Background: Epididymis displays decreased proliferation during postnatal development.
Results: miR-29a and nuclear autoantigenic sperm protein (NASP) were dramatically up-regulated and down-regulated, respectively, during postnatal epididymal development. Moreover, miR-29a can inhibit epididymal cell proliferation by targeting Nasp in vitro.
Conclusion: miR-29a may inhibit epididymal epithelial cell proliferation during postnatal epididymal development by targeting Nasp.
Significance: miR-29a may be necessary for epididymal maturation.
Keywords: Development, Epididymis, Epithelial Cell, MicroRNA, Proliferation, NASP
Abstract
Cell proliferation often decreases gradually during postnatal development of some organs. However, the underlying molecular mechanisms remain unclear. Epididymis, playing important roles in sperm maturation, is a typical organ of this type, which displays a decreased proliferation during postnatal development and even ceased at the adult stage. Here, epididymis was employed as a model to explore the underlying mechanisms. We profiled the microRNA and mRNA expression of newborn (1 day) and adult (90 day) rat epididymis by microarray analysis, and found that the level of miR-29a was dramatically up-regulated during postnatal development of rat epididymis. Subsequent investigations demonstrated that overexpression of miR-29a inhibited the proliferation of epididymal epithelial cells in vitro. The nuclear autoantigenic sperm protein (NASP), a novel target of miR-29a, was significantly down-regulated during postnatal development of rat epididymis. Further analysis showed that silence of NASP mimicked the anti-proliferation effect of miR-29a, whereas overexpression of this protein attenuated the effect of miR-29a. As in rat epididymis, miR-29a was up-regulated and Nasp was down-regulated during postnatal development of mouse epididymis, heart, liver, and lung. Moreover, miR-29a can also inhibit the proliferation of cancer cells by targeting Nasp. Thus, an increase of miR-29a, and hence decrease of Nasp, may contribute to inhibit cell proliferation during postnatal organ development.
Introduction
Cell proliferation is required for normal organ development as well as tissue repair/regeneration after damage. During postnatal development of some organs such as heart, lung, liver, and epididymis, cell proliferation deceases gradually and even becomes undetectable in the adult stage (1–4). Nevertheless, the underlying molecular and cellular mechanisms by which the cell proliferation is strictly modulated remain ambiguous.
As an important male reproductive organ, epididymis plays roles in sperm maturation, protection, concentration, and storage; therefore it is vital for male fertility (5). These can only be accomplished following considerable postnatal remodeling including duct elongation and convolution. By puberty, the epididymis has acquired its fully differentiated state consisting of a highly tortuous tubule lined by epithelial cells (6). These postnatal remodeling processes were accompanied by a series of cell fate decisions such as cell differentiation, cell proliferation, and size expansion (4). For example, from postnatal days 1 to 14, the rat epididymal cells remain un-differentiation and contain numerous mitotic figures; from days 15 to 49, the epididymal cells go through the process of differentiation, and the Halo cells, basal cells, principal cells, narrow cells, and clear cells appear sequentially. Accompanying cell differentiation, the proliferation of epididymal cells decreases gradually. By day 49, all epididymal epithelial cells are fully differentiated and by day 90, the proliferation of all cell types throughout the epididymal epitheliums has ceased (4). Therefore, epididymis is an ideal model to explore the potential mechanisms and candidate molecules involved in the regulation of cell proliferation during postnatal development.
Cell proliferation was intertwined with numerous factors. One known regulatory machinery is the microRNA (miRNA)2 family (7). miRNAs are a class of single-stranded, noncoding small RNAs consisting of ∼22 nucleotides (8). The available evidences have clearly demonstrated that miRNAs are intertwined with cell fate decisions such as cell differentiation, proliferation, and apoptosis (9) and play multiple roles in organ development (10). Williams et al. (11) reported two maternally imprinted miRNAs (miR-154 and miR-335) are down-regulated, whereas miR-29a and miR-29b are up-regulated in adult compared with neonatal or fetal lung. Our previous study revealed there were significant differences of miRNA expression between the newborn and young adult human epididymis (12). In addition, a recent study showed there were 25 miRNAs changed significantly from postnatal day 7 to 49 in the epididymis of rats (13). These data suggest that miRNAs may be involved in regulation of postnatal remodeling of the epididymis.
Therefore, the epididymis was employed as a model to further examine the contribution of miRNAs to cell proliferation controlling during postnatal organ development. The expression of miRNAs and mRNAs of newborn and adult rat epididymis were profiled. We found that the miR-29 family, including miR-29a, -b, and -c, were dramatically up-regulated during postnatal development of the rat epididymis. Previous studies revealed that the miR-29 family are also dramatically up-regulated during postnatal development of aorta (14), lung (11), brain (15), cornea (16), skeletal muscle (17), and human epididymis (12), indicating that up-regulation of the miR-29 family during postnatal development may be a common phenomena. Herein, miR-29a was selected for further study. Overexpression of miR-29a inhibited the growth of epididymal cells, which was achieved by directly down-regulating Nasp that is required for cell proliferation, suggesting that miR-29a fine-tunes Nasp expression to control epithelial cell proliferation during postnatal epididymal remodeling. Furthermore, miR-29a was up-regulated and Nasp was down-regulated during postnatal development of mouse heart, liver, lung, and epididymis, suggesting that miR-29a inhibited cell proliferation by targeting NASP may be a common regulatory machinery.
EXPERIMENTAL PROCEDURES
Animals, Tissue Preparation, and Cell Culture
Male Sprague-Dawley rats of 1, 7, 15, 28, 30, 45, 49, 60, and 90 days old, and male C57BL/6 mice of 1, 7, 14, 21, 63, and 70 days old were purchased from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). Experiments were conducted according to a protocol approved by the Institute Animal Care and Use Committee. The protocol conforms to internationally accepted guidelines for the humane care use of laboratory animals. The epididymidis were excised and frozen immediately in liquid nitrogen, or fixed in 4% paraformaldehyde for further analysis.
Two immortalized mouse epididymal cell lines PC-1 and DC-2 (PC1 from proximal caput and DC2 from distal caput) were kindly provided by Dr. M. Orgebin-Crist (Department of Obstetrics and Gynecology, Vanderbilt University School of Medicine, Nashville, TN). The two cell lines were grown in Iscove's modified Dulbecco's medium supplemented with 10% (v/v) fetal bovine serum (FBS), 1 mm sodium pyruvate, 0.1 mm nonessential amino acids, 4 mm glutamine, penicillin-streptomycin (25,000 units of penicillin G sodium, 25 mg of streptomycin sulfate), and 1 nm 5α-dihydrotestosterone (18). PC-1 and DC-2 cells were cultured at 33 °C with 5% CO2. HEK 293T, NIH3T3, HeLa, PC-3, DU-145, HepG2, SMMC-7721, A549, BEAS-2B (kindly provided by Dr. Ji, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences), and A375 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) FBS and cultured at 37 °C with 5% CO2. All cell culture reagents were bought from Invitrogen.
miRNA and mRNA Microarray
RNA samples were isolated, size fractionated, and labeled with Cy3 or Cy5. Paired samples were hybridized to dual-channel microarray using the μParaflo microfluidics chips of LC Sciences (Houston, TX). This array contained probes for rat, mouse, and human miRNAs listed in Sanger miRBase Release 11.0. Raw data were normalized by the LOWESS method on the background-subtracted data. A Student's t test was performed to analyze the statistical significance of the signal differences between the two groups. RNA isolation, reverse transcription, and cRNA synthesis, labeling, and hybridization with Affymetrix GeneChip Rat Genome 230 2.0 Array were conducted following the standard Affymetrix protocol.
