface exposure of glycolytic enzymes has been noted previously in a variety of enteric bacteria and pathogens and is responsible for specific plasminogen binding (17–27). This striking commonality of glycolytic enzyme externalization raises the possibility that the exposure of glycolytic enzymes on microorganisms reflects a subversion of innate apoptotic immunity though apoptotic mimicry that facilitates commensalism or pathogenesis. In this light, it may be appropriate to reevaluate the significance of reported plasminogen-binding activities of glycolytic enzymes.
EXPERIMENTAL PROCEDURES
Cells and Death Induction
Primary murine splenocytes (from C57BL/6 mice), S49 murine thymoma cells, DO11.10 murine T cell hybridomas, RAW 264.7 murine macrophages, Jurkat human T leukemia cells, and U937 human monocytic (histiocytic) leukemia cells were cultured at 37 °C in a humidified 5% (v/v) CO2 atmosphere in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with heat-inactivated 10% (v/v) FBS (HyClone Laboratories, Logan, UT), 2 mm l-glutamine, and 50 μm 2-mercaptoethanol. HeLa human cervical carcinoma cells and B2 cells, a transfectant reporter clone of 293T human transformed kidney epithelial cells (13), were grown in DMEM with 4.5 g/liter glucose (Mediatech) supplemented with 10% (v/v) FBS and 2 mm l-glutamine. Physiological cell death (apoptosis) was induced by treatment of cells with the macromolecular synthesis inhibitor actinomycin D (200 ng/ml, 12 h) (28), by irradiation (20 mJ/cm2) with UVC (254 nm) light, or with staurosporine (1 μm in serum-free medium for 3 h). Autophagy was induced by serum starvation with l-canavanine (1 mm) in the presence of the pan-caspase inhibitor quinolyl-valyl-aspartyl-difluorophenoxy methyl ketone (10 μm; R&D Systems, Minneapolis, MN) and was confirmed by the development of LC3-GFP puncta in transfected cells (29). Pathological cell death (necrosis) was triggered by incubation of cells at 56 °C for 10–20 min (until trypan blue uptake indicated compromise of membrane integrity). Iron-depleted medium was prepared by treatment with Chelex 100 resin (Bio-Rad) as described (30). In some experiments, apoptotic and viable target cells were digested with 0.1% (w/v) trypsin (Sigma) in PBS at 37 °C for 15 min and/or fixed by incubation with 125 mm formaldehyde (Polysciences, Inc., Warrington, PA) in PBS at 25 °C for 20 min as described previously (13).
Vesicles
Plasma membrane vesicles were prepared from HeLa cells as described previously (13). Monolayers of cells, either untreated or induced to die for 4 h with actinomycin D (and still adherent), were stimulated to vesiculate by incubation at 37 °C in vesiculation buffer (10 mm HEPES (pH 7.4), 150 mm NaCl, 2 mm CaCl2, 2 mm DTT, and 25 mm formaldehyde). Supernatants were collected after ∼2.5 h (when abundant small membrane vesicles were apparent in the culture fluid). Non-adherent cells were removed by centrifugation at 1000 × g for 10 min at 4 °C, and vesicles were pelleted from the cleared supernatant by centrifugation at 30,000 × g for 60 min at 4 °C. Cytofluorometric analysis indicated that vesicles were ∼0.8 μm in diameter and that the level of contaminating intact cells was less than one cell/100 vesicles. Viable vesicles are comparable in size. Protein content was determined by the Bradford method (Bio-Rad); phospholipid content was quantified by the method of Rouser et al. (31).
Cytofluorometric Analyses
For staining with primary antibodies conjugated to FITC or phycoerythrin (PE) or for staining with FITC-conjugated plasminogen (BioMac, Leipzig, German), cells were washed twice with cold PBS containing FBS (1%) before resuspension and staining in the same buffer for 40 min at 4 °C in the dark prior to washing and cytofluorometric analysis. Staining involving unconjugated primary antibodies followed the same procedure and was followed by a second incubation with an appropriate FITC- or PE-conjugated secondary antibody. The vendors of the antibodies and staining reagents used are Abcam (Cambridge, MA), BD Biosciences, Enzo Life Sciences (Farmingdale, NY), Novus Biologicals (Littleton, CO), and Santa Cruz Biotechnology (Santa Cruz, CA).
The accessibility of phosphatidylserine was revealed by the binding of FITC- or PE-conjugated annexin V (BD Biosciences). Cells that had been washed twice with PBS were resuspended in 100 μl of annexin V binding buffer (10 mm HEPES (pH 7.4), 150 mm NaCl, and 2.5 mm CaCl2) and incubated with 5 μl of the conjugated annexin V for 40 min in the dark at 25 °C. Propidium iodide and 7-aminoactinomycin D (7-AAD) were employed to assess plasma membrane integrity. Propidium iodide (λex = 488 nm and λem = 610 nm) or 7-AAD (λex = 488 nm and λem = 647 nm) was added to cells (at final concentrations of 1 or 4 μg/ml, respectively) immediately before cytofluorometric analysis.
For intracellular staining, cells were washed twice with cold PBS and fixed and permeabilized in a solution of 4% formaldehyde and 0.1% and saponin in PBS for 30 min at 4 °C in the dark. After fixation, cells were washed twice with PBS buffer containing 0.1% saponin and 1% FBS and stained in this same buffer.
Cells were analyzed cytofluorometrically on a FACSCalibur instrument (BD Biosciences). The excitation wavelength (λex) in all cases was at 488 nm. The fluorescence emission wavelength (λem) from fluorescein was collected at 530 ± 15 nm, from propidium iodide and PE at 610 ± 15 nm, and from 7-AAD at 650 ± 20 nm. Cytofluorometric data were processed with WinMDI software (Joe Trotter, The Scripps Research Institute, La Jolla, CA) or Summit version 4.3 software (Dako, Carpentaria, CA). Where appropriate, fluorescence data are expressed as mean fluorescence intensity (MFI). Attributes of cell death, including changes in forward-angle and side-angle light scatter, were also evaluated as described previously (12).
Transcriptional Modulation
Apoptotic immunosuppressive activity was assessed with respect to NF-κB-dependent transcription utilizing a clone of the B2 reporter cell line (B2.1) as described previously (13).
Protein Identification by Two-dimensional Gel Electrophoresis
Vesicles were lysed by sonication in isoelectric focusing rehydration buffer (7 m urea, 2 m thiourea, 4% CHAPS, 100 mm DTT, 0.2% Bio-Lyte (pH 5–8), 0.01% bromphenol blue, and protease inhibitor). Seventy-five μg of protein in a total of 185 μl of rehydration buffer was applied to 11-cm Bio-Rad ReadyStrip immobilized pH gradient strips (pH 5–8) for overnight rehydration. First-dimension isoelectric focusing was carried out on a Bio-Rad PROTEAN isoelectric focusing system for a total focusing time of 75,000 V-h. After focusing, strips were equilibrated with equilibration buffer I (6 m urea, 0.375 m Tris-HCl (pH 8.8), 2% SDS, 20% glycerol, and 2% (w/v) DTT) for 15 min. The strips were further equilibrated with equilibration buffer II (6 m urea, 0.375 m Tris-HCl (pH 8.8), 2% SDS, 20% glycerol, and 2.5% (w/v) iodoacetamide) for 15 min and directly applied to a 12.5% isocratic SDS-polyacrylamide gel for the second dimension. The resulting gel was then fixed in 10% acetic acid and 40% ethanol for 30 min and stained overnight with SYPRO Ruby. Gels were destained with 10% methanol and 7.5% acetic acid for 60 min. After washing with water, gels were scanned on a 9400 Typhoon variable mode imager (GE Healthcare) using a green laser (532 nm) and 610BP30 emission filter. Quantitative analysis of spots on gels was performed using PDQuest (Bio-Rad) and visually confirmed.
Protein spots from SYPRO Ruby-stained gels were picked for protein identification. The gel spots were diced into 1-mm3 pieces and washed with 30% acetonitrile in 50 mm ammonium bicarbonate prior to DTT reduction and iodoacetamide alkylation. Trypsin was used for overnight digestion at 37 °C. The resulting peptides were extracted with 30 μl of 1% trifluoroacetic acid, followed by desalting with ZipTip C18 pipette tips (Millipore, Bedford, MA). For MS analysis, the peptides were mixed with 7 mg/ml α-cyano-4-hydroxycinnamic acid matrix (in 60% acetonitrile) in a 1:1 ratio and spotted onto a MALDI plate. The peptides were analyzed on a 4800 MALDI TOF/TOF analyzer (Applied Biosystems, Framingham, MA). Mass spectra (m/z 880–3200) were acquired in positive ion reflector mode. The 15 most intense ions were selected for subsequent MS/MS sequencing analysis in 1-keV mode. Protein identification was performed by searching the combined MS and MS/MS spectra against the human NCBI database using a local MASCOT search engine (version 1.9) on a GPS server (version 3.5, Applied Biosystems). Proteins containing at least two peptides identified with confidence interval values of no less than 95% were considered as being identified.
Protein Quantification by iTRAQ Analysis
For iTRAQ labeling, proteins from vesicles were extracted in iTRAQ lysis buffer (1% Nonidet P-40, 1% Triton X-100, 10 mm HEPES, and 500 mm triethylammonium bicarbonate buffer) using probe sonication at 50% duty for three cycles of 15 s with a 60-s incubation in ice-cold water between cycles. The lysate was cleared by centrifugation at 16,100 × g for 15 min. The pH of samples was adjusted to 8.0 with 1.0 m triethylammonium bicarbonate buffer. The iTRAQ labeling procedures were performed according to the manufacturer's instructions as further described (32). Briefly, after reduction with tris(2-carboxyethyl)phosphine hydrochloride and alkylation with methyl methanethiosulfonate, tryptic digestion of each sample (100 μg) was initiated by the addition of 10 μg of trypsin (Promega, Madison, WI), and each sample was incubated overnight at 37 °C. Peptides derived from viable samples were labeled with iTRAQ tags 114 and 115, whereas peptides derived from apoptotic samples were labeled with iTRAQ tags 116 and 117. The labeled samples were then mixed together and fractionated via strong cation exchange and reverse phase chromatography according to a procedure described previously (33). The HPLC eluate was mixed with a matrix solution (7 mg/ml α-cyano-4-hydroxycinnamic acid in 60% acetonitrile, 5 mm ammonium monobasic phosphate, and internal mass calibrants (50 fmol/μl each [Glu1]fibrinopeptide B and ACTH(18–39)) through a 30-nl mixing tee and directly spotted onto the MALDI plates. The peptides were analyzed on a 4800 Proteomics Analyzer MALDI TOF/TOF tandem mass spectrometer (Applied Biosystems) in a data-dependent fashion using job-wide interpretation. MS spectra (m/z 800–3600) were acquired in positive ion reflectron mode with internal mass calibration. A maximum of the 15 most intense ions (signal-to-noise ratio > 50) per spot were selected for subsequent MS/MS analysis in 1.0-keV mode. Each spectrum was averaged over 2000 laser shots.
Protein Database Search and Bioinformatics
TS2Mascot (Matrix Science Inc., Boston, MA) was used to generate peak lists in Mascot generic file format from the MS/MS spectra using the following parameters: mass range from 20 to 60 Da below precursor and signal-to-noise ratio ≥ 10. The peak lists was submitted for automated search using a local Mascot server (version 2.2) against 20,244 proteins in the Swiss-Prot human protein sequence database. The following parameters were used for the search: iTRAQ 4plex (K), iTRAQ 4plex (N-terminal), and methylthio (C) as fixed modifications; iTRAQ 4plex (Y) and oxidation (M) as variable modifications; trypsin as the digestive enzyme with up to two missed cleavages allowed; monoisotopic mass with peptide precursor mass tolerance of 50 ppm; and MS/MS ion mass tolerance of 0.3 Da. Scaffold (version 3_03_01, Proteome Software Inc., Portland, OR) was used to filter MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at or greater than 95.0% probability and ≤1.0% false discovery rate as specified by the Peptide Prophet algorithm (34). Protein identifications were accepted if they could be established at or greater than 95.0% probability and contained at least one identified peptide at 95% confidence. Protein probabilities were assigned by the Protein Prophet algorithm (35). Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Peptides were quantified using the centroided iTRAQ reporter ion peak intensity. Protein quantitative values were derived from only uniquely assigned peptides. Protein quantitative ratios were calculated as the median of all relevant peptide ratios for each protein. A log 2-fold ratio for each protein reported by Scaffold was transformed to normal relative protein abundance -fold ratios in Excel (Microsoft, Redmond, WA). A two-tailed t test was performed using Excel for final statistical evaluation. In addition, only proteins altered in abundance by at least 20% from the viable vesicles were considered significant. For each identified protein, associated gene ontology terms were automatically fetched from the European Bioinformatics Institute by Scaffold software and plotted with respect to enrichment.
