Abstract
The role of basic science in the development of health care has received more and more attention. In my own area of research involving the so-called eicosanoids, there are many examples of how studies of structure and function of small molecules, as well as proteins and genes, have led to new therapeutic agents for treatment of a variety of diseases. In most of the cases, the discoveries have resulted in the recognition of novel therapeutic targets amenable to modulation by small molecules. However, there are also examples in which the molecular mechanisms of actions of drugs, discovered by phenotypic screening, have been elucidated. The majority of the examples in this article consist of approved drugs; however, in some cases, ongoing developments of potential therapeutics are cited.
Keywords: Eicosanoid, Prostaglandins, Drug Development, Lipoxygenase Pathway, leukotriene
Introduction
In the 1930s, Kurzrok and Lieb, and later Goldblatt and von Euler, discovered material in semen and accessory genital glands that contracted smooth muscle and had vasodepressor activity (1–3). It would take almost three decades until prostaglandins were isolated in pure form and the structures of prostaglandins E1 and E2 and the corresponding F derivatives were elucidated in Sune Bergström's laboratory at the Karolinska Institutet (Fig. 1) (4). This was followed by intense activities in both academic and industrial laboratories to develop synthetic methods to synthesize both the parent compounds and derivatives. Studies of their biological actions led to the recognition of many potential therapeutic applications. Over several decades, a number of drugs have been approved (Table 1). These include the antiulcer drug misoprostol, dinoproston for use in obstetrics, alprostadil for acute treatment of congenital heart disease, and latanoprost, travoprost, and bimatoprost for treatment of glaucoma.
FIGURE 1.
Ulf von Euler (left) and Sune Bergström (middle) with the author at an international conference on prostaglandins held in Florence, Italy, in 1975.
TABLE 1.
Some medicines based on discoveries related to prostaglandins, thromboxanes, and leukotrienes
| Low-dose acetylsalicylic acid (for prevention of stroke and myocardial infarction) |
| Misoprostol (for prevention of gastric ulcer) |
| Celecoxib (COX-2 inhibitor for treatment of inflammation and pain) |
| Dinoproston (for obstetric use) |
| Alprostadil (for acute treatment of congenital heart defects) |
| Iloprost and treprostinil (for treatment of arterial pulmonary hypertension) |
| Latanoprost, travoprost, and bimatoprost (for treatment of glaucoma) |
| Montelukast and zafirlukast (leukotriene antagonists for treatment of asthma and rhinitis) |
| Zileuton (5-lipoxygenase inhibitor for treatment of asthma) |
In this article, I will first allude to some aspects of my early development as a scientist because it is relevant to the understanding of the science I came to pursue. However, the main part of this article deals with examples of basic research in the eicosanoid area, in my own laboratory together with many talented collaborators, that have resulted in the development of new medicines.
After we had determined the structures of the prostaglandins in 1961, as mentioned above, and I had obtained my M.D. degree, I was looking for more training in organic chemistry. Thus, I felt very fortunate when I was accepted as a postdoctoral fellow with E. J. Corey in the chemistry department at Harvard University.
I had met E. J. Corey earlier while I was a graduate student at the University of Lund. One of my first studies was concerned with the stereochemical course of the hydroxylation of cholesterol. It had been shown by Hayaishi and others that hydroxylation of steroids involves incorporation of oxygen from molecular oxygen and not by the addition of water. The question was whether there is a direct replacement of hydrogen by oxygen with retention of configuration or some other mechanism. To solve this problem, E. J. Corey, then at the University of Illinois in Urbana, synthesized stereochemically pure 7α- and 7β-tritium-labeled cholesterol. I showed that, during conversion to cholic acid (involving 7α-hydroxylation), the hydroxylation occurs by direct replacement of the hydrogen in the position that is hydroxylated. We published the work in the Journal of the American Chemical Society (5). This study had a strong impact on my future development. First and foremost, I got to know E. J. Corey, and since this joint study, we have been personal friends, exchanged ideas, and collaborated for more than fifty years. Moreover, I became interested in biochemical mechanisms and used the findings as a tool in several studies of reaction mechanisms in cholesterol and bile acid metabolism, which was the main theme of my dissertation for a Ph.D. degree.
My stay at Harvard University had a profound effect on my future research. At this time, Konrad Bloch, E. J. Corey, Paul Doty, M. S. Meselson, Frank Westheimer, Robert Woodward, and several other prominent scientists were among the faculty members of the department. It was indeed a stimulating place for a young M.D. interested in chemistry, and I probably spent more time going to lectures and seminars than doing experiments (Fig. 2).
FIGURE 2.
E. J. Corey (left) and Robert B. Woodward (right) with the author at a conference in Uppsala, Sweden.
After I had returned to the Karolinska Institutet, I decided to try to determine the structure of a prostaglandin that contained three double bonds. Degradation studies failed to give any conclusive results; however, by employing NMR spectrometry, which I had used extensively in Corey's laboratory, I was able to determine the structure (6). The third double bond of this prostaglandin (PGE3) turned out to be in the ω-3 position. We did not have an NMR instrument in the department at the time, and before I could do the experiment, I had to write a grant application and buy the instrument.
The structure of PGE3 had a decisive effect on the future direction of our own research and that of others. When the location of the third double bond had been established, it seemed almost obvious to us that polyunsaturated fatty acids were precursors of the prostaglandins. This also occurred to van Dorp's group in the Unilever Research Laboratories in The Netherlands (7, 8).
The biogenetic link between polyunsaturated fatty acids and the prostaglandins was of considerable biological interest; both the so-called essential fatty acids and the prostaglandin activity were discovered in the 1930s. With the new findings, there seemed to be many possibilities to study functional aspects of this relationship.
