Background: It is expected that the transport pathway of CFTR should alternate between internally and externally accessible conformations.
Results: Access to the channel from both sides of the membrane is regulated by opening and closing.
Conclusion: The open state is outwardly facing, the closed state inwardly facing.
Significance: These findings prompt reevaluation of prevailing models of CFTR conformational changes.
Keywords: ABC Transporter, Anion Transport, CFTR, Chloride Channels, Ion Channels, Ion Channel Structure and Function
Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel is a member of the ATP-binding cassette (ABC) protein family, most members of which act as active transporters. Actively transporting ABC proteins are thought to alternate between “outwardly facing” and “inwardly facing” conformations of the transmembrane substrate pathway. In CFTR, it is assumed that the outwardly facing conformation corresponds to the channel open state, based on homology with other ABC proteins. We have used patch clamp recording to quantify the rate of access of cysteine-reactive probes to cysteines introduced into two different transmembrane regions of CFTR from both the intracellular and extracellular solutions. Two probes, the large [2-sulfonatoethyl]methanethiosulfonate (MTSES) molecule and permeant Au(CN)2− ions, were applied to either side of the membrane to modify cysteines substituted for Leu-102 (first transmembrane region) and Thr-338 (sixth transmembrane region). Channel opening and closing were altered by mutations in the nucleotide binding domains of the channel. We find that, for both MTSES and Au(CN)2−, access to these two cysteines from the cytoplasmic side is faster in open channels, whereas access to these same sites from the extracellular side is faster in closed channels. These results are consistent with alternating access to the transmembrane regions, however with the open state facing inwardly and the closed state facing outwardly. Our findings therefore prompt revision of current CFTR structural and mechanistic models, as well as having broader implications for transport mechanisms in all ABC proteins. Our results also suggest possible locations of both functional and dysfunctional (“vestigial”) gates within the CFTR permeation pathway.
Introduction
ATP-binding cassette (ABC)3 transporters form a large group of membrane proteins that couple ATP hydrolysis to the movement of a diverse range of substrates across the cell membrane. ABC transporters share a common minimal architecture, with two cytoplasmic nucleotide binding domains (NBDs) that hydrolyze ATP and two transmembrane domains (TMDs) that come together to form the substrate translocation pathway. As is conventional in active transporter models (1), it is assumed that ABC proteins function by an “alternating access” mechanism in which the transmembrane transport pathway transitions between “inward facing” and “outward facing” conformations, alternately exposing the transported substrate to the intracellular and extracellular solution respectively (2–4). Indeed, the structures of various prokaryotic ABC proteins revealed by x-ray crystallography have identified both inward facing (closed to the extracellular side) and outward facing (closed to the intracellular side) orientations of the TMDs (2–4). Generally speaking, the ATP-bound state of ABC proteins is thought to be the outward facing and the nucleotide-free state inward facing, suggesting that ATP binding drives the conformational switch in TMD orientation, although this speculation is subject to concerns relating to dynamic protein flexibility, substrate dependence, structural and possibly functional diversity between different transporters, and incomplete understanding of transport mechanisms (2–4).
The alternating access model can also be formulated in terms of “gates” in the transport pathway that can be either permissive (open) or prohibitive (closed) to substrate translocation (5). To achieve active transport, a transporter protein must have at least two gates in the transport pathway that are never both open simultaneously. Instead, the gates open and close alternately, so that one state (inner gate open, outer gate closed) corresponds to the “inward facing” conformation, and the other (inner gate closed, outer gate open) corresponds to “outward facing.” Such a gating formulism emphasizes local, rather than global, structural rearrangements in the transport pathway. However, the location of such gates has not been identified in ABC proteins.
The black sheep of the ABC protein family is the cystic fibrosis transmembrane conductance regulator (CFTR; ABCC7), the protein that is mutated in cystic fibrosis (6). Although CFTR shares the general architecture of other ABC proteins, it functions not as an active transporter but as an ion channel, in which ATP binding and hydrolysis at the NBDs lead to the opening of an ion channel pore formed by the TMDs that mediates the rapid electrodiffusional transport of Cl− and other small anions (6, 7). Ion channels are thought to differ from active transporters in that they have only a single functional gate in the transport pathway, allowing the channel to switch rapidly between “open” (conductive) and “closed” (nonconductive) conformations (5). This has led to the suggestion that CFTR is a “broken pump” that evolved from an actively transporting ABC ancestor due to dysfunction of one of its gates (5, 8).
The structure of the TMDs in CFTR has been observed directly only at low resolution (9–11). The structure of the entire CFTR protein has been modeled at the atomic level using bacterial ABC proteins as templates (12–15), although these proteins show the lowest sequence identity to CFTR (10–15%) in the TMDs and presumably also show low functional similarity as they are active transporters and not ion channels. CFTR has been modeled in the outward facing conformation using the bacterial ABC protein Sav1866, a multidrug efflux transporter, as a template (12–14), and in the inward facing conformation using another bacterial ABC protein template, the putative lipid translocase MsbA (15). Based on the nucleotide binding status of the bacterial templates, it was suggested that the outward facing model of CFTR represents the channel open state, and the inward facing model the closed state (15). Despite a lack of functional evidence and some major inconsistencies with longstanding functional data (see “Discussion”), the idea that CFTR TMDs are outward facing when the channel is open and inward facing when it is closed has been incorporated into recent work on CFTR channel gating (16) and reiterated in reviews of CFTR channel function (5, 8). This model has also led to the suggestion that it is the inner (cytoplasmic) gate of CFTR that has been lost functionally (5, 8).