Enzyme Assays
Enolase activity was assessed as the fluoride-inhibitable (36) conversion of 2-phosphoglycerate to phosphoenolpyruvate by a modification of an assay described by Pancholi and Fischetti (20). Intact viable and apoptotic HeLa cells were washed once with 1× PBS. Graded numbers of cells (ranging from 1 × 106 to 3 × 104) were added to a 200-μl reaction in enolase buffer (10 mm MgCl2 in 1× PBS). Reactions were started with the addition of 2-phosphoglycerate (to a final concentration of 3 mm). After 4 min of incubation at 25 °C, reactions were terminated by the addition of 800 μl of enolase stop buffer (10 mm MgCl2 and 3 mm NaF in 1× PBS). Cells were removed by centrifugation at 600 × g for 5 min, and phosphoenolpyruvate in supernatants was quantified spectrophotometrically (λ = 240 nm). The molar extinction coefficient of phosphoenolpyruvate at 240 nm is 1.164 × 103. Enolase activity in cell extracts (comparably graded cell equivalents) was assessed similarly. Extracts were prepared from viable and apoptotic HeLa cells by sonication for 6 × 10 s at 60 watts (Vibra-Cell sonicator, Sonics and Materials, Danbury, CT) after allowing the cells to swell on ice in 0.1× PBS for 30 min. Enolase activity in cell supernatants was assessed after incubating graded numbers of intact cells (as described above) in mock reactions in enolase buffer without 2-phosphoglycerate. After 4 min, cells were removed by centrifugation. 2-Phosphoglycerate was then added to the supernatant, and the 2-phosphoglycerate-dependent production of phosphoenolpyruvate was assessed after 4 min as described above.
The determination of GAPDH activity followed a similar set of procedures and was assessed as the conversion of NAD to NADH dependent on glyceraldehyde 3-phosphate by a modification of an assay described by Pancholi and Fischetti (17). In particular, the reaction buffer was adjusted to iso-osmolarity. Graded numbers of intact, washed, apoptotic, and viable HeLa cells (ranging from 3 × 105 to 1 × 104) were added to a 200-μl reaction in GAPDH buffer (60 mm NaCl, 50 mm Na2HPO4, 40 mm triethanolamine, and 1 mm NAD (pH 8.6)). Reactions were started by the addition of glyceraldehyde 3-phosphate to a final concentration of 2 mm. After 4 min of incubation at 25 °C, reactions were terminated by the addition of 800 μl of GAPDH stop buffer (60 mm NaCl, 50 mm Na2HPO4, and 40 mm triethanolamine (pH 10.0)). Cells were removed by centrifugation, and NADH in supernatants was quantified spectrophotometrically (λ = 340 nm). The molar extinction coefficient of NADH at 340 nm is 6.22 × 103.
RESULTS
Apoptotic Suppressive Determinants, Enriched in Membrane Vesicles, Are Protease-sensitive
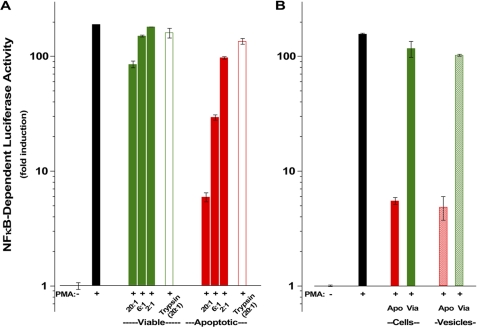
We have demonstrated that apoptotic determinants for recognition and immune modulation are evolutionarily conserved and arise on the surface of cells during the process of apoptotic death without a requirement for ensuing new gene expression (10, 12). The ability of apoptotic cells to modulate inflammatory responses occurs primarily at the level of transcription (10) and can be assessed reliably with transcriptional reporters that disclose primary inflammatory responses (i.e. transcriptional promoters linked to the firefly luciferase gene and responsive to critical transcriptional activators involved in inflammatory responses, such as NF-κB) (10, 13). Fig. 1 provides examples of the specific dose-dependent effects of apoptotic cells on a responsive cell line harboring such a transcriptional reporter. B2.1 is a highly responsive clone of stably transfected human HEK293T reporter cells (13). The results show that apoptotic cells repressed NF-κB-dependent transcription, whereas viable cells did not and recapitulated cytokine responses of those cells (13). Importantly, apoptotic cells of distinct species and tissues of origin (S49 (in Fig. 1A) is a murine T lymphocyte cell line, and HeLa (in Fig. 1B) is a human epithelial cell line) triggered equivalent responses.
FIGURE 1.
Apoptotic suppressive determinants are protease-sensitive and enriched in membrane vesicles. Apoptotic suppression of NF-κB-dependent transcription was assessed with respect to NF-κB-dependent luciferase activity in B2.1 reporter cells (13). A, suppression of phorbol 12-myristate 13-acetate (PMA; 1.25 ng/ml)-induced NF-κB-dependent luciferase activity by graded numbers (indicated as the target/responder ratio) of viable (Via; green) or apoptotic (Apo; actinomycin D, 200 ng/ml; red) S49 murine T cells (closed bars) and targets that had been digested with trypsin (0.1%, 15 min, 37 °C; open bars) was assessed. Trypsin was removed from targets by extensive washing, and residual activity was quenched by incubation in 10% serum-containing medium. B, human epithelial (HeLa) cell targets were left untreated or treated with actinomycin D. The suppressive activity of cells (closed bars; target/responder ratio = 8:1) and vesicles (hatched bars; target/responder ratio = 30:1), prepared by incubation of untreated and treated cells in vesiculation buffer (see “Experimental Procedures”), was assessed independently as described for A.
Previously, we found that the phospholipid phosphatidylserine is neither a sufficient determinant (12) nor a necessary component (13) for specific apoptotic recognition and immune modulation. We wondered whether apoptotic immunosuppressive activity involves protein determinants and would therefore be susceptible to proteolytic digestion. Indeed, we found that when apoptotic cells were digested with trypsin, their immunomodulatory activity was lost (Fig. 1A). Interestingly, we found that upon extended incubation following tryptic digestion, apoptotic cells recovered modulatory activity (data not shown). Fixation with formaldehyde after protease treatment precludes this recovery, although apoptotic immunomodulatory activity is stable to fixation with formaldehyde (13). We interpret these results to suggest that apoptotic immunomodulatory determinants are protease-sensitive molecules (or molecular complexes including essential protein components) that are resident in all cells prior to cell death and that some fraction of the intracellular stores of the relevant molecules becomes surface-exposed (and susceptible to tryptic digestion) due to apoptosis-specific post-translational modification. For brevity, the acronym SUPER (surface-exposed (during apoptotic cell death), ubiquitously expressed, protease-sensitive, evolutionarily conserved, and resident normally in viable cells) serves to emphasize these defining properties of apoptotic determinants for recognition and immune modulation.
We have shown that membrane vesicles prepared from apoptotic cells expose these determinants (13). In fact, assays for specific NF-κB-dependent transcriptional suppression as a function of the dose of apoptotic vesicles reveal that apoptotic membrane vesicles are enriched in immunomodulatory determinants, relative to whole apoptotic cells and comparable vesicles prepared from viable cells. As shown in Fig. 1B, titration of the immunomodulatory activity of intact apoptotic HeLa cells and apoptotic vesicles prepared from them demonstrated that vesicles have ∼25% of the immunomodulatory activity of whole cells. (The low level at which whole cells contaminate vesicle preparations does not account for this activity.) Given that the surface area of whole cells is ∼125-fold greater than that of these vesicles (we estimate the ratio of surface areas as 4πrc2/4πrv2 ≅ 500, where rc (the radius of an intact HeLa cell) is ∼9 μm and rv (the average radius of a vesicle) is 0.8 μm) and that the membrane-associated protein content/nmol of phospholipid of a vesicle is ∼30-fold greater than that of a whole cell (4.70 μg of protein/nmol of phospholipid for intact cells versus 0.15 μg of protein/nmol of phospholipid for vesicles), we calculate the vesicle enrichment of immunomodulatory protein determinants to be ∼1000-fold (i.e. 0.25 × 125 × 30 = 938).
Proteomic Analyses of Apoptotic Membrane Vesicles Reveal Membrane Association of Glycolytic Enzyme Molecules
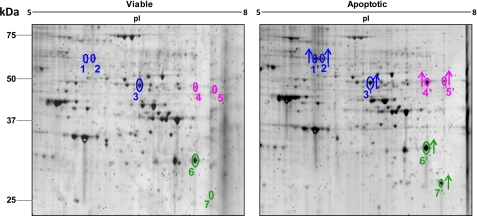
The implication that determinants of innate apoptotic immunity necessarily include essential protein components and that membrane vesicles provide an enriched source of those determinants led us to undertake a systematic analysis of the proteome of apoptotic plasma membrane vesicles. We performed a comparative analysis of membrane vesicle proteins prepared from apoptotic and viable cells by two-dimensional gel electrophoresis (Fig. 2 and Table 1). After electrophoretic resolution, spots of proteins that were distinctly altered in abundance (over- or underrepresented) were excised and subjected to tryptic digestion, followed by nanoflow LC and MS/MS analysis.
FIGURE 2.
Analysis of membrane vesicle proteins by two-dimensional gel electrophoresis. Shown are the results from comparative analysis of membrane vesicle preparations from viable and apoptotic HeLa cells by two-dimensional gel electrophoresis. Multiple gel spots corresponding to pyruvate kinase (blue), EnoA (magenta), and TPI (green) are indicated. The increased abundance of protein spots among apoptotic (relative to viable) membrane vesicles is indicated by arrows. Details are listed in Table 1.
TABLE 1.
Isoforms of identified membrane proteins
Interestingly, among the most prominent of the overrepresented species we observed were proteins known to be involved as enzymes in the terminal stages of glucose metabolism, including pyruvate kinase (Fig. 2, highlighted in blue), α-enolase (EnoA; shown in magenta), and triose-phosphate isomerase (TPI; shown in green). Each of these proteins was identified in multiple gel spots (with distinct pI values), and the abundance of each of the distinct spots was found to be increased among proteins from the apoptotic (relative to viable) membrane vesicles (Fig. 2). The multiple spots may be indicative of isoforms of each of these proteins harboring post-translational modifications. Notably, apoptosis-associated proteolysis does not appear generally to underlie these modifications; only in the case of TPI (spot 7′) is an apoptosis-enriched isoform of distinctly lower apparent molecular mass (presumably due to proteolytic cleavage) evident.
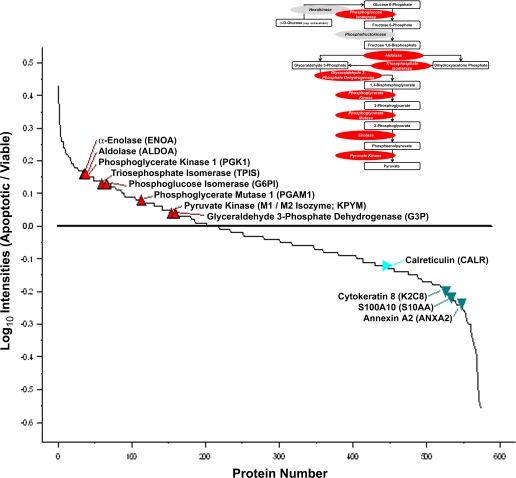
Independently, we undertook a complete quantitative proteomic characterization of apoptotic and viable membrane vesicles employing iTRAQ technology, which permits the analysis of less abundant species. Apoptotic and viable vesicle extracts were denatured, alkylated, and labeled with isobaric iTRAQ reagent tags (see “Experimental Procedures”). Duplicate viable vesicle peptides were labeled with iTRAQ reagent tags 114 and 115 (differential tags of 114 and 115 Da, respectively); duplicate apoptotic membrane peptides were labeled with iTRAQ tags 116 and 117. Samples were then digested with trypsin, and the labeled peptides were mixed in even ratios and quantified by LC-MS/MS. The relative abundance (enrichment or depletion) of distinct peptides in apoptotic and viable samples was determined by replicate comparisons between the labeled samples. From these data, we identified a total of 564 proteins (supplemental Table I). A graphic representation of this distribution, highlighting selected proteins, is shown in Fig. 3. Tables 2 and 3 list 56 overrepresented and 105 underrepresented proteins, respectively, that varied by at least 20% between apoptotic and viable membrane vesicle preparations at a false discovery rate of <1%. We have previously established, with known standards, that the analytical coefficient of variance is <10%; therefore, changes between apoptotic and viable membrane proteins of >20% are reliably significant. Notably, the overrepresented population included proteins from all intracellular locales (see below).
