Background: Lectins are carbohydrate-binding proteins that exert their activity by binding to specific glycoreceptors.
Results: Clitocybe nebularis lectin (CNL) showed biological activity, although its nonsugar-binding and monovalent mutants were inactive.
Conclusion: The bivalent carbohydrate-binding property of CNL is essential for its activity.
Significance: Understanding the interactions of lectins with glycans and elucidating their modes of action are necessary for their application in biomedicine.
Keywords: Fungi, Glycobiology, Lectin, Recombinant Protein Expression, Structural Biology, β-Trefoil Fold, Bivalent Carbohydrate-binding Property, Clitocybe nebularis, LacdiNAc, Ricin B-like Lectin
Abstract
Lectins are carbohydrate-binding proteins that exert their biological activity by binding to specific cell glycoreceptors. We have expressed CNL, a ricin B-like lectin from the basidiomycete Clitocybe nebularis in Escherichia coli. The recombinant lectin, rCNL, agglutinates human blood group A erythrocytes and is specific for the unique glycan N,N′-diacetyllactosediamine (GalNAcβ1–4GlcNAc, LacdiNAc) as demonstrated by glycan microarray analysis. We here describe the crystal structures of rCNL in complex with lactose and LacdiNAc, defining its interactions with the sugars. CNL is a homodimeric lectin, each of whose monomers consist of a single ricin B lectin domain with its β-trefoil fold and one carbohydrate-binding site. To study the mode of CNL action, a nonsugar-binding mutant and nondimerizing monovalent CNL mutants that retain carbohydrate-binding activity were prepared. rCNL and the mutants were examined for their biological activities against Jurkat human leukemic T cells and the hypersensitive nematode Caenorhabditis elegans mutant strain pmk-1. rCNL was toxic against both, although the mutants were inactive. Thus, the bivalent carbohydrate-binding property of homodimeric CNL is essential for its activity, providing one of the rare pieces of evidence that certain activities of lectins are associated with their multivalency.
Introduction
Lectins are carbohydrate-binding proteins that exert their biological activity by binding to specific glycoreceptors. They act as general recognition molecules in cell-molecule and cell-cell interactions and elicit diverse biological responses in various cells and organisms. However, the exact modes of their action are rarely defined (1). Different types of lectins are distinguished according to their overall structure; for example, merolectins contain a single carbohydrate-binding domain, whereas hololectins are composed of two or more such domains (2). However, lectins usually possess multiple carbohydrate-binding sites, which enable their agglutinating activity. Moreover, multivalent lectin-glycan interactions on the cell surface constitute a possible mechanism for signal transduction through cross-linking and clustering of specific glycoreceptors (3). For example, human homodimeric galectin-1 was shown to induce apoptosis of human T cells by cross-linking and segregating specific receptors on the cell surface (4).
Basidiomycete fungi constitute a rich source of lectins exhibiting various carbohydrate-binding properties and structures (5). By binding to specific glycoreceptors, mushroom lectins can elicit toxic effects against various organisms, suggesting their defensive role (6, 7), in addition to other biological functions in fungal growth and development (8). So far, six different structural families of mushroom lectins have been identified as follows: the novel lectin fold observed for fungal immunomodulatory protein from Flammulina velutipes; the mushroom lectin (XCL) family resembling actinoporins; β-propeller-fold lectins; the galectin; the jacalin-related lectin families; and the ricin family with the β-trefoil fold (5). Ricin B-like lectins are carbohydrate-binding proteins similar to the B chain domains of ricin, a toxin from the castor bean (Ricinus communis) (9). The main characteristic of these lectin domains is that they consist of three repeated subdomains, referred to as α-, β-, and γ-repeats, each containing a more or less well conserved QXW motif (10). Several mushroom ricin B-like lectins have been identified (5), and crystal structures have been solved for Laetiporus sulfureus lectin (LSL)2 (11), a lectin from mushroom Marasmius oreades (MOA) (12) and for Polyporus squamosus lectin (PSL) (13). Similarly to toxic proteins like ricin (14), abrin (15), and related plant toxins (16), these mushroom proteins are chimerolectins, containing a nonlectin domain at the C terminus in addition to the lectin domain at the N terminus. LSL contains a pore-forming module (11), whereas a cysteine protease domain, which also serves as a dimerization interface, is present in MOA and PSL (12, 13, 17). The toxic activities of these modular proteins have been attributed to their catalytic domains, whereas the lectin domain facilitates internalization and intracellular transport of the protein by binding to glycolipids or glycoproteins (16, 17). The β-trefoil fold is also present in another ascomycete lectin, Sclerotinia sclerotiorum agglutinin (18).
Recently, we reported a novel, biologically active ricin B-like lectin isolated from mushroom Clitocybe nebularis, named C. nebularis lectin (nCNL), that showed antiproliferative activity against Jurkat human leukemic T cells (19). This lectin also exhibits insecticidal and anti-nutritional activities against the fruit fly (Drosophila melanogaster) and Colorado potato beetle (Leptinotarsa decemlineata) (20). It is toxic against the mosquito Aedes aegypti and the amoebozoa Acanthamoeba castellanii and against a hypersensitive strain of nematode Caenorhabditis elegans (7), suggesting its defensive role in the mushroom against predators and parasites. nCNL preferentially agglutinates blood group A human erythrocytes and is specific for terminal, nonreducing N-acetylgalactosamine (GalNAc)-containing carbohydrates including N,N′-diacetyllactosediamine (GalNAcβ1–4GlcNAc, LacdiNAc or LDN) (19).
LDN is a unique N-glycan structure rarely found in mammals, although it is abundant in invertebrates such as parasitic helminths and insects (21, 22). It has been proposed that LDN-glycans constitute a molecular pattern for immune recognition of parasites mediated by mammalian galectin-3 (23). In humans, LDN is restricted to certain glycoproteins, particularly hormones (21); moreover, it has been claimed that the glycan is observed in leukemic cells but not in multiple normal tissues (24). Other reports also suggest that LDN is associated with tumor malignancy (25–27). Therefore, LDN-specific lectins and studies of the molecular basis of their specificity could be of interest in biotechnological and biomedical applications.
Here, we used recombinant CNL (rCNL) expressed in Escherichia coli to further characterize the lectin at the molecular, structural, and functional levels. We determined its specificity by glycan microarray analysis and solved the crystal structure of the lectin in complex with lactose and LDN to study its carbohydrate-binding properties and the molecular basis of the interactions. Several CNL mutants were constructed to study the mode of CNL action against two biological systems, human Jurkat cell line and nematode C. elegans.
EXPERIMENTAL PROCEDURES
Expression and Purification of Recombinant CNL
The gene and cDNA sequences of CNL isolated from basidiomycete C. nebularis fruiting bodies were obtained previously (19). For heterologous expression of the recombinant lectin, the CNL-encoding cDNA sequence (GenBankTM/EMBL accession number FJ477895) was amplified using primers incorporating NdeI and BamHI restriction sites to the 5′ and 3′ ends, respectively. After NdeI/BamHI (New England Biolabs) digestion of both insert and vectors, the resulting insert was subcloned into pET3a and pET11a expression vectors (Novagen). Both vectors were transformed into BL21(DE3) (Invitrogen) and BL21(DE3) pLysS (Novagen) strains of E. coli to determine the optimal expression system. Recombinant CNL was expressed in BL21(DE3) strain harboring the pET11a construct. Five hours after induction of expression at 37 °C, bacteria were harvested by centrifugation, resuspended in 50 mm Tris-HCl buffer, pH 7.5, 2 mm EDTA, 0.1% Triton X-100, frozen and thawed, and then sonicated at 4 °C. The insoluble fraction was separated by centrifugation (10,000 × g, 15 min), resuspended in the same buffer, and then subjected to successive cycles of centrifugation and solubilization in buffers containing increasing concentrations of urea (1, 3, 6, and 8 m). Solutions containing rCNL were collected, and the lectin was purified and refolded by removing urea using gel filtration chromatography (Sepharose S-200 column) followed by affinity chromatography on Sepharose-immobilized lactose.