Discovery of Cyclooxygenase Reaction and Development of Drugs to Treat Inflammation and Pain
At this time, I had established my own laboratory at the Karolinska Institutet and decided to study the biochemical mechanisms involved in the transformation of polyunsaturated fatty acids into prostaglandins. I used the transformation of 8,11,14-eicosatrienoic acid into PGE1 as a model to limit the number of double bonds in the product. With 18O2, I could show that the oxygens of the alcohol groups originated in O2; however, because of exchange with water, the lack of 18O2 in the keto group was inconclusive. By employing short-term incubations with rapid reduction of the keto group with sodium borohydride to an alcohol group, it was possible to demonstrate that all three oxygens in the prostaglandin molecule originated in O2. With this information, I developed the hypothesis that an endoperoxide structure was involved in the transformation. I tested this idea on several of my chemistry friends, and the one who was most enthusiastic about this mechanism was Duilio Arigoni (ETH Zürich), whom I met in a smoky bar in Zürich. The problem was getting experimental evidence for the hypothesis. On the way back to Stockholm, I designed an experiment that would prove that the two oxygen atoms in the five-membered ring originated in the same molecule of oxygen. I was lucky because two experimental tools that I needed had just become available. Isotopic 18O2 of high purity (99%) could be obtained from Israel, and I had access to gas chromatography-mass spectrometry at the Karolinska Institutet. The incubation of eicosatrienoic acid with the enzyme preparation was carried out in a mixture of 18O2 and 16O2, the reduced product was converted into a trimethoxy derivative, and the side chain carrying a hydroxyl group was cleaved off by oxidation. The resulting dicarboxylic acid ester contained the two oxygens that were introduced into the five-membered ring during the biosynthesis. Analysis of this derivative by mass spectrometry showed that it had either two atoms of 18O or two atoms of 16O in the ring and that molecules with one atom of 18O and one atom of 16O were essentially absent. The experiment demonstrated that the oxygen atom of the hydroxyl group at C-11 and the keto group at C-9 originated in the same molecule of oxygen (9).
The mechanistic studies were continued in collaboration with Mats Hamberg. After having excluded introduction of oxygen at C-15 as the initial step, we concentrated on mechanisms for introduction of the two oxygen atoms in the ring. Two different mechanisms seemed possible. One involved the addition of oxygen across C-9 and C-11 with the concomitant formation of the new carbon–carbon bond between C-8 and C-12. The other mechanism consisted of a lipoxygenase-like reaction with the formation of 11-peroxy-8,12,14-eicosatrienoic acid as the initial step. In the latter pathway, a hydrogen at C-13 was presumably removed as the first step. Hence, we needed eicosatrienoic acid stereospecifically labeled with tritium at C-13. To synthesize such a compound seemed almost insurmountable; however, I recalled that Konrad Bloch had shown that Tetrahymena pyriformis introduced three double bonds into stearic acid without degradation. Using this tool, we could convert stereospecifically tritium-labeled stearic acids into 13-l- and 13-d-tritium-labeled 8,11,14-eicosatrienoic acid after chemical elongation of the C18 acids.
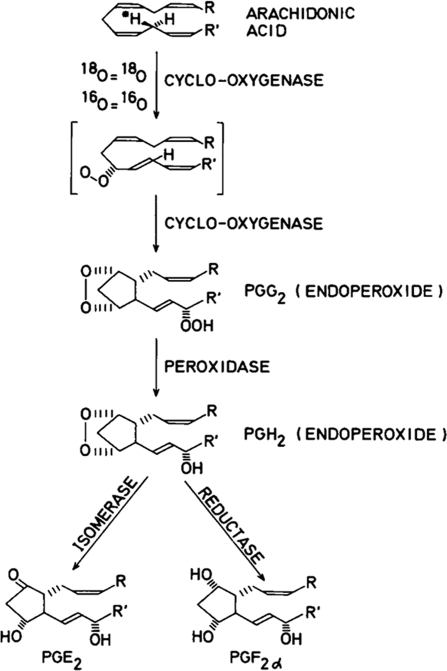
The 13-l-tritium-labeled eicosatrienoic acid was transformed into PGE1 with essentially complete loss of tritium, and the remaining precursor acid was significantly enriched with respect to tritium. This indicated that 11-peroxy-8,12,13-eicosatrienoic acid is formed in the initial step and converted into an endoperoxide by a concerted reaction involving the addition of oxygen at C-15, isomerization of a double bond, formation of a new carbon–carbon bond between C-8 and C-12, and attack by the oxygen radical at C-9 (see Fig. 3, which shows corresponding reactions for arachidonic acid). The endoperoxide is transformed into PGE1 by isomerization or into PGF1α by reductive cleavage of the peroxide. Additional experiments showing formation of 11-dehydro-PGF1α and retention of the tritium label at C-9 provided strong evidence for the existence of endoperoxide intermediates (10).
FIGURE 3.
Mechanism of prostaglandin biosynthesis.
Encouraged by these mechanistic studies, we decided to try to isolate the intermediate endoperoxides. Using an enzyme preparation from vesicular glands and short-term incubations with arachidonic acid, we were able to detect an endoperoxide. The incubation mixtures were treated with stannous chloride to reduce endoperoxide into PGF2α. This was followed by sodium borodeuteride reduction and determination of the resulting PGF2 species by multiple-ion analysis using gas chromatography-mass spectrometry. The method allowed us to analyze PGE2, PGF2α, and 11-dehydro-PGF1α. An initial burst of PGF2α, which disappeared when SnCl2 and borodeuteride reduction was omitted, indicated the formation of an endoperoxide structure. After additional studies, two endoperoxide structures could be isolated and characterized (Fig. 3) (11, 12). One compound, PGH2, had a hydroxyl group at C-15. The less polar compound, PGG2, gave PGF2α as the major product when treated with mild reducing agents such as SnCl2 and triphenylphosphine. This showed the presence of a peroxide bridge between C-9 and C-11 but did not discriminate between a hydroxyl and a peroxy group at C-15 because these agents would reduce the latter group into the former. In a second experiment, PGG2 was treated with lead tetraacetate in benzene followed by triphenylphosphine. In this case, 15-keto-PGF2 was the major product. Lead tetraacetate causes dehydration of hydroperoxides into ketones, and therefore, formation of a 15-ketoprostaglandin from PGG2 by this treatment strongly indicated the presence of a 15-peroxy group at C-15.