Previously we identified three sites in the sixth transmembrane α-helix (TM6) at which introduced cysteine residues could be modified by cysteine-reactive methanethiosulfonate (MTS) reagents applied to either the intracellular or extracellular sides of the membrane, even though these reagents are not permeant in CFTR (17). We suggested that conformational changes in the pore might allow these sites (Phe-337, Thr-338, Ser-341) to be alternately accessible to different sides of the membrane (17). Interestingly, cysteines substituted into another pore-lining TM, TM1, did not show access to both sides of the membrane, with the outermost site in this TM that could be modified by intracellular MTS reagents (L102C) being insensitive to extracellular MTS reagents (18). In the present work, we have compared changes in the accessibility of T338C in TM6 with L102C in TM1 to both intracellular and extracellular cysteine-reactive reagents, both large, impermeant [2-sulfonatoethyl] MTS (MTSES) and smaller, permeant Au(CN)2− ions, under conditions in which ATP-dependent channel gating is altered. Our results suggest that sites within the CFTR pore show alternate access to the two sides of the membrane in open and closed channels that is unexpected from current structural models of the pore.
EXPERIMENTAL PROCEDURES
Experiments were carried out on baby hamster kidney cells transiently transfected with CFTR. In this study we have used a human CFTR variant in which all cysteines had been removed by mutagenesis (as described in Ref. 19), and which includes a mutation in the first NBD (V510A) to increase protein expression in the cell membrane (20). This Cys-less variant, which we have used in previous studies of substituted cysteine accessibility inside the pore (17, 18, 21, 22), has channel pore properties very similar to those of wild-type CFTR (23). Additional mutations were introduced into the Cys-less background using the QuikChange site-directed mutagenesis system (Agilent Technologies, Santa Clara, CA) and verified by DNA sequencing. Two reporter cysteines in the pore were studied: T338C in TM6, which is modified by both intracellular and extracellular MTS reagents (17), and L102C in TM1, which is modified by intracellular, but not extracellular MTS reagents (18). Based on their locations and relative sensitivities to intracellular and extracellular MTS reagents, we hypothesized that these two residues might be located closest to the “boundary” between externally and internally accessible regions of the pore within these two important TMs, and as such the most sensitive reporters of changes in pore orientation during channel gating. These two reporter cysteine substitutions were combined with mutations in the NBDs that affect ATP-dependent channel gating: K464A (NBD1) and E1371Q (NBD2). As discussed in detail in our recent study (22), these NBD mutations are expected either to decrease (K464A) or increase (E1371Q) overall CFTR channel activity via well characterized effects on ATP-dependent channel gating.
Modification of individual cysteine residues introduced into Cys-less CFTR by cysteine-reactive reagents was monitored as a change in macroscopic current amplitude carried by CFTR channels using patch clamp recording. To allow the rate of modification on application of cysteine-reactive reagents to either the intracellular or extracellular face of the membrane to be compared, modification by intracellular reagents was studied using excised, inside-out membrane patches, whereas modification by extracellular reagents was studied in intact cells using whole cell current recording. Inside-out patch experiments were carried out exactly as described in our recent study (22). Following patch excision and recording of background currents (where appropriate), CFTR channels were activated by exposure to protein kinase A (PKA) catalytic subunit (20 nm) plus MgATP (1 mm) in the cytoplasmic solution. In some experiments, channels bearing the NBD2 mutation E1371Q were used. This mutation results in constitutive, high levels of activity when expressed in baby hamster kidney cells (24). Both intracellular (bath) and extracellular (pipette) solutions contained 150 mm NaCl, 2 mm MgCl2, 10 mm TES, pH 7.4. Following channel activation, CFTR macroscopic current amplitude was monitored during brief voltage deflections (to ±50 mV) from a holding potential of 0 mV applied every 6 s (see supplemental Fig. S1).
Conventional whole cell patch clamp recordings were made by rupturing the membrane patch following seal formation. Bath (extracellular) solution contained 145 mm NaCl, 15 mm sodium glutamate, 4.5 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 10 mm HEPES, 5 mm glucose, pH 7.4, and pipette (intracellular) solution contained 139 mm CsCl, 2 mm MgCl2, 5 mm EGTA, 10 mm HEPES, 5 mm glucose, 1 mm ATP, 0.1 mm GTP, pH 7.2. Patch pipettes had a resistance of 4–8 megohms when filled with these solutions. Overall calculated Cl− concentrations were 155.5 mm intracellular and 143 mm extracellular. Whole cell currents were monitored continuously at a membrane potential of +30 mV. Following attainment of the whole cell configuration and recording of stable base-line currents, CFTR channels were activated by extracellular application of a cyclic AMP stimulatory mixture containing 10 μm forskolin, 100 μm 3-isobutyl-1-methylxanthine, and 100 μm 8-(4-chlorophenylthio) cyclic AMP. At the end of the experiment, remaining currents were confirmed as being carried by CFTR by their sensitivity to the specific CFTR inhibitor GlyH-101. As described above, channels bearing the E1371Q mutation were constitutively active, and whole cell currents carried by such channels were not further increased in amplitude by application of cAMP mixture, although they were sensitive to GlyH-101 (see supplemental Fig. S2).