Site-directed Mutagenesis
Several mutants of CNL were constructed by the following substitutions: D20R for a nonsugar-binding mutant (0-CNL); N110D for nondimerizing monovalent single mutant (Mono1-CNL); and N110D/L54R and N110D/L54W for nondimerizing monovalent double mutants Mono2R-CNL and Mono2W-CNL, respectively. In PCR site-directed mutagenesis using KOD Hot Start DNA polymerase (Novagen) and appropriate mutagenic primers, pET11a::rCNL vector was used as a template. Plasmids containing mutated inserts were then subjected to digestion with DpnI endonuclease (Fermentas) followed by recovery of the vectors containing substitutions (28). The mutant constructs were verified by DNA sequencing and then expressed at 37 °C in BL21(DE3) strain of E. coli as inclusion bodies, solubilized in urea, and purified using gel filtration chromatography.
Biochemical Characterization of rCNL and Its Mutants
The proteins were characterized as described previously (19). The molecular masses of RP-HPLC-purified rCNL and its mutants were determined by electrospray ionization mass spectrometry on Q-Tof premier mass spectrometer (Micromass, UK). The N-terminal sequence of rCNL was examined by automated Edman degradation with Procise Protein Sequencing System 492 (PE Applied Biosystems, CA). Molecular masses of rCNL and the mutants under nondenaturing conditions were estimated by gel filtration fast protein liquid chromatography (ÄKTA FPLC System, Amersham Biosciences) and nondenaturing PAGE (NativePAGE Novex BisTris Gel System, Invitrogen) following the manufacturer's protocol. Isoelectric focusing was performed using a PhastSystem (Pharmacia).
Circular Dichroism Spectroscopy Analysis
To compare conformations of nCNL, rCNL, and mutants of the latter, far-UV circular dichroism spectra were recorded from 185 to 250 nm. The lectins in 10 mm phosphate buffer, pH 7.5, with concentrations of ∼0.2 mg/ml were analyzed on an AVIV 62A DS spectropolarimeter using 1-mm path length cell. Buffer base lines were measured and subtracted from the corresponding spectra. The data were transformed to mean residue ellipticity.
Hemagglutination Assays
The specificity of rCNL for human blood groups A, AB, B, and O and for bovine erythrocytes was studied by hemagglutination assay. Agglutinating activity of the mutants was examined using group A erythrocytes. Preliminary study of specificity of rCNL for sugars (glucose, galactose, sucrose, lactose, fructose, and mannose) and the glycoprotein asialofetuin was performed by hemagglutination inhibition assay using group A erythrocytes and an agglutinating concentration of rCNL of 0.49 μm. The hemagglutination assays were performed in triplicate as described previously (19), and the mean lowest agglutinating concentrations with standard deviations were calculated.
Glycan Microarray Analysis
Specificities of rCNL and the mutants 0-CNL, Mono1-CNL, Mono2R-CNL, and Mono2W-CNL, were determined by glycan microarray analysis performed by the Consortium for Functional Glycomics (Core H). Mammalian Printed Array, version 5.0, consisting of 611 glycan targets in replicates of six was used. Before the assay, the proteins were biotinylated using No-WeightTM NHS-PEO4-Biotin (Pierce, IL) according to the manufacturer's instructions. The labeled lectins in binding buffer (20 mm Tris-HCl, pH 7.4, containing 150 mm sodium chloride, 2 mm calcium chloride, 2 mm magnesium chloride, 0.05% Tween 20 and 1% BSA) were analyzed as described previously (29). Briefly, 70 μl of rCNL or its mutants with concentrations of 100, 10, 1, and 0.1 μg/ml were applied to the microarray slides that were incubated at room temperature in a humidified chamber for 1 h. After the slides were washed four times in binding buffer containing 0.05% Tween 20 and four times in the buffer without Tween 20, 70 μl of fluorescently labeled streptavidin in binding buffer was added to the slides and incubated for 1 h. The slides were then washed again as described above, followed by four washes in distilled water. Bound lectins were detected using a fluorescence reader ProScanArray (PerkinElmer Life Science), and images were analyzed using ImaGene software (BioDiscovery). To determine the glycan-binding specificity of rCNL, a detailed analysis of concentration-dependent binding of the lectin to glycan microarrays was performed as described (29). Results obtained for 100, 10, 1, and 0.1 μg/ml concentrations of rCNL were used to calculate its average binding for each target, and glycans were then ranked and sorted in descending order. The glycans that were not bound by the lectin in a concentration-dependent manner exhibited a nonspecific mechanism of binding and were eliminated from evaluation, thus the actual glycan-binding specificity of rCNL was revealed.
Structure Solution and Refinement
Recombinant CNL was concentrated to 30 mg/ml in 10 mm Tris-HCl buffer, pH 7.0, containing 5 mm lactose. The crystals for CNL-LAC/4.4 structure were grown in 0.1 m sodium acetate, pH 4.4, containing 29% PEG 550 MME and for CNL-LAC/7.1 structure in 0.085 m HEPES, pH 7.1, containing 1.7% PEG 400, 2.0 m AmSO4, and 19% glycerol. To determine the CNL structure in complex with LDN (the compound was kindly provided by the Carbohydrate Synthesis/Protein Expression Core of the Consortium for Functional Glycomics), CNL-LAC/7.1 crystals were washed three times in 5 μl of reservoir solution to remove the lactose and then soaked for 4 h in reservoir solution containing 10 mm LDN. Diffraction data for CNL-LAC/7.1 and CNL-LAC/4.4 were collected at Synchrotron Elletra, Trieste, Italy, and the data for CNL-LDN structure were collected on an in-house copper rotating anode Rigaku (RU 200). All data were processed using the HKL2000 package (30). The CNL structure was solved by single wavelength anomalous diffraction phasing using the data collected from the CNL-LAC/4.4 crystal briefly soaked in the reservoir solution containing 0.5 m sodium iodide. The data set was collected to 1.8 Å on the in-house Rigaku rotating anode (RU 200) using Xenox mirrors. From a single crystal with the linear R-merge of 5% and redundancy of 3, 190 images were collected. Single wavelength anomalous diffraction phasing was based on 20 iodine positions with occupancy ranging from 0.5 to 0.02 using automated SOLVE/RESOLVE scripts incorporated in the AutoSol module of the PHENIX suite (31). Automated model building and docking to the CNL amino acid sequence gave a solution with ∼30 of the 148 amino acids built (data not shown). The high resolution data set was phased with the partial structure of CNL from the iodine-soaked crystal, using molecular replacement program AMoRe (32). Automated building with ARP/wARP (33) produced an almost complete structure (142 residues of 148). The structures were refined with REFMAC (34) and MAIN (35), whereas geometric parameters for bound ligands were obtained from PURY server (36). The crystallization conditions, data collection, and refinement statistics are summarized in Table 1.
TABLE 1.
Data collection and refinement statistics
Numbers in parentheses refer to the highest resolution shells. Data sets from only one crystal were used for determination of each structure.