Northern Blotting
Total RNA was resolved by a 15% denatured polyacrylamide gel containing 8 m urea. The RNA was then transferred to GeneScreen Plus Hybridization Transfer Membrane (PerkinElmer Life Sciences). After baking at 80 °C for one-half hour and UV cross-linking, the membranes were stained by methylene blue to evaluate the transfer efficiency. The membranes were incubated in hybridization buffer (Toyobo, Osaka, Japan) at 42 °C for 30 min, then hybridized with specific γ-32P-labeled oligonucleotide probes (Sangon, Shanghai) complementary to each miRNA at 42 °C for 16 h. The membranes were washed twice with washing buffer (2× SSC with 0.1% SDS) and images were visualized with a Fuji phosphorimaging system. The hybridized membranes were striped with 1% SDS at 100 °C for 15 min and re-hybridized with specific γ-32P-labeled U6 snRNA or 5 S rRNA probes (Sangon, Shanghai), which were used as loading controls. The oligonucleotide probes for Northern blotting are listed in supplemental Table S1.
PCR and Quantitative Real-time PCR
PCR regents of 2× Taq PCR Master Mix were from Lifefeng (Shanghai). Quantitative real-time RT-PCR was performed using SYBR Green Real-time PCR Mix (Toyobo, Osaka, Japan) according to the manufacturer's protocol. Levels of mRNAs were normalized to GAPDH mRNA. The primers used for PCR and real-time PCR are listed in supplemental Table S2.
miRNAs, Small Interfering RNAs, and Transfection
RNA oligonucleotides were transfected into cells by using Lipofectamine RNAi MAX Transfection Reagent (Invitrogen) according to the manufacturer's protocol. miR-29a duplex (pre-miR-29a) or miR-29a specific inhibitor (anti-miR-29a) molecules and appropriate negative control molecules (pre-miR-negative control, pre-miR-NC; or anti-miR- negative control, anti-miR-NC) were purchased from Ambion. Small interfering RNAs (siRNA) against NASP were synthesized by GenePharma (GenePharma, Shanghai, China): siNASP (human), 5′-GCACAGUUCAGCAAAUCUAdTdT-3′ (19), siNasp (mouse), 5′-GGCCUAUGGUUACAACUCUTT-3′, 5′-GUAGCACAGUUUGGCAAAUTT-3′, and 5′-GUCUGCAGCUACAAUUAAATT-3′; and negative control siRNA, 5′-UUCUCCGAACGUGUCACGU-3′.
Cell Growth Assay
For quantitative analysis of the cell proliferation rate, 10 μl of the Cell Counting Kit-8 (CCK-8 Kit, Dojindo, Kumamoto, Japan) solution was added to each well. After incubation at 37 °C for 4 h in a humidified CO2 incubator, absorbance at 450 nm was monitored with a microplate reader (Multiskan MK3, Thermo Lab systems, Shanghai, China). The obtained values were normalized to those from control cells transfected with scramble oligonucleotides. All experiments were performed in triplicates.
miRNAs Target Prediction
Targets of differentially expressed miRNAs were predicted using TargetScan 5.1 (www.targetscan.org), combined with differentially expressed genes (≧2-fold). Target prediction sets of TargetScan are typically ranked, with the assertion that the better scoring predictions are more likely to be authentic or effective (20). Two important parameters of TargetScan prediction were total context score and Aggregate Pct. Total context score quantitatively measured the overall target efficacy (21). Aggregate Pct, the probability of conserved targeting, provides a useful criterion for assessing the biological relevance of predicted miRNA-target interactions (22). miRanda (www.microrna.org/microrna), Pictar (pictar.mdc-berlin.de/), and TargetScan 5.1 were combined to predict the potential miRNAs that may target NASP.
Western Blotting
Proteins were extracted from cells using RIPA buffer (25 mm Tris, pH 7.4, 150 mm NaCl, 1% (v/v) Nonidet P-40, 1% (w/v) sodium deoxycholate, 0.1% (w/v) SDS). Each protein sample (30–50 μg) was separated by 10% SDS-polyacrylamide gels and semi-dry blotted to polyvinylidene difluoride membranes (Amersham Biosciences). Immunodetection of proteins was carried out by standard procedures using antibody against NASP (ProteinTech Group, Inc., Chicago, IL; 1:500 dilution). The primary Abs were probed with goat anti-rabbit secondary Abs conjugated to HRP (Sigma) and detected using ECL (GE Healthcare, Piscataway, NJ) reagents. β-Actin (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a loading control.
DNA Constructs and Site-directed Mutagenesis
The 3′ UTR of the human NASP were amplified using primers 5′-GACctcgagGTAAGCAAAGGTTGAGGCT-3′ (XhoI) and 5′-GACgcggccgcAACTCAACCAGCCAGCCT-3′ (NotI). After being cut with XhoI and NotI, the amplified products were subcloned to downstream of the Renilla luciferase gene of the psiCHECKTM-2 plasmid (Promega, Madison, WI). Site-directed mutagenesis was performed using QuikChange Site-directed Mutagenesis kit (Stratagene). The primers for mutation of the upstream target site of miR-29a at human NASP 3′ UTR (position 275–296) were 5′-GGCCTTTGTTTTCCAATGGGTAGATATTCTGTTTTCAAACACTTCACTGAACCC-3′ (forward) and 5′-GGGTTCAGTGAAGTGTTTGAAAACAGAATATCTACCCATTGGAAAACAAAGGCC-3′ (reverse). The primers for mutation of the downstream target site (position 334–355) were 5′-GCTGTCTTGCAAACTTTCAGTGGGTAGGTCCCTGGATGGGG-3′ (forward) and 5′-CCCCATCCAGGGACCTACCCACTGAAAGTTTGCAAGACAGC-3′ (reverse). The cDNA of human tNASP was amplified using the forward primer 5′-GACTaagcttGCCATTTTCTGTCCCTGAGTGA-3′ (HindIII) and reverse primer 5′-GACggatccGCTGTGCCCCCTCTTAACATG-3′ (BamHI) and this cDNA was subcloned to pcDNA3.1(+) using HindIII and BamHI. Then the wild type and mutated human NASP 3′ UTR (above mentioned) were amplified with forward primer 5′-GACggatccCCTCCTCCCAAGGGAAAGTGT-3′ (BamHI) and reverse primer 5′-GACctcgagGCAGATGGGTAACAATTCCAAGTG-3′ (XhoI). The amplified 3′ UTRs were cloned downstream of the NASP coding sequence in pcDNA3.1(+) vector using BamHI and XhoI.
