Background: The role of dental epithelium in stem cell differentiation has not been clearly elucidated.
Results: SP cells differentiated into odontoblasts by epithelial BMP4, whereas iPS cells differentiated into ameloblasts when cultured with dental epithelium.
Conclusion: Stem cells can be induced to odontogenic cell fates when co-cultured with dental epithelium.
Significance: This is the first report to show induction of ameloblasts from iPS cells.
Keywords: Epithelium, Extracellular Matrix, Induced Pluripotent Stem (iPS) Cell, Stem Cells, Tooth Development, Ameloblastin
Abstract
Epithelial-mesenchymal interactions regulate the growth and morphogenesis of ectodermal organs such as teeth. Dental pulp stem cells (DPSCs) are a part of dental mesenchyme, derived from the cranial neural crest, and differentiate into dentin forming odontoblasts. However, the interactions between DPSCs and epithelium have not been clearly elucidated. In this study, we established a mouse dental pulp stem cell line (SP) comprised of enriched side population cells that displayed a multipotent capacity to differentiate into odontogenic, osteogenic, adipogenic, and neurogenic cells. We also analyzed the interactions between SP cells and cells from the rat dental epithelial SF2 line. When cultured with SF2 cells, SP cells differentiated into odontoblasts that expressed dentin sialophosphoprotein. This differentiation was regulated by BMP2 and BMP4, and inhibited by the BMP antagonist Noggin. We also found that mouse iPS cells cultured with mitomycin C-treated SF2-24 cells displayed an epithelial cell-like morphology. Those cells expressed the epithelial cell markers p63 and cytokeratin-14, and the ameloblast markers ameloblastin and enamelin, whereas they did not express the endodermal cell marker Gata6 or mesodermal cell marker brachyury. This is the first report of differentiation of iPS cells into ameloblasts via interactions with dental epithelium. Co-culturing with dental epithelial cells appears to induce stem cell differentiation that favors an odontogenic cell fate, which may be a useful approach for tooth bioengineering strategies.
Introduction
Tooth morphogenesis is characterized by reciprocal interactions between dental epithelium and mesenchymal cells derived from the cranial neural crest, which result in formation of the proper number and shapes of teeth. Multiple extracellular signaling molecules, including BMPs, FGFs, WNTs, and SHH, have been implicated in these interactions for tooth development (1). Epithelial cells then subsequently give rise to enamel-forming ameloblasts, while mesenchymal stem cells (MSCs)3 form dentin-forming odontoblasts and dental pulp cells. Initial tooth development is also regulated by extracellular matrices (ECMs), such as basement membrane components that include laminin, collagen, fibronectin, and perlecan (2, 3). These matrices control proliferation, polarity, and attachment, and also determine tooth germ size and morphology. At later stages of tooth development, the basement membrane components disappear and odontogenic cells begin to secrete a variety of tooth-specific extracellular matrices that give rise to layers of enamel and dentin, produced by epithelial-derived ameloblasts and mesenchymal-derived odontoblasts, respectively. Ameloblastin (Ambn) is one of the enamel matrix proteins expressed by differentiating ameloblasts, and is essential for dental epithelial cell differentiation into ameloblasts and enamel formation (2, 4). Dentin sialophosphoprotein (DSPP) is a member of the SIBLING (Small Integrin-Binding Ligand N-linked Glycoprotein) family of extracellular matrix glycophosphoproteins, and is expressed by differentiating ameloblasts and odontoblasts (5). These extracellular matrices are important for the formation of enamel and dentin (2).
Stem cell research has identified and established several types of stem cells, including induced pluripotent stem (iPS) cells, which are generated from a variety of somatic cell types via introduction of transcription factors that mediate pluripotency (6). Direct reprogramming of somatic cells into iPS cells by forced expression of a small number of defined factors (e.g. Oct3/4, Sox2, Klf4, and c-Myc) has great potential for tissue-specific regenerative therapies. In addition, this process also avoids ethical issues surrounding the use of embryonic stem (ES) cells, as well as problems with rejection following implantation of non-autologous cells (7). A variety of cell types, including hematopoietic precursor cells (8, 9), endothelial cells, MSCs, neuronal cells (10), reproductive cells (11), and cardiomyocytes (12, 13), undergo in vitro differentiation. However previous studies of dental cell differentiation are not adequate to explain this process. Several dental stem cell populations have been identified in different parts of the tooth, including cells from the periodontal ligament that links the tooth root with the bone, tips of developing roots, and tissue (dental follicle) that surrounds an unerupted tooth. In addition, dental pulp stem cells (DPSCs) have been identified in the pulp of exfoliated deciduous teeth of both children and adults (14). These different cell types likely share a common lineage, being derived from neural crest cells, and all have generic MSC-like properties.
Transplantation of in vitro expanded DPSCs mixed with hydroxyapatite/tricalcium phosphate particles results in the formation of dental pulp and dentin-like tissue complexes in immunocompromised mice (15). Similar results have been observed with an MSC population obtained from human exfoliated deciduous teeth (SHED) (14). DPSCs also express the putative stem cell marker STRO-1 and perivascular cell marker CD146, while a proportion co-expresses smooth muscle actin and the pericyte-associated antigen 3G5 (16). These findings suggest that a population of DPSCs may reside in this perivascular niche within the pulp of adult teeth.
Side population (SP) cells were identified by flow cytometry analysis with a Hoechst 33342 efflux assay and found to have stem cell characteristics (17). SP cells are a small population that show low levels of Hoechst dye staining for the expression of Abcg2, an ATP-binding cassette transporter (18), which is strongly expressed in dental pulp in human adult and deciduous teeth (19). Dental pulp contains multipotent stem cells and is viewed as a potential source of iPS cells (14, 20, 21). In tooth germ development, undifferentiated neural crest-derived MSCs interact with dental epithelium and differentiate into dentin matrix-secreting odontoblasts. However, the interactions between stem cells and dental epithelium have not been clearly elucidated.
In this study, we established an SP cell line from mouse dental papilla. We then cultured these SP cells with rat dental epithelial cells to investigate epithelial-mesenchymal interactions. SP cells were induced to differentiate into DSPP expressing odontoblasts via the action of epithelial BMP4. Furthermore, mouse iPS cells differentiated into Ambn-expressing dental epithelium when cultured with dental epithelial cells. Thus, these undifferentiated stem cells can be induced to an odontogenic cell fate when co-cultured with dental epithelial cells. These findings may be useful for analysis of dental cell differentiation in vitro and for procurement of odontogenic cells for use in regenerative medicine.