The rate of modification of introduced cysteine residues by two cysteine-reactive reagents, MTSES and Au(CN)2−, was quantified as described recently (22). For both intracellular and extracellular application, the time-dependent change in current amplitude following addition of MTSES or Au(CN)2− was fitted by a single exponential function. The measured exponential time constant (τ) was then used to calculate the apparent second order reaction rate constant, k, from the equation k = 1/([R] τ), where [R] is the concentration of reagent (MTSES or Au(CN)2−) applied. Cys-less CFTR is not sensitive to either intracellular or extracellular MTSES or Au(CN)2− under the conditions used in this study (see Fig. 3) (14, 17, 20, 22).
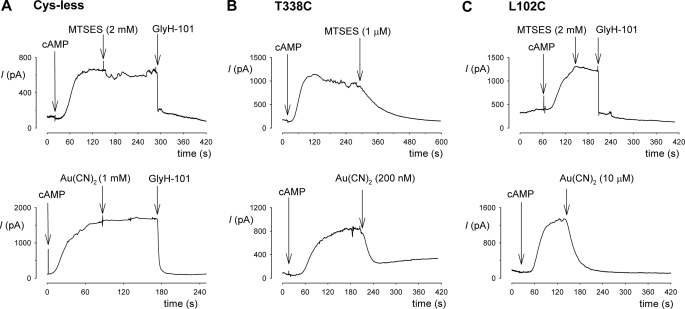
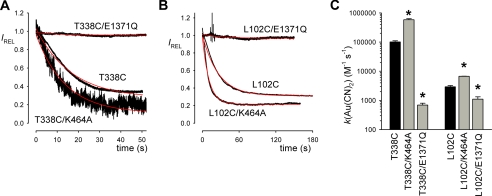
FIGURE 3.
Modification by external MTSES and Au(CN)2−. Sample whole cell currents were recorded at +30 mV for Cys-less (A), T338C (B), and L102C (C). CFTR currents were activated by application of cAMP stimulatory mixture (indicated as cAMP) in each case. A, Cys-less CFTR currents were insensitive to high concentrations of MTSES (2 mm) and Au(CN)2− (1 mm) but were confirmed as being carried by CFTR by their sensitivity to GlyH-101 (50 μm). B, T338C currents were inhibited by low concentrations of MTSES (1 μm) or Au(CN)2− (200 nm). C, L102C currents were insensitive to MTSES (2 mm) but were inhibited by GlyH-101 (50 μm) and Au(CN)2− (10 μm). All substances were added directly to the extracellular side of the membrane.
Experiments were carried out at room temperature, 21–24 °C. Values are presented as mean ± S.E. Unless stated otherwise, tests of significance were carried out using an unpaired t test, with p < 0.05 being considered statistically significant. All chemicals were from Sigma-Aldrich, except for PKA (Promega), MTSES (Toronto Research Chemicals, North York, ON, Canada), and GlyH-101 (EMD Chemicals, Gibbstown, NJ).
RESULTS
Regulated Access from Cytoplasm to Pore
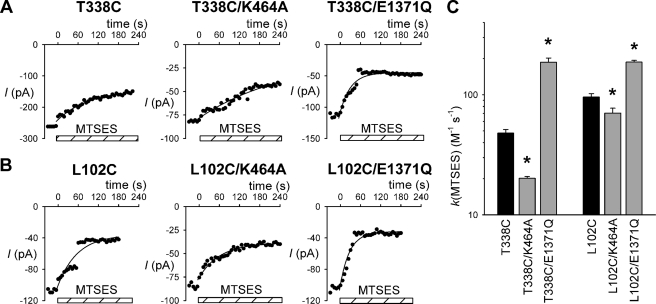
Recently we have used pharmacological and mutagenic approaches to correlate the rate of modification of cysteine residues introduced into the CFTR channel pore by intracellular MTS reagents with ATP-dependent channel gating (22). Specifically, following the lead of other groups (25, 26), we used the NBD1 mutation K464A to decrease overall channel activity, and the NBD2 mutation E1371Q to increase channel activity. Previously we showed that these mutations mimic the effects of decreasing channel activity with low cytoplasmic ATP concentrations (K464A) or by “locking” channels in the open state by treatment with 2 mm sodium pyrophosphate (E1371Q) (22). Fig. 1 shows the influence of these NBD mutations on the rate of modification of two cysteines introduced deep into the channel pore from the inside, T338C in TM6 and L102C in TM1. The rate of modification by cytoplasmically applied MTSES was quantified by measuring the rate of change of macroscopic current amplitude in fully activated channels in inside-out membrane patches. As described previously (17, 18), application of intracellular MTSES caused a decrease in current amplitude in these two mutants. In both cases, the rate of modification by MTSES was significantly decreased by the K464A mutation and significantly increased by the E1371Q mutation, effects that are quantified in Fig. 1C. For modification of T338C, the mean modification rate constant was decreased ∼2.4-fold in a K464A background and increased ∼3.9-fold in E1371Q, whereas the modification rate constant for L102C was decreased by ∼26% in K464A and increased ∼2.0-fold in E1371Q. As in our previous work (22), the effects of the E1371Q mutation were mimicked by locking channels open by treatment with 2 mm pyrophosphate (data not shown; ∼3.9-fold increase for T338C and ∼1.6-fold increase for L102C). These results suggest that access of MTSES to these sites in the pore, as with sites located closer to the intracellular ends of TMs 1 and 6 (22), is accelerated by ATP-dependent channel opening.