FIGURE 3.
Relative abundance of apoptotic and viable membrane vesicle proteins determined by iTRAQ analysis. Shown is a compilation of the relative abundance of membrane vesicle proteins from apoptotic versus viable preparations (Apoptotic/Viable) determined by iTRAQ analysis (see supplemental Table I). The relative abundance of selected proteins is indicated. Proteins involved as enzymes in the glycolytic pathway (see inset; shown in red) are found to be overrepresented. Other molecules identified as plasminogen receptors (see text; shown in green) are underrepresented.
TABLE 2.
Overrepresented apoptotic membrane-associated proteins
Proteins identified by iTRAQ analysis and found to be overrepresented among apoptotic membrane vesicles by at least 20% at a confidence level of >95% are listed. SUMO, small ubiquitin-like modifier.
| Identified proteins | Accession ID | Ratio | t test |
|---|---|---|---|
| Cytochrome c | CYC_HUMAN | 2.71 | 0.01 |
| Stathmin | STMN1_HUMAN | 2.07 | 0.00 |
| Macrophage migration inhibitory factor | MIF_HUMAN | 2.00 | 0.01 |
| Acyl-CoA-binding protein | ACBP_HUMAN | 1.94 | 0.05 |
| SUMO-conjugating enzyme UBC9 | UBC9_HUMAN | 1.81 | 0.02 |
| Profilin-1 | PROF1_HUMAN | 1.80 | 0.01 |
| T-complex protein 1 subunit ϵ | TCPE_HUMAN | 1.74 | 0.01 |
| Peptidylprolyl cis,trans-isomerase A | PPIA_HUMAN | 1.74 | 0.00 |
| Calpastatin | ICAL_HUMAN | 1.68 | 0.03 |
| Glutathione S-transferase Ω1 | GSTO1_HUMAN | 1.68 | 0.00 |
| Aldose reductase | ALDR_HUMAN | 1.68 | 0.02 |
| Heat shock protein HSP90α | HS90A_HUMAN | 1.68 | 0.05 |
| Transgelin-2 | TAGL2_HUMAN | 1.68 | 0.05 |
| Plastin-3 | PLST_HUMAN | 1.62 | 0.01 |
| Nucleoside-diphosphate kinase B | NDKB_HUMAN | 1.62 | 0.03 |
| 14-3-3 protein β/α | 1433B_HUMAN | 1.57 | 0.02 |
| Thioredoxin | THIO_HUMAN | 1.56 | 0.03 |
| Myristoylated alanine-rich C-kinase substrate | MARCS_HUMAN | 1.52 | 0.05 |
| Importin-7 | IPO7_HUMAN | 1.52 | 0.01 |
| Glutathione S-transferase P | GSTP1_HUMAN | 1.51 | 0.02 |
| α-Actinin-1 | ACTN1_HUMAN | 1.46 | 0.01 |
| Spectrin β chain, brain 1 | SPTB2_HUMAN | 1.46 | 0.01 |
| Cytosolic phospholipase A2 | PA24A_HUMAN | 1.46 | 0.01 |
| ATP synthase subunit d, mitochondrial | ATP5H_HUMAN | 1.46 | 0.01 |
| Nuclear migration protein NudC | NUDC_HUMAN | 1.46 | 0.01 |
| α-Enolase | ENOA_HUMAN | 1.46 | 0.03 |
| Fructose-bisphosphate aldolase A | ALDOA_HUMAN | 1.46 | 0.03 |
| T-complex protein 1 subunit θ | TCPQ_HUMAN | 1.46 | 0.03 |
| Phosphoglycerate kinase 1 | PGK1_HUMAN | 1.46 | 0.03 |
| Heat shock cognate 71-kDa protein | HSP7C_HUMAN | 1.37 | 0.01 |
| Actin-related protein 2/3 complex subunit 3 | ARPC3_HUMAN | 1.37 | 0.01 |
| Nascent polypeptide-associated complex subunit α | NACA_HUMAN | 1.37 | 0.01 |
| Cu,Zn-superoxide dismutase | SODC_HUMAN | 1.36 | 0.05 |
| Proteasome subunit β type 3 | PSB3_HUMAN | 1.36 | 0.05 |
| Triose-phosphate isomerase | TPIS_HUMAN | 1.36 | 0.05 |
| Heat shock protein HSP90β | HS90B_HUMAN | 1.36 | 0.05 |
| 14-3-3 protein ϵ | 1433E_HUMAN | 1.36 | 0.05 |
| Tyrosyl-tRNA synthetase, cytoplasmic | SYYC_HUMAN | 1.36 | 0.05 |
| Ubiquitin-like modifier-activating enzyme 1 | UBA1_HUMAN | 1.36 | 0.05 |
| Ezrin | EZRI_HUMAN | 1.32 | 0.03 |
| Cofilin-1 | COF1_HUMAN | 1.32 | 0.03 |
| Transgelin | TAGL_HUMAN | 1.32 | 0.03 |
| Proteasome subunit α type 5 | PSA5_HUMAN | 1.32 | 0.00 |
| T-complex protein 1 subunit γ | TCPG_HUMAN | 1.32 | 0.04 |
| l-Lactate dehydrogenase A chain | LDHA_HUMAN | 1.32 | 0.04 |
| Ras-related protein Rab-1B | RAB1B_HUMAN | 1.28 | 0.02 |
| Actin-related protein 2/3 complex subunit 2 | ARPC2_HUMAN | 1.27 | 0.02 |
| Eukaryotic peptide chain release factor GTP-binding subunit ERF3A | ERF3A_HUMAN | 1.27 | 0.02 |
| Fatty acid synthase | FAS_HUMAN | 1.23 | 0.00 |
| Protein SET | SET_HUMAN | 1.19 | 0.04 |
| Phosphoglycerate mutase 1 | PGAM1_HUMAN | 1.19 | 0.04 |
| Elongation factor 1γ | EF1G_HUMAN | 1.19 | 0.04 |
| Proliferation-associated protein 2G4 | PA2G4_HUMAN | 1.19 | 0.03 |
| Elongation factor 2 | EF2_HUMAN | 1.19 | 0.03 |
| Acidic leucine-rich nuclear phosphoprotein 32 family member A | AN32A_HUMAN | 1.19 | 0.03 |
| Importin subunit β1 | IMB1_HUMAN | 1.19 | 0.03 |
TABLE 3.
Underrepresented apoptotic membrane-associated proteins
Proteins identified by iTRAQ analysis and found to be underrepresented among apoptotic membrane vesicles by at least 20% at a confidence level of >95% are listed.
| Identified proteins | Accession ID | Ratio | t test |
|---|---|---|---|
| Histone H2A type 2B | H2A2B_HUMAN | 0.28 | 0.01 |
| Large neutral amino acids transporter small subunit 1 | LAT1_HUMAN | 0.29 | 0.03 |
| Dipeptidyl peptidase 1 | CATC_HUMAN | 0.31 | 0.02 |
| Small nuclear ribonucleoprotein-associated proteins B and B′ | RSMB_HUMAN | 0.32 | 0.03 |
| Alkaline phosphatase, tissue-nonspecific isozyme | PPBT_HUMAN | 0.42 | 0.03 |
| Asparagine synthetase (glutamine-hydrolyzing) | ASNS_HUMAN | 0.42 | 0.02 |
| 60 S ribosomal protein L19 | RL19_HUMAN | 0.45 | 0.03 |
| Annexin A5 | ANXA5_HUMAN | 0.45 | 0.02 |
| Annexin A6 | ANXA6_HUMAN | 0.47 | 0.02 |
| 60 S ribosomal protein L24 | RL24_HUMAN | 0.50 | 0.02 |
| Cytochrome bc1 complex subunit 7 | QCR7_HUMAN | 0.50 | 0.02 |
| 40 S ribosomal protein S17 | RS17_HUMAN | 0.52 | 0.00 |
| Keratin, type I cytoskeletal 10 | K1C10_HUMAN | 0.54 | 0.04 |
| 60 S ribosomal protein L34 | RL34_HUMAN | 0.56 | 0.04 |
| 40 S ribosomal protein S2 | RS2_HUMAN | 0.57 | 0.03 |
| Annexin A2 | ANXA2_HUMAN | 0.57 | 0.03 |
| Heat shock protein β1 | HSPB1_HUMAN | 0.57 | 0.00 |
| 40 S ribosomal protein S23 | RS23_HUMAN | 0.57 | 0.01 |
| Protein S100A4 | S10A4_HUMAN | 0.57 | 0.03 |
| 60 S ribosomal protein L32 | RL32_HUMAN | 0.58 | 0.01 |
| Programmed cell death protein 6 | PDCD6_HUMAN | 0.59 | 0.03 |
| 60 S ribosomal protein L36a | RL36A_HUMAN | 0.59 | 0.00 |
| DNA replication licensing factor MCM5 | MCM5_HUMAN | 0.60 | 0.02 |
| 60 S ribosomal protein L9 | RL9_HUMAN | 0.60 | 0.02 |
| Protein transport protein Sec31A | SC31A_HUMAN | 0.60 | 0.02 |
| Tubulin β4 chain | TBB4_HUMAN | 0.60 | 0.05 |
| Protein S100A10 | S10AA_HUMAN | 0.61 | 0.03 |
| Small nuclear ribonucleoprotein SmD3 | SMD3_HUMAN | 0.61 | 0.03 |
| Polypeptide N-acetylgalactosaminyltransferase 2 | GALT2_HUMAN | 0.62 | 0.00 |
| Non-POU domain-containing octamer-binding protein | NONO_HUMAN | 0.62 | 0.01 |
| 60 S ribosomal protein L14 | RL14_HUMAN | 0.62 | 0.04 |
| Endothelin-converting enzyme 1 | ECE1_HUMAN | 0.62 | 0.04 |
| Keratin, type II cytoskeletal 8 | K2C8_HUMAN | 0.64 | 0.04 |
| Interleukin enhancer-binding factor 3 | ILF3_HUMAN | 0.64 | 0.04 |
| Histone H2B type 1 J | H2B1J_HUMAN | 0.64 | 0.01 |
| DNA replication licensing factor MCM4 | MCM4_HUMAN | 0.64 | 0.02 |
| Histone H1.5 | H15_HUMAN | 0.64 | 0.02 |
| 40 S ribosomal protein S18 | RS18_HUMAN | 0.66 | 0.04 |
| Annexin A1 | ANXA1_HUMAN | 0.66 | 0.04 |
| 60 S ribosomal protein L26 | RL26_HUMAN | 0.66 | 0.00 |
| Lysosome-associated membrane glycoprotein 1 | LAMP1_HUMAN | 0.66 | 0.01 |
| Δ3,5,Δ2,4-Dienoyl-CoA isomerase, mitochondrial | ECH1_HUMAN | 0.66 | 0.01 |
| Splicing factor, arginine/serine-rich 6 | SFRS6_HUMAN | 0.66 | 0.01 |
| Mitochondrial carrier homolog 2 | MTCH2_HUMAN | 0.66 | 0.01 |
| Probable ATP-dependent RNA helicase DDX17 | DDX17_HUMAN | 0.66 | 0.01 |
| 40 S ribosomal protein S16 | RS16_HUMAN | 0.68 | 0.05 |
| 40 S ribosomal protein S3a | RS3A_HUMAN | 0.68 | 0.05 |
| Clathrin light chain A | CLCA_HUMAN | 0.68 | 0.05 |
| EGF-like repeat- and discoidin I-like domain-containing protein 3 | EDIL3_HUMAN | 0.68 | 0.05 |
| Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit β1 | GBB1_HUMAN | 0.68 | 0.05 |
| 40 S ribosomal protein S9 | RS9_HUMAN | 0.68 | 0.01 |
| N-Acetylgalactosaminyltransferase 7 | GALT7_HUMAN | 0.68 | 0.01 |
| 40 S ribosomal protein S15a | RS15A_HUMAN | 0.68 | 0.01 |
| Aspartyl-tRNA synthetase, cytoplasmic | SYDC_HUMAN | 0.68 | 0.01 |
| X-ray repair cross-complementing protein 5 | XRCC5_HUMAN | 0.68 | 0.03 |
| Interleukin enhancer-binding factor 2 | ILF2_HUMAN | 0.68 | 0.03 |
| Protein ERGIC-53 | LMAN1_HUMAN | 0.69 | 0.05 |
| 60 S ribosomal protein L23 | RL23_HUMAN | 0.71 | 0.05 |
| Heterogeneous nuclear ribonucleoproteins A2 and B1 | ROA2_HUMAN | 0.71 | 0.00 |
| Peroxiredoxin-2 | PRDX2_HUMAN | 0.71 | 0.00 |
| 60 S ribosomal protein L13 | RL13_HUMAN | 0.71 | 0.02 |
| Reticulon-4 | RTN4_HUMAN | 0.71 | 0.02 |
| 60 S ribosomal protein L18 | RL18_HUMAN | 0.71 | 0.02 |
| 60 S ribosomal protein L15 | RL15_HUMAN | 0.71 | 0.03 |
| Myosin light polypeptide 6 | MYL6_HUMAN | 0.73 | 0.02 |
| 60 S ribosomal protein L6 | RL6_HUMAN | 0.73 | 0.02 |
| Heterogeneous nuclear ribonucleoproteins C1 and C2 | HNRPC_HUMAN | 0.73 | 0.02 |
| 60 S ribosomal protein L35 | RL35_HUMAN | 0.73 | 0.01 |
| Calumenin | CALU_HUMAN | 0.73 | 0.01 |
| DNA topoisomerase 2α | TOP2A_HUMAN | 0.73 | 0.01 |
| Heterogeneous nuclear ribonucleoprotein G | HNRPG_HUMAN | 0.73 | 0.01 |
| 60 S ribosomal protein L3 | RL3_HUMAN | 0.73 | 0.05 |
| ADP/ATP translocase 2 | ADT2_HUMAN | 0.73 | 0.05 |