EXPERIMENTAL PROCEDURES
Preparation of Mouse Dental Papilla Cells
Dental papilla tissues were isolated from incisors from newborn ICR mice by digesting with 0.1% collagenase D (Roche) and 2.5% trypsin for 30 min at 37 °C. Enzymatically digested tissues were minced into 2–4 mm pieces using micro-scissors and washed three times with Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) containing 10% fetal bovine serum (FBS) (Invitrogen) and an antibiotic-antimycotic mixture (Invitrogen), then filtered through a cell strainer (40 μm) to eliminate clumps and debris. Mouse dental papilla (mDP) cells were cultured in 60-mm culture dishes and immortalized by expression of a mutant human papilloma virus type 16 E6 gene lacking the PDZ-domain-binding motif (22). mDP cells were maintained with DMEM supplemented with 10% FBS and an antibiotic-antimycotic mixture at 37 °C in a humidified atmosphere containing 5% CO2.
Generation of Dental Epithelial Cell Line SF2-24 and Cell Culture
Rat dental epithelial cells were enzymatically isolated from the cervical loop at the apical end of the lower incisors from a Sprague-Dawley rat with 1% collagenase. Dental epithelial cells were cultured with DMEM (Invitrogen) supplemented with 10% FBS for 4 weeks, then, maintained in serum-free keratinocyte synthetic medium (Keratinocyte-SFM, Invitrogen) for 1 year. An established cell line, SF2 was maintained as previously described (4). SF2 cells were transfected with a pEF6/GFP-PDGFtm-myc-HA vector expressing the GFP-PDGF receptor-transmembrane fusion protein with myc-HA tag using Lipofectamine 2000 (Invitrogen). Transfected cells were selected as SF2 subclones by culturing with media containing 400 μg/ml of G418. Twenty-five clones were selected as a stable transfected cell line, with one of them designated as SF2-24 (Ambn high expression) and another SF2-7 (Ambn low expression).
SP and MP Cell Analysis and Flow Cytometry
Hoechst staining of mDP cells for SP cell analysis was conducted as previously described (17). Subconfluent mDP cells were stained with Hoechst dye for 90 min at 37 °C. After staining, all cells were resuspended in 100 μl of Hanks' balanced salt solution (HBSS) with calcium/magnesium medium and kept on ice. The SP and MP gates were defined as previously described (17). For analysis, the cells were resuspended in ice-cold HBSS with 2% FBS containing propidium iodide (Sigma) at a final concentration of 2 μg/ml to identify dead cells, then filtered through a cell strainer. Sorting and analyses were carried out with an EPICS ALTRA flow cytometer (Beckman Coulter, Fullerton, CA). The SP cell fraction was enriched by repeating cell sorting 3 times. The expression of stem cell markers in SP cells was confirmed by flow cytometry using anti-Sca-1 and Oct3/4 antibodies (Santa Cruz Biotechnology).
Differentiation of SP Cells
For odontoblastic induction, SP cells were plated at 6 × 104 cells in 60-mm dishes. After the cells had reached 50–60% confluence, we replaced the control medium with induction medium containing 100 ng/ml of BMP2 or BMP4 (Wako Pure Chemical Industries), and cells were incubated for 2 days. For blocking BMP signaling, recombinant mouse Noggin protein (R&D systems) was used. Total RNA was isolated and real time RT-PCR was performed using mouse Bcrp1 (18) and DSPP primer sets (supplemental Table S1).
For adipogenic differentiation, SP cells were seeded at 1 × 105 cells per well in 6-well plates and cultured in DMEM supplemented with 10% FBS. Adipogenic differentiation was induced with induction medium from a Poietics hMSC Media Bullet kit (Cambrex Bio Science Walkersville, Inc., Walkersville, MD) for 3 days and incubated in maintenance medium for 3 days, then the cells were cultured for an additional 7 days in maintenance medium. As a control, cells were cultured in only maintenance medium. Adipogenesis was confirmed by staining with Oil-Red-O and the expression of PPARγ was analyzed by RT-PCR.
For osteogenic differentiation, SP cells were seeded at 1.5 × 104 cells per well in 6-well plates and cultured in DMEM supplemented with 10% FBS, 10 mm β-glycerophosphate, 0.2 mm ascorbic acid, 2-phosphate, and 10−8 m dexamethasone. Induction and control media were replaced every 2 days. Osteogenesis was determined by alkaline phosphatase (ALP) and von Kossa staining for calcium deposition, as previously described (23). After 4 weeks culturing with osteoblast induction medium, the expressions of osteocalcin, osteonectin, and Runx2 in osteogenesis-induced SP cells were analyzed by RT-PCR.
For neurogenic differentiation, we modified a neuronal induction protocol using recombinant nerve growth factor (NGF) (Chemicon). SP cells were seeded at 1 × 105 cells per well in 6-well plates. After reaching 80–90% confluence, neurogenic differentiation was induced by culturing the cells in DMEM supplemented 2% FBS, 1.25% dimethyl sulfoxide, 10−6 m retinoic acid, 2.5 μg/ml insulin, and 50 ng/ml NGF. Two weeks later, neurogenesis was characterized by Western blot analysis using an anti-neurofilament-M specific antibody (Cell Signaling Technology).
Odontoblastic Induction of SP Cells by Co-culturing with Dental Epithelial Cells
We investigated the role of dental epithelial cells in specification of odontogenic cell lineage using two types of co-culture systems: feeder and chamber types with a cell culture insert (BD Falcon). We used confluent SF2 cells, or SF2 cells treated with 4% paraformaldehyde (PFA) or ammonium (denudation) as feeder cells. SF2 and SP cells were harvested and placed into either 6-well plates or cell culture inserts (chamber), then cultured until reaching confluence.
Screening of Co-culture Conditions for Ameloblastic Induction of iPS Cells
A mouse iPS cell line (iPS-MEF-Ng-20D-17), carrying the Nanog-GFP/IRES/puromycin resistant gene, was established by Yamanaka (Kyoto University, Japan), and obtained from RIKEN Cell Bank (Saitama, Japan) (6). Mouse iPS cells were cultured with rat dental epithelial cells (SF2-24), which predominantly express Ambn mRNA, as feeder cells. Preparatory co-culture experiments were performed as follows: iPS cells were cultured with mouse embryonic fibroblasts (MEFs) treated with mitomycin C (MMC) or with three different types of SF2-24 feeder cells (confluent cells, cells treated with MMC, cells treated with 4% PFA). MMC was supplied at 9 μg/ml (final concentration) for 2 h to arrest SF2-24 cell proliferation.
Induction of iPS Cell-derived Ameloblasts
iPS cells (plated 1.5 × 103/cm2) were cultured on sheets of MMC-treated SF2-24 cells for 7, 10, and 14 days in the same medium used for the SF2-24 culture without leukemia inhibitory factor and 2-mercaptoethanol. The culture medium was changed every day throughout the co-culture period. After 7 and 10 days, the co-cultured iPS cells were subjected to RT-PCR, while those after 14 days of culture were analyzed by immunocytochemistry. Total RNA from iPS cells co-cultured with MMC-treated MEFs was isolated after 3 days of culture. Conditioned media from cultures of SF2-24 and SF2-7 were collected after 2 days of incubation. The procedures used for transfection of Ambn-expressing vectors, as well as their construction and isolation of recombinant proteins have been previously described (2, 24). K252a (Trk inhibitor, Calbiochem), PD98059 (MEK inhibitor, Cell signaling), anti-NT-4 neutralizing antibody (Applied Biological Materials), and Noggin (R&D systems) were added to conditioned medium obtained from SF2-24 cells.