FIGURE 1.
Rate of modification of T338C and L102C by internal MTSES. A and B, sample time courses of macroscopic currents (measured at −50 mV) carried by different CFTR channel variants as indicated in inside-out membrane patches. Current amplitudes were measured every 6 s following attainment of stable current amplitude after channel activation. In each panel, 200 μm MTSES was applied to the cytoplasmic face of the patch at time zero as indicated by the hatched bars. The decline in current amplitude following MTSES application has been fitted by a single exponential function. C, average modification rate constants (k) for MTSES, calculated from fits to data such as those shown in A and B. Asterisks indicate a significant difference from the cysteine mutants T338C and L102C (black bars) (p < 0.05). Data are mean from three or four patches.
To investigate whether permeant anions show the same regulated access from the cytoplasm to T338C and L102C, we investigated channel modification by Au(CN)2−, a highly permeant anion that has been used previously to modify cysteine side chains in the CFTR pore (14, 22). As shown in Fig. 2, application of a low concentration of Au(CN)2− (2 μm) caused a rapid inhibition of current carried by both T338C and L102C. As with MTSES, the rate of modification by Au(CN)2− was significantly decreased by the K464A mutation (by ∼1.8-fold for T338C and ∼3.4-fold for L102C) and significantly increased by the E1371Q mutation (by ∼5.6-fold for T338C and ∼1.8-fold for L102C), as well as by pyrophosphate treatment (by ∼6.0-fold for T338C and ∼2.0-fold for L102C; data not shown). Thus, permeant anion access from the cytoplasm to these residues also appears to be regulated by ATP-dependent channel opening.
FIGURE 2.
Rate of modification by internal Au(CN)2−. A and B, sample time courses of macroscopic currents carried by different CFTR channel variants as indicated in inside-out membrane patches. In each panel, 2 μm Au(CN)2− was applied to the cytoplasmic face of the patch at time zero as indicated by the hatched bars. The decline in current amplitude following Au(CN)2− application has been fitted by a single exponential function. C, average modification rate constants (k) for Au(CN)2−, calculated from fits to data such as those shown in A and B. Asterisks indicate a significant difference from the cysteine mutants T338C and L102C (black bars) (p < 0.02). Data are mean from three or four patches.
Regulated Access from Extracellular Solution to Pore
T338C is modified not only by intracellular, but also by extracellular MTS reagents (14, 17, 26), whereas L102C was reported to be insensitive to extracellular MTS reagents (18). To investigate the rate of modification of introduced cysteines by extracellular cysteine-reactive reagents (MTSES and Au(CN)2−) under different channel gating conditions, we used whole cell patch clamp recording of the same CFTR constructs used for inside-out patch recording and cytoplasmic modification. Expression of all CFTR constructs (except those containing the E1371Q mutation, see below) in baby hamster kidney cells led to the appearance of cAMP-activated whole cell currents that were inhibited by the specific CFTR inhibitor GlyH-101 (Fig. 3 and supplemental Fig. S2) and which were not observed in cells transfected with vector alone (supplemental Fig. S2). Expression of all E1371Q-CFTR constructs led to the appearance of constitutive, cAMP-insensitive but GlyH-101-inhibited whole cell currents (supplemental Fig. S2). Cys-less CFTR was insensitive to application of high concentrations of MTSES (2 mm) or Au(CN)2− (1 mm) to the extracellular solution (Fig. 3A). In contrast, T338C was strongly inhibited by very much lower concentrations of MTSES (1 μm) and Au(CN)2− (200 nm) (Fig. 3B). L102C was insensitive to 2 mm MTSES, but was strongly inhibited by intermediate concentrations of Au(CN)2− (10 μm) (Fig. 3C).
The rate of modification by extracellularly applied cysteine-reactive reagents was quantified by measuring the rate of change of whole cell current amplitude following stable cAMP stimulation (Figs. 4 and 5). Fig. 4A shows examples of the rate of current inhibition in response to application of a common concentration of MTSES (1 μm) in T338C, T338C/K464A, and T338C/E1371Q. Quantification of the rate constant for modification (Fig. 4B) suggests an increase of ∼3.7-fold in T338C/K464A and a dramatic decrease of ∼35-fold in T338C/E1371Q compared with T338C alone. Note that, because MTSES modification was so slow in T338C/E1371Q, the rate constant for modification of this construct was calculated from experiments using a higher concentration of MTSES (200 μm). L102C was not apparently modified by extracellular MTSES (Fig. 3C), consistent with previous findings (18).