| Tubulin β chain | TBB5_HUMAN | 0.76 | 0.00 |
| DNA-dependent protein kinase catalytic subunit | PRKDC_HUMAN | 0.76 | 0.03 |
| 40 S ribosomal protein S10 | RS10_HUMAN | 0.76 | 0.03 |
| Vigilin | VIGLN_HUMAN | 0.76 | 0.03 |
| 60 S ribosomal protein L4 | RL4_HUMAN | 0.78 | 0.02 |
| 40 S ribosomal protein S19 | RS19_HUMAN | 0.78 | 0.02 |
| 60 S ribosomal protein L10 | RL10_HUMAN | 0.78 | 0.02 |
| 60 S ribosomal protein L21 | RL21_HUMAN | 0.78 | 0.02 |
| 60 S ribosomal protein L7a | RL7A_HUMAN | 0.78 | 0.02 |
| Heterogeneous nuclear ribonucleoprotein D0 | HNRPD_HUMAN | 0.78 | 0.02 |
| Heterogeneous nuclear ribonucleoprotein R | HNRPR_HUMAN | 0.78 | 0.02 |
| 40 S ribosomal protein S4, X isoform | RS4X_HUMAN | 0.81 | 0.05 |
| 60 S ribosomal protein L13a | RL13A_HUMAN | 0.81 | 0.05 |
| 60 S ribosomal protein L23a | RL23A_HUMAN | 0.81 | 0.05 |
| 60 S ribosomal protein L18a | RL18A_HUMAN | 0.81 | 0.05 |
| Heterogeneous nuclear ribonucleoprotein L | HNRPL_HUMAN | 0.81 | 0.05 |
| Serpin H1 | SERPH_HUMAN | 0.81 | 0.05 |
| Thymidylate kinase | KTHY_HUMAN | 0.81 | 0.05 |
| Trifunctional enzyme subunit α, mitochondrial | ECHA_HUMAN | 0.81 | 0.05 |
| ATP-dependent RNA helicase A | DHX9_HUMAN | 0.84 | 0.04 |
| 26 S protease regulatory subunit 4 | PRS4_HUMAN | 0.84 | 0.04 |
| 60 S acidic ribosomal protein P0 | RLA0_HUMAN | 0.84 | 0.04 |
| Actin-related protein 2 | ARP2_HUMAN | 0.84 | 0.04 |
| Cytochrome b5 | CYB5_HUMAN | 0.84 | 0.04 |
| Filamin-A | FLNA_HUMAN | 0.84 | 0.04 |
| Nucleolin | NUCL_HUMAN | 0.84 | 0.04 |
| Signal recognition particle 9-kDa protein | SRP09_HUMAN | 0.84 | 0.04 |
| 60 S ribosomal protein L7 | RL7_HUMAN | 0.84 | 0.03 |
| 40 S ribosomal protein S8 | RS8_HUMAN | 0.84 | 0.03 |
| 60 S ribosomal protein L35a | RL35A_HUMAN | 0.84 | 0.03 |
| Histone H2A.V | H2AV_HUMAN | 0.84 | 0.03 |
| Splicing factor, proline- and glutamine-rich | SFPQ_HUMAN | 0.84 | 0.03 |
With the vesiculation protocol, as many as 8% of the cells in an otherwise untreated control (“viable”) cell culture become apoptotic. Thus, we would expect that an idealized candidate SUPER molecule (i.e. a protein that is not associated with viable cell membranes and that is associated specifically with apoptotic cell membranes (and that actually is overrepresented by x-fold among apoptotic cell membrane proteins)) would appear to be enriched among apoptotic vesicle proteins by no more than ∼12-fold (i.e. x/0.08x). Although the membrane vesicles that we prepared have no ultrastructure and are depleted for non-membrane-associated molecules, the non-surface-exposed intravesicular contents of viable vesicles may contribute further to a diminution of the apparent enrichment of apoptosis-specific membrane proteins in apoptotic vesicle preparations. Indeed, the maximum enrichment of a protein that we observed among apoptotic vesicles (Fig. 3) was <3-fold.
Inventory of Apoptotic Membrane Proteins
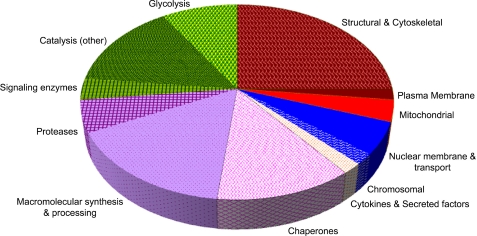
We categorized the identified molecules according to previous descriptions of their major molecular functions (Fig. 4; see below). Molecules characterized for their catalytic activities involved in metabolism constituted the largest group of overrepresented proteins present in apoptotic cell vesicles (15/56). Among these, glycolytic enzymes (including aldolase, TPI, phosphoglycerate kinase, EnoA, and pyruvate kinase) were highly represented, consistent with our two-dimensional gel electrophoresis analysis. An example of this enrichment, observed by iTRAQ analysis, is presented for EnoA peptides (Fig. 5). Indeed, it is striking that almost all members of the glycolytic pathway are enriched among apoptotic cell membranes (see Fig. 3, inset). Other major classes among the overrepresented proteins present in apoptotic vesicles are structural (including cytoskeletal) molecules (14/56), those involved in macromolecular synthesis (especially translation) and processing (including proteases) (12/56), and molecular chaperones (7/56).
FIGURE 4.
Functional categorization of overrepresented proteins from apoptotic membrane vesicles. Shown is the functional categorization (gene ontology) of proteins found to be enriched among apoptotic membrane vesicles by iTRAQ analysis (see Table 2). The two largest groups of proteins (26.8% each) are those characterized as membrane proteins and structural and cytoskeletal elements (shown in brown) and as catalytic proteins (shown in green). Proteins associated with the glycolytic pathway are the largest coherent cohort (9.0%) within either group. The next largest groups are proteins associated with macromolecular synthesis (especially translation) and processing and proteases (21.4%; shaded in violet) and molecular chaperones (12.5%; cross-hatched magenta area).
FIGURE 5.
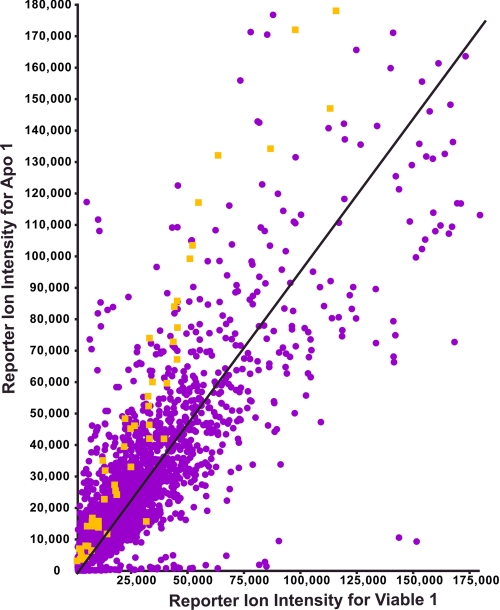
Detail of EnoA data from iTRAQ analysis. Shown is the overrepresentation of EnoA ascertained from the consistent iTRAQ ratios obtained from all related precursor ions. The plot shows reporter ion 114 (Viable 1) versus 116 (Apo 1) intensities for all peptides (purple dots) identified in iTRAQ experiment. Yellow dots represent the iTRAQ signals derived from spectra matched to EnoA (Apo 1).
Importantly, some of the proteins identified here as enriched among apoptotic vesicles (for example, the actin-associated proteins cofilin and Arp2/3 (37), numerous heat shock proteins, and other chaperones associated with stress (38–40)) fulfill expectations derived from other studies. Similarly, the mitochondrial protein cytochrome c is known to be released from mitochondria during apoptotic cell death (41, 42). It is significant that not all of the vesicle-enriched molecules are membrane-exposed; in particular, we did not detect surface-exposed cytochrome c (data not shown).
On the other hand, some proteins expected to be enriched in apoptotic vesicles were not identified in this array. Actin was expected to be among the apoptotic vesicle proteins (43–45), along with actin-associated and other structural molecules. Histones also were expected to be enriched among apoptotic vesicle proteins based on previous studies documenting their unique presence on the apoptotic cell surface (1). The observation that vesicles have immunomodulatory activity (like intact apoptotic cells) but lack externalized histones (unlike intact apoptotic cells) allows us to exclude histones definitively as apoptotic recognition determinants. Annexin A1, which has been suggested previously to be involved in apoptotic recognition (46), was relatively depleted in our apoptotic vesicle preparation (only ∼70% representation) (Table 3) relative to the viable vesicle preparation. Other proteomic studies also have not found annexin A1 to be present generally among apoptotic membrane proteins (see, for example, Ref. 47).
It is most striking that both of the independent proteomic analyses we employed identified the preferential membrane vesicle association of glycolytic enzymes with apoptotic cell death. Because the issue of surface exposure is a defining criterion with regard to apoptotic determinants for recognition and immune modulation (SUPER) candidates, we sought to evaluate whether glycolytic enzyme molecules are present on the apoptotic cell surface.
Cytofluorometric Confirmation of Apoptotic Externalization of Glycolytic Enzyme Molecules
We examined independently the preferential enrichment of glycolytic enzyme molecules among apoptotic membrane proteins and tested specifically whether those molecules are exposed on the apoptotic cell surface. We analyzed apoptotic cells for the externalization of three glycolytic enzymes (EnoA, GAPDH, and TPI) by immunofluorescence and cytofluorometric analyses. We examined a variety of cell types and cell lines for cell death-associated externalization of these proteins. (In addition to the HeLa cells used to prepare the membrane vesicles subjected to our protein analyses, we analyzed human and murine epithelial, lymphoid, and myeloid cell lines and primary cells induced to undergo apoptotic death with a variety of suicidal stimuli.)
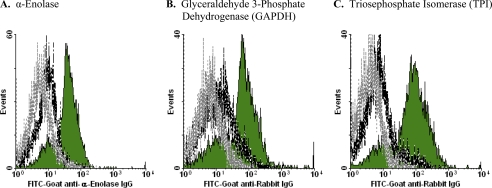
By immunofluorescence staining analysis, we found that EnoA is displayed generally on the surface of apoptotic (but not viable) cells. An example of these analyses (with primary murine splenocytes) is presented in Fig. 6A. Completely analogous staining patterns were observed for the two other glycolytic enzymes that were analyzed, GAPDH (Fig. 6B) and TPI (Fig. 6C). Thus, glycolytic enzyme molecules not only are enriched among apoptotic membrane proteins but are exposed specifically on the apoptotic cell surface. Externalized glycolytic enzyme molecules fulfill the critical criteria for apoptotic determinants of recognition and immune modulation: they are evolutionarily conserved proteins that are resident in all cells ubiquitously and, although unexposed on the surface of viable cells, are membrane-exposed on apoptotic cells.
FIGURE 6.