| Crystal structure | CNL-LAC/7.1 | CNL-LAC/4.4 | CNL-LDN |
|---|---|---|---|
| Data collection | |||
| Protein Data Bank code | 3NBD | 3NBC | 3NBE |
| Crystallization conditions | 0.085 m HEPES, pH 7.1 | 0.1 m sodium acetate, pH 4.4 | 0.085 m HEPES, pH 7.1 |
| 1.7% PEG 400 | 29% PEG 550 MME | 1.7% PEG 400 | |
| 2.0 m AmSO4 | 2.0 m AmSO4 | ||
| 19% glycerol | 19% glycerol | ||
| Space group cell dimensions | P212121 | P212121 | P212121 |
| a, b, c | 34.12, 85.08, 97.63 Å | 54.79, 50.90, 112.41 Å | 41.89, 80.96, 95.78 Å |
| α, β, γ | 90, 90, 90° | 90, 90, 90° | 90, 90, 90° |
| Resolution | 23.0 to 1.15 Å | 50.0 to 1.01 Å | 50.0 to 1.85 Å |
| Rmerge | 6.0% (32.6%) | 4.6% (40.3%) | 8.3% (35.2%) |
| I/σI | 11.5 (3.3) | 17.5 (3.5) | 13.6 (2.0) |
| Completeness | 98.3% (65.6%) | 98.4% (94.8%) | 96.5% (48.6%) |
| Redundancy | 8.5 (3.0) | 8.6 (3.0) | 7.5 (1.8) |
| Refinement | |||
| Resolution | 23.0 to 1.15 Å | 27.9 to 1.01 Å | 16.5 to 1.86 Å |
| No. of reflections (work/free) | 122,034/6458 | 168,717/8906 | 26,123/1396 |
| Rwork/Rfree | 14.2/16.0 | 15.7/17.2 | 17.8/21.9 |
| B factors | |||
| Protein | 12.6 | 12.3 | 13.0 |
| Water | 28.9 | 28.1 | 36.5 |
| Ligand | 31.3 | 14.4 | 31.2 |
| No. of atoms | |||
| Protein | 2238 | 2238 | 2238 |
| Water | 528 | 596 | 384 |
| Ligand | 48 | 24 | 68 |
| Root mean square deviation | |||
| Bond lengths | 0.050 Å | 0.051 Å | 0.025 Å |
| Bond angles | 2.23° | 2.105° | 2.19° |
Cell Viability Assay
The effect of rCNL and its mutants on Jurkat cell line (derived from human leukemic T cells; ATCC number TIB-152) was studied as described previously (19). The lectins in PBS, pH 7.4, with concentrations of 25, 50, and 100 μg/ml, were added to Jurkat cells with final concentrations of 4 × 105 cells/ml. In control samples, appropriate volumes of PBS devoid of the lectins or lectins preincubated with 0.1 m lactose were added to cells, and all the cells were incubated for 4 days at 37 °C. Their viability was then assessed using CellTiter 96 AQueous one solution cell proliferation assay kit (Promega) as described previously (19). The experiment was performed in triplicate and mean values of treated versus untreated cells were compared using a two-tailed, unpaired t test.
C. elegans Biotoxicity Assay
Biotoxicity assays with the nematode C. elegans wild-type isolate Bristol N2 and hypersensitive mutant strain pmk-1(km25) were performed as described previously (37), with the exception that the assays were executed in flat bottom 96-well plates. 20–50 first stage larvae (L1) were mixed with the E. coli BL21(DE3) culture that expressed rCNL, 0-CNL, or Mono2R-CNL recombinant proteins overnight at 23 °C with the A600 adjusted to 2, and the number of L1 larvae was recorded. After 48 h of incubation at 20 °C, the fraction of animals that reached the L4 stage was determined, and after 72 h the fraction of gravid adult animals was determined by visual inspection, using an inverted microscope (Axiovert 25, Zeiss). Five replicates were performed for each recombinant protein, and an empty vector in E. coli BL21(DE3) was used as control. Expression of the recombinant proteins after overnight induction at 23 °C was analyzed in whole cell extracts and in soluble protein fractions as described previously (37).
RESULTS
Expression and Biochemical Properties of rCNL and Its Mutants
The wild-type recombinant CNL and its mutants were successfully expressed in E. coli at 37 °C, mainly as insoluble inclusion bodies as revealed by SDS-PAGE analysis of the supernatant and pellet of disrupted cells (supplemental Fig. S1). The analysis of the supernatants obtained from successive solubilization of the pellets with increasing concentrations of urea showed the lectins dissolved in 1 and 3 m urea (supplemental Fig. S1). After the gel filtration chromatography refolding process and purification, ∼190 mg of the lectins was obtained from 1 liter of bacterial culture.
Biochemical characterization of rCNL revealed properties corresponding to those of isolated nCNL (19). SDS-PAGE analysis of purified rCNL revealed a single band corresponding to ∼19 kDa (supplemental Fig. S1), whereas mass spectrometry analysis revealed two molecular masses that correspond exactly to theoretical masses with or without initiating Met (15,992 and 15,861 Da). This was confirmed by N-terminal sequencing, which showed two parallel sequences, one starting with Met and the other with the following Ser. Similar characteristics were also observed for the mutants of CNL. Molecular masses of nondenatured rCNL and the mutants were estimated using gel filtration and nondenaturing electrophoresis. Both methods gave similar results, showing a molecular mass of ∼33 kDa for rCNL, indicating its dimeric structure. In contrast, Mono1-CNL, Mono2R-CNL, and Mono2W-CNL mutants showed ∼20 or ∼15-kDa molecular mass, indicating monomers (Fig. 1). The isoelectric point of rCNL was at pH 4.8, which corresponds to the theoretical value (4.87) calculated from the amino acid sequence, whereas nCNL has an isoelectric point at 4.3 (19), which could be the result of its acetylation that confers a negative charge to the lectin.
FIGURE 1.
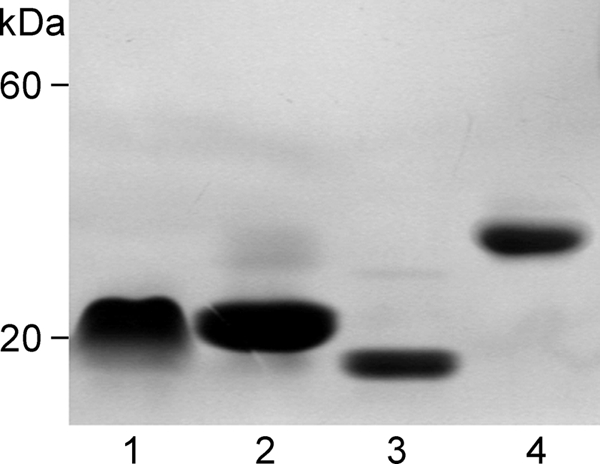
Nondenaturing PAGE analysis of nondimerizing CNL mutants and rCNL. Lane 1, Mono1-CNL mutant; lane 2, Mono2R-CNL; lane 3, Mono2W-CNL; and lane 4, wild-type rCNL.
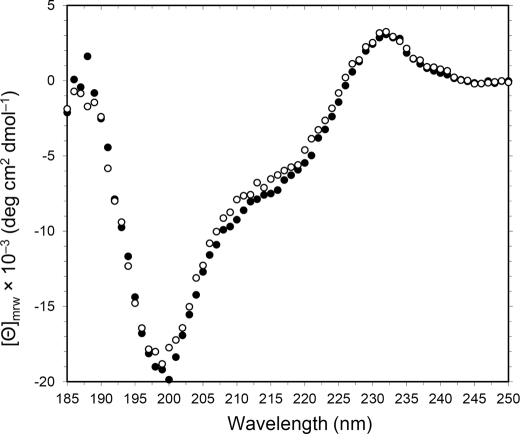
CD spectroscopy analysis revealed that the spectrum of rCNL was closely similar to that of nCNL (Fig. 2), both having the unusual peak at 232 nm associated with the tryptophan tertiary environment (38). The mutant rCNLs also displayed such CD spectra (data not shown), indicating the recombinant proteins were folded correctly during the refolding process.
FIGURE 2.
Far-UV CD spectra of nCNL (○) and rCNL (●). Spectra were measured at 25 °C in a 1-mm cell with lectin concentrations of 0.14 mg/ml. [ϴ]mrw represents mean residue ellipticity.
Specificity of rCNL for Different Types of Erythrocyte and Sugars
Hemagglutination assays, using human and bovine erythrocytes, showed that rCNL, like nCNL (19), is specific for human blood group A erythrocytes, the lowest concentration that exhibited agglutination being 0.32 ± 0.04 μm, followed by group AB (0.49 ± 0.01 μm). The lectin has low specificity for blood group B (55.00 ± 4.75 μm) and O (60.12 ± 8.68 μm) erythrocytes, whereas no agglutination of bovine erythrocytes occurred up to the highest concentration of rCNL tested (121.46 μm). In the hemagglutination inhibition assay rCNL was inhibited by lactose, the lowest concentration of the sugar exhibiting inhibition being 0.34 ± 0.02 mm, followed by galactose (0.92 ± 0.05 mm). Glucose (60.77 ± 5.59 mm) and sucrose (463.64 ± 12.86 mm) showed low inhibition, whereas fructose did not inhibit the agglutination up to the highest concentration tested (909.09 mm). The glycoprotein asialofetuin also showed strong inhibition of agglutination with the lowest concentration that inhibited rCNL being 0.44 ± 0.10 μg/ml.