Reverse Transcription-PCR
Total RNA was isolated using TRIzol (Invitrogen) and first-strand cDNA was synthesized at 50 °C for 50 min using oligo(dT) or random primers with the SuperScript III First-strand Synthesis System (Invitrogen). PCR was performed with Takara Ex Taq HotStart Version (Takara) or a PCR Additives Kit (Jena Bioscience, Germany). The primer sequences are presented in supplemental Table S1. PCR amplicons were separated and visualized on 1.5% agarose gels with SYBR Green staining using the LAS-4000 mini image analyzing system (Fujifilm). For semi-quantitative PCR analysis, the band intensities of PCR amplicons were quantified using MultiGauge software (Fujifilm) and normalized by dividing the intensity of the band of GAPDH. Because of the high degree of homology between the Ambn gene in mice and rats (94.2%), we designed a species-specific mouse Ambn primer that encoded locked nucleic acid (LNA) at a different base sequence between the mouse and rat Ambn gene in a conserved region. The specificity of the mouse Ambn primer was confirmed by PCR using mouse and rat tooth germ cDNA. Statistical analysis of gene expression was performed using the Student's t test.
Immunocytochemistry
For immunocytochemistry, cells were fixed with 4% PFA for 5 min at room temperature. After washing with PBS three times for 5 min, the cells were treated with Power Block Universal Reagent (BioGenex) for 5 min at room temperature, followed by three washes with PBS. The cells were incubated with the anti-Ambn primary antibody included in the kit (1:200, M-300, Santa Cruz Biotechnology). The primary antibody was visualized with Alexa Fluor 594 donkey anti-rabbit antibody (1:500, A21207, Invitrogen). Nuclei were stained with Hoechst 33258 (Invitrogen). Immunocytochemistry and phase images were captured using a BZ-8000 microscopic system (KEYENCE Co, Osaka, Japan), and images of the sections were analyzed using a BZ analyzer (KEYENCE).
RESULTS
Establishment of SP Cell Line from Mouse Dental Papilla Cells
Side population (SP) cells, which displayed stem cell ability, make up less than 1% of total cells in the mouse dental papilla (mDP) from postnatal tooth germs. Thus, biochemical and biomolecular analyses of SP cells are difficult to perform because of the limited numbers of cells available. We enriched an SP cell population and established an SP cell line using a cell sorting technique. mDP cells were obtained from mouse incisor tooth germs and immortalized, as previously described (22). The cells were then stained with Hoechst dye and sorted to enrich the SP cell fraction. Cell sorting was repeated three times and SP cells were enriched from about 0.9% to 82.3% in the gated area (Fig. 1A). This SP cell line showed high expression levels of the stem cell markers Sca-1 and Oct3/4 when compared with the majority population (MP) cells, which was comprised of a greater number dental papilla cells in various differentiation stages (Fig. 1B).
FIGURE 1.
Isolation of SP cells from mDP cell line. A, flow cytometry analysis of SP cells. mDP cells made up ∼0.9% of the total cell population with a relatively lower level of Hoechst 33342 fluorescence (SP cells), while 13.8% of the population was maintained as MP cells. Using repeated cell sorting, the SP cell population was enriched by 11.6% at the first sorting, 30.3% at the second sorting, and 82.3% at the third sorting. B, expression of the stem cell markers Sca-1 and Oct3/4 in dental pulp, SP, and MP cells.
Because the SP cells expressed a set of stem cell markers, we examined their multipotency. Using an odontoblast differentiation medium containing BMP2 or BMP4, the SP cells were induced to express DSPP, a marker of odontoblasts, whereas the expression of the undifferentiated cell marker Bcrp1 was decreased (Fig. 2A). In osteoblast differentiation medium, the SP cells showed increased levels of ALP and von Kossa staining, as well as expressions of the osteoblast marker genes osteocalcin, osteonectin, and Runx2, whereas the MP cells showed no induction of expression of those genes (Fig. 2, B and C). When SP cells were cultured in differentiation medium for adipogenesis or neurogenesis, they were Oil-Red-O positive or showed neurite outgrowths, along with high levels of adipogenic expression and protein expressions of neurogenic markers, such as PPARγ and Neurofilament-M, respectively (supplemental Fig. S1, A--D). These results suggest that the SP cell line established in this study has a high level of multipotency.
FIGURE 2.
Odontoblast and osteoblast differentiation in SP cells. A, differentiation of SP cells to odontoblasts. Expression of the odontoblast marker DSPP and the undifferentiated mesenchymal marker Bcrp1 in dental pulp (black bar) and SP cells (gray bar) cultured with or without BMP2 or BMP4. B, differentiation of SP cells to osteoblasts in osteoblast induction medium (Osteogenic cond.). ALP and von Kossa staining of dental pulp, SP, and MP cells. C, expressions of osteoblast markers in dental pulp, SP, and MP cells cultured in regular (−) or osteoblast induction medium (+). *, p < 0.05.
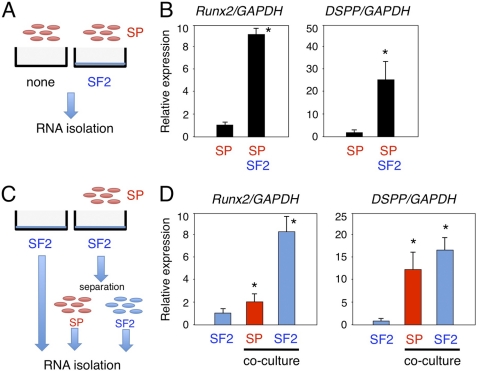
Expressions of Runx2 and DSPP in SP Cells Cultured with SF2 Cells
We analyzed epithelial and mesenchymal stem cell interactions by culturing SP cells with rat dental epithelial SF2 cells that had been engineered to express a GFP-myc-HA tag on the cell membrane surface. This allowed us to distinguish between SP and SF2 cell types (supplemental Fig. S2). SP cells were cultured with or without SF2 cells for 48 h, and total RNA was isolated from the mixed cell cultures (Fig. 3A). The expressions of Runx2 and DSPP were increased in SP cells that had been cultured with SF2 cells, as compared with those cultured without SF2 cells (Fig. 3B). Because Runx2 and DSPP are expressed by both odontoblasts and ameloblasts, co-cultured SP and SF2 cells were separated into individual cell populations using the anti-HA antibody, which specifically recognizes SF2 cells (Fig. 3C). We found a dramatic increase in the expression level of Runx2 in SF2 cells as compared with SP cells (Fig. 3D). No epithelial marker was detected in SP cells co-cultured with SF2 cells, suggesting that the SP cells had differentiated into odontoblasts (data not shown). Runx2 is expressed in enamel matrix-secreting ameloblasts, but not in the pre-secretion stage of ameloblasts (25). Our results suggest that the SF2 cells had fully differentiated into enamel matrix-secreting ameloblasts by co-culturing with SP cells. The expression of DSPP was up-regulated in both cell types. However, in MP cells, which are fully differentiated dental papilla cells, no expression of Runx2 or DSPP was induced by co-culturing with SF2 cells (data not shown). These results indicate that epithelial and mesenchymal stem cell interactions promote individual differential states in SF2 and SP cells.