Cytofluorometric analysis of externalization of glycolytic enzyme molecules. Murine splenocytes that had undergone apoptosis spontaneously in culture (12 h) and freshly isolated, viable splenocytes were analyzed cytofluorometrically following staining with FITC-conjugated goat anti-EnoA peptide IgG polyclonal antibody (Santa Cruz Biotechnology) (A), rabbit anti-GAPDH IgG polyclonal antibody (Abcam) and FITC-conjugated goat anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology) (B), and rabbit anti-TPI IgG polyclonal antibody (Novus Biologicals) and FITC-conjugated goat anti-rabbit IgG secondary antibody (C). Profiles shown are for apoptotic (solid green histograms) and viable (black dashed lines) cells stained with the specific FITC-conjugated reagents and for apoptotic cells stained with the secondary antibody alone (gray dotted lines). (The profile of viable cells is identical.) Cells that had lost membrane integrity (propidium iodide-positive, reduced forward- and expanded side-angle light scatter) were excluded from these analyses by electronic gating.
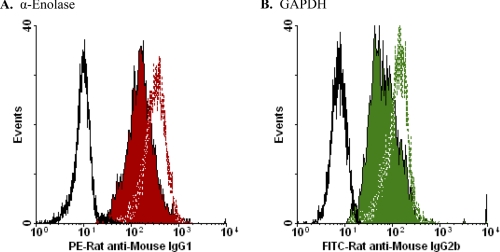
We evaluated the extent of glycolytic enzyme molecule externalization by comparing immunofluorescence staining of membrane-intact and permeabilized apoptotic cells. Examples of these analyses are presented in Fig. 7. Permeabilized viable and apoptotic cells stained identically and homogeneously for EnoA (Fig. 7A), GAPDH (Fig. 7B), and TPI (data not shown). The extent of externalization of these molecules ranged from 10 to 50% of the total cellular protein for each species during apoptotic cell death (by comparison of mean fluorescence intensities; see Fig. 7 legend). Importantly, these data indicate that the observed apoptosis-specific externalization of glycolytic enzyme molecules is distinct from a general accessibility to intracellular molecules that would result from plasma membrane compromise. In contrast, the detection of glycolytic enzyme molecules on necrotic cells is not distinguishable from plasma membrane compromise (see below). Our data also demonstrate that there is no net elevation of cellular glycolytic enzyme concentration with apoptosis, but rather a preferential externalization of those molecules.
FIGURE 7.
Quantification of glycolytic enzyme molecule externalization. Apoptotic cells (induced with actinomycin D) were analyzed cytofluorometrically without (solid red or green histogram) or following (red or green dashed lines) permeabilization. A, HeLa cells were stained with mouse anti-EnoA monoclonal antibody (L-27, Santa Cruz Biotechnology) and PE-conjugated rat anti-mouse IgG1 secondary monoclonal antibody (BD Biosciences). The MFI of staining for non-permeabilized cells is 156.5; the MFI for permeabilized cells is 307.3; and the MFI for staining with secondary antibody alone (black solid line) is 10.0. We calculated the fraction of total cellular EnoA that was externalized in this experiment as 49%. The profile of staining of permeabilized viable HeLa cells (MFI = 319.5) was very similar to that of permeabilized apoptotic HeLa cells. B, DO11.10 cells were stained with mouse anti-GAPDH monoclonal antibody (Abcam) and FITC-conjugated rat anti-mouse IgG2b secondary antibody (BD Biosciences). The MFI for non-permeabilized cells is 67.2; the MFI for permeabilized cells is 142.0; and the MFI for staining with secondary antibody alone (black solid line) is 15.2. The fraction of total cellular GAPDH externalized is 41%. The profile of staining of permeabilized viable DO11.10 cells (MFI = 138.5) was indistinguishable from that of permeabilized apoptotic DO11.10 cells.
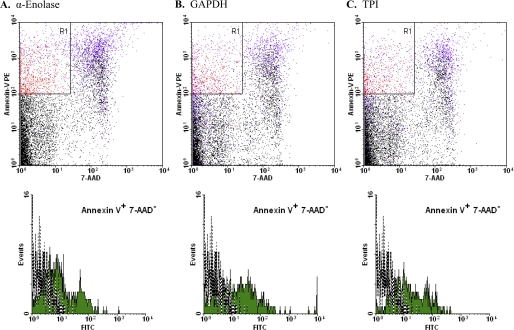
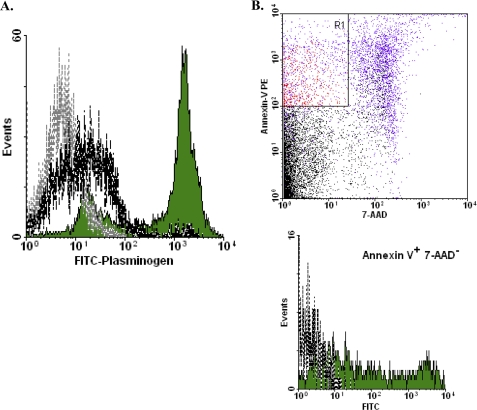
We explored the kinetics of the appearance of these glycolytic enzyme molecules by examining the earliest apoptotic cells (annexin V+/7-AAD−) following brief treatment with staurosporine, a potent inducer of apoptosis (Fig. 8). Externalized EnoA, GAPDH, and TPI could be found already externalized on these early apoptotic cells. Interestingly, the extent of exposure ranged as high as the levels found on later apoptotic cells. By comparison, neither autophagic or necrotic cells exposed glycolytic enzyme molecules (supplemental Fig. 1; note that we examined “early” (annexin V+/7-AAD−) necrotic cells (12) to preclude the detection of intracellular glycolytic molecules that become accessible with the loss of membrane integrity), consistent with the notion that the externalization of glycolytic enzyme molecules is a specific attribute of the apoptotic cell death process. Furthermore, we found that the process of glycolytic enzyme externalization, like apoptosis itself, is caspase-dependent (supplemental Fig. 2).
FIGURE 8.
Characterization of appearance of exposed glycolytic enzyme molecules. Murine splenocytes that were induced to undergo apoptosis with staurosporine were analyzed cytofluorometrically following staining with PE-conjugated annexin V, 7-AAD, and FITC-conjugated goat anti-EnoA peptide IgG polyclonal antibody (A), rabbit anti-GAPDH IgG polyclonal antibody and FITC-conjugated goat anti-rabbit IgG secondary antibody (B), and rabbit anti-TPI IgG polyclonal antibody and FITC-conjugated goat anti-rabbit IgG secondary antibody (C). Cells that met the criteria of staining positively with annexin V and negatively with 7-AAD (Annexin V+ 7-AAD−; the R1 region indicated in red in the upper dot plots) were gated electronically, and the fluorescein signal of those cells was analyzed (shown as solid green histograms in the lower panels). The fluorescein signal of annexin V+/7-AAD− cells stained with secondary antibody alone also is presented (gray dotted lines).
These staining data involve a variety of mono- and polyclonal antibodies. Although all apoptotic cells reacted with polyclonal sera specific for the glycolytic enzymes, we noted differences among apoptotic cell populations with regard to reactivity with glycolytic enzyme-specific monoclonal antibodies (data not shown). Some glycolytic enzyme epitopes appear not to be exposed in some cell lines (although those epitopes are immunologically detectable intracellularly). These data suggest that the process of apoptosis-specific externalization may be constrained conformationally (see below).
In summary, our data from three independent approaches reveal that the externalization of glycolytic enzyme molecules is a dramatic event that occurs reliably and early during the process of apoptotic cell death. The surface-exposed, membrane-associated forms of glycolytic enzyme proteins represent novel and unambiguous apoptosis-specific biomarkers.
Externalized Glycolytic Enzyme Molecules Lack Enzymatic Activity
We assessed enolase and GAPDH activities in apoptotic and viable cells. Although apoptotic cells had elevated levels of apparently externalized activity, accounting for 40–50% of the total cellular activity observed in whole cell extracts (Table 4), virtually all of that externalized activity can be attributed to activity leaking from broken cells, i.e. we found equivalent activity in cell supernatants prepared by incubation of cells in reaction mixtures (that are of physiological osmolarity) without substrate. Thus, we have no evidence that the “externalized” activities we observed represent the enzymatic activity of enzyme molecules localized to the cell surface as opposed to the activities of intracellular enzyme molecules released due to plasma membrane leakage. Indeed, we saw similar absolute levels of enolase and GAPDH activities associated with undisrupted cells from untreated cultures (although total cellular activities in apoptotic cultures were 40–60% lower than in viable cultures) (Table 4).
TABLE 4.
Cell-associated enzymatic activities
Enolase and GAPDH activities were assessed as described under “Experimental Procedures.” Graded numbers of undisrupted viable or apoptotic HeLa cells or the sonicated extracts of equivalent cell numbers (disrupted cells) were incubated in 200-μl reactions for 4 min at 25 °C. Cells and cell debris were then removed by centrifugation, and reaction products were quantified spectrophotometrically. Activity in cell supernatants was assessed by incubating cells as described above in mock reactions without substrate, removing cells by centrifugation, and then incubating supernatants with substrate for an additional 4 min at 25 °C.
| Enolase activity (HeLa cells)a |
||
|---|---|---|
| Cells from viable populations | Cells from apoptotic populations | |
| nmol/104 cells | ||
| Disrupted cell activity | 9.7 ± 1.1 | 3.7 ± 0.8 |
| Undisrupted cell activity | 1.9 ± 0.4 | 1.9 ± 0.5 |
| Undisrupted cell supernatant activity | 1.4 ± 0.2 | 1.2 ± 0.2 |
| GAPDH activity (HeLa cells)b |
||
|---|---|---|
| Cells from viable populations | Cells from apoptotic populations | |
| nmol/104 cells | ||
| Disrupted cell activity | 5.5 ± 0.8 | 3.4 ± 0.1 |
| Undisrupted cell activity | 0.8 ± 0.1 | 1.4 ± 0.4 |
| Undisrupted cell supernatant activity | 1.2 ± 0.3 | 1.2 ± 0.1 |
a Conversion of 2-phosphoglycerate to phosphoenolpyruvate.
b Glyceraldehyde 3-phosphate-dependent formation of NADH.
We conclude that apoptosis-specific externalized enzyme molecules are not enzymatically active. This conclusion is consistent with our suggestion that the process of apoptosis-specific externalization may be conformationally constrained (see above).
Externalized Glycolytic Enzyme Molecules Bind Plasminogen
Several of the glycolytic enzyme molecules that we have shown to be externalized with apoptosis (especially EnoA, GAPDH, and phosphoglycerate kinase) have been implicated previously in the binding of plasminogen (48–53). Elevated plasminogen binding associated with apoptotic cell death also has been described (54). Consistent with these observations, we observed robust plasminogen binding to apoptotic cells (Fig. 9A). Plasminogen binding, like glycolytic enzyme molecule externalization, was evident on the earliest apoptotic cells (Fig. 9B).
FIGURE 9.
Cytofluorometric analysis of plasminogen binding to apoptotic cells. A, murine splenocytes that had undergone apoptosis spontaneously in culture (12 h) and freshly isolated, viable splenocytes were analyzed cytofluorometrically as described in the legend of Fig. 6 following staining with FITC-conjugated plasminogen. B, murine splenocytes that were induced to undergo apoptosis with staurosporine were analyzed cytofluorometrically following staining with PE-conjugated annexin V, 7-AAD, and FITC-conjugated plasminogen and analyzed as described in the legend of Fig. 8.
Pre-binding of plasminogen to apoptotic cells precluded the binding of EnoA-specific antibodies, indicating that EnoA serves as a plasminogen-binding “receptor” (data not shown), although it appears that EnoA is not the only species responsible for plasminogen binding to apoptotic cells. Conversely, plasminogen binding to the apoptotic cell surface was inhibited competitively with the lysine analog ϵ-aminocaproic acid (data not shown), consistent with the characterization of cell surface lysine residues as targets for plasminogen binding (48).
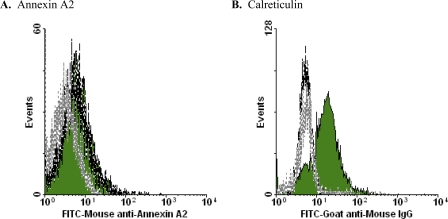
In contrast to the glycolytic enzyme molecules, annexin A2, which also has been implicated as a plasminogen-binding receptor (52), is not exposed preferentially during apoptotic cell death. There is concordance between iTRAQ and cytofluorometric analyses in this regard (Figs. 3 and 10A). By comparison, cytofluorometric analysis revealed the apoptosis-specific externalization of calreticulin (Fig. 10B), although our iTRAQ analysis did not identify calreticulin among apoptotic membrane vesicle-enriched molecules (Fig. 3). The exposure of calreticulin in association with apoptotic cell death has been noted previously (Ref. 55, but see Ref. 56).