The agglutinating activities of CNL mutants were tested using human group A erythrocytes. 0-CNL, Mono2R-CNL, and Mono2W-CNL did not agglutinate erythrocytes up to the highest concentrations tested (419.07, 171.78, and 96.74 μm, respectively), whereas Mono1-CNL agglutinated erythrocytes down to 1.43 ± 0.36 μm, indicating that the latter mutant dimerizes, but to a lesser extent than the wild-type CNL.
Glycan-binding Specificity of rCNL
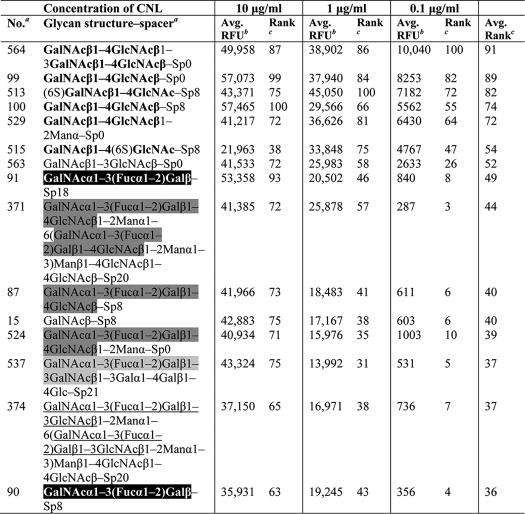
A detailed analysis of concentration-dependent binding of rCNL to glycan microarrays (Table 2) showed that the lectin has a high affinity for LDN. It shows the strongest binding to the tandem repeat of the LDN motif, followed by LDN and 6-sulfated LDN (3- and 4-sulfated LDNs show negligible affinity, the binding being apparently hindered by the modifications at these positions). rCNL also binds a unique carbohydrate structure GalNAcβ1–3GlcNAc with the monosaccharides linked by a β1,3-glycosidic bond; however, the β1,4 linkage in LDN is preferred. The recombinant lectin also shows relatively high affinity for a series of carbohydrates containing terminal human blood group A determinants and for GalNAc (Table 2). rCNL recognizes terminal nonreducing GalNAc, and in addition, GlcNAc at the reducing end apparently increases the binding of the lectin. In contrast, galactose and lactose, in which the 2′-acetamido groups are absent, show considerably lower affinity for rCNL than LDN and GalNAc.
TABLE 2.
Specificity of recombinant CNL for glycans determined by glycan microarray analysis
Specificity was defined using concentration-dependent binding of the lectin to glycan microarrays with concentrations of 10, 1, and 0.1 μg/ml (29). Glycans with the highest affinities for rCNL are listed. LDN motif is printed in boldface; human blood group A determinant trisaccharide is shaded black; tetrasaccharide A type 2 is shaded dark gray; A type 4 is shaded light gray; and A type 1 is underlined.
a Mammalian printed array version 5.0 consisting of 611 glycan structures in replicates of six was used. A list of glycans and designation of spacer arms are available at the Consortium for Functional Glyconics website.
b Relative fluorescence units (RFU) represent the degree of binding of fluorescently labeled streptavidin to biotin-labeled rCNL, which indicates the specificity of rCNL for individual glycans. The value represents the mean of four replicates (the highest and lowest points from six replicates have been removed).
c At each concentration, a rank was determined for each glycan as 100 × (RFU of individual glycan/highest RFU value in the assay). Finally, average ranks were calculated for individual glycans, which are sorted in descending order.
Glycan microarray analysis of CNL mutants revealed that 0-CNL has no carbohydrate binding activity, whereas Mono1-CNL, Mono2R-CNL, and Mono2W-CNL monovalent mutants show very similar binding patterns to that of wild-type rCNL (supplemental Fig. S2).
Crystal Structure of rCNL
Crystal structures were determined for rCNL in complex with lactose at pH 4.4 and 7.1 and with LDN. All three structures, CNL-LAC/4.4, CNL-LAC/7.1, and CNL-LDN, crystallized in P212121 space group with two monomers in the asymmetric unit. The data collection and refinement statistics are summarized in Table 1. The complete amino acid sequence of CNL, from Ser1 to Val148, was accounted for in the analysis, and the position of nearly all the residues is clearly revealed by the electron density maps. The exceptions in all three crystals are the short loop Asn100–Gly102, which is only loosely defined, and the C-terminal part (Ser146–Val148), where no interpretable electron density was observed. The structures of the two monomers in the asymmetric units of all three crystals are closely related, with a root mean square deviation of 130 eq Cα atoms less than 0.25 Å. The root mean square deviation is slightly larger when monomers from different crystals are compared but are never larger than 0.5 Å.
Monomeric Structure
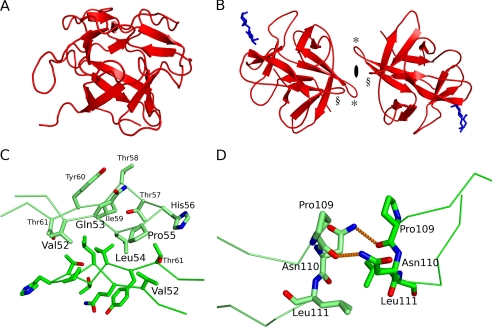
CNL has a β-trefoil fold consisting of three structurally similar subdomains, the α-, β-, and γ-repeats (Figs. 4C and 5) that are related by pseudo-3-fold symmetry around the hydrophobic core. The CNL fold is reminiscent of a tree with a short thick trunk and a crown with branches expanding far from the center (Fig. 3A), similar to that in the β-trefoil fold of fungal protease inhibitors, mycocypins (39). The trunk part is an up-and-down β-barrel composed of six antiparallel β-strands (β1, β4, β5, β8, β9, and β12). The strands are laid at an angle of less than 45° to the axis of the barrel. The N and C termini are at the bottom. On the top, the tree crown is composed of three long regions, each containing a pair of antiparallel β-strands; one region is between the β1 and β4 strands, the second is between the β5 and β8 strands, and the third is between the β9 and β12 strands.
FIGURE 4.
Carbohydrate-binding pocket of CNL with bound lactose (A) and LDN (B) is shown. The sugars are presented as stick models. The amino acid residues involved in the formation of hydrogen bonds (shown as orange dashed lines) are labeled. C, superimposition of α-, β-, and γ-repeats of CNL. The repeats are shown in yellow, blue, and green, respectively. The amino acid residues involved in hydrogen bond formations in α-repeat are labeled and colored yellow, and structurally equivalent residues in β- and γ-repeats (see Fig. 5) are colored blue and green, respectively. It is evident that, in contrast to Asp20 in the α-repeat, the corresponding Arg72 in β- and Thr116 in γ-repeats cannot serve as the anchoring point for a lactose molecule.
FIGURE 5.
Structural alignment of partial amino acid sequences of α-, β-, and γ-repeats of CNL. The alignment was performed with the protein structure comparison service PDBeFold (SSM) (53) at the European Bioinformatics Institute. Structurally aligned β-strands are shaded gray, amino acid residues involved in lactose and LDN binding are in boldface, and the Ser residue involved in LDN binding is underlined.
FIGURE 3.
Three-dimensional structures of CNL and dimer-forming interactions. A, structure of the monomer in a tree-like orientation. B, structure of the dimer with bound lactose molecules (blue) and a 2-fold symmetry axis. Interacting β4-β5 loops are indicated by * and β10-β11 loops by §. C, hydrophobic interactions between the two β4-β5 loops. D, interaction between the two β10-β11 loops. Hydrogen bonds are shown as orange dashed lines.