Luciferase Reporter Assay
HEK 293T or NIH3T3 cells were seeded to 24-well plates 24 h before transfection. HEK 293T cells were transiently transfected with 100 ng if psiCHECK-2 (Promega) vector containing the wild-type NASP 3′ UTR (named as NASP-3′UTR-WT) or NASP 3′ UTR double mutants (named as NASP-3′UTR-Mut1 and -2), and together with 30 nm pre-miR-29a or pre-miR-NC, respectively. The luciferase activities were measured 24 h after transfection. NIH3T3 cells were transiently transfected with 100 ng of psiCHECK-2 vector containing the wild-type NASP 3′ UTR or mutated NASP 3′ UTR (NASP-3′UTR-Mut1 and -2) together with anti-miR-29a or anti-miR-NC. The luciferase activities were measured 36 h after transfection. Luciferase activity was measured using the Dual Luciferase Assay kit (Promega). Renilla luciferase activity was normalized to Firefly luciferase activity. Lipofectamine 2000 transfection reagent was used for co-transfection of RNA oligonucleotides and plasmids.
Immunohistochemical Staining
Immunohistochemical staining was performed as described previously (23). Primary and secondary antibodies were diluted in PBS containing 10% normal goat serum. The 1:50 diluted anti-NASP antiserum (ProteinTech Group, Inc., Chicago, IL) was applied to the tissues overnight at 4 °C and the 1:200 diluted biotin-conjugated goat anti-rabbit IgG was incubated for 1 h at room temperature. The expression was visualized using horseradish peroxidase (HRP) substrate. As a negative control, serial sections were subjected to the same procedure, with normal rabbit serum replacing the primary antibody. The expression was visualized using HRP substrate. The sections were mounted in 80% glycerol and examined with an Olympus BX-52 microscope.
In Situ Hybridization
In situ hybridization was performed as described previously (24, 25). Briefly, the epididymal tissues were fixed in 4% paraformaldehyde and then embedded in paraffin. The embedded epididymidis were sectioned. The slides were dewaxed, then digested with proteinase K (500 ng/ml) hybridized with locked nucleic acid probes (Exiqon), special for rno-miR-29a, which were labeled with digoxigenin (DIG). The temperature of hybridization was 65 °C. The expression of miR-29a was visualized using alkaline phosphatase substrate.
RESULTS
miR-29 Was Dramatically Up-regulated during Postnatal Epididymal Development
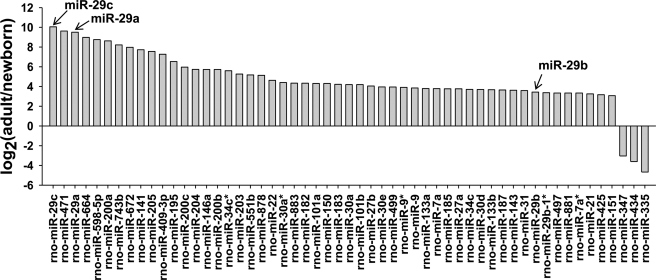
Epididymal cells exhibited totally different cell proliferative activities between the newborn and adult stage. LC Science MiRNA Arrays and Affymetrix GeneChip Rat Genome 230 2.0 Arrays were used here to identify the miRNAs and genes potentially associated with this biological process. According to the signal difference of miRNA array, 52 miRNAs were found to be significantly up-regulated and 3 miRNAs were dramatically down-regulated (≧8-fold) in the rat adult (90 days) compared with the neonatal (1 day) epididymis (Fig. 1). To validate the results obtained from the miRNA arrays, the expression patterns of nine miRNAs were examined by Northern blotting. As shown in supplemental Fig. S1, all the expression patterns of miRNAs detected by Northern blotting were consistent with the results of microarray analysis. Interestingly, of these up-regulated miRNAs, miR-29 family members, especially miR-29c and miR-29a, were most up-regulated during postnatal epididymis development (Fig. 1). These results were consistent with previous reports that the miR-29 family including miR-29a, miR-29b, and miR-29c were dramatically up-regulated during development of several tissues such as aorta, lung, brain, cornea, and skeletal muscle (11, 14–17). Our previous study also showed that the miR-29 family was most significantly up-regulated in adult epididymis compared with the newborn epididymis of human (supplemental Fig. S2, the data were retrieved online). On the contrary, the proliferative activities of epididymal epithelial cells were decreased significantly in adult compared with newborn stage cells (4). These data suggested that miR-29 may function in epididymal postnatal remolding.
FIGURE 1.
Differentially expressed miRNAs between newborn (1 day) and adult (90 day) rat epididymis. The upper part of the horizontal axis represents up-regulated miRNAs (≧8-fold), and the lower part represents down-regulated miRNAs (≧8-fold) in adult rat epididymis compared with newborn rat epididymis.
miR-29a Inhibited the Proliferation of Epididymal Cells in Vitro
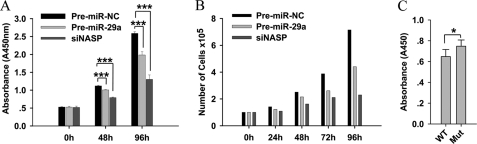
Further analysis of the top 40 highly expressed miRNAs in adult human (data from the humanet website), mouse, rat epididymis as well as PC-1 cells, showed miR-29a was expressed at a relative high levels in these tissues or cells (supplemental Fig. S3). Among the miR-29 family, miR-29a was relatively highly expressed in adult rat epididymis compared with the other two members, miR-29b and miR-29c (Fig. 2A and supplemental Fig. S3). Previous studies demonstrated that miR-29a was tightly related to the proliferation of cancer cells (26). To investigate whether miR-29a can influence the proliferation of epididymal cells, we overexpressed miR-29a in PC-1 and DC-2 cells (two cell lines from mouse epididymis) by transfected pre-miR-29a, and measured the cell growth rate at 48 h post-transfection using the CCK-8 kit. The results showed that PC-1 and DC-2 cells transfected with pre-miR-29a displayed a decrease of cell growth compared with those transfected with scrambled oligonucleotides (pre-miR-NC) (Fig. 2, B and C). Consistently, inhibition of endogenous miR-29a by miRNA inhibitor transfection increased the growth rate of PC-1 cells (Fig. 2D). Counting cells using a hematocytometer 48, 72, and 96 h after the initial transfection also showed that miR-29a overexpression decreased cell growth of PC-1 cells (Fig. 2E). Taken together, miR-29a negatively regulated the proliferation of epididymal cells in vitro.
FIGURE 2.
miR-29a inhibited the proliferation of epididymal epithelial cells in vitro. A, the relative signal intensity of miR-29 family including miR-29a, miR-29b, and miR-29c. B and C, quantitative analysis of the proliferation of PC-1 (B) and DC-2 (C) cells in which miR-29a was overexpressed. Samples were incubated with CCK-8 regents for 4 h, and the absorbance was detected at A450 nm, 48 h after miRNA mimics transfection. Data were expressed as mean ± S.D. ***, p < 0.001. D, quantitative analysis of the proliferation of PC-1 cells in which endogenous miR-29a was inhibited. Samples were incubated with CCK-8 regents for 4 h, and the absorbance was detected at A450 nm, 72 h after miRNA inhibitor transfection. Data were expressed as mean ± S.D. **, p < 0.01. E, PC-1 cell proliferation assay using cell counting. The cell number was counted using blood cell counting chambers 48, 72, and 96 h after the miRNA mimics transfection. The experiment repeated twice, and data were expressed as mean ± S.D.