FIGURE 10.
Cytofluorometric analysis of externalization of other molecules. Human transformed (Jurkat) T lymphocytes that had been induced to undergo apoptosis by treatment with actinomycin D or that had been left untreated were analyzed cytofluorometrically following staining with FITC-conjugated mouse anti-human annexin II monoclonal antibody (BD Biosciences) (A) or mouse anti-calreticulin monoclonal and FITC-conjugated goat anti-rabbit IgG secondary antibodies (Enzo Life Sciences) (B). Apoptotic (solid green histograms) and viable (dashed lines) cells were identified by scatter properties and gated electronically.
DISCUSSION
Efficient recognition of the corpse, coupled to immunomodulation, is perhaps the ultimate functional consequence of apoptotic cell death, and the identification of the relevant molecules that determine those outcomes is of fundamental importance. On the basis of results from a variety of our studies indicating that apoptotic immunomodulatory determinants, which can be evaluated by the transcriptional responses they elicit, appear relatively early in the process of cell death, are enriched in plasma membrane vesicles from apoptotic cells, and are protease sensitive, we undertook distinct, independent, and unbiased proteomic approaches to characterize apoptosis-specific (compared with viable) membrane vesicle-associated proteins. We elaborated criteria that represent critical features of the molecules that function as apoptotic determinants for recognition and immune response modulation (denoted SUPER): molecules that become surface-exposed (during apoptotic cell death), ubiquitously expressed, protease-sensitive, evolutionarily conserved, and resident normally in viable cells (albeit not on the cell surface of non-apoptotic cells).
There were intriguing and informative surprises in the array of proteins identified. For example, the absence of histones, which meet the SUPER criteria for apoptotic determinants for recognition and immune modulation, is striking. The absence of histones from apoptotic vesicles may reflect their loose association with the apoptotic cell membrane and loss as a consequence of the extensive washing and ultracentrifugation involved in vesicle preparation. The apparent absence of actin (as distinct from molecules with which it shares significant sequence identity (e.g. actinin, etc.)) may simply represent an artifact of the informatic paradigm by which protein assignments from identified peptides are made, although, by immunofluorescence staining, we did not detect actin associated with apoptotic vesicles (data not shown). Presumably, the enrichment of cytochrome c among apoptotic vesicle proteins reflects the release of soluble cytochrome c from mitochondria during the apoptotic process, whereas the absence of cytochrome c from the cell surface underscores the selectivity of apoptosis-specific protein externalization.
Unexpectedly, we identified a group of glycolytic enzyme molecules that become redistributed and membrane-associated during apoptotic cell death, and we demonstrated cytofluorometrically their externalization to the apoptotic cell surface. With the exception of two of the upstream members (hexokinase and phosphofructokinase), all of the enzymes of the aerobic glycolytic pathway (phosphoglucose isomerase, aldolase, TPI, GAPDH, phosphoglycerate kinase, EnoA, and pyruvate kinase) were identified as enriched in the membrane vesicle fraction from apoptotic cells. Previous work (57, 58) has documented that glycolytic enzymes are not (normally) associated with the plasma membrane. Still, our results are consistent with findings from other proteomic studies. For example, Gu et al. (59) observed that several glycolytic enzymes (including EnoA and GAPDH) were up-regulated at late times of death (triggered by activation of p53), and Jin et al. (45) found EnoA and GAPDH in microparticles recovered from human plasma, including apoptotic blebs. Sunaga et al. (60) also noted that GAPDH is overexpressed during the apoptotic death of neuronal cells and that it is exposed in amyloid plaques. Interestingly, these molecules have been shown to form multimeric complexes intracellularly on cytoskeletal and membrane elements in viable cells (61–64); we do not know if they exist in an aggregated form when externalized.
Our data demonstrate that glycolytic enzyme molecule externalization is a common and early aspect of apoptotic cell death in different cell types triggered to die with distinct suicidal stimuli. Although all apoptotic cells expose glycolytic enzyme molecules, only a fraction of those molecules within a cell are externalized to the cell surface; the externalized molecules lack enzymatic activity. Numerous metabolic processes and enzymes have been shown to be important for many aspects of apoptosis (65–68); however, the redistribution of a large subset of glycolytic enzymes to the apoptotic cell membrane has not been characterized previously. Our findings demonstrate that the externalization of glycolytic enzyme molecules is a unique feature and a definitive marker of apoptotic cell death.
GAPDH and EnoA externalization has been noted previously in particular cases. For example, GAPDH has been reported to serve as a receptor for transferrin (30). Raje et al. (30) found that GAPDH was externalized on cells of a macrophage cell line cultured in iron-depleted medium. We repeated those experiments (with the same macrophage cell lines and others) and found that the iron-deficient conditions employed trigger apoptotic cell death, leading to the exposure of GAPDH (and other glycolytic enzyme molecules) (data not shown). Thus, these findings reiterate that externalized GAPDH is a definitive marker of the apoptotic cell, independent of transferrin-binding activity. The case of plasminogen binding to externalized EnoA is discussed below.
The cohort of externalized glycolytic enzyme molecules that we identified by several approaches fulfills the critical SUPER criteria. Others of the molecules enriched among apoptotic membrane vesicles and identified in our iTRAQ analysis also meet SUPER criteria. Those found not to be externalized (e.g. actin, cytochrome c, and histones; discussed above) likely can be excluded from further consideration. In addition, the group of chaperones likely does not function as apoptotic immunosuppressive determinants; heat shock proteins, in particular, have been implicated as immunostimulatory “danger signals” (69).
Furthermore, because exposure of resident intracellular molecules from necrotic cells does not confer apoptosis-like immunosuppressive activity (10, 12), protein externalization per se (in the absence of apoptosis-specific post-translational modification) likely is not sufficient for the appearance of apoptotic determinants for recognition and immune modulation. At least with regard to EnoA, our data demonstrating that the appearance of apoptotic determinants for recognition and immune response modulation is not dependent on de novo gene expression (10, 12) are consistent with the suggestion of Redlitz et al. (49) that the surface-exposed form of that molecule does not arise as the product of a specific or altered gene transcript.
It is not clear how apoptosis-specific protein externalization occurs, although our data indicate that it occurs in a caspase-dependent manner. The externalization of glycolytic enzymes has been observed in a wide variety of cells and among many species, independent of cell death (17–27, 48–50, 53). Among these examples are bacterial and protist pathogens, some of which have been shown to mimic apoptotic cell immune responses (70, 71). The export signals or mechanisms by which these normally intracellular molecules become membrane-exposed have not been identified. The enzymatically inactive forms of externalized glycolytic enzyme molecules on apoptotic cells contrast with the enzymatically active glycolytic enzymes exposed on the bacterial surface (17, 18, 20), suggesting that different processes for membrane externalization may pertain.
As discussed above, we observed similar mobilities (both relative molecular mass and pI) (Fig. 2) for non-externalized glycolytic enzyme molecules that are derived from viable cells and for the glycolytic enzyme molecules of apoptotic cells, at least a large fraction of which are externalized. With the exception of TPI, where there does appear to be apoptosis-enhanced proteolysis (Fig. 2, spot 7′), it is clear from these data that apoptosis-specific post-translational modifications that give rise to glycolytic enzyme molecule externalization generally are not proteolytic. Our analysis of such modifications, including the possibility of oxidation-dependent modification (72), is under way. Notably, apoptotic externalization appears to target proteins selectively and independently of protein abundance. The process of blebbing (73–76), which is characteristic of apoptosis, involves protein relocalization (1). We do not know whether blebbing per se is sufficient for the protein externalization that we have characterized.
Externalization of EnoA has been noted in the context of its ability to bind plasminogen (48–51). The nature of the cells exposing EnoA on the cell surface was not explored in those studies. Our data show that significant binding of plasminogen occurs specifically to apoptotic (and not viable) cells and that EnoA is involved in (at least some of) this binding. These results confirm and extend previous observations (54). In particular, independent studies have implicated externalized glycolytic enzymes (especially EnoA and GAPDH) on mammalian and pathogen surfaces as sites for plasminogen binding and activation (18, 27, 77, 78), although no compelling physiological rationale for the presence of this activity on such disparate cells has been offered.
Plasminogen binding, leading to proteolytic cleavage and plasmin activation, has been suggested to be important for pathogen invasiveness (27, 79). With mammalian cells, several (but not all) species of plasminogen receptor molecules are enriched on the surface of apoptotic cells. It seems unlikely that migration and invasiveness (e.g. extracellular matrix degradation) are selected attributes for apoptotic cells, although plasmin-dependent proteolytic activation of latent matrix-associated TGF-β may be significant (see Ref. 80). Plasminogen also appears to be a component of serum that enhances phagocytosis of apoptotic cells.4 Although molecules identified as plasminogen receptors that are externalized in a non-cell death-related manner, such as annexin A2, may be physiologically relevant for plasminogen binding and its consequences (81, 82), plasminogen-binding molecules exposed in an apoptosis-specific manner may function in an entirely distinct capacity on the apoptotic cell surface.
Several molecular species of “plasminogen receptors” other than glycolytic enzyme molecules have been identified. For example, Plow and co-workers (83, 84) have suggested that histone H2B is a plasminogen receptor, and cell surface actin has been implicated similarly (44, 52, 85). In this context, it is interesting that our iTRAQ analysis indicated that these and other molecules characterized as (or associated with) plasminogen receptors (S100A10, annexin A2, and cytokeratin-8) (82, 86, 87) are not preferentially enriched on the apoptotic cell surface (Fig. 3).
We take our data to suggest that plasminogen binding per se may not represent the primary functional consequence of apoptosis-specific protein externalization; rather, the externalization of glycolytic enzyme molecules may be significant functionally in apoptotic cell recognition and immune modulation. In this context, the significance of the expression of orthologous molecules on bacterial (and other pathogenic) surfaces may relate to apoptotic mimicry, leading to immune suppression (attenuation of inflammatory and other immune responses) rather than to plasminogen binding. Our results may then serve to integrate diverse previous findings to suggest that the observed binding of plasminogen to glycolytic enzyme molecules exposed on the mammalian cell surface is a consequence of the apoptosis-specific externalization of determinants for recognition and immune modulation and that apoptotic mimicry is the primary effect of exposed glycolytic molecules on commensal bacterial and pathogens.
Independently of their intracellular roles in metabolism (as well as more recently identified functions in transcription, cytoskeletal trafficking, and autophagy and cell death) (88–92), our findings proffer glycolytic enzyme molecules, in an extracellular context, as candidate determinants of immunomodulation and suggest that they may fulfill an immunological “moonlighting” (93) role. It is not surprising that, in cases of autoimmune pathology, where normal innate apoptotic immunity and tolerance are broken, these molecules are recognized as autoantigens to which antibodies are generated. Indeed, glycolytic enzymes have been identified as potent autoantigens in several autoimmune syndromes, e.g. EnoA in Hashimoto encephalopathy, Behçet disease, systemic lupus erythematosus, inflammatory bowel disease, vasculitis, mixed cryoglobulinemia, and rheumatoid arthritis (94–100); TPI in neuropsychiatric lupus, systemic lupus erythematosus, and osteoarthritis (101, 102); and pyruvate kinase in rheumatoid arthritis and systemic lupus erythematosus (103). Further characterization of the apoptosis-specific mechanism by which glycolytic enzymes are post-translationally modified and externalized and their role in immune modulation holds promise for understanding and addressing causes of autoimmune and inflammatory pathology.
Supplementary Material
Acknowledgments
D. S. U. gratefully acknowledges Michael Federle and Nancy Freitag for recognizing a parallel between apoptotic and bacterial glycolytic enzyme externalization.
This work was supported in part by National Institutes of Health Grants AG029633 (to D. S. U.) and NS046593 (to H. L., for the support of a UMDNJ Neuroproteomics Core Facility).

This article contains supplemental Figs. 1 and 2 and Table I.
K. Lauber, personal communication.
- iTRAQ
- isobaric tagging for relative and absolute quantification
- PE
- phycoerythrin
- 7-AAD
- 7-aminoactinomycin D
- MFI
- mean fluorescence intensity
- EnoA
- α-enolase
- TPI
- triose-phosphate isomerase.