FIGURE 3.
In vitro epithelial-mesenchymal interaction system using dental epithelial cells (SF2) and dental mesenchymal stem cells (SP) to promote odontogenic cell differentiation. A and C, schematic diagram of the co-culture system. B, comparisons of Runx2 and DSPP gene expressions between the SP monolayer culture and SP and SF2 cell co-culture system. C, total RNA samples were separately prepared from SP and SF2 cells, using the anti-HA antibody. D, expressions of Runx2 and DSPP in co-cultured SF2 (blue) and SP (red) cells. The expression level of GAPDH was used an internal control. *, p < 0.05.
Involvement of Exogenous Factors from Dental Epithelium in DSPP Expression of SP Cells
We attempted to identify the factors in dental epithelial cells involved in SP cell differentiation by treating SF2 cells with 4% PFA to inhibit extracellular signaling, including the effects of growth factors (Fig. 4A). Ammonia treatment, through a process known as denudation, removes all cell components except the extracellular matrices and is often used for three-dimensional matrix cell culture experiments (26). DSPP expression in SP cells was partially inhibited by PFA treatment, while they retained the extracellular matrix network. This result suggests that the extracellular environment including extracellular matrices, growth factors, and cell-cell interaction produced by SF2 cells contributes to odontoblast induction. Denuded SF2 cells were also incapable of inducing DSPP expression in SP cells (Fig. 4B). Odontoblast induction of SP cells was observed in co-cultures with living SF2 cells, indicating that some types of soluble secreted molecules and matrices from SF2 cells are required to induce SP cells to undergo odontogenic differentiation.
FIGURE 4.
Co-culture conditions for screening of odontogenic cell differentiation using in vitro cell-cell interaction system. A, SP cells were cultured on SF2 cells in monolayers, then fixed with 4% paraformaldehyde (PFA) or treated with ammonia (denudation). B, DSPP expression in SP cells co-cultured under different conditions. C, four sets of co-culture conditions using cell chambers were analyzed. D, DSPP expression in SF2 cells (blue) and SP cells (red) cultured in lower dishes, with co-culture partner cells in the upper chambers. The expression level of GAPDH was used an internal control. *, p < 0.05.
Next, we screened the factors secreted from SF2 cells that promote odontogenic cell differentiation from epithelial and mesenchymal cells using cell culture chambers, which allowed the factors to be secreted into cell culture medium (Fig. 4C). Heterologous combinations of SF2 and SP cells were important for promotion of DSPP expression in both types of cells. We found that co-cultures consisting of SF2 cells in the upper chamber and SP cells in the lower chamber were most effective for stimulation of DSPP gene expression in SP cells (Fig. 4D). These results suggest that secreted factors are important for induction of DSPP expression in SP cells co-cultured with dental epithelial cells.
Regulation of DSPP Expression in SP Cells via BMP2-BMP4 Crosstalk
The involvement of several different types of growth factors has been reported in epithelial-mesenchymal interactions, for example, BMPs were shown to promote dental mesenchymal cell differentiation (27). We examined the potential involvement of BMPs in SP cell differentiation by adding soluble Noggin, which antagonizes BMP activity, to cell chamber cultures that contained SP cells in the lower chambers (Fig. 5A). The presence of Noggin in culture medium resulted in down-regulation of the expression of DSPP in SP cells as compared with the control cells (Fig. 5B). Therefore, BMPs are required for induction of DSPP expression in SP cells co-cultured with dental epithelial cells. In tooth germ development, BMP4 is involved in epithelial-mesenchymal interaction, and also regulates the mesenchymal expression of Msx1 and Msx2, which are important for tooth development, whereas BMP2 promotes dental mesenchymal differentiation (27). However, details regarding crosstalk between BMP2 and BMP4 in dental epithelial and mesenchymal stem cell interactions have not been elucidated. We sought to clarify the role of BMPs in these interactions by examining the expressions of BMP2 and BMP4 in SF2 and SP cells using a separated chamber assay (Fig. 5C). The expression of BMP2 was higher in SP cells than SF2 cells under the heterologous combination culture condition, whereas BMP2 was not detected in homologous cultures (Fig. 5D). In contrast, the expression of BMP4 was higher in SF2 cells than in SP cells in the heterologous combinations (Fig. 5D). Taken together, these results suggest that the interactions between dental epithelium and dental mesenchymal stem cells induce BMP4 and BMP2, which, in turn, promote odontogenic cell differentiation via paracrine and autocrine signaling.
FIGURE 5.
In vitro epithelial-mesenchymal interaction system shows that crosstalk BMP signaling is essential for odontogenic cell differentiation. A, total RNA was isolated from SP cells co-cultured with SF2 cells in the presence or absence of Noggin recombinant protein. B, DSPP expression in SP cells co-cultured with SF2 cells after blocking BMP signaling. C, four sets of culture conditions using cell chambers were analyzed. D, BMP2 and BMP4 expressions in SF2 (blue) and SP (red) cells, with co-culture partner cells in the upper chambers. *, p < 0.05.
Optimization of Co-culture Conditions for Differentiation of iPS Cells into Ambn-expressing Dental Epithelial Cells
Following interaction with SF2 cells, SP cells differentiated into DSPP expressing cells, but not ameloblasts (Figs. 3, 4, and 5). This may be because SP cells are mesenchymal stem cells and committed to differentiate into mesenchyme lineage cell types. Therefore, we used mouse iPS cells to examine whether these cells can be differentiated into ameloblasts when cultured with SF2 cells. However, SF2 cells did not effectively promote their differentiation (data not shown), which may be due to the necessity of factors from differentiated dental epithelial cells for differentiation of iPS cells into ameloblasts. To test this possibility, we subcloned 25 different SF2 cell lines and examined the expression levels of the Ambn gene. Of these lines, the SF2-24 cell line expressed Ambn at the highest level (supplemental Fig. S3A). Dental epithelium SF2-24 cells grew tightly together in a square or cuboidal shape (Fig. 6A), and expressed Ambn and cytokeratin-14 (CK14), but not the reprogrammed factors Sox2, Klf4, and Oct3/4 (supplemental Fig. S3B). On the other hand, iPS cells formed colonies that expressed Nanog promoter-driven GFP (data not shown) as well as Klf4, Sox2, Oct3/4, and Nanog, but not Ambn or CK14 (supplemental Fig. S3B).
FIGURE 6.