NASP Expression Was Negatively Correlated with miR-29a Expression
miRNAs function by repressing mRNA translation or reducing mRNA stability. To find targets that mediated the anti-proliferation effect of miR-29a during epididymal postnatal remolding, we performed mRNA microarray, and confirmed the expression profiles of mRNAs by real-time PCR (supplemental Fig. S4). Analysis of the mRNA profiles of newborn and adult rat epididymis showed that 162 proliferation-associated genes were down-regulated and 116 proliferation-associated genes were up-regulated (≧2-fold) in adult stage epididymis compared with the newborn stage (supplemental Tables S3 and S4, cell proliferation associated rat genes being retrieved from GOstat data base: GO:0042127). Of which, the dramatically regulated (≧8-fold) genes are listed in supplemental Fig. S5. Ki-67, usually used as a marker to assess the proliferative capacity of cells, was significantly down-regulated in adult epididymis (supplemental Fig. S5), further confirming that cell proliferation of newborn epididymis was higher than the adult epididymis.
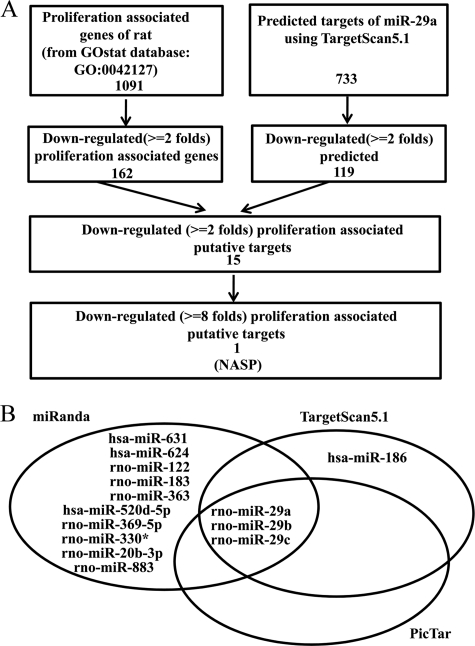
miRNAs modulate the expression of their targets by mRNA degradation or translation block, therefore, the expression pattern of miRNAs and their targets usually showed a reverse relationship. Thus the mRNAs down-regulated (≧2-fold) in adult stage were selected by integrated analysis with putative targets of miR-29a predicted by TargetScan 5.1. The results of the integrated analysis are shown in supplemental Table S5. Of these putative targets, Sp1, Dnmt3a, Hdac4, Col1a1, Col3a1, Col4a1, Col5a1, Col5a2, Col5a3, Col7a1, and Mmp2 etc. were validated previously (27–30). Further analysis showed that the putative targets included 15 proliferation associated genes (supplemental Table S6 and Fig. 3A). Among them, NASP, a highly conserved gene and which is required for cell proliferation and cell cycle (19, 31), displayed the best prediction scoring and significant change during postnatal epididymal development (supplemental Table S6). In addition, of those significantly down-regulated cell proliferation-associated genes (≧8-fold) listed in supplemental Fig. S5, Nasp was the only potential target of miR-29a. Considering that miR-29a controlled epididymal cell proliferation, we therefore hypothesized that Nasp may mediate the anti-proliferation effect of miR-29a during postnatal development of rat epididymis. Consistent with the hypothesis, combined using online software, miRanda, TargetScan, and Pictar, to predict those miRNAs that potentially target NASP, we found that the miR-29 family, including miR-29a, miR-29b, and miR-29c, were the overlap miRNAs (Fig. 3B).
FIGURE 3.
Nasp is a potential target of miR-29a. A, cell proliferation-related putative targets of miR-29a. There are 1091 cell proliferation-associated rat genes (data retrieved from GOstat data base: GO:0042127) and 162 of that are down-regulated (≧2-fold) in adult epididymis. Meanwhile, there are 733 conserved targets of miR-29a (predicted by TargetScan5.1 software) and 119 were down-regulated in adult epididymis. There are 15 overlapping genes between 162 down-regulated proliferation-associated genes and 119 down-regulated putative targets of miR-29a. Among them, Nasp was the most dramatically down-regulated (8-fold). B, miR-29a, -b, and -c were predicted to targeting NASP by using on-line software, including miRanda, TargetScan, and Pictar.
We confirmed the association of miR-29a and Nasp by detecting their expression patterns during postnatal development of rat epididymis. Northern blotting showed that miR-29a was up-regulated gradually in postnatal 1-, 7-, 15-, 30-, 45-, 60-, and 90-day rat epididymis (Fig. 4A), whereas Nasp was down-regulated gradually during this period (Fig. 4B). Furthermore, in situ hybridization revealed miR-29a levels were extremely low in un-differentiated epididymis (days 1 and 7), whereas it was highly expressed in fully differentiated epididymis (day 49) (Fig. 4C). On the other hand, using immunohistochemical staining Nasp was highly expressed in the un-differentiated epididymis (days 1 and 7) and lowly expressed in fully differentiated epididymis (day 49) (Fig. 4C). Thus, miR-29a and Nasp were reversely expressed during postnatal development of the rat epididymis. The negative relationship between NASP and miR-29a also existed among different cell lines, including NIH3T3, PC-1, and HEK 293T cells (Fig. 4D). Moreover, the miR-29a expression level was much higher in 60-day-old rat epididymis with poor Nasp than in 60-day-old rat testis with abundant Nasp (Fig. 4E). In summary, the expression of Nasp was negatively correlated with miR-29a levels in vivo and in vitro, strongly supporting that the Nasp was a potential target of miR-29a.
FIGURE 4.
NASP expression was negatively correlated with miR-29a levels in vivo. A, Northern blotting detection of miR-29a levels in postnatal epididymis of days 1, 7, 15, 30, 45, 60, and 90. U6 snRNA was used as a loading control. B, Western blotting detection of Nasp expression in postnatal epididymis of days 1, 7, 15, 30, 45, 60, and 90. β-Actin was used as a loading control. C, detection of miR-29a and Nasp expression in postnatal rat epididymis by in situ hybridization and immunohistochemistry, respectively. miR-29a and Nasp expression were measured in the rat epididymis of days 1, 7, 28, and 49 by staining the paraffin-embedded tissue sections. Probes specific for miR-29a were labeled with digoxigenin (DIG) and expression was visualized using alkaline phosphatase substrate. Positive-expressing miR-29a cells stain blue (arrow). The rabbit polyclonal antibody toward Nasp was used for immunohistochemistry and the secondary antibody was labeled with biotin, and the expression was visualized using horseradish peroxidase (HRP). The brown staining represents the positive immunoreactivity for the Nasp as indicated (arrowhead). All pictures were taken at an original magnification of ×400. D, Northern blotting detection of miR-29a (left), and Western blotting detection of NASP (right) in NIH3T3, PC-1, and HEK 293T cells. E, Northern blotting detection of miR-29a (left), and Western blotting detection of Nasp (right) in 60-day-old rat epididymis and testis. 5 S rRNA was used as a loading control for Northern blotting and β-actin was used as a loading control for Western blotting.