REFERENCES
- 1. Cocca B. A., Cline A. M., Radic M. Z. (2002) Blebs and apoptotic bodies are B cell autoantigens. J. Immunol. 169, 159–166 [DOI] [PubMed] [Google Scholar]
- 2. Kerr J. F., Wyllie A. H., Currie A. R. (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Savill J., Dransfield I., Gregory C., Haslett C. (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2, 965–975 [DOI] [PubMed] [Google Scholar]
- 4. Birge R. B., Ucker D. S. (2008) Innate apoptotic immunity: the calming touch of death. Cell Death Differ. 15, 1096–1102 [DOI] [PubMed] [Google Scholar]
- 5. Herrmann M., Voll R. E., Zoller O. M., Hagenhofer M., Ponner B. B., Kalden J. R. (1998) Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 41, 1241–1250 [DOI] [PubMed] [Google Scholar]
- 6. Cohen P. L., Caricchio R., Abraham V., Camenisch T. D., Jennette J. C., Roubey R. A., Earp H. S., Matsushima G., Reap E. A. (2002) Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 196, 135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rothlin C. V., Ghosh S., Zuniga E. I., Oldstone M. B., Lemke G. (2007) TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131, 1124–1136 [DOI] [PubMed] [Google Scholar]
- 8. Thorp E. B. (2010) Mechanisms of failed apoptotic cell clearance by phagocyte subsets in cardiovascular disease. Apoptosis 15, 1124–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ucker D. S. (2009) in Phagocytosis of Dying Cells: From Molecular Mechanisms to Human Diseases (Krysko D. V., Vandenabeele P., eds), pp. 163–187 Springer, New York [Google Scholar]
- 10. Cvetanovic M., Ucker D. S. (2004) Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J. Immunol. 172, 880–889 [DOI] [PubMed] [Google Scholar]
- 11. Patel V. A., Longacre-Antoni A., Cvetanovic M., Lee D. J., Feng L., Fan H., Rauch J., Ucker D. S., Levine J. S. (2007) The affirmative response of the innate immune system to apoptotic cells. Autoimmunity 40, 274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cocco R. E., Ucker D. S. (2001) Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol. Biol. Cell 12, 919–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cvetanovic M., Mitchell J. E., Patel V., Avner B. S., Su Y., van der Saag P. T., Witte P. L., Fiore S., Levine J. S., Ucker D. S. (2006) Specific recognition of apoptotic cells reveals a ubiquitous and unconventional innate immunity. J. Biol. Chem. 281, 20055–20067 [DOI] [PubMed] [Google Scholar]
- 14. Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Savill J. (1998) Apoptosis. Phagocytic docking without shocking. Nature 392, 442–443 [DOI] [PubMed] [Google Scholar]
- 16. Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., Henson P. M. (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 [PubMed] [Google Scholar]
- 17. Pancholi V., Fischetti V. A. (1992) A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate dehydrogenase with multiple binding activity. J. Exp. Med. 176, 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bergmann S., Rohde M., Chhatwal G. S., Hammerschmidt S. (2001) α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40, 1273–1287 [DOI] [PubMed] [Google Scholar]
- 19. Bergmann S., Rohde M., Hammerschmidt S. (2004) Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect. Immun. 72, 2416–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pancholi V., Fischetti V. A. (1998) α-Enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 273, 14503–14515 [DOI] [PubMed] [Google Scholar]
- 21. Candela M., Biagi E., Centanni M., Turroni S., Vici M., Musiani F., Vitali B., Bergmann S., Hammerschmidt S., Brigidi P. (2009) Bifidobacterial enolase, a cell surface receptor for human plasminogen involved in the interaction with the host. Microbiology 155, 3294–3303 [DOI] [PubMed] [Google Scholar]
- 22. Vanegas G., Quiñones W., Carrasco-López C., Concepción J. L., Albericio F., Avilán L. (2007) Enolase as a plasminogen-binding protein in Leishmania mexicana. Parasitol. Res. 101, 1511–1516 [DOI] [PubMed] [Google Scholar]
- 23. Lama A., Kucknoor A., Mundodi V., Alderete J. F. (2009) Glyceraldehyde-3-phosphate dehydrogenase is a surface-associated, fibronectin-binding protein of Trichomonas vaginalis. Infect. Immun. 77, 2703–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhowmick I. P., Kumar N., Sharma S., Coppens I., Jarori G. K. (2009) Plasmodium falciparum enolase: stage-specific expression and subcellular localization. Malar. J. 8, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gil-Navarro I., Gil M. L., Casanova M., O'Connor J. E., Martínez J. P., Gozalbo D. (1997) The glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is a surface antigen. J. Bacteriol. 179, 4992–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goudot-Crozel V., Caillol D., Djabali M., Dessein A. J. (1989) The major parasite surface antigen associated with human resistance to schistosomiasis is a 37-kDa glyceraldehyde-3-phosphate dehydrogenase. J. Exp. Med. 170, 2065–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jong A. Y., Chen S. H., Stins M. F., Kim K. S., Tuan T. L., Huang S. H. (2003) Binding of Candida albicans enolase to plasmin(ogen) results in enhanced invasion of human brain microvascular endothelial cells. J. Med. Microbiol. 52, 615–622 [DOI] [PubMed] [Google Scholar]
- 28. Chang S. H., Cvetanovic M., Harvey K. J., Komoriya A., Packard B. Z., Ucker D. S. (2002) The effector phase of physiological cell death relies exclusively on the post-translational activation of resident components. Exp. Cell Res. 277, 15–30 [DOI] [PubMed] [Google Scholar]
- 29. Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. (2000) LC3, a mammalian homolog of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raje C. I., Kumar S., Harle A., Nanda J. S., Raje M. (2007) The macrophage cell surface glyceraldehyde-3-phosphate dehydrogenase is a novel transferrin receptor. J. Biol. Chem. 282, 3252–3261 [DOI] [PubMed] [Google Scholar]
- 31. Rouser G., Fleischer S., Yamamoto A. (1970) Two-dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5, 494–496 [DOI] [PubMed] [Google Scholar]
- 32. Jain M. R., Bian S., Liu T., Hu J., Elkabes S., Li H. (2009) Altered proteolytic events in experimental autoimmune encephalomyelitis discovered by iTRAQ shotgun proteomics analysis of spinal cord. Proteome Sci. 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jain M. R., Liu T., Hu J., Darfler M., Fitzhugh V., Rinaggio J., Li H. (2008) Quantitative proteomic analysis of formalin-fixed paraffin-embedded oral HPV lesions from HIV patients. Open Proteomics J. 1, 40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keller A., Nesvizhskii A. I., Kolker E., Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 [DOI] [PubMed] [Google Scholar]
- 35. Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 [DOI] [PubMed] [Google Scholar]
- 36. Kanapka J. A., Hamilton I. R. (1971) Fluoride inhibition of enolase activity in vivo and its relationship to the inhibition of glucose-6-P formation in Streptococcus salivarius. Arch. Biochem. Biophys. 146, 167–174 [DOI] [PubMed] [Google Scholar]
- 37. Mannherz H. G., Gonsior S. M., Gremm D., Wu X., Pope B. J., Weeds A. G. (2005) Activated cofilin colocalizes with Arp2/3 complex in apoptotic blebs during programmed cell death. Eur. J. Cell Biol. 84, 503–515 [DOI] [PubMed] [Google Scholar]
- 38. Buttyan R., Zakeri Z., Lockshin R., Wolgemuth D. (1988) Cascade induction of c-fos, c-myc, and heat shock 70K transcripts during regression of the rat ventral prostate gland. Mol. Endocrinol. 2, 650–657 [DOI] [PubMed] [Google Scholar]
- 39. Zinszner H., Kuroda M., Wang X., Batchvarova N., Lightfoot R. T., Remotti H., Stevens J. L., Ron D. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huot J., Houle F., Rousseau S., Deschesnes R. G., Shah G. M., Landry J. (1998) SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J. Cell Biol. 143, 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kluck R. M., Bossy-Wetzel E., Green D. R., Newmeyer D. D. (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275, 1132–1136 [DOI] [PubMed] [Google Scholar]
- 42. D'Herde K., De Prest B., Mussche S., Schotte P., Beyaert R., Van Coster R., Roels F. (2000) Ultrastructural localization of cytochrome c in apoptosis demonstrates mitochondrial heterogeneity. Cell Death Differ. 7, 331–337 [DOI] [PubMed] [Google Scholar]
- 43. Laster S. M., Mackenzie J. M., Jr. (1996) Bleb formation and F-actin distribution during mitosis and tumor necrosis factor-induced apoptosis. Microsc. Res. Tech. 34, 272–280 [DOI] [PubMed] [Google Scholar]
- 44. Dudani A. K., Ganz P. R. (1996) Endothelial cell surface actin serves as a binding site for plasminogen, tissue plasminogen activator, and lipoprotein(a). Br. J. Haematol. 95, 168–178 [DOI] [PubMed] [Google Scholar]
- 45. Jin M., Drwal G., Bourgeois T., Saltz J., Wu H. M. (2005) Distinct proteome features of plasma microparticles. Proteomics 5, 1940–1952 [DOI] [PubMed] [Google Scholar]
- 46. Arur S., Uche U. E., Rezaul K., Fong M., Scranton V., Cowan A. E., Mohler W., Han D. K. (2003) Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 4, 587–598 [DOI] [PubMed] [Google Scholar]
- 47. Miguet L., Pacaud K., Felden C., Hugel B., Martinez M. C., Freyssinet J. M., Herbrecht R., Potier N., van Dorsselaer A., Mauvieux L. (2006) Proteomic analysis of malignant lymphocyte membrane microparticles using double ionization coverage optimization. Proteomics 6, 153–171 [DOI] [PubMed] [Google Scholar]
- 48. Miles L. A., Dahlberg C. M., Plescia J., Felez J., Kato K., Plow E. F. (1991) Role of cell surface lysines in plasminogen binding to cells: identification of α-enolase as a candidate plasminogen receptor. Biochemistry 30, 1682–1691 [DOI] [PubMed] [Google Scholar]
- 49. Redlitz A., Fowler B. J., Plow E. F., Miles L. A. (1995) The role of an enolase-related molecule in plasminogen binding to cells. Eur. J. Biochem. 227, 407–415 [DOI] [PubMed] [Google Scholar]
- 50. Nakajima K., Hamanoue M., Takemoto N., Hattori T., Kato K., Kohsaka S. (1994) Plasminogen binds specifically to α-enolase on rat neuronal plasma membrane. J. Neurochem. 63, 2048–2057 [DOI] [PubMed] [Google Scholar]
- 51. López-Alemany R., Suelves M., Muñoz-Cánoves P. (2003) Plasmin generation dependent on α-enolase-type plasminogen receptor is required for myogenesis. Thromb. Haemost. 90, 724–733 [DOI] [PubMed] [Google Scholar]
- 52. Kwon M., MacLeod T. J., Zhang Y., Waisman D. M. (2005) S100A10, annexin A2, and annexin A2 heterotetramer as candidate plasminogen receptors. Front. Biosci. 10, 300–325 [DOI] [PubMed] [Google Scholar]
- 53. Wygrecka M., Marsh L. M., Morty R. E., Henneke I., Guenther A., Lohmeyer J., Markart P., Preissner K. T. (2009) Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood 113, 5588–5598 [DOI] [PubMed] [Google Scholar]
- 54. O'Mullane M. J., Baker M. S. (1998) Loss of cell viability dramatically elevates cell surface plasminogen binding and activation. Exp. Cell Res. 242, 153–164 [DOI] [PubMed] [Google Scholar]
- 55. Obeid M., Tesniere A., Ghiringhelli F., Fimia G. M., Apetoh L., Perfettini J. L., Castedo M., Mignot G., Panaretakis T., Casares N., Métivier D., Larochette N., van Endert P., Ciccosanti F., Piacentini M., Zitvogel L., Kroemer G. (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 [DOI] [PubMed] [Google Scholar]
- 56. Gardai S. J., McPhillips K. A., Frasch S. C., Janssen W. J., Starefeldt A., Murphy-Ullrich J. E., Bratton D. L., Oldenborg P. A., Michalak M., Henson P. M. (2005) Cell surface calreticulin initiates clearance of viable or apoptotic cells through transactivation of LRP on the phagocyte. Cell 123, 321–334 [DOI] [PubMed] [Google Scholar]