Dimeric Structure
The homodimer is formed by interactions between the two β4-β5 loops and between the two β10-β11 loops, the monomers being related by pseudo-2-fold rotational symmetry (Fig. 3B). The β4-β5 loop from one monomer lies over the β4-β5 loop of the second monomer. The interactions are exclusively hydrophobic and involve Val52, Leu54, Pro55, and Ile59 (Fig. 3C). A hydrogen bond could be formed between the hydroxyl groups of Thr57 from one monomer and Thr61 from the other monomer; however, the orientations of the side chains, which are clearly defined in the electron density, exclude that possibility. In contrast, the interaction between the two β10-β11 loops is a consequence of the two hydrogen bonds shown in Fig. 3D. They are formed between the amine nitrogen of Asn110 in the first monomer and the carbonyl oxygen of Pro109 in the second monomer, and between the carbonyl oxygen of Pro109 in the first monomer and the amine nitrogen of Asn110 in the second monomer. These interactions can thus be described by a square, defined by the two hydrogen bonds on two opposite sides and the Pro109–Asn110 residues on the other two sides (Fig. 3D).
Carbohydrate-binding Site
The dimeric structure CNL-LAC/7.1 revealed two bound lactose molecules, one to each monomer. The position of one molecule is resolved unambiguously in the 2Fo − Fc map at 3.5 σ, and the second is at 1.5 σ. The second lactose molecule also lacked well defined electron density for the outer sugar ring, a reducing glucose. The positions of the two LDN molecules in the CNL-LDN structure were clearly revealed in the 2Fo − Fc map at 2.5 σ. In contrast, only one lactose molecule was observed per dimer in the CNL-LAC/4.4 structure. Its position was resolved unambiguously in the 2Fo − Fc map at 1.4 σ and modeled with an occupancy of 0.7. Electron density is totally absent around the second potential binding pocket, although the crystal packing allows enough space for binding of the lactose molecule. The weaker binding of lactose to CNL at pH 4.4 is most probably a consequence of the partial protonation of Asp20 that serves as an anchor for lactose binding.
Lactose binds through nonreducing galactose to a shallow pocket on the surface of CNL in the α-repeat, opposite the dimer interacting area (Fig. 3B). The β2 and β3 strands form the bottom of the binding pocket, whereas the walls are constituted by the β2-β3 and β3-β4 loops. Lactose is bound to the pocket by five hydrogen bonds (Fig. 4A) formed between two hydroxyl groups of galactose and four amino acid residues in CNL (Asp20 in the β2 strand, Gly23 in the β2-β3 loop, and Asn38 and Asn46 in the β3-β4 loop; Fig. 5). Asp20 forms the bottom of the pocket and acts as an anchor for docking the lactose. With its two carboxyl oxygen atoms, Asp20 forms two hydrogen bonds with the O3 and O4 atoms of galactose. Atom O4 forms one additional hydrogen bond with the amide nitrogen in Gly23, whereas the O3 atom is connected to two side chain amide nitrogen atoms in Asn38 and Asn46. The binding of LDN is similar to that of lactose; however, one additional hydrogen bond is formed between the O2-bound carbonyl oxygen in the acetate group of GalNAc and the hydroxyl oxygen of Ser24 (Figs. 4B and 5). Moreover, analysis of the hydrogen bond lengths revealed that, besides the additional hydrogen bond, they are in general shorter than those in structures of CNL in complex with lactose (Table 3). This explains the considerably higher affinity of CNL for LDN than for lactose, as shown by the glycan microarray analysis.
TABLE 3.
The lengths of hydrogen bonds (Å) between CNL and lactose or LDN
Interactions between oxygens or nitrogens of amino acid residues in CNL and oxygen atoms of lactose or LDN (O4 and O3) or carbonyl oxygen (CO) in the acetate group of LDN are presented. Values in the upper and lower lines of individual structures are those of hydrogen bond lengths in Å for both binding sites in a dimer, except in the CNL-LAC/4.4 structure, where only one lactose molecule was observed.
| Lactose/LDN O4a |
Lactose/LDN O3a |
LDN COa |
||||
|---|---|---|---|---|---|---|
| Asp-20 Ob | Gly-23 Nb | Asp-20 Ob | Asn-38 Nb | Asn-46 Nb | Ser-24 Ob | |
| CNL-LAC/7.1 | 2.69 | 2.93 | 2.68 | 2.91 | 3.07 | / |
| 2.69 | 2.93 | 2.64 | 2.94 | 3.01 | / | |
| CNL-LAC/4.4 | 2.68 | 2.97 | 2.69 | 2.94 | 3.22 | / |
| CNL-LDN | 2.36 | 2.99 | 2.56 | 2.81 | 3.03 | 2.77 |
| 2.29 | 2.96 | 2.48 | 2.90 | 3.18 | 2.80 | |
a Interacting sugar atom.
b Interacting amino acid atom.
The shape of the binding pocket in the α-repeat of CNL is also structurally conserved in the β- and γ-repeats (Fig. 4C), although Asp20 in the α-repeat, which serves as an anchor for carbohydrate docking in the bottom of the binding pocket, is replaced in the β- and γ-repeats by Arg72 and Thr116 (Fig. 5). Being larger, and with only one oxygen atom in the side chain, these residues cannot serve as an anchor for lactose. Additionally, there are no residues in the β- and γ-repeats that could replace the role of Asn38 in the α-repeat.
In CNL, the structure of the lactose-binding site is not altered on binding lactose, as seen from the structures of occupied and unoccupied binding sites in the CNL-LAC/4.4 structure. The reason is the hydrogen bond network between the side chains of Asp20, Asn38, and Asn46 and the main chain atoms in Leu21 and Tyr44, which demands the same orientation of the side chains, regardless of the presence of lactose.
Mutants of CNL
On the basis of structural analysis of rCNL and its interactions with the sugars, a nonsugar-binding mutant 0-CNL and nondimerizing monovalent mutants Mono1-CNL, Mono2R-CNL, and Mono2W-CNL were generated.
0-CNL was constructed by substituting Asp20 by Arg, whose long side chain prevents carbohydrate docking. The D20R mutant lacked agglutinating activity, even at very high concentrations, and did not bind to any glycans on the glycan microarray at 200 μg/ml (supplemental Fig. S2A).
To construct monovalent CNL mutants containing only one carbohydrate-binding site, substitutions were introduced to disrupt the dimer-forming interactions. In the Mono1-CNL single mutant, Asn110 was substituted by Asp to prevent the formation of hydrogen bonds between the monomers via the amine group of Asn. The mutant retained the dimeric structure and the activity, although the latter was lower than that of the wild-type rCNL. Therefore, additional substitutions were introduced to give double mutants in which Leu54 was substituted with either Arg or Trp, to disrupt the hydrophobic interactions. The monomeric nature of the resulting mutants Mono2R-CNL and Mono2W-CNL was shown by gel filtration and nondenaturing electrophoresis, and no agglutinating activity of the double mutants was observed. Nevertheless, glycan microarray analysis showed that they retained the carbohydrate binding activity of the wild-type rCNL (supplemental Fig. S2, B–D).
Cytotoxicity of rCNL against Jurkat Cells
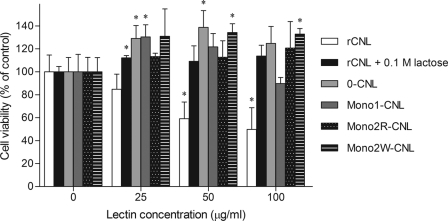
A viability assay on Jurkat cells showed a dose-dependent inhibitory effect of rCNL on cell growth. The number of viable cells was significantly reduced at rCNL concentrations of 50 μg/ml and above, with a more than 50% decrease at the highest concentration of 100 μg/ml (Fig. 6). When rCNL was preincubated with lactose, the effect was abolished. The nonsugar-binding mutant 0-CNL was similarly not inhibitory. Mono1-CNL showed slight inhibition at the highest concentration (100 μg/ml) used, whereas the Mono2R-CNL and Mono2W-CNL nondimerizing mutants were not toxic to Jurkat cells (Fig. 6). In the presence of the mutants, greater viability of the cells was observed, as also noted at low concentrations of human recombinant galectin-1 (40, 41).
FIGURE 6.