NASP Is a Bona Fide Target of miR-29a
To confirm miR-29a targeting NASP directly, the dual luciferase reporter assay was performed. The 3′ UTR of human NASP mRNA contained two conserved potential target sites of miR-29a (Fig. 5A). The wild-type or mutated 3′ UTR of NASP mRNA was cloned to downstream of the Renilla luciferase gene and co-transfected with pre-miR-29a or scrambled oligonucleotides into HEK 293T cells. Luciferase activities were measured 24 h after transfection. Cells co-transfected with the reporter gene carrying wild-type NASP 3′ UTR and pre-miR-29a exhibited apparent reduction of the luciferase activities compared with cells transfected with the scrambled oligonucleotide (Fig. 5B, left). Then, four nucleotides (TGCT → GTAG) in “seed” sequences of both miR-29a potential target sites were mutated (Fig. 5A). Luciferase assay of HEK 293T cells co-transfected with the reporter gene carrying double mutated NASP mRNA 3'UTR and pre-miR-29a showed that the reduction of luciferase activity of the reporter by miR-29a was abrogated by the mutations (Fig. 5B, right). Furthermore, inhibition of endogenous miR-29a expression using antisense oligonucleotides increased luciferase activity in NIH3T3 cells 36 h after transfection (Fig. 5C, left), whereas there was no apparent changes of the luciferase activities when the 3′ UTR was mutated (Fig. 5C, right). These data indicated that the NASP 3′ UTR was truly targeted by miR-29a.
FIGURE 5.
NASP is a bona fide target of miR-29a. A, sequences of miR-29a target sites in NASP 3′ UTR. Mutated sequences were underlined. There were two conserved target sites of miR-29a in human, mouse, and rat NASP 3′ UTR. The wild-type and mutant 3′ UTR was inserted downstream of the Renilla luciferase reporter gene in psiCHECK2 vector, and the Firefly luciferase reporter worked as a control. B, HEK 293T cells carrying the wild-type or double mutated NASP 3′ UTR were transfected with pre-miR-29a or negative control (pre-miR-NC), respectively. The luciferase activities were analyzed 24 h after transfection. The data represented the average of three independent experiments ± S.D. C, inhibition of endogenous miR-29a by antisense oligonucleotides. NIH3T3 cells carrying the wild-type or double mutated NASP 3′ UTR were transfected with anti-miR-29a or negative control (anti-miR-NC), respectively. The luciferase activities were analyzed 36 h after transfection. The data represent the average of three independent experiments ± S.D. D, PCR analysis of the mRNA levels of NASP in PC-1 (left) and HEK 293T (right) 48 h after miR-29a transfection. E, Western blotting detection of the protein levels of NASP in PC-1 cells (left) and HEK 293T cells (right) 48 h after miR-29a transfection. F, Western blotting detection of the protein levels of Nasp in PC-1 cells (left) and NIH3T3 cells (right) 48 h after inhibition of endogenous miR-29a with antisense RNA oligonucleotides. GAPDH was used as a loading control for PCR and β-actin was used as a loading control for Western blotting.
Furthermore, we investigated whether miR-29a modulated the expression of NASP at mRNA and protein levels. PC-1 cells and HEK 293T cells were transfected with pre-miR-29a or a scrambled oligonucleotide, and then NASP mRNAs and protein levels were measured by RT-PCR and Western blotting, respectively. Transfection with the pre-miR-29a, but not the scrambled control, resulted in a remarkable reduction of the endogenous NASP mRNA (Fig. 5D) and protein (Fig. 5E) in the two cell lines. On the contrary, inhibition of endogenous miR-29a using antisense oligonucleotides increased Nasp protein levels in PC-1 cells (Fig. 5F, left panel). As described above, NIH3T3 cells with a high basal miR-29a level had low NASP expression (Fig. 4D). Also, suppressing endogenous miR-29a resulted in elevation of the NASP protein in NIH3T3 cells (Fig. 5F, right panel). Taken together, miR-29a can directly target NASP via mRNA degradation and/or translation. NASP was a bona fide target of miR-29a.
miR-29a Regulated Proliferation through NASP
The above facts that miR-29a negatively regulated the proliferation of epididymal cells, and directly targeted NASP, suggest that NASP may be involved in proliferation inhibition of miR-29a on epididymal epithelia. To confirm this hypothesis, PC-1 cells were transfected with siRNAs against mouse Nasp, which efficiently reduce the expression of NASP at mRNA and protein levels (supplemental Fig. S6). Silencing of NASP significantly decreased the proliferative rate of PC-1 cells, which mimicked the effect of overexpression of miR-29a (Fig. 6, A and B). Then we investigated whether NASP could counteract the anti-proliferation effect of miR-29a. We generated two expression vectors, pcDNA3.1(+)-NASP-3′UTR-WT and pcDNA3.1(+)-NASP-3′UTR-Mut1 and -2, which encoded the full-length tNASP mRNA with wild-type 3′ UTR or mutated 3′ UTR at the two miR-29a binding sites, respectively. We demonstrated that both vectors could encode the tNASP protein (supplemental Fig. S7). Then, each tNASP expression vector was co-transfected with pre-miR-29a into PC-1 cells using Lipofectamine 2000 transfection reagent and cell viability was measured 72 h after transfection. Notably, co-transfection of the tNASP expression vector with mutated 3′ UTR attenuated the anti-proliferation effect of miR-29a compared with that of wild-type 3′ UTR (Fig. 6C), indicating that miR-29a regulated PC-1 cell proliferation through Nasp. Thus, these results indicate that miR-29a may inhibit the proliferation of epididymal epithelial cells by targeting Nasp.
FIGURE 6.
miR-29a inhibited PC-1 cell proliferation by targeting Nasp. A, CCK-8 assay of proliferation of PC-1 cells at 0, 48, or 96 h after transfection with pre-miR-NC, pre-miR-29a, or siRNA against Nasp, respectively. B, cell counting analysis of proliferation of PC-1 cells at 0, 24, 48, 72, or 96 h after transfection with pre-miR-NC, pre-miR-29a, or siRNA against Nasp, respectively. C, rescue experiments in PC-1 cells. PC-1 cells were co-transfected with pre-miR-29a and the tNASP expression vectors, together with wild-type (pcDNA3.1(+)-tNASP-3′UTR-WT) or mutant (pcDNA3.1(+)-tNASP-3′UTR-Mut1 and -2) 3′ UTR, respectively. The proliferation activities of PC-1 cells were measured with the CCK-8 kit 72 h after transfection. The result was represented as mean ± S.D. *, p < 0.05; ***, p < 0.001.
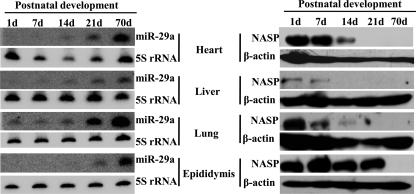
We further demonstrated that miR-29a was also up-regulated during postnatal development of mouse heart, liver, lung, and epididymis. Consistently, Nasp was down-regulated (Fig. 7). These data indicated that miR-29a up-regulation and hence Nasp down-regulation during postnatal development was a universal phenomenon.
FIGURE 7.