Two reactions are involved in the conversion of PGG2 into PGE2, viz. reduction of the hydroperoxyl group at C-15 into a hydroxyl group (peroxidase) and isomerization of the endoperoxide structure into a β-hydroxyketone (endoperoxide isomerase). We coined the name cyclooxygenase (COX) for the enzyme that catalyzes the conversion of arachidonic acid into PGG2 by oxygenation at C-11 and C-15 (12).
The cyclooxygenase was subsequently isolated and cloned. It could then be demonstrated that aspirin and other nonsteroidal anti-inflammatory drugs, which John Vane and collaborators had shown to inhibit the formation of prostaglandins from arachidonic acid in an enzyme preparation from lungs, exert their action by inhibiting the cyclooxygenase.
An interesting development occurred when it was discovered that an isoform of COX (COX-2) is induced during inflammation. The pharmaceutical industry rapidly developed COX-2 inhibitors (coxibs) because they had lower incidence of gastrointestinal side effects. Two coxibs reached the market, Vioxx and Celebra; however, Vioxx had to be taken off the market because long-term safety studies showed that it caused increased incidence of cardiovascular side effects.
Discovery of Membrane Prostaglandin E Synthase-1 and Development of Anti-inflammatory Drugs
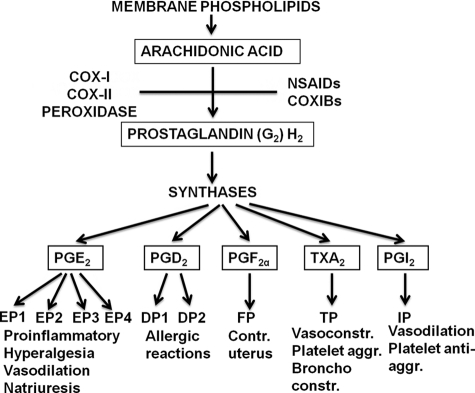
The formation of PGE2 from PGH2, formed by COX-1 or COX-2, is catalyzed by isomerases named PGE synthases (Fig. 4). In a search for enzymes catalyzing the metabolism of PGH2 and in collaboration with Per-Johan Jakobsson, we discovered that human MGST1-L1 (MGST-1-like-1) expressed in Escherichia coli displayed strong PGE2 synthase activity (13). The protein belongs to a superfamily of proteins termed MAPEG (membrane-associated proteins in eicosanoid and glutathione metabolism). This family of proteins also consists of leukotriene C4 synthase and 5-lipoxygenase-activating protein, as well as three glutathione transferases/peroxidases called microsomal glutathione transferases 1–3.
FIGURE 4.
Formation and biological effects of prostaglandins and thromboxane A2. NSAIDs, nonsteroidal anti-inflammatory drugs; TXA2, thromboxane A2.
MGST1-L1 was renamed membrane PGE synthase-1 (mPGES-1). mPGES-1 is a glutathione-dependent integral membrane protein occurring as a trimer. The thiolate ion of glutathione in a U-shaped form together with Arg-126 participate in the isomerase reaction (14, 15).
The discovery of mPGES-1 led to a rapid development of our understanding of these enzymes. Now, three different PGE synthases are known, viz. cytosolic PGE synthase (cPGES) and two membrane-bound PGE synthases, mPGES-1 and mPGES-2 (16). Of these isomerases, cPGES and mPGES-2 are constitutive enzymes, whereas mPGES-1 is mainly an induced isomerase. cPGES uses PGH2 produced by COX-1, whereas mPGES-1 uses COX-2-derived endoperoxide. mPGES-2 can use both sources of PGH2. mPGES-1 is up-regulated in response to various proinflammatory stimuli with a concomitant increased expression of COX-2. The coordinate increased expression of COX-2 and mPGES-1 is inhibited by glucocorticoids.
In inflammation, the formation of PGE2 is increased through increased arachidonic acid release and induction of COX-2. The discovery of mPGES-1 and its induction by proinflammatory cytokines explain the selective formation of PGE2 among several endoperoxide-derived products during inflammation. Inflammation, pain, fever, and cancer are pathological conditions in which disruption of the mPGES-1 gene shows beneficial effects and in which increased formation of PGE2 is linked to augmented expression of mPGES-1.
The increased cardiovascular safety observed after disruption of the mPGES-1 gene in mice compared with COX-2 inhibition has made mPGES-1 an attractive target for development of therapeutic agents for treatment of several diseases. This is actively being pursued in several pharmaceutical companies today.
Discovery of Thromboxanes Leads to Development of Low-dose Aspirin for Prevention of Heart Attacks and Strokes
When the endoperoxides had been isolated in pure form, we found that they had biological actions that were different from the prostaglandins known at that time. They contracted strips of rabbit aorta, and they caused aggregation of human platelets. Their activities were reminiscent of labile aggregating material and rabbit aorta-contracting substance (17, 18). None of these factors had been characterized chemically.