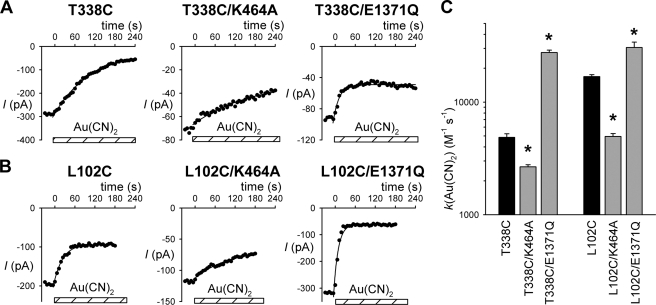
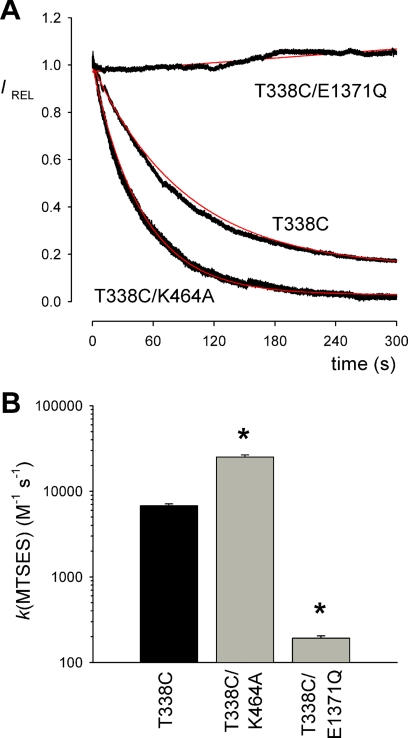
FIGURE 4.
Rate of modification of T338C by external MTSES. A, time course of whole cell current amplitude decay (measured at +30 mV, see Fig. 3) following application of 1 μm MTSES in different channel constructs. Current amplitudes have been scaled to amplitude prior to MTSES application (IREL). The decline in current amplitude following MTSES application has been fitted by a single exponential function (red line). B, average modification rate constants (k) for MTSES, calculated from fits to data such as those shown in A. Modification rate constant for T338C/E1371Q was quantified from experiments using a higher concentration of MTSES (200 μm). Asterisks indicate a significant difference from T338C alone (p < 0.0005). Data are mean from three or four patches.
FIGURE 5.
Rate of modification of T338C and L102C by external Au(CN)2−. A and B, time course of whole cell current amplitude decay (measured at 30 mV, see Fig. 3) following application of Au(CN)2− (200 nm in A, 10 μm in B) in different channel constructs. Current amplitudes have been scaled to amplitude prior to Au(CN)2− application (IREL). The decline in current amplitude following Au(CN)2− application has been fitted by a single exponential function (red line). C, average modification rate constants (k) for Au(CN)2−, calculated from fits to data such as those shown in A and B. Modification rate constants for T338C/E1371Q and L102C/E1371Q were quantified from experiments using a higher concentration of Au(CN)2− (100 μm). Asterisks indicate a significant difference from T338C and L102C alone as applicable (black bars) (p < 0.01).Data are mean from three or four patches.
Fig. 5 shows a similar analysis of the rate of modification by extracellular Au(CN)2−, both for T338C (Fig. 5A; 200 nm Au(CN)2−) and for L102C (Fig. 5B; 10 μm Au(CN)2−). Quantification of the rate constant for modification (Fig. 5C) suggests, for modification of T338C, an increase of ∼5.7-fold in K464A and a decrease of ∼150-fold in E1371Q, and for modification of L102C, an increase of ∼2.3-fold in K464A and a decrease of ∼2.7-fold in E1371Q. As with extracellular MTSES modification of T338C/E1371Q (Fig. 4), the rate constant for Au(CN)2− modification of E1371Q channels was calculated from experiments using higher concentrations of Au(CN)2− (100 μm).
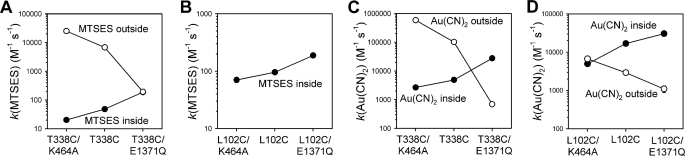
Changing Patterns of Accessibility Suggest Marker Cysteine Residues “Switch Sides” of Membrane during Gating
The effects of NBD mutations on the rate of modification of T338C and L102C by internal cysteine-reactive reagents (estimated from experiments on inside-out membrane patches) and by external cysteine-reactive reagents (estimated from whole cell current recording experiments) are compared in Fig. 6. It should be pointed out that these two types of experiments were not carried out under the same conditions; for example, differences in voltage protocols and methods of channel stimulation are likely to influence the overall modification rate constants calculated, and as such we believe that there is no information contained in the relative rate of modification for internal versus external reagents in the same channel construct. It is also possible that NBD mutations might differentially affect channel function under different channel stimulation conditions. However, what is most relevant here is the relative change in modification rate constant for the same cysteine residue in the presence of different NBD mutations that are expected to alter ATP-dependent channel gating. In each panel, it can be seen that the rate of modification by internal MTSES and Au(CN)2− increases in the order K464A < Cys-less < E1371Q, whereas modification by extracellular MTSES (in T338C) and Au(CN)2− shows the opposite pattern, K464A > Cys-less > E1371Q. Thus, the same molecular manipulation (NBD mutation) that causes a decrease in the rate of modification by internal reagents causes an increase in the rate of modification by external reagents, and vice versa. This suggests that the reporter cysteines in the pore that we have used, T338C and L102C, are capable of “moving” from a relatively internally accessible position to a relatively externally accessible position. Given the known effects of the K464A and E1371Q mutations on ATP-dependent channel gating, it seems reasonable to us to infer that the factor causing this movement from one side of the membrane to the other is channel gating: when the channel is closed (enriched in the K464A constructs), accessibility of these cysteines from the outside is increased, and when the channel is open (enriched in the E1371Q constructs), their accessibility from the inside is increased.