Epithelial cell shapes of iPS cells after co-culturing with SF2-24 cells. A, phase micrographs of monolayer SF2-24 cells and iPS cells cultured with SF2-24 feeder cells for 4 days, followed by DAPI staining. B and C, low and high magnification phase micrographs of iPS cells on MMC-treated SF2-24 feeder cells after 6 (6Day) and 10 days (10Day). Enlarged image shows a part of the iPS cells with epithelial cell shapes. C, epithelial cell cluster formed by iPS cell-derived epithelial cells (area within yellow dashed line). Bar, 50 mm.
We also examined the effects of differentiation by co-culturing iPS cells with MMC-treated non-proliferating SF2-24 feeder cells (Fig. 6A). The shape of the co-cultured iPS cells was clearly rounded along the boundary of the clusters after 6 days (Fig. 6B). These cells had migrated and formed what appeared to be epithelium after 10 days (area surrounded by yellow dashed line, Fig. 6C).
The differentiation of iPS cells was then determined by RT-PCR analysis. First, we examined the specificity of mouse Ambn locked nucleic acid (LNA) primer sets (supplemental Fig. S4). A mouse Ambn LNA primer set specifically detected the mouse Ambn gene, but not the rat Ambn gene (supplemental Fig. S4A). Using this primer set, Ambn expression was not detected in mouse iPS cells or MEFs (supplemental Fig. S4B). Next, we examined co-culture conditions for the differentiation of iPS cells into dental epithelium (Fig. 7A). iPS cells co-cultured with MMC-treated SF2-24 cells showed a high expression of the mouse Ambn gene, while those co-cultured with PFA-treated or non-treated SF2-24 cells did not (Fig. 7B). SF2-24 feeder cells expressed rat Ambn when co-cultured with iPS cells, while that expression was reduced at 10 days (Fig. 7C).
FIGURE 7.
Effects of culture conditions on ameloblast induction of iPS cells. A, iPS cells were co-cultured with SF2-24 cells, MMC-treated (MMC) MEFs, MMC-treated SF2-24 cells or PFA-treated SF2-24 cells. B, Ambn expression in mouse iPS (upper panel) and rat-derived SF2-24 (bottom panel) cells in different co-culture conditions for 10 days. C, time course analysis of gene expressions of stem cell (blue), endo/mesoderm (black), and ameloblast (red) markers in iPS cells co-cultured with SF2-24 cells for 7 (7Day) and 10 days (10Day).
Interestingly, expressions of the stem cell markers Sox2, Oct3/4, Nanog, Fgf4, and Gdf3 were not changed throughout the co-culture period, because of the existence of undifferentiated iPS cells (Fig. 7C), while those of the endodermal markers Cdx2 and Gata6 were also not increased. Furthermore, the mesodermal marker Brachyury was highly expressed in iPS cells, because of technical contamination resulting RNA extraction from MEFs used for maintenance of the iPS cells, and then gradually decreased over time. We also observed increased expressions of the mouse ameloblast markers Ambn and Enamelin (Enam), as well as the epithelial markers CK14 and p63, in iPS cells after 7 and 10 days (Fig. 7C). Furthermore, the expression of epiprofin/Sp6, a transcription factor highly expressed in dental epithelium (28), was increased in those cells (supplemental Fig. S5). A similar expression pattern was observed in co-cultured iPS cells separated from SF2-24 cells using the anti-HA antibody (data not shown).
Differentiation of iPS Cells into Ambn-expressing Dental Epithelial Cells
We then examined the protein expression of Ambn in iPS cells using immunostaining. Approximately 95% of the epithelial-like cells were positive for Ambn (Fig. 8A), while the immunofluorescence intensity of Ambn was stronger in iPS cells than in SF2-24 cells (Fig. 8B). Therefore, mouse iPS cells differentiated into dental epithelium, but not into endodermal or mesodermal cells.
FIGURE 8.
Expression of Ambn, an ameloblast specific protein, in iPS cells co-cultured with SF2-24 cells. A, phase micrographs of iPS cell colonies cultured with mitomycin C-treated SF2-24 cells. Hoechst staining (blue), Ambn staining (red), and merged images. B, high magnifications of phase and merged images in A. Bottom panel, relative expression levels of Ambn protein in SF2-24 and iPS cells cultured in ameloblast induction system. *, p < 0.05; Bar, 100 mm.
We attempted to identify the factors involved in differentiation of iPS cells into dental epithelium by culturing with MEFs in medium conditioned by SF2-24 cell cultures (Fig. 9A). Culturing with SF2-24 condition medium induced the expression of Ambn in iPS cells, indicating an involvement of soluble factors including growth factors, and extracellular matrices derived from SF2-24 cells (Fig. 9B). Next, we examined the effect of Ambn on differentiation of iPS cells into dental epithelial cells. Expression vectors for the full-length (AB1), C-terminal (AB2), and N-terminal (AB3) half of Ambn (Fig. 9C) were separately transfected into Ambn low-expressing cells (SF2-7), then conditioned media from those cells or recombinant Ambn proteins (AB1, -2, or -3) were added to cultures of iPS cells. Conditioned media from SF2-24 cells and full-length AMBN-expressing cells, but not from other transfectants or recombinant Ambn proteins, induced Ambn expression in iPS cells (Fig. 9D), indicating that Ambn may be necessary for differentiation of iPS cells into dental epithelium. Previously, we showed that neurotrophic factor NT-4 is important for the differentiation of ameloblasts (29). To examine the effect of NT-4 on dental epithelial cell differentiation by iPS cells, we analyzed the expressions of Ambn and CK14 in iPS cells cultured with SF2-24-conditioned medium in the presence of K252a (inhibitor of neurotrophic receptor Trk), PD98059 (MEK inhibitor), anti-NT-4 neutralizing antibody, or Noggin (BMP antagonist). K252a, PD98059, anti-NT-4, and Noggin each inhibited the expression of Ambn in iPS cells. Furthermore, CK14 expression in iPS cells was not inhibited by K252a, anti-NT-4, or Noggin (Fig. 9E). These results indicate that NT-4 and BMP signaling are important for differentiation into dental epithelial cells, but not CK14-positive epithelial cells.
FIGURE 9.
Promotion of ameloblast induction of iPS cells using conditioned SF2-24 cells. A, iPS cells were cultured on mitomycin C-treated MEFs in iPS cell culture medium supplemented with (CM) or without (M) conditioned medium from SF2-24 cells. B, expression of mouse Ambn gene in iPS cells cultured in iPS cell culture medium supplemented with (CM) or without (M) conditioned medium from SF2-24 cells. C, creation of Ambn deletions. All recombinant Ambn proteins have V5 and His tags at the C terminus. D, expression of mouse Ambn gene in iPS cells cultured in iPS cell culture medium supplemented with (CM) or without (M) condition medium from SF2-24 cells, recombinant Ambn-expressing SF2-7 cells or recombinant Ambn proteins. *, p < 0.05 (compared with non-transfected SF2-7 cells). E, expression of mouse Ambn and CK14 genes in iPS cells cultured in SF2-24 conditioned medium supplemented with K252a, PD98059, anti-NT-4, or Noggin. *, p < 0.05 (compared with CM only).