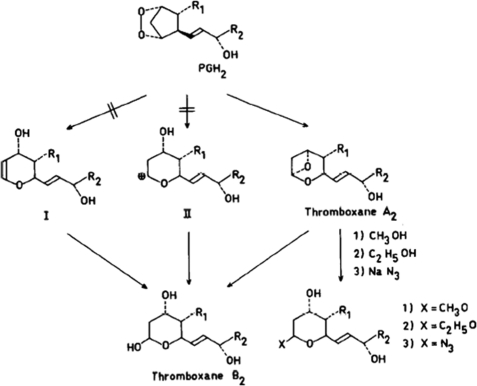
In collaboration with Mats Hamberg, we incubated labeled arachidonic acid with suspensions of human platelets and isolated and determined the structures of the three major metabolites. One was 12-l-hydroxy-5,8,10,14-eicosatetraenoic acid, a more polar metabolite was identified as 12-l-hydroxy-5,8,10-heptadecatrienoic acid, and a third component was found to be the hemiacetal derivative of 8-(1-hydroxy-3-oxopropyl)-9,12-l-dihydroxy-5,10-heptadecadienoic acid (now called thromboxane B2) (Fig. 5). Small amounts of PGE2 and PGF2α could also be detected. All of the compounds identified were stable compounds and did not cause platelet aggregation (19).
FIGURE 5.
Transformations of PGH2 into thromboxane derivatives.
Additional biological work involving characterization of the material formed from arachidonic acid during incubation with platelets was therefore carried out. An aliquot of the incubate was transferred to a suspension of platelets preincubated with indomethacin (preventing transferred arachidonic acid from being metabolized). The resulting aggregation was recorded, and the amount of endoperoxides was determined after different time intervals. This showed that the amount of endoperoxides was highest in the very early phase of the incubation period, whereas the aggregating factor had a maximum later. The half-life of the aggregating factor was ∼30 s, whereas the half-life of the endoperoxides determined in separate experiments was 4–5 min (20).
Further work involving 18O studies indicated that thromboxane B2 was formed from PGG2 by rearrangement and incorporation of one molecule of water. It was therefore conceivable that, if the rearranged intermediate had an appreciable lifetime, it should be trapped in the presence of nucleophilic reagents. This was found to be the case. The addition of 25 volumes of methanol to platelets incubated for 30 s with arachidonic acid gave rise to two epimers of thromboxane B2, methylated at the hemiacetal hydroxyl group (Fig. 5). Similarly, the addition of ethanol gave rise to the corresponding ethylated derivatives, and when 5 volumes of 5 m sodium azide was added, an azido alcohol was formed. These trapping experiments suggested the existence of a very unstable intermediate in the conversion of PGG2 to thromboxane B2. The half-life of the intermediate was determined to be 32 s. Fig. 5 shows the proposed structure of the unstable intermediate. The acetal carbon atom binding two oxygens should be susceptible to attack by nucleophiles. The addition of CH3O2H to platelets incubated with arachidonic acid led to formation of mono-O-methylthromboxane B2 lacking carbon-bound deuterium. This finding excluded an alternative structure of the unstable intermediate, i.e. an unsaturated oxane (structure I in Fig. 5). Moreover, the half-life of thromboxane A2 seemed to exclude a carbonium ion structure (structure II in Fig. 5), which, in aqueous medium, should be considerably less stable (20).
The new oxane derivatives were named thromboxanes because of their origin and structures (thrombocytes, oxane ring). Thromboxane A2 is the highly unstable bicyclic compound, and thromboxane B2 is the stable derivative. It took about ten years until the unusual structure of thromboxane A2 could be confirmed by synthesis.
Thromboxane A2 has been shown to possess a variety of strong biological effects. The best known of these are platelet aggregation and constricting effects on vascular smooth muscle. The dual effects of thromboxane A2, vasoconstriction and platelet aggregation, both come into operation after a vessel has been injured. They indicate that thromboxane A2 plays a role in normal hemostasis as well as in pathological conditions with an increased tendency to vasospasm and/or thrombosis. Thromboxane A2 also has potent contractile effects on airways.
The structures and biological effects of the endoperoxides and thromboxane A2 were presented for the first time in 1975 at a prostaglandin conference in Florence. After my talk, John Vane (then at Wellcome Research Laboratories) asked if he could have some endoperoxide because he was interested in developing inhibitors of the thromboxane synthase for use as antithrombotic agents (Fig. 6). I sent some endoperoxide; however, when he incubated the material with an arterial preparation, he found an arterial relaxing factor instead of the expected contractile factor. The chemical structure of the factor was determined in collaboration with chemists at The Upjohn Company in the United States and was called prostacyclin. It had vasodilating and antiplatelet aggregating properties (21).
FIGURE 6.
John Vane with the author at an international conference on prostaglandins held in 1975 in Florence, Italy.
Prostacyclin Analogs (e.g. Iloprost and Treprostinil) Are Now Used for Treatment of Arterial Pulmonary Hypertension
Thromboxane A2 and prostacyclin probably form a homeostatic mechanism for control of the tonus of blood vessels and the aggregation of platelets in vivo. The discovery of thromboxane A2 stimulated many laboratories to develop therapeutic agents that inhibit thromboxane A2 formation for use as antithrombotic therapies. A breakthrough came when it was discovered that low-dose aspirin (75 mg/day) inhibits the formation of thromboxane A2 because of irreversible inhibition of the platelet cyclooxygenase (COX-1) by acetylation of the enzyme and the absence of resynthesis of enzyme during the lifetime of the platelet (41). This therapeutic regimen is used by millions of people to prevent heart attacks and strokes.