FIGURE 6.
Relationship between modification rates and NBD function. Each panel illustrates the change in modification rate constant for the same reporter cysteine (T338C in A and C, L102C in B and D) in three different backgrounds (K464A, Cys-less, and E1371Q), for modification by MTSES (A and B) or Au(CN)2− (C and D) applied to the intracellular (●, inside) or extracellular (○, outside) side of the membrane. Modification rate constants under different conditions are as shown in Figs. 1C (internal MTSES), 2C (internal Au(CN)2−), 4B (external MTSES), and 5C (external Au(CN)2−). Note that L102C was not apparently modified by extracellular MTSES (B; see Fig. 3C).
DISCUSSION
ABC proteins are thought to achieve active transmembrane transport by an alternating access mechanism, whereby substrate bound to the TMDs can access either the extracellular or intracellular solution depending on whether the TMDs are in an outward facing or inward facing configuration. Transport is then thought to be driven by ATP binding and hydrolysis at the NBDs controlling the switch between outward and inward facing conformations. We have taken advantage of the unique functional properties of CFTR as an ion channel to monitor changes in access from the extracellular and intracellular solutions to sites within the TMDs. As an ion channel, modification of CFTR can be monitored rapidly and with high temporal resolution as changes in electrical current. Because of the unique ion channel function of CFTR, different conformations of the TMDs are recognized as functionally distinct open and closed channel states.
The simplest model of ion channel gating would be that access of ions to the pore increases upon opening and decreases upon closing. We would suggest that the most straightforward interpretation of our results (summarized in Fig. 6) is contrary to this simple model. For residues supposed to be located near the extracellular ends of TM6 (Thr-338) and TM1 (Leu-102), access from the cytoplasm is increased by manipulations that increase channel open probability by altering interactions between the NBDs and ATP, the normal mechanism of channel gating. However, these same manipulations have exactly the opposite effect on access from the other side of the membrane, suggesting that these amino acids paradoxically become less accessible from the outside in open channels than they are in closed channels. These findings have clear parallels to a classical alternating access model of the permeation pathway, with the channel closed state facing outward and the open state facing inward (Fig. 7A).
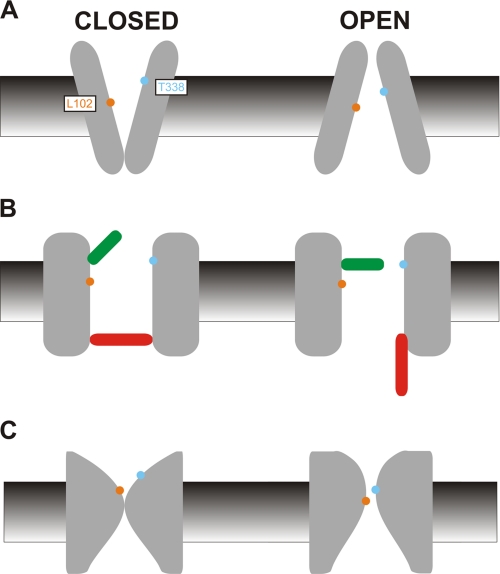
FIGURE 7.
Models of CFTR pore structure during gating. The implications of the current experimental findings, that T338C and L102C in the CFTR pore show increased access to the extracellular solution in the closed state and increased access to the intracellular solution in the open state, can be interpreted according to a number of different simple diagram models of channel function. A, in the simplest interpretation of the inward facing/outward facing configurations of the TMDs, the closed state is designated as outward facing, and the open state as inward facing, with physical exposure of Leu-102 (orange) and Thr-338 (blue) alternating between the extracellular (closed) and intracellular (open) side of the membrane. B, as an ABC protein, the channel can be envisioned as having two gates, one of which controls channel opening (the activation gate, red) and a vestigial gate (green) that closes when the activation gate opens. The gates are positioned to allow opening of the channel to be associated with an increase in accessibility from the cytoplasmic side of the membrane and a decrease of accessibility from the extracellular side of the membrane for residues located between the two gates. Possible physical locations of these two putative gates are discussed under “Discussion.” C, this slightly less schematic view incorporates key aspects of the first two models. Opening of the channel is suggested to be associated with a physical dilation of the intracellular part of the narrow pore region and a physical constriction of the outer mouth of the pore, causing Leu-102 and Thr-338 to move from a position that is exposed to the extracellular solution in closed channels to a physically constricted, more cytoplasmically exposed position in open channels.