DISCUSSION
Tooth development progresses through a number of stages, and the differentiation of dentin matrix-secreting odontoblasts and enamel matrix-producing ameloblasts results in formation of the crown. Ameloblasts and odontoblasts are central cell types involved in tooth development. In developing molars, restricted dental mesenchymal cells interact with the inner dental epithelium through the matrix and differentiate into odontoblasts. In the present study, we established an SP cell line from dental papilla mDP cells using cell sorting with Hoechst staining. SP cells are known to retain multipotency characteristics and can differentiate into various cell types, such as odontoblasts, osteoblasts, adipocytes, and neural cells. Our method for obtaining multipotent SP cells from a single cell line may be useful for development of novel therapeutic strategies that aim at regeneration of oral tissues.
Our co-culture assay of SP cells with dental epithelial cells showed that dental epithelial cells promote SP cell differentiation into DSPP-expressing cells via BMP2 and BMP4, which are secreted from dental epithelial cells (Fig. 5B, 5D, and 10A). Because BMP2 is not highly expressed in dental epithelium, BMP4 may be the dominant signaling regulator during odontoblast differentiation. In the early stages of tooth development, BMP4 is expressed in dental epithelium and induces the transcription factor Msx1 (30). The expression of DSPP is induced via the BMP signaling pathway in cooperation with Runx2, Dlx5, and Msx1 in undifferentiated mesenchymal cells (31). Previously, a bead soak assay of mandibular organ culture showed that BMP4 induced dental mesenchymal cell differentiation (32). Also, a transgenic approach revealed that inhibition of BMP4 by Noggin overexpression, driven by a keratin 14 promoter (K14-Noggin), resulted in the absence of all molars in the mandible. This indicates that BMP4 is essential for tooth bud formation by inducing dental mesenchymal cells (33). As demonstrated, in the present study odontoblastic differentiation of SP cells is completely disturbed by the blocking of BMP signaling. Thus, our finding strongly support the notion that BMP4 signaling is a key factor in induction of dental mesenchymal cells and their differentiation.
FIGURE 10.
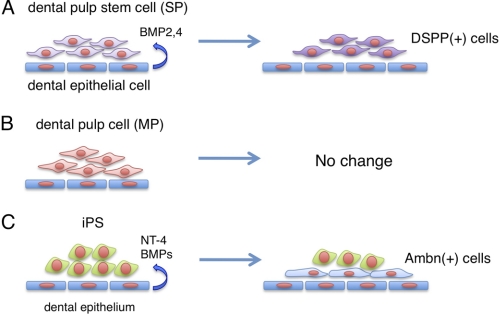
Proposed models of odontogenic induction from dental mesenchymal stem cells and iPS cells by co-culturing with dental epithelial cells. A, dental epithelial cells induce DSPP-expressing odontoblasts from SP cells. B, no odontogenic induction was observed in differentiated (MP) cells co-cultured with dental epithelial cells. C, dental epithelial cells induce Ambn-expressing ameloblasts from iPS cells.
Differential synchronization between dental epithelial and mesenchymal cells has been observed during tooth development. Dental epithelial and mesenchymal cells are separated by a basement membrane, which is an essential regulator for epithelial-mesenchymal interaction (34). Both crown and root odontoblasts are induced by interactions with epithelial cells, such as those of the inner dental epithelium, epithelial rest, and epithelial diaphragm (35). Similar to in vivo situations, physical cell attachment of dental epithelial cells was not required for odontogenic induction of SP cells in our experiments, indicating that soluble factors including BMPs are important for odontogenic induction by dental epithelial cells in culture. We also found that MP cells from dental papilla did not differentiate into DSPP-expressing cells, indicating that epithelial-mesenchymal interactions are important for cell fate determination of dental pulp stem cells, but not for differentiated dental pulp cells (Fig. 10, A and B). It was recently reported that Ambn protein, or a synthetic peptide based on the N-terminal region of the Ambn protein, induced osteoblastic cell differentiation (36). In addition to BMPs, Ambn may also be one of the factors involved in the odontogenic induction process, because the sharing of signaling pathways underlies the mechanism of odontoblastic and osteoblastic induction.
Ameloblasts secrete enamel-specific extracellular matrices including Ambn, which are lost upon tooth eruption. This makes it impossible to repair or replace damaged enamel in an erupted tooth. Therefore, identifying alternative sources of these cells becomes important. Bone marrow-derived cells can give rise to different types of epithelial cells. In mixed cultures with c-Kit+-enriched bone marrow cells, embryonic dental epithelial cells, and dental mesenchyme, bone marrow cells might be reprogrammed to give rise to ameloblast-like cells (37). Our strategy to create ameloblasts from mouse iPS cells may have direct application in tooth regeneration. We succeeded in establishing a co-culture system using cells derived from two different species, mouse iPS cells and rat derived enamel matrix secreting ameloblasts. This is the first demonstration of differentiation of iPS cells into ameloblasts through interactions with dental epithelium (Fig. 10C). However, a set of stem cell markers was continuously expressed in iPS cells after 7 days of co-culturing (Fig. 7C), indicating that a portion of the iPS cells had differentiated into enamel-secreting ameloblasts and some still retained stem cell potential. Thus, the efficacy of iPS cell differentiation into ameloblasts by enamel-secreting ameloblasts feeder cells must to be improved prior to for clinical application.
A number of factors are thought to give iPS cells the capacity for direct or indirect differentiation into ameloblasts. Possible direct effectors include gap junctions, intercellular binding molecules, adhesion factors, and extracellular matrices secreted by dental epithelium. Growth factors might also be involved, because conditioned medium from SF2-24 cells induced Ambn expression in iPS cells. Ambn is also a candidate factor for dental cell differentiation of iPS cells, as SF2 cells expressing low levels of Ambn did not induce differentiation of iPS cells. Furthermore, overexpression of full-length Ambn in cells expressing low levels of Ambn induced iPS cells into ameloblast-like differentiation (Fig. 9D). Ambn has diverse functions in various cellular physiologies, such as cell growth, differentiation, cell polarization, and attachment, though the detailed mechanisms of Ambn signaling require additional investigation. Ambn-null mice display severe enamel hypoplasia due to impaired dental epithelial cell proliferation, polarization, and differentiation into ameloblasts, as well as loss of cell attachment activity with immature enamel matrix (2). These results suggest that Ambn, especially full-length, is necessary for both in vivo and in vitro ameloblast differentiation.