Cytotoxicity of rCNL against Jurkat cells. Differently shaded bars represent viability of Jurkat human leukemic T cells treated with rCNL, rCNL preincubated with 0.1 m lactose, or with the mutants. Cell viability is represented as the percentage of control cell viability devoid of the lectins, taken as 100%. Data are means with error bars indicating standard deviations of triplicate experiments. Statistically significant differences (p < 0.05) between treated groups compared with the untreated are indicated by an asterisk above bars.
Toxicity of rCNL against C. elegans
In the biotoxicity assays, the nematodes were fed on E. coli expressing rCNL, 0-CNL, or Mono2R-CNL. All were expressed at 23 °C as soluble proteins at similar levels (supplemental Fig. S3).
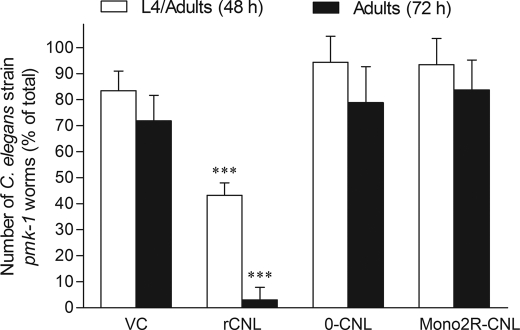
rCNL was found to have no effect on wild-type C. elegans nematodes (supplemental Fig. S4) but was toxic to the hypersensitive mutant strain pmk-1 (Fig. 7). Development to larval stage L4 was impaired in ∼50% of larvae, and further development to adult animals was almost completely arrested. In contrast, the nonsugar-binding and monovalent mutants of CNL showed no effects on the nematodes (Fig. 7).
FIGURE 7.
Toxicity of rCNL against hypersensitive C. elegans mutant strain pmk-1. Bars represent means of percentage of total numbers of larvae or adult animals in five replicates, and error bars represent standard deviations. VC stands for the vector control, E. coli containing empty vector. Groups of treated larvae or adults were compared pairwise with vector controls using the two-sample t test. Statistically significant differences (p < 0.001) are indicated by three asterisks above bars.
DISCUSSION
From E. coli, an active recombinant CNL was obtained with biochemical properties corresponding to the isolated nCNL. rCNL retained the ability of binding to lactose-Sepharose and agglutinating erythrocytes. Moreover, the native conformations of rCNL and its mutants were ascertained by CD analysis showing the same spectra as nCNL indicating that the proteins have the same pattern of secondary structure elements and were properly refolded during purification. Similar CD spectra with the unusual peak at 232 nm were also noted for basidiomycete ricin B-like lectins such as MOA (42), which also adopts the β-trefoil fold (12), and RSA from Rhizoctonia solani (43). In addition, the β-trefoil fold protease inhibitors, clitocypin from C. nebularis and macrocypin from Macrolepiota procera, also showed similar CD spectra (39, 44–46). This type of CD spectrum appears to be characteristic of proteins adopting β-trefoil folds.
The carbohydrate-binding specificity of recombinant CNL is similar to that of nCNL (19); however, it shows more restricted specificity for LDN than the natural counterpart. The broader specificity of the isolated nCNL could be the consequence of natural isolate heterogeneity, as it presumably contains various isolectins, showing slightly different carbohydrate-binding properties. CNL recognizes terminal nonreducing GalNAc glycans such as LDN and human blood group A determinants, which is consistent with the observed agglutination of this type of erythrocyte by the lectin. Increased binding of glycans containing GlcNAc at the reducing end was also noted; however, interactions of the lectin with GlcNAc at the molecular level could not be corroborated by the structural analysis of the LDN-rCNL complex. Nevertheless, the interactions are not excluded.
CNL exhibits the β-trefoil fold, consisting of three structurally similar repeats, α, β, and γ, characteristic of ricin B-like lectins. Similar folds are also present in proteins like Kunitz-type soybean trypsin inhibitor (47), interleukins-1α and -1β (48), fibroblast growth factors (49), and mushroom protease inhibitors, mycocypins (39) and cospin (50). In contrast to other mushroom ricin B-like lectins with known structures, monomeric CNL is composed of only one domain, the ricin B lectin domain, whereas LSL, MOA, and PSL are modular proteins. The biological activity of the latter proteins is mediated by the nonlectin domain, whereas the lectin domain facilitates their internalization (16, 17). In contrast, the effects shown for CNL were elicited solely by the carbohydrate-binding lectin domain. However, additional functions of rCNL cannot be excluded, because the β-trefoil fold is a conserved structural scaffold that allows diverse protein functions (39, 51).
Structural analysis showed that CNL contains a single carbohydrate-binding site per monomer in the α-repeat. In contrast, LSL has two binding sites per subunit, in β- and γ-repeats (11), and MOA has three binding sites, one in each repeat (12). PSL contains only one binding site in the β-repeat, although amino acid residues from the α-repeat are also involved (13). An attempt was made to introduce a further carbohydrate-binding site in the γ-repeat of CNL. A triple mutant, T116D/D120S/D132N, was constructed to imitate the LDN-binding site in the α-repeat (see Fig. 5). However, its crystal structure with lactose at 1.9 Å resolution showed no sugar binding at the introduced site, and the agglutinating activity and glycan-binding pattern of the mutant, as well as its inhibitory effect on Jurkat cells, were comparable with those of the wild-type lectin, indicating that the introduced potential binding site was inactive. Apparently, the structure of the γ-repeat does not allow an orientation that would allow amino acid residues to form hydrogen bonds with the sugar, despite the introduced substitutions.
Although CNL possesses only one carbohydrate-binding site per monomer, as a homodimeric structure it is bivalent. The carbohydrate-binding activity of CNL is essential for its biological activity, and moreover, the two sugar-binding sites are required, as shown for Jurkat cells and the hypersensitive strain of nematode C. elegans. The toxicity of rCNL for the cells was comparable with that of nCNL (19), both showing more than 50% decrease in cell viability at the highest concentration (100 μg/ml). Because the lectin did not affect other cells tested (U-937 human monocytic, MCF-10A neoT fibrocystic breast, and human embryonic kidney 293 cell lines; data not shown), we conclude that the effect is specific to Jurkat cells and not the result of general cytotoxicity. When rCNL was preincubated with lactose, the toxic effect was abolished; moreover, a nonsugar-binding mutant and nondimerizing monovalent mutants did not show the effect. Similar results were obtained in biotoxicity assays with the nematodes, where the mutants did not show the toxicity of the wild-type CNL. Taken together, these results imply that the activity of CNL is associated with the bivalent carbohydrate-binding properties of the dimeric lectin. The lectin presumably recognizes and cross-links specific glycan ligands on Jurkat cells and in the nematodes, which can lead to signal transduction and subsequent toxic effects. The biological activity of lectins could be ascribed to multivalent lectin-carbohydrate interactions (3), as shown for human homodimeric galectin-1, which induces apoptosis of human T cells by cross-linking and segregating specific receptors on the cell surface (4).
The specificity of CNL for LDN suggests that the sugar-containing carbohydrates constitute target glycan for the lectin in Jurkat human leukemic cells and nematodes. The LDN motif has been found in human leukemic cells (24) but is generally rare in mammals, whereas the glycan is abundant in invertebrates, such as parasitic helminths and insects (21, 22). The glycan was thus proposed as a parasite pattern for mammalian galectin-3-mediated immune recognition (23). However, in contrast to CNL (7, 20), CGL3, a galectin from Coprinopsis cinerea, which binds chitooligosaccharides and also LDN (52), did not show any toxicity against D. melanogaster,3 and A. aegypti, A. castellanii, or C. elegans (7). Still, although both lectins bind LDN, they show differences in specificity, with CNL recognizing the terminal GalNAc glycans, whereas CGL3 displays preference for oligomers of GlcNAc.
In conclusion, the crystal structure of recombinant CNL with marked carbohydrate-binding specificity for LDN was determined in complex with the specific sugars, which revealed details of the lectin-carbohydrate interactions and the molecular basis of its specificity. In contrast to other ricin B-like lectins, CNL exerts its activity by the sole carbohydrate-binding ricin B lectin domain. Moreover, utilizing several CNL mutants, the bivalent carbohydrate-binding property of CNL was shown to be essential for its activity, providing one of the rare pieces of evidence that certain activities of lectins are associated with their multivalency. This has not been demonstrated for any other mushroom lectin. This study provides the groundwork for further investigations concerning the cellular mechanisms of CNL action and its potential application in biomedicine and biotechnology, as well as its function in the mushroom.