- 57. Shui W., Sheu L., Liu J., Smart B., Petzold C. J., Hsieh T. Y., Pitcher A., Keasling J. D., Bertozzi C. R. (2008) Membrane proteomics of phagosomes suggests a connection to autophagy. Proc. Natl. Acad. Sci. U.S.A. 105, 16952–16957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rogers L. D., Foster L. J. (2007) The dynamic phagosomal proteome and the contribution of the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 104, 18520–18525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gu S., Liu Z., Pan S., Jiang Z., Lu H., Amit O., Bradbury E. M., Hu C. A., Chen X. (2004) Global investigation of p53-induced apoptosis through quantitative proteomic profiling using comparative amino acid-coded tagging. Mol. Cell. Proteomics 3, 998–1008 [DOI] [PubMed] [Google Scholar]
- 60. Sunaga K., Takahashi H., Chuang D. M., Ishitani R. (1995) Glyceraldehyde-3-phosphate dehydrogenase is overexpressed during apoptotic death of neuronal cultures and is recognized by a monoclonal antibody against amyloid plaques from Alzheimer brain. Neurosci. Lett. 200, 133–136 [DOI] [PubMed] [Google Scholar]
- 61. Srere P. A., Knull H. R. (1998) Location-location-location. Trends Biochem. Sci. 23, 319–320 [DOI] [PubMed] [Google Scholar]
- 62. Singh P., Salih M., Leddy J. J., Tuana B. S. (2004) The muscle-specific calmodulin-dependent protein kinase assembles with the glycolytic enzyme complex at the sarcoplasmic reticulum and modulates the activity of glyceraldehyde-3-phosphate dehydrogenase in a Ca2+/calmodulin-dependent manner. J. Biol. Chem. 279, 35176–35182 [DOI] [PubMed] [Google Scholar]
- 63. Minaschek G., Gröschel-Stewart U., Blum S., Bereiter-Hahn J. (1992) Microcompartmentation of glycolytic enzymes in cultured cells. Eur. J. Cell Biol. 58, 418–428 [PubMed] [Google Scholar]
- 64. Campanella M. E., Chu H., Low P. S. (2005) Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc. Natl. Acad. Sci. U.S.A. 102, 2402–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ishitani R., Chuang D. M. (1996) Glyceraldehyde-3-phosphate dehydrogenase antisense oligodeoxynucleotides protect against cytosine arabinonucleoside-induced apoptosis in cultured cerebellar neurons. Proc. Natl. Acad. Sci. U.S.A. 93, 9937–9941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Eguchi Y., Shimizu S., Tsujimoto Y. (1997) Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 57, 1835–1840 [PubMed] [Google Scholar]
- 67. Leist M., Single B., Castoldi A. F., Kühnle S., Nicotera P. (1997) Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 185, 1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Robey R. B., Hay N. (2006) Mitochondrial hexokinases, novel mediators of the anti-apoptotic effects of growth factors and Akt. Oncogene 25, 4683–4696 [DOI] [PubMed] [Google Scholar]
- 69. Manjili M. H., Park J., Facciponte J. G., Subjeck J. R. (2005) HSP110 induces “danger signals” upon interaction with antigen-presenting cells and mouse mammary carcinoma. Immunobiology 210, 295–303 [DOI] [PubMed] [Google Scholar]
- 70. de Freitas Balanco J. M., Moreira M. E., Bonomo A., Bozza P. T., Amarante-Mendes G., Pirmez C., Barcinski M. A. (2001) Apoptotic mimicry by an obligate intracellular parasite down-regulates macrophage microbicidal activity. Curr. Biol. 11, 1870–1873 [DOI] [PubMed] [Google Scholar]
- 71. Barcinski M. A., Moreira M. E., de Freitas Balanco J. M., Wanderley J. L., Bonomo A. C. (2003) The role of apoptotic mimicry in host-parasite interplay: is death the only alternative for altruistic behavior? Kinetoplastid Biol. Dis. 2, 6–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sambrano G. R., Steinberg D. (1995) Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc. Natl. Acad. Sci. U.S.A. 92, 1396–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mills J. C., Stone N. L., Erhardt J., Pittman R. N. (1998) Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J. Cell Biol. 140, 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Coleman M. L., Sahai E. A., Yeo M., Bosch M., Dewar A., Olson M. F. (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 3, 339–345 [DOI] [PubMed] [Google Scholar]
- 75. Sebbagh M., Renvoizé C., Hamelin J., Riché N., Bertoglio J., Bréard J. (2001) Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 3, 346–352 [DOI] [PubMed] [Google Scholar]
- 76. Leverrier Y., Ridley A. J. (2001) Apoptosis: caspases orchestrate the ROCK “n” bleb. Nat. Cell Biol. 3, E91–E93 [DOI] [PubMed] [Google Scholar]
- 77. Felez J., Chanquia C. J., Fabregas P., Plow E. F., Miles L. A. (1993) Competition between plasminogen and tissue plasminogen activator for cellular binding sites. Blood 82, 2433–2441 [PubMed] [Google Scholar]
- 78. Miles L. A., Hawley S. B., Baik N., Andronicos N. M., Castellino F. J., Parmer R. J. (2005) Plasminogen receptors: the sine qua non of cell surface plasminogen activation. Front. Biosci. 10, 1754–1762 [DOI] [PubMed] [Google Scholar]
- 79. Lähteenmäki K., Edelman S., Korhonen T. K. (2005) Bacterial metastasis: the host plasminogen system in bacterial invasion. Trends Microbiol. 13, 79–85 [DOI] [PubMed] [Google Scholar]
- 80. Plow E. F., Herren T., Redlitz A., Miles L. A., Hoover-Plow J. L. (1995) The cell biology of the plasminogen system. FASEB J. 9, 939–945 [DOI] [PubMed] [Google Scholar]
- 81. Odaka C., Tanioka M., Itoh T. (2005) Matrix metalloproteinase-9 in macrophages induces thymic neovascularization following thymocyte apoptosis. J. Immunol. 174, 846–853 [DOI] [PubMed] [Google Scholar]
- 82. Falcone D. J., Borth W., Khan K. M., Hajjar K. A. (2001) Plasminogen-mediated matrix invasion and degradation by macrophages is dependent on surface expression of annexin II. Blood 97, 777–784 [DOI] [PubMed] [Google Scholar]
- 83. Das R., Burke T., Plow E. F. (2007) Histone H2B as a functionally important plasminogen receptor on macrophages. Blood 110, 3763–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Herren T., Burke T. A., Das R., Plow E. F. (2006) Identification of histone H2B as a regulated plasminogen receptor. Biochemistry 45, 9463–9474 [DOI] [PubMed] [Google Scholar]
- 85. Hawley S. B., Green M. A., Miles L. A. (2000) Discriminating between cell surface and intracellular plasminogen-binding proteins: heterogeneity in profibrinolytic plasminogen-binding proteins on monocytoid cells. Thromb. Haemost. 84, 882–890 [PubMed] [Google Scholar]
- 86. Ling Q., Jacovina A. T., Deora A., Febbraio M., Simantov R., Silverstein R. L., Hempstead B., Mark W. H., Hajjar K. A. (2004) Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J. Clin. Invest. 113, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cesarman G. M., Guevara C. A., Hajjar K. A. (1994) An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA). II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J. Biol. Chem. 269, 21198–22203 [PubMed] [Google Scholar]
- 88. Feo S., Arcuri D., Piddini E., Passantino R., Giallongo A. (2000) ENO1 gene product binds to the c-myc promoter and acts as a transcriptional repressor: relationship with Myc promoter-binding protein 1 (MBP-1). FEBS Lett. 473, 47–52 [DOI] [PubMed] [Google Scholar]
- 89. Colell A., Ricci J. E., Tait S., Milasta S., Maurer U., Bouchier-Hayes L., Fitzgerald P., Guio-Carrion A., Waterhouse N. J., Li C. W., Mari B., Barbry P., Newmeyer D. D., Beere H. M., Green D. R. (2007) GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 129, 983–997 [DOI] [PubMed] [Google Scholar]
- 90. Tisdale E. J. (2002) Glyceraldehyde-3-phosphate dehydrogenase is phosphorylated by protein kinase Cι/λ and plays a role in microtubule dynamics in the early secretory pathway. J. Biol. Chem. 277, 3334–3341 [DOI] [PubMed] [Google Scholar]
- 91. Glaser P. E., Han X., Gross R. W. (2002) Tubulin is the endogenous inhibitor of the glyceraldehyde-3-phosphate dehydrogenase isoform that catalyzes membrane fusion: implications for the coordinated regulation of glycolysis and membrane fusion. Proc. Natl. Acad. Sci. U.S.A. 99, 14104–14109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tisdale E. J., Azizi F., Artalejo C. R. (2009) Rab2 utilizes glyceraldehyde-3-phosphate dehydrogenase and protein kinase Cι to associate with microtubules and to recruit dynein. J. Biol. Chem. 284, 5876–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jeffery C. J. (2009) Moonlighting proteins–an update. Mol. BioSyst. 5, 345–350 [DOI] [PubMed] [Google Scholar]
- 94. Sabbatini A., Dolcher M. P., Marchini B., Chimenti D., Moscato S., Pratesi F., Bombardieri S., Migliorini P. (1997) α-Enolase is a renal-specific antigen associated with kidney involvement in mixed cryoglobulinemia. Clin. Exp. Rheumatol. 15, 655–658 [PubMed] [Google Scholar]
- 95. Roozendaal C., Zhao M. H., Horst G., Lockwood C. M., Kleibeuker J. H., Limburg P. C., Nelis G. F., Kallenberg C. G.(1998) Catalase and α-enolase: two novel granulocyte autoantigens in inflammatory bowel disease (IBD). Clin. Exp. Immunol. 112, 10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pratesi F., Moscato S., Sabbatini A., Chimenti D., Bombardieri S., Migliorini P. (2000) Autoantibodies specific for α-enolase in systemic autoimmune disorders. J. Rheumatol. 27, 109–115 [PubMed] [Google Scholar]
- 97. Moscato S., Pratesi F., Sabbatini A., Chimenti D., Scavuzzo M., Passatino R., Bombardieri S., Giallongo A., Migliorini P. (2000) Surface expression of a glycolytic enzyme, α-enolase, recognized by autoantibodies in connective tissue disorders. Eur. J. Immunol. 30, 3575–3584 [DOI] [PubMed] [Google Scholar]
- 98. Saulot V., Vittecoq O., Charlionet R., Fardellone P., Lange C., Marvin L., Machour N., Le Loët X., Gilbert D., Tron F. (2002) Presence of autoantibodies to the glycolytic enzyme α-enolase in sera from patients with early rheumatoid arthritis. Arthritis Rheum. 46, 1196–1201 [DOI] [PubMed] [Google Scholar]
- 99. Lee K. H., Chung H. S., Kim H. S., Oh S. H., Ha M. K., Baik J. H., Lee S., Bang D. (2003) Human α-enolase from endothelial cells as a target antigen of anti-endothelial cell antibody in Behçet disease. Arthritis Rheum. 48, 2025–2035 [DOI] [PubMed] [Google Scholar]
- 100. Fujii A., Yoneda M., Ito T., Yamamura O., Satomi S., Higa H., Kimura A., Suzuki M., Yamashita M., Yuasa T., Suzuki H., Kuriyama M. (2005) Autoantibodies against the amino terminal of α-enolase are a useful diagnostic marker of Hashimoto encephalopathy. J. Neuroimmunol. 162, 130–136 [DOI] [PubMed] [Google Scholar]
- 101. Xiang Y., Sekine T., Nakamura H., Imajoh-Ohmi S., Fukuda H., Nishioka K., Kato T. (2004) Proteomic surveillance of autoimmunity in osteoarthritis: identification of triose-phosphate isomerase as an autoantigen in patients with osteoarthritis. Arthritis Rheum. 50, 1511–1521 [DOI] [PubMed] [Google Scholar]
- 102. Sasajima T., Watanabe H., Sato S., Sato Y., Ohira H. (2006) Anti-triose-phosphate isomerase antibodies in cerebrospinal fluid are associated with neuropsychiatric lupus. J. Neuroimmunol. 181, 150–156 [DOI] [PubMed] [Google Scholar]
- 103. Oremek G. M., Müller R., Sapoutzis N., Wigand R. (2003) Pyruvate kinase-type tumor M2 plasma levels in patients afflicted with rheumatic diseases. Anticancer Res. 23, 1131–1134 [PubMed] [Google Scholar]
- 104. Kato H., Fukuda T., Parkison C., McPhie P., Cheng S. Y. (1989) Cytosolic thyroid hormone-binding protein is a monomer of pyruvate kinase. Proc. Natl. Acad. Sci. U.S.A. 86, 7861–7865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Giallongo A., Feo S., Moore R., Croce C. M., Showe L. C. (1986) Molecular cloning and nucleotide sequence of a full-length cDNA for human α-enolase. Proc. Natl. Acad. Sci. U.S.A. 83, 6741–6745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gracy R. W. (1975) Triose-phosphate isomerase from human erythrocytes. Methods Enzymol. 41, 442–447 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.