Discovery of Leukotrienes Leads to Development of Drugs to Treat Asthma and Rhinitis
At the time I was planning the following experiments, it had been proposed that anti-inflammatory steroids partly exert their action by inhibiting the release of arachidonic acid from phospholipids. Because nonsteroidal anti-inflammatory drugs and steroids have different anti-inflammatory effects, it seemed conceivable that some of these differences might be due to the formation of additional proinflammatory derivatives of arachidonic acid. I therefore decided to study the transformation of arachidonic acid in leukocytes in collaboration with a newly arrived Canadian postdoctoral fellow, Pierre Borgeat. These studies resulted in the discovery of a novel group of compounds, the leukotrienes. These compounds are of importance in immediate hypersensitivity reactions such as asthma and in inflammation.
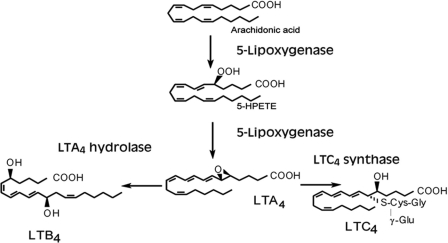
When arachidonic acid was incubated with polymorphonuclear leukocytes, it was found that the major metabolite was a new lipoxygenase product, viz. (5S)-hydroxy-6,8,11,14-eicosatetraenoic acid. Additional experiments led to the identification of (5S,12R)-dihydroxy-6,8,10,14-eicosatetraenoic acid (now leukotriene B4) (Fig. 7), two additional (5S,12R)-dihydroxyeicosatetraenoic acids (epimeric at C-12), and two isomeric 5,6-dihydroxyeicosatetraenoic acids (22–24).
FIGURE 7.
Enzymes and metabolites in leukotriene biosynthesis. 5-HPETE, 5-hydroperoxyeicosatetraenoic acid.
Stereochemical studies showing formation of two acids with all-trans-conjugated trienes and epimeric at C-12 and one major isomer (12R) with a different configuration of the triene raised the question of the mechanism of formation. With isotopic oxygen, it was shown that the oxygen of the alcohol group at C-5 originated in molecular oxygen, whereas the oxygen at C-12 was derived from water. These observations led us to develop the hypothesis that leukocytes generated an unstable intermediate that could undergo nucleophilic attack by water, alcohols, and other nucleophiles. The half-life of the unstable intermediate was calculated to be 3–4 min. On the basis of these and additional experiments, the structure 5,6-oxido-7,9,11,14-eicosatetraenoic acid (leukotriene A4 (LTA4)) (Fig. 7) was proposed for the intermediate (25). This structure was subsequently confirmed by chemical synthesis, and the stereochemistry was elucidated in collaboration with E. J. Corey and his associates (26).
5-Lipoxygenase
The enzyme responsible for the formation of the unstable intermediate (LTA4) is 5-lipoxygenase (5-LO). It catalyzes both the initial oxygenation at C-5, forming a 5-hydroperoxy derivative, and the second step involving formation of LTA4. Human 5-LO is a monomeric enzyme with 673 amino acids. It consists of an N-terminal β-sandwich and a C-terminal catalytic domain. The catalytic domain, composed of several α-helices, binds the prosthetic iron. In the lipoxygenase reaction, the iron acts as an electron acceptor and donor during hydrogen abstraction and peroxide formation. Residues in the β-sandwich bind calcium, cellular membranes, and coactosin-like protein (CLP). Most of the studies on 5-LO have been carried out in collaboration with Olof Rådmark (27).
The enzymatic activity of 5-LO from human leukocytes depends on microsomal membranes or synthetic phosphatidylcholine vesicles. Using the yeast two-hybrid system, we found that human CLP binds to 5-LO with a 1:1 binding stoichiometry. CLP is a member of the ADF/cofilin group of actin-binding proteins. Both phosphatidylcholine or membranes and CLP are required for efficient LTA4 production in vitro (28).
Upon cell stimulation, cytosolic phospholipase A2, 5-LO, and CLP translocate to the nuclear membrane, where cytosolic phospholipase A2 releases arachidonic acid from phospholipids, and it has been proposed that 5-LO-activating protein (FLAP) assists in the transfer of the precursor to 5-LO.
5-LO can be phosphorylated at three residues: Ser-271 by MAPK-activated protein kinases, Ser-663 by ERK2, and Ser-523 by protein kinase A (PKA). Phosphorylations at Ser-271 and Ser-663 are stimulatory, whereas phosphorylation at Ser-523 by PKA suppresses the activity of 5-LO. The phosphorylation by PKA provides a molecular basis for the suppressive effects of exogenous adenosine and increased cAMP, which activate PKA.
Because of the pivotal role of 5-LO in the formation of leukotrienes, 5-LO inhibitors have been developed as therapeutics. Zileuton is used for treatment of asthma (see “Slow-reacting Substance of Anaphylaxis (SRS-A)”). FLAP has a critical role at the nuclear membrane in the synthesis of LTA4, and FLAP inhibitors are currently in clinical development for treatment of respiratory and atherosclerotic diseases. The discovery of the role of CLP in the formation of LTA4 provides yet another target to develop therapeutics based on inhibition of leukotrienes. Thus, it has been found that hyperforin (from St. John's wort) interferes with the binding of 5-LO to CLP and inhibits the formation of leukotrienes.
Leukotriene B4
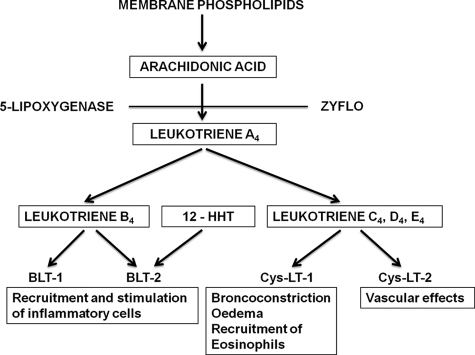
LTB4 is a potent chemoattractant through BLT1 (B leukotriene receptor 1), which also displays leukocyte-activating functions (Fig. 8). It mediates the recruitment of neutrophils, mast cells, monocytes or macrophages, and T cells. LTB4 has been suggested to play a role in sepsis, chronic obstructive pulmonary disease (COPD), cystic fibrosis, asthma, pulmonary emphysema, and other inflammatory diseases. LTB4 also stimulates cancer cell proliferation.