Supplementary Material
Acknowledgments
We thank Dr. Jože Brzin (Dept. of Biotechnology, Jožef Stefan Institute, Ljubljana, Slovenia) for assistance with the work on lectins; Prof. Roger H. Pain (Dept. of Biotechnology, Jožef Stefan Institute, Ljubljana, Slovenia) for assistance with CD spectroscopy analysis and for critical review of the manuscript; Dr. Dušan Žigon (Dept. of Environmental Sciences, Jožef Stefan Institute, Ljubljana, Slovenia) for performing the mass spectrometry analysis; Silvia Bleuler-Martínez (Dept. of Biology, Institute of Microbiology, ETH, Zürich, Switzerland) for technical assistance with nematode biotoxicity assays; and Dr. Jamie Heimburg-Molinaro (Dept. of Biochemistry, Emory University School of Medicine, Atlanta, GA) for work on glycan microarrays. The glycan microarray analyses were conducted in collaboration with the Protein-Carbohydrate Interaction Core H, and the carbohydrate compound LDN was provided by the Carbohydrate Synthesis/Protein Expression Core, both of the Consortium for Functional Glycomics funded by National Institutes of Health NIGMS Grant GM62116.
This work was supported by Research Agency of the Republic of Slovenia Grants P4-0127 and J4-4123 (to J. K.) and Grants P1-0048 and PR-02266 (to D. T.), ETH Zürich, and Swiss National Science Foundation Grant 31003A-130671 (to M. K.).

This article contains supplemental Figs. S1–S4.
The atomic coordinates and structure factors (codes 3NBC, 3NBD, and 3NBE) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/). The nucleotide sequence(s) reported in this paper has been submitted to the DDBJ/GenBankTM/EBI Data Bank with accession number(s) FJ477895.
M. Künzler, unpublished results.
- LSL
- L. sulfureus lectin
- CNL
- C. nebularis lectin
- 0-CNL
- nonsugar-binding mutant of CNL
- LDN
- N,N′-diacetyllactosediamine (GalNAcβ1–4GlcNAc, LacdiNAc)
- MOA
- M. oreades agglutinin
- Mono1-CNL
- Mono2R-CNL and Mono2W-CNL, nondimerizing monovalent mutants of CNL
- PSL
- P. squamosus lectin
- rCNL
- recombinant CNL.
REFERENCES
- 1. Varki A., Cummings R. D., Esko J. D., Freeze H. H., Hart G. W., Marth J. D. (eds) (1999) Essentials of Glycobiology, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York: [PubMed] [Google Scholar]
- 2. Peumans W. J., Van Damme E. J. (1995) Lectins as plant defense proteins. Plant Physiol. 109, 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sacchettini J. C., Baum L. G., Brewer C. F. (2001) Multivalent protein-carbohydrate interactions. A new paradigm for supermolecular assembly and signal transduction. Biochemistry 40, 3009–3015 [DOI] [PubMed] [Google Scholar]
- 4. Pace K. E., Lee C., Stewart P. L., Baum L. G. (1999) Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J. Immunol. 163, 3801–3811 [PubMed] [Google Scholar]
- 5. Goldstein I. J., Winter H. C. (2007) in Comprehensive Glycoscience (Kamerling J. P., Boons G. J., Suzuki A., Taniguchi N., Voragen A. G., eds) pp. 601–622, Elsevier Ltd., Oxford, UK [Google Scholar]
- 6. Butschi A., Titz A., Wälti M. A., Olieric V., Paschinger K., Nöbauer K., Guo X., Seeberger P. H., Wilson I. B., Aebi M., Hengartner M. O., Künzler M. (2010) Caenorhabditis elegans N-glycan core β-galactoside confers sensitivity toward nematotoxic fungal galectin CGL2. PLoS Pathog. 6, e1000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bleuler-Martínez S., Butschi A., Garbani M., Wälti M. A., Wohlschlager T., Potthoff E., Sabotiĉ J., Pohleven J., Lüthy P., Hengartner M. O., Aebi M., Künzler M. (2011) A lectin-mediated resistance of higher fungi against predators and parasites. Mol. Ecol. 20, 3056–3070 [DOI] [PubMed] [Google Scholar]
- 8. Guillot J., Konska G. (1997) Lectins in higher fungi. Biochem. Syst. Ecol. 25, 203–230 [Google Scholar]
- 9. Rutenber E., Ready M., Robertus J. D. (1987) Structure and evolution of ricin B chain. Nature 326, 624–626 [DOI] [PubMed] [Google Scholar]
- 10. Hazes B. (1996) The (QxW)3 domain: a flexible lectin scaffold. Protein Sci. 5, 1490–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mancheño J. M., Tateno H., Goldstein I. J., Martínez-Ripoll M., Hermoso J. A. (2005) Structural analysis of the Laetiporus sulphureus hemolytic pore-forming lectin in complex with sugars. J. Biol. Chem. 280, 17251–17259 [DOI] [PubMed] [Google Scholar]
- 12. Grahn E., Askarieh G., Holmner A., Tateno H., Winter H. C., Goldstein I. J., Krengel U. (2007) Crystal structure of the Marasmius oreades mushroom lectin in complex with a xenotransplantation epitope. J. Mol. Biol. 369, 710–721 [DOI] [PubMed] [Google Scholar]
- 13. Kadirvelraj R., Grant O. C., Goldstein I. J., Winter H. C., Tateno H., Fadda E., Woods R. J. (2011) Structure and binding analysis of Polyporus squamosus lectin in complex with the Neu5Ac α2–6Galβ1–4GlcNAc human-type influenza receptor. Glycobiology 21, 973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montfort W., Villafranca J. E., Monzingo A. F., Ernst S. R., Katzin B., Rutenber E., Xuong N. H., Hamlin R., Robertus J. D. (1987) The three-dimensional structure of ricin at 2.8 Å. J. Biol. Chem. 262, 5398–5403 [PubMed] [Google Scholar]
- 15. Tahirov T. H., Lu T. H., Liaw Y. C., Chen Y. L., Lin J. Y. (1995) Crystal structure of abrin-a at 2.14 Å. J. Mol. Biol. 250, 354–367 [DOI] [PubMed] [Google Scholar]
- 16. Olsnes S. (2004) The history of ricin, abrin, and related toxins. Toxicon 44, 361–370 [DOI] [PubMed] [Google Scholar]
- 17. Wohlschlager T., Butschi A., Zurfluh K., Vonesch S. C., auf dem Keller U., Gehrig P., Bleuler-Martinez S., Hengartner M. O., Aebi M., Künzler M. (2011) Nematotoxicity of Marasmius oreades agglutinin (MOA) depends on glycolipid binding and cysteine protease activity. J. Biol. Chem. 286, 30337–30343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sulzenbacher G., Roig-Zamboni V., Peumans W. J., Rougé P., Van Damme E. J., Bourne Y. (2010) Crystal structure of the GalNAc/Gal-specific agglutinin from the phytopathogenic ascomycete Sclerotinia sclerotiorum reveals novel adaptation of a β-trefoil domain. J. Mol. Biol. 400, 715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pohleven J., Obermajer N., Sabotic J., Anzlovar S., Sepcić K., Kos J., Kralj B., Strukelj B., Brzin J. (2009) Purification, characterization, and cloning of a ricin B-like lectin from mushroom Clitocybe nebularis with antiproliferative activity against human leukemic T cells. Biochim. Biophys. Acta 1790, 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pohleven J., Brzin J., Vrabec L., Leonardi A., Cokl A., Strukelj B., Kos J., Saboti J. (2011) Basidiomycete Clitocybe nebularis is rich in lectins with insecticidal activities. Appl. Microbiol. Biotechnol. 91, 1141–1148 [DOI] [PubMed] [Google Scholar]
- 21. Van den Eijnden D. H., Neeleman A. P., Van der Knaap W. P., Bakker H., Agterberg M., Van Die I. (1995) Novel glycosylation routes for glycoproteins. The lacdiNAc pathway. Biochem. Soc. Trans. 23, 175–179 [DOI] [PubMed] [Google Scholar]