There were differences in cell lineage determination of the dental pulp stem cells and iPS cells when co-cultured with dental epithelial cells. RT-PCR analysis showed that co-culturing induced SP cells to form odontoblastic cells, whereas iPS cells were induced to form ameloblastic cells. In addition, the expression of Brachyury, a mesodermal marker, in iPS cells was down-regulated by co-culturing with SF2-24 cells (Fig. 7C). Conversely, expressions of the epithelial markers p63 and CK14, as well as the dental epithelial marker epiprofin/Sp6 were up-regulated (Fig. 7C, supplemental Fig. S5) (28). These results suggest that the cell lineage of the iPS cells in our co-culturing system was effectively guided into an epithelial cell lineage. It has been reported that the default cell lineage of ES cells is the ectodermal cells, except when cultured in the presence of BMP antagonists (38, 39). Because BMPs promote ectodermal differentiation of ES cells, the expression of BMP observed in SF2 cells (Fig. 5D) may also contribute to dental epithelial cell differentiation of iPS cells. A previous our reported that NT-4 induced Ambn expression in dental epithelium, while NT-4 knock-out mice showed delayed expression of enamel matrices in the early stage of ameloblast differentiation (29). In the present study, the presence of the anti-NT-4 neutralizing antibody or Noggin in conditioned medium from SF2-24 cells inhibited Ambn expression, but not that of CK14 (Fig. 9E). On the other hand, SP cells strongly expressed the endogenous Sox2 protein, one of the reprogramming factors involved in generation of iPS cells (data not shown). Recently, iPS cells were generated from human dental pulp cells with a high level of efficiency in comparison to dermal fibroblasts, possibly due to a high expression level of Sox2 in dental pulp stem cells. However, additional reprogramming factors are required for creation of iPS cells from dental pulp cells. Thus, SP cells themselves did not have the same degree of multipotency as seen with ES and iPS cells. SP cells are considered to be mesenchymal stem cells that originate from dental pulp cells, which are derived from cranial neural crest cells. Neural crest cells can differentiate into several different cell lineages, such as neuron, glia, melanocyte, osteoblast, chondrocyte, and odontoblast cells (40, 41). We believe that SP cells are not able to gain multipotency beyond the potential of neural crest cells. Thus, SP cells preserve some degree of multipotency that is different in an undifferentiated state as compared with ES and iPS cells. In co-cultures with SF2-24 cells, SP cells did not differentiate into ameloblasts, whereas iPS cells did (Fig. 10). Comparative analysis between SP and iPS cells is essential to clarify the mechanisms involved in directional cell fate determination.
In this study, we sought to clarify the role of dental epithelium and stem cell interactions by culturing rat dental epithelium with mouse iPS cells and SP cells. Rodent incisors grow throughout the lifespan of the animal by maintaining stem cells in the cervical loop, located at the end of incisor. A dental epithelial cell niche also exists in the cervical loop of the incisor. Analysis of gene knock-out mice for epiprofin/Sp6, an essential transcription factor for dental epithelial cell differentiation and enamel formation, has revealed that supernumerary teeth are formed by interactions between dental mesenchyme and undifferentiated dental epithelium (4, 42). In addition, those studies showed continuous signals from dental epithelial cells of mutant mice induced the continued differentiation of dental mesenchymal cells into odontoblasts (4, 42). Together these findings suggest that dental epithelial cells can induce dental mesenchymal cells to differentiate into odontoblasts. Therefore, rat dental epithelial cells may provide an in vitro niche environment for surrounding mouse iPS cells and SP cells. Elucidation of the mechanism of cell fate determination by dental epithelial cells may facilitate development of novel therapeutic approaches for regenerative dentistry.
Supplementary Material
This work was supported, in whole or in part, by the Intramural Research Program of the NIDCR, National Institutes of Health (to Y. Y.). This work was also supported by Grants-in-aid 20679006 (to S. F.), 21792054 (to A. Y.), 21792154 (to E. F.) from the Ministry of Education, Science, and Culture of Japan, and the NEXT program (LS010, to S. F.), and by grants from the Takeda Science Foundation.

This article contains supplemental Figs. S1–S5 and Table S1.
- MSC
- mesenchymal stem cell
- mDP
- mouse dental pulp
- Ambn
- Ameloblastin
- DSPP
- dentin sialophosphoprotein
- iPS
- induced pluripotent stem
- DPSC
- dental pulp stem cell
- SP
- side population
- MP
- majority population
- ALP
- alkaline phosphatase
- MEF
- mouse embryonic fibroblasts
- MMC
- mitomycin C.
REFERENCES
- 1. Thesleff I. (2003) Epithelial-mesenchymal signaling regulating tooth morphogenesis. J. Cell Sci. 116, 1647–1648 [DOI] [PubMed] [Google Scholar]
- 2. Fukumoto S., Kiba T., Hall B., Iehara N., Nakamura T., Longenecker G., Krebsbach P. H., Nanci A., Kulkarni A. B., Yamada Y. (2004) Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J. Cell Biol. 167, 973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yuasa K., Fukumoto S., Kamasaki Y., Yamada A., Fukumoto E., Kanaoka K., Saito K., Harada H., Arikawa-Hirasawa E., Miyagoe-Suzuki Y., Takeda S., Okamoto K., Kato Y., Fujiwara T. (2004) Laminin α2 is essential for odontoblast differentiation regulating dentin sialoprotein expression. J. Biol. Chem. 279, 10286–10292 [DOI] [PubMed] [Google Scholar]
- 4. Nakamura T., de Vega S., Fukumoto S., Jimenez L., Unda F., Yamada Y. (2008) Transcription factor epiprofin is essential for tooth morphogenesis by regulating epithelial cell fate and tooth number. J. Biol. Chem. 283, 4825–4833 [DOI] [PubMed] [Google Scholar]
- 5. Fisher L. W., Fedarko N. S. (2003) Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 44, Suppl. 1, 33–40 [PubMed] [Google Scholar]
- 6. Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- 7. Lewitzky M., Yamanaka S. (2007) Reprogramming somatic cells towards pluripotency by defined factors. Curr. Opin. Biotechnol. 18, 467–473 [DOI] [PubMed] [Google Scholar]
- 8. Xu D., Alipio Z., Fink L. M., Adcock D. M., Yang J., Ward D. C., Ma Y. (2009) Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc. Natl. Acad. Sci. U.S.A. 106, 808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanna J., Wernig M., Markoulaki S., Sun C. W., Meissner A., Cassady J. P., Beard C., Brambrink T., Wu L. C., Townes T. M., Jaenisch R. (2007) Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318, 1920–1923 [DOI] [PubMed] [Google Scholar]