FIGURE 8.
Formation and biological effects of leukotrienes. 12-HHT, 12-l-hydroxy-5,8,10-heptadecatrienoic acid; Cys-LT, cysteinyl leukotriene.
LTB4 is formed from the unstable epoxide intermediate, LTA4, by hydrolysis involving introduction of a hydroxyl group at C-12. The enzyme catalyzing this reaction (LTA4 hydrolase (LTA4H)) is a soluble monomeric enzyme. After the amino acid sequence of the enzyme had been determined, we recognized a consensus zinc-binding motif (His-295, His-299, and Glu-318) and, in collaboration with Bert Vallee's group at Harvard Medical School, demonstrated peptidase activity and found it to be a member of a family of zinc-containing aminopeptidases (29, 30). The zinc atom is required for both the epoxide hydrolase activity and the aminopeptidase activity, whereas Asp-375 is required for the former reaction and Glu-296 and chloride ions for the latter reaction. The crystal structure of LTA4H and the detailed enzymatic mechanisms have subsequently been reported by Haeggström et al. (31).
What seems to be the natural peptide substrate for LTA4H has recently been reported (32). A tripeptide, proline-glycine-proline (PGP), generated from collagen, is degraded by LTA4H. PGP is a potent chemoattractant for neutrophils. Thus, LTA4H has opposing proinflammatory (LTB4 formation) and anti-inflammatory (PGP degradation) effects. Neutrophils and lung epithelial cells can release extracellular LTA4H, which can degrade PGP. This mechanism has been suggested to resolve neutrophilic inflammation and limit tissue damage. Neutrophilia is associated with persistent pulmonary inflammation such as COPD, cystic fibrosis, and severe asthma. It is of interest in this context that cigarette smoke, which is a risk factor in the development of COPD, modified PGP by acetylation into acetyl-PGP. This modification increased the potency of the peptide to recruit neutrophils and also protected it from degradation by LTA4H. The cigarette smoke selectively inhibited the peptidase activity of LTA4H responsible for PGP degradation, whereas the epoxide activity of LTA4H that generates LTB4 was essentially intact. Cigarette smoke thus seems to transform LTA4H into a phenotype in which LTB4 and PGP can persistently drive the inflammation without resolution, as in COPD. Our finding that chloride ions stimulate the peptidase activity of LTA4H is of particular interest in relation to cystic fibrosis, in which there is impaired chloride ion transport and increased concentrations of PGP/acetyl-PGP.
Inhibitors of LTA4H are being developed for treatment of diseases in which LTB4 seems to play a role: these include inflammatory pulmonary diseases and atherosclerosis. With our current knowledge of the role of the two enzymatic activities of LTA4H, it seems highly attractive to develop potential therapeutic agents that selectively inhibit the epoxide hydrolase activity, leaving the aminopeptidase activity intact.
Slow-reacting Substance of Anaphylaxis (SRS-A)
The occurrence of a smooth muscle-stimulating factor (SRS) appearing in the perfusate of guinea pig lung treated with cobra venom was described in 1938 (33). The factor was shown to be released also by immunological challenge. Biological studies of SRS suggested that it might be an important mediator of anaphylactic and other immediate hypersensitivity reactions. Characterization of SRS indicated that it was a low molecular weight acidic molecule (34) with UV absorption (35). Studies with labeled arachidonic acid suggested that it might be incorporated into SRS (36).
Studies in our laboratory showed that treatment of human neutrophils with the ionophore A23187 stimulated the synthesis of the 5,12-dihydroxy acid (LTB4) described above (24). On the basis of the stimulatory effect of the ionophore on both SRS production and LTB4 formation, the UV absorbance data, and other considerations, I developed the hypothesis that there was a biogenetic relationship between the unstable allylic epoxide intermediate involved in LTB4 formation in neutrophils and SRS generated in a variety of systems. I was fortunate in having two very able collaborators for the project: Sven Hammarström from our laboratory, who had extensive experience in eicosanoid research, and Robert C. Murphy, a visiting scientist on sabbatical leave who had a strong interest in analytical methods, especially mass spectrometry.
We found a simpler system for generation of SRS than those employed before. A murine mastocytoma cell line (CXBGABMCT-1) produced substantial amounts of SRS when stimulated with the calcium ionophore A23187 and was suitable for studies of the incorporation of tentative precursors. The purified SRS showed UV absorption characteristics resembling those of the dihydroxy acids we had found in neutrophils; however; the maximum was shifted 10 nm to a higher wavelength. This was in agreement with a sulfur substituent α to a conjugated triene. Labeled arachidonic acid and cysteine were incorporated in the product.
Degradation of SRS by Raney nickel desulfurization gave 5-hydroxyarachidic acid, indicating that the arachidonic acid derivative and cysteine were linked by a thioether bond. This finding supported the hypothesis that there was a biogenetic relationship between the 5-LO pathway in leukocytes and SRS. Derivatization of cysteine was suggested by the failure to isolate alanine after desulfurization. Additional studies demonstrated that the structure of SRS from the mastocytoma cells was 5-hydroxy-(6S)-glutathionyl-7,9,11,14-eicosatetraenoic acid (Fig. 7) (37, 38). Its structure was confirmed, and the detailed stereochemical features were determined in collaboration with E. J. Corey and co-workers by comparing various isomers with our biological material (39). Because of the cumbersome systematic names of the compounds involved and the biological significance of the biosynthetic pathway, we introduced the term leukotriene. This was based on the same principle as thromboxane and was chosen because the compounds were discovered in leukocytes, and the common structural feature is a conjugated triene (Fig. 7).