- 22. Sasaki N., Yoshida H., Fuwa T. J., Kinoshita-Toyoda A., Toyoda H., Hirabayashi Y., Ishida H., Ueda R., Nishihara S. (2007) Drosophila β1,4-N-acetylgalactosaminyltransferase-A synthesizes the LacdiNAc structures on several glycoproteins and glycosphingolipids. Biochem. Biophys. Res. Commun. 354, 522–527 [DOI] [PubMed] [Google Scholar]
- 23. van den Berg T. K., Honing H., Franke N., van Remoortere A., Schiphorst W. E., Liu F. T., Deelder A. M., Cummings R. D., Hokke C. H., van Die I. (2004) LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J. Immunol. 173, 1902–1907 [DOI] [PubMed] [Google Scholar]
- 24. Saarinen J., Helin J., Satomaa T. (August 16, 2003) International Patent WO/2003/016464
- 25. Hirano K., Nakamura T., Amano J. (December 3, 2009) International Patent WO/2010/064683
- 26. Huang J., Liang J. T., Huang H. C., Shen T. L., Chen H. Y., Lin N. Y., Che M. I., Lin W. C., Huang M. C. (2007) β1,4-N-Acetylgalactosaminyltransferase III enhances malignant phenotypes of colon cancer cells. Mol. Cancer Res. 5, 543–552 [DOI] [PubMed] [Google Scholar]
- 27. Machado E., Kandzia S., Carilho R., Altevogt P., Conradt H. S., Costa J. (2011) N-Glycosylation of total cellular glycoproteins from the human ovarian carcinoma SKOV3 cell line and of recombinantly expressed human erythropoietin. Glycobiology 21, 376–386 [DOI] [PubMed] [Google Scholar]
- 28. Weiner M. P., Costa G. L. (1994) Rapid PCR site-directed mutagenesis. PCR Methods Appl. 4, S131–S136 [DOI] [PubMed] [Google Scholar]
- 29. Smith D. F., Song X., Cummings R. D. (2010) Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol. 480, 417–444 [DOI] [PubMed] [Google Scholar]
- 30. Otwinowski Z., Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 31. Adams P. D., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., Terwilliger T. C. (2002) PHENIX. Building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 [DOI] [PubMed] [Google Scholar]
- 32. Navaza J., Saludjian P. (1997) AMoRe: An automated molecular replacement program package. Methods Enzymol. 276, 581–594 [DOI] [PubMed] [Google Scholar]
- 33. Perrakis A., Morris R., Lamzin V. S. (1999) Automated protein model building combined with iterative structure refinement. Nat. Struct. Biol. 6, 458–463 [DOI] [PubMed] [Google Scholar]
- 34. Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 35. Turk D. (1992) Weiterentwicklung eines Programms für Molekülgraphik und Elektrondichte-Manipulation und Seine Anwendung auf Verschiedene Protein-Strukturaufklerungen. Ph.D thesis, Technische Universität München, Germany [Google Scholar]
- 36. Andrejasic M., Praaenikar J., Turk D. (2008) PURY. A database of geometric restraints of hetero compounds for refinement in complexes with macromolecular structures. Acta Crystallogr. D Biol. Crystallogr. 64, 1093–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Künzler M., Bleuler-Martinez S., Butschi A., Garbani M., Lüthy P., Hengartner M. O., Aebi M. (2010) Biotoxicity assays for fruiting body lectins and other cytoplasmic proteins. Methods Enzymol. 480, 141–150 [DOI] [PubMed] [Google Scholar]
- 38. Woody R. W. (1994) Contributions of tryptophan side chains to the far-ultraviolet circular dichroism of proteins. Eur. Biophys. J. 23, 253–262 [DOI] [PubMed] [Google Scholar]
- 39. Renko M., Sabotic J., Mihelic M., Brzin J., Kos J., Turk D. (2010) Versatile loops in mycocypins inhibit three protease families. J. Biol. Chem. 285, 308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adams L., Scott G. K., Weinberg C. S. (1996) Biphasic modulation of cell growth by recombinant human galectin-1. Biochim. Biophys. Acta 1312, 137–144 [DOI] [PubMed] [Google Scholar]
- 41. Vas V., Fajka-Boja R., Ion G., Dudics V., Monostori E., Uher F. (2005) Biphasic effect of recombinant galectin-1 on the growth and death of early hematopoietic cells. Stem Cells 23, 279–287 [DOI] [PubMed] [Google Scholar]
- 42. Tateno H., Goldstein I. J. (2004) Partial identification of carbohydrate-binding sites of a Galα1,3Galβ1,4GlcNAc-specific lectin from the mushroom Marasmius oreades by site-directed mutagenesis. Arch. Biochem. Biophys. 427, 101–109 [DOI] [PubMed] [Google Scholar]
- 43. Candy L., Peumans W. J., Menu-Bouaouiche L., Astoul C. H., Van Damme J., Van Damme E. J., Erard M., Rougé P. (2001) The Gal/GalNAc-specific lectin from the plant pathogenic basidiomycete Rhizoctonia solani is a member of the ricin-B family. Biochem. Biophys. Res. Commun. 282, 655–661 [DOI] [PubMed] [Google Scholar]
- 44. Kidric M., Fabian H., Brzin J., Popovic T., Pain R. H. (2002) Folding, stability, and secondary structure of a new dimeric cysteine proteinase inhibitor. Biochem. Biophys. Res. Commun. 297, 962–967 [DOI] [PubMed] [Google Scholar]
- 45. Sabotic J., Galesa K., Popovic T., Leonardi A., Brzin J. (2007) Comparison of natural and recombinant clitocypins, the fungal cysteine protease inhibitors. Protein Expr. Purif. 53, 104–111 [DOI] [PubMed] [Google Scholar]
- 46. Sabotic J., Popovic T., Puizdar V., Brzin J. (2009) Macrocypins, a family of cysteine protease inhibitors from the basidiomycete Macrolepiota procera. FEBS J. 276, 4334–4345 [DOI] [PubMed] [Google Scholar]
- 47. Song H. K., Suh S. W. (1998) Kunitz-type soybean trypsin inhibitor revisited. Refined structure of its complex with porcine trypsin reveals an insight into the interaction between a homologous inhibitor from Erythrina caffra and tissue-type plasminogen activator. J. Mol. Biol. 275, 347–363 [DOI] [PubMed] [Google Scholar]
- 48. Graves B. J., Hatada M. H., Hendrickson W. A., Miller J. K., Madison V. S., Satow Y. (1990) Structure of interleukin 1α at 2.7-Å resolution. Biochemistry 29, 2679–2684 [DOI] [PubMed] [Google Scholar]
- 49. Ago H., Kitagawa Y., Fujishima A., Matsuura Y., Katsube Y. (1991) Crystal structure of basic fibroblast growth factor at 1.6-Å resolution. J. Biochem. 110, 360–363 [DOI] [PubMed] [Google Scholar]
- 50. Sabotic J., Bleuler-Martinez S., Renko M., Avanzo Caglic P., Kallert S., Strukelj B., Turk D., Aebi M., Kos J., Künzler M. (2012) Structural basis of trypsin inhibition and entomotoxicity of Cospin, serine protease inhibitor involved in defense of Coprinopsis cinerea fruiting bodies. J. Biol. Chem. 287, 3898–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chavez L. L., Gosavi S., Jennings P. A., Onuchic J. N. (2006) Multiple routes lead to the native state in the energy landscape of the β-trefoil family. Proc. Natl. Acad. Sci. U.S.A. 103, 10254–10258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wälti M. A., Walser P. J., Thore S., Grünler A., Bednar M., Künzler M., Aebi M. (2008) Structural basis for chitotetraose coordination by CGL3, a novel galectin-related protein from Coprinopsis cinerea. J. Mol. Biol. 379, 146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krissinel E., Henrick K. (2004) Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.