The expression of miR-29a and Nasp during postnatal development of mouse heart, liver, lung, and epididymis. The left side shows Northern blotting detection of miR-29a levels during postnatal development of mouse heart, liver, lung, and epididymis of days 1, 7, 14, 21, and 70. 5 S rRNA was used as a loading control. The right side shows Western blotting detection of Nasp expression in postnatal epididymis of days 1, 7, 14, 21, and 70. β-Actin was used as a loading control.
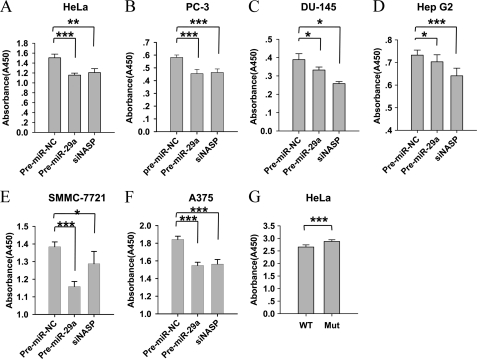
We also demonstrated miR-29a inhibited the proliferation of HeLa cells (Fig. 8A), as well as five other cancer cell lines, including PC-3, DU-145, HepG2, SMMC-7721, and A375 (Fig. 8, B–F). Moreover, the anti-proliferation effects of miR-29a were rescued by using the NASP expression vectors with mutated 3′ UTR but not the vector with wild-type 3′ UTR in HeLa cells (Fig. 8G). These results indicated that the inhibition of cell proliferation by miR-29a through NASP not only promoted a normal development processes, but might also prevent tumorigenesis.
FIGURE 8.
Overexpression of miR-29a or knockdown of NASP suppressed the proliferation of cancer cells. A–F, miR-29a mimics or siRNA against NASP were transfected into cervical cancer cells (HeLa cells), prostate cancer cells (PC-3 and DU-145 cells), hepatocellular cancer cells (HepG2 and SMMC-7721 cells), and melanoma cells (A375 cells), respectively. The cell proliferative activities were analyzed using the CCK-8 kit 72 h after transfection. G, rescue experiments in PC-1 cells. PC-1 cells were co-transfected with pre-miR-29a and the tNASP expression vectors carrying wild-type (pcDNA3.1(+)-tNASP-3′UTR-WT) or mutant (pcDNA3.1(+)-tNASP-3′UTR-Mut1 and -2) 3′ UTR, respectively. The proliferation activities of PC-1 cells were measured by the CCK-8 kit 72 h after transfection. Values were expressed as mean ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
DISCUSSION
Cell proliferation gradually decreases with maturation of many organs, especially epididymis. From neonatal to adult, the epididymis goes through un-differentiation, differentiating, and fully differentiated stages. As the differentiation proceeds, the proliferative activity of epididymal epithelial cells slows down until cell multiplication ceases at adult stage (4). Therefore, epididymis can serve as an ideal model to study the regulation of cell proliferation or cell differentiation during postnatal development.
miRNAs are pivotal regulators of post-transcriptional gene silencing, which are involved in complex biological processes during multiple developmental processes. Expression of miRNA is subjected to tight temporal and special regulation, and they exhibit specific functions in different organs and tissues. Using rat epididymis as a model, here we characterized the miRNA expression during postnatal remodeling. We found 55 miRNAs were significantly changed in the rat adult epididymis compared with the newborn stage, suggesting the miRNA expression of epididymis was regulated not only temporally but also stage differently. Our study provides a database for future research of miRNA in epididymis.
Cell fate decision during epididymal postnatal remodeling is most characterized with a dramatic decrease of epididymal epithelial cell proliferation. The miRNAs function in cell fate decisions (32). Therefore, the miRNA expression profiles of epididymis development provided information for the controlled poor proliferation situation of miRNAs of the adult epididymal epithelia. Our analysis showed many up-regulated miRNAs were related to proliferation inhibition, such as miR-205 (33), miR-195 (34), miR-22 (35), miR-145 (36, 37), and the let-7 family (38–42). Among them, the three most up-regulated miRNAs were miR-29c, miR-471, and miR-29a. Considering that miR-29a and miR-29c were also most significantly up-regulated in adult epididymis compared with the newborn epididymis of human, we believed that miR-29 played critical roles in regulation of the proliferation during epididymal remodeling. Furthermore, miR-29a was also up-regulated during postnatal development of mouse epididymis, heart, liver, and lung (Fig. 7), indicating miR-29a is involved in the development of multiple organs.
Exogenous overexpression of miR-29a in both lung and pancreatic cancer cell lines resulted in a significant reduction in the invasion phenotype, as well as in proliferation (43). miR-29a also functioned in promoting proliferation (44, 45), suggesting miR-29a promoted or inhibited proliferation depending on cellular contexts. We demonstrated here that miR-29a was expressed at high abundance in adult epididymis with poor proliferation. Exogenous overexpression of miR-29a resulted in attenuated proliferation of PC-1 and DC-2 cells, confirming that, in epididymis, miR-29a served as a critical negative regulator of cell proliferation.
miRNAs function involves a modest regulation of multiple target genes in a common biological process by a single miRNA. miR-29a inhibited cell proliferation or promoted cell apoptosis by targeting DNMT3A, DNMT3B, CDK6, BCL2, MCL1, TCL-1, CDC42, PIK3R1 (P85α) etc. (26, 28, 46–49). We analyzed the expression of all these demonstrated miR-29a target genes in newborn and adult rat epididymis by microarray, and found that Dnmt3b, Cdk6, and Bcl2 were not expressed in either stage. Although Cdc42 and Pik3r1 showed no change and Dnmt3a, Mcl1, and Hdac4 changed weakly between the two stages (supplemental Table S7) suggests that miR-29a may regulate the epididymal epithelial proliferation by novel targets. As expected, we identified NASP as a bona fide target gene of miR-29a, and demonstrated that NASP mediated the anti-proliferative effect of miRNA-29a on the epididymal epithelia.
Being a nuclear autoantigenic sperm protein, NASP had two isoforms, tNASP (testis type) and sNASP (somatic type) sharing the same 3′ UTR. The tNASP was mainly expressed in testis, a variety of malignant tumors, stem cells, and embryonic tissues, whereas sNASP existed in all somatic mitosis cells (50). Both types were associated with DNA replication, cell proliferation, and cell cycle progression by specifically binding to histone H1, H3, and H4, and affecting chromatin assembly (50). Either overexpression or down-regulation of NASP will affect progression through the cell cycle (31, 51). We demonstrated here, in the newborn epididymis with high proliferation and low miR-29, the level of tNasp was high, whereas in the adult epididymis with poor proliferation and high miR-29, the level of tNasp was very low. Moreover, Nasp was also down-regulated during postnatal development of the mouse heart, liver, lung, and epididymis, indicating that miR-29a up-regulation and hence NASP down-regulation during postnatal development were universal. NASP was not only regulated by miR-29a, but also by miR-29b and miR-29c (supplemental Fig. S8). As tNASP, sNASP was regulated by miR-29a too (supplemental Fig. S9).