LTC4 is metabolized to LTD4 by enzymatic elimination of glutamic acid by γ-glutamyl transpeptidase. The remaining peptide bond in LTD4 can be hydrolyzed by a renal dipeptidase to give LTE4. SRS-A is a mixture of leukotrienes containing cysteine (usually referred to as cysteinyl leukotrienes), viz. LTC4, LTD4, and LTE4. The cysteinyl leukotrienes are potent bronchoconstrictors and cause mucous hypersecretion, increased vascular permeability, and eosinophilic inflammation, i.e. the cardinal symptoms of bronchial asthma (Fig. 8).
Our discovery of the leukotrienes was presented at the Fourth International Prostaglandin Conference held in 1979 in Washington, D.C. Within days, Merck Sharp & Dohme Corporation initiated a program to develop antileukotrienes for treatment of asthma. This led to the development of montelukast, which is used today by millions of patients with asthma to relieve their symptoms and improve lung function. It is also used to treat allergic rhinitis. Other leukotriene-based therapeutics to treat asthma are zafirlukast, also a leukotriene antagonist, and zileuton, which inhibits the enzyme 5-LO, a pivotal enzyme in the biosynthesis of the leukotrienes.
Lipoxins
Another group of arachidonic acid derivatives, the lipoxins, formed by the action of 5-LO was discovered in collaboration with C. N. Serhan and Mats Hamberg (40). They are formed by the additional oxygenation by a 15-LO. Their biosynthesis can be initiated by either 5- or 15-LO. Both lipoxins A4 and B4 are conjugated tetraenes with alcohol groups at positions 5, 6, and 15 and at positions 5, 14, and 15, respectively. The lipoxins are formed by the interaction of several cell types.
Lipoxin A4 is a potent endogenous anti-inflammatory derivative that activates the G-protein-coupled receptor ALX/FPR2. It has been proposed to limit inflammatory reactions and promote resolution of inflammation. Analogs of the lipoxins are currently being developed as anti-inflammatory therapeutics.
Concluding Comments
The examples I have given of the relation between discoveries in basic research and the development of medicines for prevention or treatment of various diseases illustrate the power of research that is not targeted to a specific disease but rather focuses on understanding the structures and functions of the molecules constituting the human body. The new information can be used to understand pathophysiological mechanisms and to provide novel targets for therapeutic interventions.
Acknowledgments
Unfortunately, I have been able to mention only a few collaborators; however, I would like to acknowledge the important contributions of many postdoctoral fellows and graduate students to the work described in this article.
REFERENCES
- 1. Kurzrok R., Lieb C. C. (1930) Biochemical studies on human semen. II. The action of semen on the human uterus. Exp. Biol. Med. 28, 268–272 [Google Scholar]
- 2. Goldblatt M. W. (1933) A depressor substance in seminal fluid. J. Soc. Chem. Ind. 52, 1056–1057 [Google Scholar]
- 3. von Euler U. S. (1934) Zur kenntnis der pharmakologischen wirkungen von nativsekreten und extrakten männlicher accessorischer geschlechtsdrüsen. Arch. Exp. Pathol. Pharmakol. 175, 78–84 [Google Scholar]
- 4. Bergström S., Samuelsson B. (1965) Prostaglandins. Annu. Rev. Biochem. 34, 101–108 [DOI] [PubMed] [Google Scholar]
- 5. Bergström S., Lindstedt S., Samuelsson B., Corey E. J., Gregoriou G. A. (1958) The stereochemistry of 7α-hydroxylation in the biosynthesis of cholic acid from cholesterol. J. Am. Chem. Soc. 80, 2337–2338 [Google Scholar]
- 6. Samuelsson B. (1963) Prostaglandins and related factors: the structure of prostaglandin E3. J. Am. Chem. Soc. 85, 1878–1879 [Google Scholar]
- 7. Bergström S., Danielsson H., Samuelsson B. (1964) The enzymatic formation of prostaglandin E2 from arachidonic acid prostaglandins and related factors 32. Biochim. Biophys. Acta 90, 207–210 [DOI] [PubMed] [Google Scholar]
- 8. van Dorp D. A., Beerthuis R. K., Nugteren D. H., Vonkeman H. (1964) The biosynthesis of prostaglandins. Biochim. Biophys. Acta 90, 204–207 [DOI] [PubMed] [Google Scholar]
- 9. Samuelsson B. (1965) On the incorporation of oxygen in the conversion of 8,11,14-eicosatrienoic acid to prostaglandin E1. J. Am. Chem. Soc. 87, 3011–3013 [DOI] [PubMed] [Google Scholar]
- 10. Hamberg M., Samuelsson B. (1967) Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J. Biol. Chem. 242, 5344–5354 [PubMed] [Google Scholar]
- 11. Hamberg M., Samuelsson B. (1973) Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 70, 899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. (1974) Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc. Natl. Acad. Sci. U.S.A. 71, 345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jakobsson P. J, Thorén S., Morgenstern R., Samuelsson B. (1999) Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. U.S.A. 96, 7220–7225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hammarberg T., Hamberg M., Wetterholm A., Hansson H., Samuelsson B., Haeggström J. Z. (2009) Mutation of a critical arginine in microsomal prostaglandin E synthase-1 shifts the isomerase activity to a reductase activity that converts prostaglandin H2 into prostaglandin F2α. J. Biol. Chem. 284, 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jegerschöld C., Pawelzik S. C., Purhonen P., Bhakat P., Gheorghe K. R., Gyobu N., Mitsuoka K., Morgenstern R., Jakobsson P. J., Hebert H. (2008) Structural basis for induced formation of the inflammatory mediator prostaglandin E2. Proc. Natl. Acad. Sci. U.S.A. 105, 11110–11115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samuelsson B., Morgenstern R., Jakobsson P. J. (2007) Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol. Rev. 59, 207–224 [DOI] [PubMed] [Google Scholar]