- 10. Soldner F., Hockemeyer D., Beard C., Gao Q., Bell G. W., Cook E. G., Hargus G., Blak A., Cooper O., Mitalipova M., Isacson O., Jaenisch R. (2009) Parkinson disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136, 964–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okita K., Ichisaka T., Yamanaka S. (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 [DOI] [PubMed] [Google Scholar]
- 12. So K. H., Han Y. J., Park H. Y., Kim J. G., Sung D. J., Bae Y. M., Yang B. C., Park S. B., Chang S. K., Kim E. Y., Park S. P. (2010) Int. J. Cardiol. 153, 277–285 [DOI] [PubMed] [Google Scholar]
- 13. Yoshida Y., Yamanaka S. (2011) J. Mol. Cell Cardiol. 50, 327–332 [DOI] [PubMed] [Google Scholar]
- 14. Miura M., Gronthos S., Zhao M., Lu B., Fisher L. W., Robey P. G., Shi S. (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. U.S.A. 100, 5807–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gronthos S., Mankani M., Brahim J., Robey P. G., Shi S. (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 97, 13625–13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi S., Bartold P. M., Miura M., Seo B. M., Robey P. G., Gronthos S. (2005) The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod. Craniofac Res. 8, 191–199 [DOI] [PubMed] [Google Scholar]
- 17. Goodell M. A., Brose K., Paradis G., Conner A. S., Mulligan R. C. (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 183, 1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou S., Schuetz J. D., Bunting K. D., Colapietro A. M., Sampath J., Morris J. J., Lagutina I., Grosveld G. C., Osawa M., Nakauchi H., Sorrentino B. P. (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 7, 1028–1034 [DOI] [PubMed] [Google Scholar]
- 19. Li L., Kwon H. J., Harada H., Ohshima H., Cho S. W., Jung H. S. (2011) Expression patterns of ABCG2, Bmi-1, Oct-3/4, and Yap in the developing mouse incisor. Gene Expr. Patterns 11, 163–170 [DOI] [PubMed] [Google Scholar]
- 20. Nam H., Lee G. (2009) Identification of novel epithelial stem cell-like cells in human deciduous dental pulp. Biochem. Biophys. Res. Commun. 386, 135–139 [DOI] [PubMed] [Google Scholar]
- 21. Yan X., Qin H., Qu C., Tuan R. S., Shi S., Huang G. T. (2010) iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem. Cells Dev. 19, 469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yokoi T., Saito M., Kiyono T., Iseki S., Kosaka K., Nishida E., Tsubakimoto T., Harada H., Eto K., Noguchi T., Teranaka T. (2007) Establishment of immortalized dental follicle cells for generating periodontal ligament in vivo. Cell Tissue Res. 327, 301–311 [DOI] [PubMed] [Google Scholar]
- 23. Klotz O. (1905) Studies upon calcareous degeneration: I. The process of pathological calcification. J. Exp. Med. 7, 633–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sonoda A., Iwamoto T., Nakamura T., Fukumoto E., Yoshizaki K., Yamada A., Arakaki M., Harada H., Nonaka K., Nakamura S., Yamada Y., Fukumoto S. (2009) Critical role of heparin binding domains of ameloblastin for dental epithelium cell adhesion and ameloblastoma proliferation. J. Biol. Chem. 284, 27176–27184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen S., Gu T. T., Sreenath T., Kulkarni A. B., Karsenty G., MacDougall M. (2002) Spatial expression of Cbfa1/Runx2 isoforms in teeth and characterization of binding sites in the DSPP gene. Connect. Tissue Res. 43, 338–344 [DOI] [PubMed] [Google Scholar]
- 26. Cukierman E., Pankov R., Stevens D. R., Yamada K. M. (2001) Taking cell-matrix adhesions to the third dimension. Science 294, 1708–1712 [DOI] [PubMed] [Google Scholar]
- 27. Nakashima M. (1994) Induction of dentin formation on canine amputated pulp by recombinant human bone morphogenetic proteins (BMP)-2 and -4. J. Dent. Res. 73, 1515–1522 [DOI] [PubMed] [Google Scholar]
- 28. Nakamura T., Unda F., de-Vega S., Vilaxa A., Fukumoto S., Yamada K. M., Yamada Y. (2004) The Krüppel-like factor epiprofin is expressed by epithelium of developing teeth, hair follicles, and limb buds and promotes cell proliferation. J. Biol. Chem. 279, 626–634 [DOI] [PubMed] [Google Scholar]
- 29. Yoshizaki K., Yamamoto S., Yamada A., Yuasa K., Iwamoto T., Fukumoto E., Harada H., Saito M., Nakasima A., Nonaka K., Yamada Y., Fukumoto S. (2008) Neurotrophic factor neurotrophin-4 regulates ameloblastin expression via full-length TrkB. J. Biol. Chem. 283, 3385–3391 [DOI] [PubMed] [Google Scholar]
- 30. Chen Y., Bei M., Woo I., Satokata I., Maas R. (1996) Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 122, 3035–3044 [DOI] [PubMed] [Google Scholar]
- 31. Cho Y. D., Yoon W. J., Woo K. M., Baek J. H., Park J. C., Ryoo H. M. (2010) The canonical BMP signaling pathway plays a crucial part in stimulation of dentin sialophosphoprotein expression by BMP-2. J. Biol. Chem. 285, 36369–36376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vainio S., Karavanova I., Jowett A., Thesleff I. (1993) Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75, 45–58 [PubMed] [Google Scholar]
- 33. Plikus M. V., Zeichner-David M., Mayer J. A., Reyna J., Bringas P., Thewissen J. G., Snead M. L., Chai Y., Chuong C. M. (2005) Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity. Evol. Dev. 7, 440–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thesleff I., Hurmerinta K. (1981) Tissue interactions in tooth development. Differentiation 18, 75–88 [DOI] [PubMed] [Google Scholar]
- 35. Ten Cate A. R. (1996) The role of epithelium in the development, structure and function of the tissues of tooth support. Oral Dis. 2, 55–62 [DOI] [PubMed] [Google Scholar]
- 36. Iizuka S., Kudo Y., Yoshida M., Tsunematsu T., Yoshiko Y., Uchida T., Ogawa I., Miyauchi M., Takata T. (2011) Ameloblastin regulates osteogenic differentiation by inhibiting Src kinase via cross talk between integrin β1 and CD63. Mol. Cell Biol. 31, 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu B., Unda F., Bopp-Kuchler S., Jimenez L., Wang X. J., Haïkel Y., Wang S. L., Lesot H. (2006) Bone marrow cells can give rise to ameloblast-like cells. J. Dent. Res. 85, 416–421 [DOI] [PubMed] [Google Scholar]
- 38. Chang C., Hemmati-Brivanlou A. (1998) Cell fate determination in embryonic ectoderm. J. Neurobiol. 36, 128–151 [PubMed] [Google Scholar]
- 39. Muñoz-Sanjuan I., Brivanlou A. H. (2002) Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 3, 271–280 [DOI] [PubMed] [Google Scholar]
- 40. Baroffio A., Dupin E., Le Douarin N. M. (1991) Common precursors for neural and mesectodermal derivatives in the cephalic neural crest. Development 112, 301–305 [DOI] [PubMed] [Google Scholar]
- 41. Sieber-Blum M., Cohen A. M. (1980) Clonal analysis of quail neural crest cells: they are pluripotent and differentiate in vitro in the absence of noncrest cells. Dev. Biol. 80, 96–106 [DOI] [PubMed] [Google Scholar]
- 42. Nakamura T., Fukumoto S., Yamada Y. (2011) Review: diverse function of epiprofin in tooth development. J. Oral Biosci. 53, 22–30 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.