Although miR-29a was down-regulated in NASP in cells of multiple tissues, the regulatory intensity is different. Among human bronchial epithelial cells (BEAS-2B), human adenocarcinomic alveolar basal epithelial cells (A549), human cervical cancer cells (HeLa), and mouse epididymal cells (PC-1), the down-regulation of NASP was much more intense in PC-1 cells, although the targets DNMT3A, DNMT3B, and CDC42 showed different levels of down-regulation by miR-29a in different cell lines (supplemental Fig. S10). Unexpectedly, the target CDK6 was most fiercely down-regulated in these four cell lines (supplemental Fig. S10), but it was not expressed in newborn and adult rat epididymis (supplemental Table S7). So, we believe a miRNA can regulate different targets in different cells, even for the same target, the strength of regulation was different. In epididymis, the response of NASP to miR-29a seemed to be hyperintensively.
In summary, by microarray analysis and experimental confirmation, we profiled the miRNA expression of epididymis of adult and newborn rat, and found that miR-29a was dramatically up-regulated in postnatal development of rat epididymis. We further demonstrated that miR-29a inhibited epididymal epithelial cell proliferation by directly repressing its novel target, NASP. Our results revealed that miRNAs regulated the cell fate decisions of the epididymal epithelia during the postnatal remodeling. Nevertheless, how miR-29a expression was regulated during epididymis remodeling remains obscure. We are generating miR-29 engineering mouse models to better understand the truth.
Supplementary Material
This work was supported by National Basic Science Research and Development Project of China Grants 30930053 and 31101069 and Chinese Academy of Sciences (CAS) Knowledge Innovation Program Grant KSCX2-EW-R-07.

This article contains supplemental Figs. S1–S10 and Tables S1–S7.
- miRNA
- microRNA
- NASP
- nuclear autoantigenic sperm protein
- pre-miR-NC
- pre-miR-negative control
- anti-miR-NC
- anti-miR-negative control
- tNASP
- testicular type NASP
- sNASP
- somatic type NASP
- Mut1 and -2
- NASP 3′ UTR with two mutated sites.
REFERENCES
- 1. Machida N., Brissie N., Sreenan C., Bishop S. P. (1997) Inhibition of cardiac myocyte division in c-myc transgenic mice. J. Mol. Cell Cardiol. 29, 1895–1902 [DOI] [PubMed] [Google Scholar]
- 2. Crocker T. T., Teeter A., Nielsen B. (1970) Postnatal cellular proliferation in mouse and hamster lung. Cancer Res. 30, 357–361 [PubMed] [Google Scholar]
- 3. Márquez M. G., Cabrera I., Serrano D. J., Sterin-Speziale N. (2002) Cell proliferation and morphometric changes in the rat kidney during postnatal development. Anat. Embryol. 205, 431–440 [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez C. M., Kirby J. L., Hinton B. T. (2001) in The Epididymis. From Molecules to Clinical Practice (Robaire B., Hinton B. T., eds) pp. 251–267, Kluwer Academic/Plenum Publishers, New York [Google Scholar]
- 5. Robaire B., Hermo L. (1988) in The Physiology of Reproduction (Knobil E., Neill J., eds) pp. 999–1080, Raven Press, New York [Google Scholar]
- 6. Cornwall G. A. (2009) New insights into epididymal biology and function. Hum. Reprod. Update 15, 213–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ambros V. (2001) microRNAs. Tiny regulators with great potential. Cell 107, 823–826 [DOI] [PubMed] [Google Scholar]
- 8. Pauli A., Rinn J. L., Schier A. F. (2011) Non-coding RNAs as regulators of embryogenesis. Nat. Rev. Genet. 12, 136–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shenouda S. K., Alahari S. K. (2009) MicroRNA function in cancer. Oncogene or a tumor suppressor? Cancer Metastasis Rev. 28, 369–378 [DOI] [PubMed] [Google Scholar]
- 10. Alvarez-Garcia I., Miska E. A. (2005) MicroRNA functions in animal development and human disease. Development 132, 4653–4662 [DOI] [PubMed] [Google Scholar]
- 11. Williams A. E., Moschos S. A., Perry M. M., Barnes P. J., Lindsay M. A. (2007) Maternally imprinted microRNAs are differentially expressed during mouse and human lung development. Dev. Dyn. 236, 572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang J., Liu Q., Zhang W., Li J., Li Z., Tang Z., Li Y., Han C., Hall S. H., Zhang Y. (2010) Comparative profiling of genes and miRNAs expressed in the newborn, young adult, and aged human epididymides. Acta Biochim. Biophys. Sin. 42, 145–153 [DOI] [PubMed] [Google Scholar]
- 13. Wang J., Ruan K. (2010) miR-335 is involved in the rat epididymal development by targeting the mRNA of RASA1. Biochem. Biophys. Res. Commun. 402, 222–227 [DOI] [PubMed] [Google Scholar]
- 14. Ott C. E., Grünhagen J., Jäger M., Horbelt D., Schwill S., Kallenbach K., Guo G., Manke T., Knaus P., Mundlos S., Robinson P. N. (2011) MicroRNAs differentially expressed in postnatal aortic development down-regulate elastin via 3′ UTR and coding-sequence binding sites. PLoS One 6, e16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Podolska A., Kaczkowski B., Kamp Busk P., Søkilde R., Litman T., Fredholm M., Cirera S. (2011) MicroRNA expression profiling of the porcine developing brain. PLoS One 6, e14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y., Piatigorsky J. (2009) Targeted deletion of Dicer disrupts lens morphogenesis, corneal epithelium stratification, and whole eye development. Dev. Dyn. 238, 2388–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang T. H., Zhu M. J., Li X. Y., Zhao S. H. (2008) Discovery of porcine microRNAs and profiling from skeletal muscle tissues during development. PLoS One 3, e3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Araki Y., Suzuki K., Matusik R. J., Obinata M., Orgebin-Crist M. C. (2002) Immortalized epididymal cell lines from transgenic mice overexpressing temperature-sensitive simian virus 40 large T-antigen gene. J. Androl. 23, 854–869 [PubMed] [Google Scholar]
- 19. Richardson R. T., Alekseev O. M., Grossman G., Widgren E. E., Thresher R., Wagner E. J., Sullivan K. D., Marzluff W. F., O'Rand M. G. (2006) Nuclear autoantigenic sperm protein (NASP), a linker histone chaperone that is required for cell proliferation. J. Biol. Chem. 281, 21526–21534 [DOI] [PubMed] [Google Scholar]
- 20. Baek D., Villén J., Shin C., Camargo F. D., Gygi S. P., Bartel D. P. (2008) The impact of microRNAs on protein output. Nature 455, 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu J., Li C. X., Li Y. S., Lv J. Y., Ma Y., Shao T. T., Xu L. D., Wang Y. Y., Du L., Zhang Y. P., Jiang W., Li C. Q., Xiao Y., Li X. (2011) miRNA-miRNA synergistic network. Construction via co-regulating functional modules and disease miRNA topological features. Nucleic Acids Res. 39, 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friedman R. C., Farh K. K., Burge C. B., Bartel D. P. (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu C. F., Liu Q., Zhang L., Yuan H. X., Zhen W., Zhang J. S., Chen Z. J., Hall S. H., French F. S., Zhang Y. L. (2007) RNase9, an androgen-dependent member of the RNase A family, is specifically expressed in the rat epididymis. Biol. Reprod. 76, 63–73 [DOI] [PubMed] [Google Scholar]