Previously our group showed that T338C could be modified by both intracellular and extracellular MTS reagents, even though these reagents are thought to be too large to permeate through the channel pore (17). Our present results suggest that this reflects switching of this amino acid residue from one side of the membrane to the other (Fig. 7A). Thus, it is possible that T338C is modified by intracellular MTSES in open channels and by extracellular MTSES in closed channels; the measured apparent rate of modification would then be dependent on the intrinsic rate of modification and the proportion of time the channel spends in the open and closed state in different channel constructs. Importantly, the same sidedness was observed for modification by permeant Au(CN)2− ions, suggesting that the switching of Thr-338 across the membrane is relevant to Cl− access to the permeation pathway.
Although decreased accessibility to one side of the membrane in open channels might seem counterintuitive, it is not without precedent in CFTR-TM6. Zhang et al. (25) showed that R334C, located slightly closer to the outside of TM6 than Thr-338, was modified by extracellular MTS reagents only in the closed state. The rate of modification of L333C and K335C, also at the extracellular end of TM6, was also decreased in an E1371Q background, suggesting slower modification of open, compared with closed channels (26). However, these cysteines in the outer pore region (L333C, R334C, and K335C) are not modified by intracellular MTS reagents under any conditions (17, 27), suggesting that unlike T338C they cannot move to a position that is accessible to large cytoplasmic substances. Residues closer to the cytoplasmic end of TM6 show regulated access to intracellular MTS reagents without being accessible to extracellular MTS reagents, for example I344C is accessible to intracellular MTSES only in open channels (22) but is not modified by extracellular MTS reagents (14, 17). Therefore, Thr-338 may exist in a restricted “central” part of TM6 that can alternate between intracellular and extracellular access to large, impermeant substances.
Furthermore, the ability to be modified by MTS reagents applied to either side of the membrane appears exclusive to TM6 among TMs studied to date; we did not identify any sites in either TM1 (18) or TM12 (21) that could be modified by both intracellular and extracellular MTS reagents. For example, L102C in TM1 is modified by internal, but not external MTS reagents (18), a result confirmed by the present results (Figs. 1 and 3), whereas R104C, only 2 residues closer to the external end of TM1, is modified by external, but not internal MTS reagents (28). L102C, like T338C, becomes apparently more accessible to internal cysteine reactive reagents in open channels (Fig. 6B), but is inaccessible to extracellular MTSES (Fig. 3C). L102C is accessible to permeant Au(CN)2− ions applied to either side of the membrane, as expected for a permeant probe that ought to access the entire permeation pathway, and as with T338C access from the outside decreases as access from the inside increases (Fig. 6D), again consistent with easier access from the cytoplasm in open channels and from the extracellular solution in closed channels. One possible explanation for the difference in external accessibility of L102C in TM1 and T338C in TM6 is that T338C is located in a more superficial position in the outer mouth of the pore (at least in closed channels), such that it can be accessed by large extracellular MTS reagents that cannot penetrate further into the pore from the outside to modify L102C (Fig. 7A). Consistent with this differential access from the outside, the rate of modification by extracellular Au(CN)2− is approximately 35 times greater for T338C than for L102C in a Cys-less background (Fig. 5C). One apparent problem with this explanation is that a residue only slightly closer to the outer end of TM1 (R104C) is accessible to extracellular, but not intracellular MTS reagents (see above); the model shown in Fig. 7A should put this residue close to Thr-338. One possible speculative explanation for this would be that different TMs show not only changes in tilt (relative to the membrane) but also translational movement relative to each other during opening and closing. For example, TM6 could move outward (or TM1 inward) during channel closing, placing Thr-338 closer to the extracellular end of the closed channel pore, and TM6 could move inward (or TM1 outward) upon opening.
Strikingly, the association of outward facing/inward facing with closed and open channels suggested in Fig. 7A is exactly the opposite of that proposed on the basis of structural studies in other ABC proteins (see the Introduction). Of course, our model is a functional one and should not be taken in a literal structural sense. On the other hand, current structural models of the TMDs that are based on homology modeling of distantly related proteins with diverse functions are subject to a number of important caveats (as described in the Introduction) and, importantly, have not previously been subjected to direct experimental testing. In fact, the model in Fig. 7A seems more consistent with longstanding functional models that propose an inner vestibule in the open channel that is deep and wide enough to accommodate large cytoplasmic substances, such as those that enter into the pore from its cytoplasmic end to act as open channel blockers (29, 30). In contrast, in outwardly facing homology models that have been proposed to represent the open channel (12–14), the TMDs are widely separated at their extracellular end and close together at the intracellular end, such that most amino acids in the putative permeation pathway are exposed to the extracellular solution, and no inner vestibule is apparent. Furthermore, recent substituted cysteine accessibility studies have shown that many residues in the TMDs are accessible to internally applied cysteine reactive reagents, and in many cases these same residues are inaccessible to the same reagents applied extracellularly, suggesting a physical constriction near the outer end of the TMDs (17, 18, 21, 22, 27, 31). In fact, residues in the cytoplasmic half of the TMDs appear to show equivalent access to cytoplasmic substances in both open and closed channels (22), suggesting that channel gating is not associated with a major conformational change at the intracellular end of the pore. Interestingly, a recent structural study of CFTR was suggested to be consistent with an outwardly facing conformation of the TMDs, even though the channel was unstimulated and presumed to be in an inactive, closed state (11).