- 17. Willis A. L. (1974) An enzymatic mechanism for the antithrombotic and antihemostatic actions of aspirin. Science 183, 325–327 [DOI] [PubMed] [Google Scholar]
- 18. Piper P. J., Vane J. R. (1969) Release of additional factors in anaphylaxis and its antagonism by anti-inflammatory drugs. Nature 223, 29–35 [DOI] [PubMed] [Google Scholar]
- 19. Hamberg M., Samuelsson B. (1974) Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc. Natl. Acad. Sci. U.S.A. 71, 3400–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamberg M., Svensson J., Samuelsson B. (1975) Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc. Natl. Acad. Sci. U.S.A. 72, 2994–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whittaker N., Bunting S., Salmon J., Moncada S., Vane J. R., Johnson R. A., Morton D. R., Kinner J. H., Gorman R. R., McGuire J. C., Sun F. F. (1976) The chemical structure of prostaglandin X (prostacyclin). Prostaglandins 12, 915–928 [DOI] [PubMed] [Google Scholar]
- 22. Borgeat P., Hamberg M., Samuelsson B. (1976) Transformation of arachidonic acid and homo-γ-linolenic acid by rabbit polymorphonuclear leukocytes. Monohydroxy acids from novel lipoxygenases. J. Biol. Chem. 251, 7816–7820 [PubMed] [Google Scholar]
- 23. Borgeat P., Samuelsson B. (1979) Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid. J. Biol. Chem. 254, 2643–2646 [PubMed] [Google Scholar]
- 24. Borgeat P., Samuelsson B. (1979) Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J. Biol. Chem. 254, 7865–7869 [PubMed] [Google Scholar]
- 25. Borgeat P., Samuelsson B. (1979) Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc. Natl. Acad. Sci. U.S.A. 76, 3213–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rådmark O., Malmsten C., Samuelsson B., Goto G., Marfat A., Corey E. J. (1980) Leukotriene A. Isolation from human polymorphonuclear leukocytes. J. Biol. Chem. 255, 11828–11831 [PubMed] [Google Scholar]
- 27. Rådmark O., Samuelsson B. (2010) Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem. Biophys. Res. Commun. 396, 105–110 [DOI] [PubMed] [Google Scholar]
- 28. Esser J., Rakonjac M., Hofmann B., Fischer L., Provost P., Schneider G., Steinhilber D., Samuelsson B., Rådmark O. (2010) Coactosin-like protein functions as a stabilizing chaperone for 5-lipoxygenase: role of tryptophan 102. Biochem. J. 425, 265–274 [DOI] [PubMed] [Google Scholar]
- 29. Funk C. D., Rådmark O., Fu J. Y., Matsumoto T., Jörnvall H., Shimizu T., Samuelsson B. (1987) Molecular cloning and amino acid sequence of leukotriene A4 hydrolase. Proc. Natl. Acad. Sci. U.S.A. 84, 6677–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haeggström J. Z., Wetterholm A., Vallee B. L., Samuelsson B. (1990) Leukotriene A4 hydrolase: an epoxide hydrolase with peptidase activity. Biochem. Biophys. Res. Commun. 173, 431–437 [DOI] [PubMed] [Google Scholar]
- 31. Haeggström J. Z., Tholander F., Wetterholm A. (2007) Structure and catalytic mechanisms of leukotriene A4 hydrolase. Prostaglandins Other Lipid Mediat. 83, 198–202 [DOI] [PubMed] [Google Scholar]
- 32. Snelgrove R. J. (2011) Leukotriene A4 hydrolase: an anti-inflammatory role for a proinflammatory enzyme. Thorax 66, 550–551 [DOI] [PubMed] [Google Scholar]
- 33. Feldberg W., Kellaway C. H. (1938) Liberation of histamine and formation of lysocithin-like substances by cobra venom. J. Physiol. 94, 187–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Orange R. P., Murphy R. C., Karnovsky M. L., Austen K. F. (1973) The physicochemical characteristics and purification of slow-reacting substance of anaphylaxis. J. Immunol. 110, 760–770 [PubMed] [Google Scholar]
- 35. Morris H. R., Taylor G. W., Piper P. J., Sirois P., Tippins J. R. (1978) Slow-reacting substance of anaphylaxis. Purification and characterization. FEBS Lett. 87, 203–206 [DOI] [PubMed] [Google Scholar]
- 36. Jakschik B. A., Falkenhein S., Parker C. W. (1977) Precursor role of arachidonic acid in release of slow-reacting substance from rat basophilic leukemia cells. Proc. Natl. Acad. Sci. U.S.A. 74, 4577–4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy R. C., Hammarström S., Samuelsson B. (1979) Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc. Natl. Acad. Sci. U.S.A. 76, 4275–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Samuelsson B., Borgeat P., Hammarström S., Murphy R. C. (1979) Introduction of a nomenclature: leukotrienes. Prostaglandins 17, 785–787 [DOI] [PubMed] [Google Scholar]
- 39. Hammarström S., Samuelsson B., Clark D. A., Goto G., Marfat A., Mioskowski C., Corey E. J. (1980) Stereochemistry of leukotriene C1. Biochem. Biophys. Res. Commun. 92, 946–953 [DOI] [PubMed] [Google Scholar]
- 40. Serhan C. N., Hamberg M., Samuelsson B. (1984) Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. U.S.A. 81, 5335–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patrono C., Coller B., Dalen J. E., FitzGerald G. A., Fuster V., Gent M., Hirsh J., Roth G. (2001) Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest 119, 39S–63S [DOI] [PubMed] [Google Scholar]