- 24. Gupta A., Mo Y. Y. (2011) Detection of microRNAs in cultured cells and paraffin-embedded tissue specimens by in situ hybridization. Methods Mol. Biol. 676, 73–83 [DOI] [PubMed] [Google Scholar]
- 25. Nuovo G. J. (2008) In situ detection of precursor and mature microRNAs in paraffin-embedded, formalin-fixed tissues and cell preparations. Methods 44, 39–46 [DOI] [PubMed] [Google Scholar]
- 26. Park S. Y., Lee J. H., Ha M., Nam J. W., Kim V. N. (2009) miR-29 miRNAs activate p53 by targeting p85α and CDC42. Nat. Struct. Mol. Biol. 16, 23–29 [DOI] [PubMed] [Google Scholar]
- 27. Liu S., Wu L. C., Pang J., Santhanam R., Schwind S., Wu Y. Z., Hickey C. J., Yu J., Becker H., Maharry K., Radmacher M. D., Li C., Whitman S. P., Mishra A., Stauffer N., Eiring A. M., Briesewitz R., Baiocchi R. A., Chan K. K., Paschka P., Caligiuri M. A., Byrd J. C., Croce C. M., Bloomfield C. D., Perrotti D., Garzon R., Marcucci G. (2010) Sp1/NFκB/HDAC/miR-29b regulatory network in KIT-driven myeloid leukemia. Cancer Cell 17, 333–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fabbri M., Garzon R., Cimmino A., Liu Z., Zanesi N., Callegari E., Liu S., Alder H., Costinean S., Fernandez-Cymering C., Volinia S., Guler G., Morrison C. D., Chan K. K., Marcucci G., Calin G. A., Huebner K., Croce C. M. (2007) MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. U.S.A. 104, 15805–15810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Y., Taylor N. E., Lu L., Usa K., Cowley A. W., Jr., Ferreri N. R., Yeo N. C., Liang M. (2010) Renal medullary microRNAs in Dahl salt-sensitive rats. miR-29b regulates several collagens and related genes. Hypertension 55, 974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winbanks C. E., Wang B., Beyer C., Koh P., White L., Kantharidis P., Gregorevic P. (2011) TGF-β regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J. Biol. Chem. 286, 13805–13814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richardson R. T., Batova I. N., Widgren E. E., Zheng L. X., Whitfield M., Marzluff W. F., O'Rand M. G. (2000) Characterization of the histone H1-binding protein, NASP, as a cell cycle-regulated somatic protein. J. Biol. Chem. 275, 30378–30386 [DOI] [PubMed] [Google Scholar]
- 32. Ivey K. N., Srivastava D. (2010) MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 7, 36–41 [DOI] [PubMed] [Google Scholar]
- 33. Wu H., Zhu S., Mo Y. Y. (2009) Suppression of cell growth and invasion by miR-205 in breast cancer. Cell Res. 19, 439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu T., Zhu Y., Xiong Y., Ge Y. Y., Yun J. P., Zhuang S. M. (2009) MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology 50, 113–121 [DOI] [PubMed] [Google Scholar]
- 35. Li X., Liu J., Zhou R., Huang S., Chen X. M. (2010) Gene silencing of MIR22 in acute lymphoblastic leukaemia involves histone modifications independent of promoter DNA methylation. Br. J. Haematol. 148, 69–79 [DOI] [PubMed] [Google Scholar]
- 36. Akao Y., Nakagawa Y., Kitade Y., Kinoshita T., Naoe T. (2007) Down-regulation of microRNAs-143 and -145 in B-cell malignancies. Cancer Sci. 98, 1914–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin T., Dong W., Huang J., Pan Q., Fan X., Zhang C., Huang L. (2009) MicroRNA-143 as a tumor suppressor for bladder cancer. J. Urol. 181, 1372–1380 [DOI] [PubMed] [Google Scholar]
- 38. Yu F., Yao H., Zhu P., Zhang X., Pan Q., Gong C., Huang Y., Hu X., Su F., Lieberman J., Song E. (2007) let-7 regulates self-renewal and tumorigenicity of breast cancer cells. Cell 131, 1109–1123 [DOI] [PubMed] [Google Scholar]
- 39. Mayr C., Hemann M. T., Bartel D. P. (2007) Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315, 1576–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sampson V. B., Rong N. H., Han J., Yang Q., Aris V., Soteropoulos P., Petrelli N. J., Dunn S. P., Krueger L. J. (2007) MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 67, 9762–9770 [DOI] [PubMed] [Google Scholar]
- 41. Akao Y., Nakagawa Y., Naoe T. (2006) let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol. Pharm. Bull 29, 903–906 [DOI] [PubMed] [Google Scholar]
- 42. Johnson S. M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K. L., Brown D., Slack F. J. (2005) RAS is regulated by the let-7 microRNA family. Cell 120, 635–647 [DOI] [PubMed] [Google Scholar]
- 43. Muniyappa M. K., Dowling P., Henry M., Meleady P., Doolan P., Gammell P., Clynes M., Barron N. (2009) miRNA-29a regulates the expression of numerous proteins and reduces the invasiveness and proliferation of human carcinoma cell lines. Eur. J. Cancer 45, 3104–3118 [DOI] [PubMed] [Google Scholar]
- 44. Santanam U., Zanesi N., Efanov A., Costinean S., Palamarchuk A., Hagan J. P., Volinia S., Alder H., Rassenti L., Kipps T., Croce C. M., Pekarsky Y. (2010) Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc. Natl. Acad. Sci. U.S.A. 107, 12210–12215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han Y. C., Park C. Y., Bhagat G., Zhang J., Wang Y., Fan J. B., Liu M., Zou Y., Weissman I. L., Gu H. (2010) MicroRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J. Exp. Med. 207, 475–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao J. J., Lin J., Lwin T., Yang H., Guo J., Kong W., Dessureault S., Moscinski L. C., Rezania D., Dalton W. S., Sotomayor E., Tao J., Cheng J. Q. (2010) MicroRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood 115, 2630–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xiong Y., Fang J. H., Yun J. P., Yang J., Zhang Y., Jia W. H., Zhuang S. M. (2010) Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 51, 836–845 [DOI] [PubMed] [Google Scholar]
- 48. Mott J. L., Kobayashi S., Bronk S. F., Gores G. J. (2007) mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 26, 6133–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pekarsky Y., Santanam U., Cimmino A., Palamarchuk A., Efanov A., Maximov V., Volinia S., Alder H., Liu C. G., Rassenti L., Calin G. A., Hagan J. P., Kipps T., Croce C. M. (2006) Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 66, 11590–11593 [DOI] [PubMed] [Google Scholar]
- 50. Wang H., Walsh S. T., Parthun M. R. (2008) Expanded binding specificity of the human histone chaperone NASP. Nucleic Acids Res. 36, 5763–5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alekseev O. M., Bencic D. C., Richardson R. T., Widgren E. E., O'Rand M. G. (2003) Overexpression of the Linker histone-binding protein tNASP affects progression through the cell cycle. J. Biol. Chem. 278, 8846–8852 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.