Our present results can also be interpreted in terms of the location of channel gates, and a speculative model of this kind is presented in Fig. 7B. When the channel is closed, access from the cytoplasm is prohibited by a closed activation gate (red). Previously, we suggested that such a gate is located in the region of Lys-95/Gln-98 (TM1), Ile-344/Val-345 (TM6), Met-1140/Ser-1141 (TM12) (17, 18, 21, 22). Our present results are consistent with such a model, as Leu-102 and Thr-338 are expected to be on the extracellular side of this gate and as such inaccessible to the cytoplasm in closed channels. Importantly, internally applied Au(CN)2− ions also show regulated access to this part of the pore, suggesting that the activation gate can prevent passage of permeant ions as well as large MTS reagents. In this model, reduced access from the extracellular solution in open channels is due to partial closure of a vestigial open gate, which decreases the rate of entry of extracellular MTSES and Au(CN)2− to T338C and L102C, although not completely occluding the pore and thus allowing Cl− permeation. Again, Leu-102 is shown as being more deeply into the pore from the outside, perhaps beyond the vestigial gate as experienced by extracellular substances, resulting in external MTSES insensitivity. Although this simple model can explain our functional data, as well as being consistent with previous work on the functional properties of the pore, it may not be possible to prove the existence of a vestigial gate that does not contribute to an experimentally observable change in channel gating state. Nevertheless, we would tentatively speculate that the nonfunctional, vestigial outer gate in the CFTR pore is located close to Thr-338 in TM6. It is known that Thr-338 contributes to the physically narrowest part of the open channel pore (30), and this amino acid has also been shown to contribute to a region of high resistance to Cl− flux in open channels (32). Mutations that decrease amino acid side chain volume at this position increase the rate of Cl− permeation through open channels, whereas mutations that increase side chain volume dramatically reduce Cl− conductance (33). This side chain may also contribute to the channel selectivity filter that allows some discrimination between Cl− and other anions (30, 33, 34). We therefore speculate that Thr-338 contributes to a gate that closes incompletely when the channel opens, forming a narrow constriction in the channel that acts as a “selectivity filter” and so allows it to function as a Cl−-selective ion channel. The dramatic decrease in modification rate for external MTSES and Au(CN)2− seen in T338C/E1371Q (Figs. 4B, 5C, and 6) suggests that access to this narrow region from the extracellular solution is greatly decreased in open channels. When the channel closes, access from the outside is very high, suggesting that this region becomes wide open to the extracellular solution.
As with the alternating access model shown in Fig. 7A, it must be noted that the gates model of Fig. 7B is in direct contradiction of previous suggestions that the CFTR pore should have an atrophied, vestigial inner gate (5, 8, 15). Our previous work has suggested that the rate of modification by cytoplasmic MTS reagents is independent of channel gating for residues on the cytoplasmic side of the activation gate (red in Fig. 7B) (22), suggesting that there is no functionally consequential conformational change occurring on the cytoplasmic side of this gate during channel gating.
Our ideas concerning conformational changes in the pore during gating, based on present and previous functional data, are summarized in Fig. 7C. As with the other models in Fig. 7, it must be stressed that this is a scheme with strictly limited structural implications. In the closed state, the pore is occluded at the outer end of the inner vestibule, and the outer vestibule is dilated, making Thr-338 readily accessible to the extracellular solution. Leu-102 is shown as being more deeply into the pore from the outside, preventing access to large MTS reagents from the outside. On opening, the occlusion in the pore dilates, perhaps in the region of Lys-95/Gln-98 (TM1), Ile-344/Val-345 (TM6) (22). The wide inner vestibule itself does not change in conformation, consistent with equivalent access to this region in open and closed channels (22). The outer vestibule of the pore constricts, contributing to a narrow pore region that is known to exist in open channels (see above). Opening may possibly also be associated with inward translational movement of Thr-338 and/or Leu-102. In this model, only a physically restricted region of the pore alternates between inward facing and outward facing conformations.
CFTR has been described as a broken pump that at some point in evolutionary history was converted from an active transporter to an anion-selective ion channel, due to loss of function of one of the gates controlling access to the transport pathway. Although our functionally based models in Fig. 7 remain speculative at present, it is interesting to conjecture as to their implications for the relationship between CFTR and its ABC protein relatives. One possibility raised by Fig. 7B is that CFTR evolved as a transporter in which the outer gate became “leaky” to small anions even when closed, eventually leading to the formation of an open, selective, rapidly conducting (and inward facing) ion channel pore. Another possible implication is that our model suggests that both putative gates are located within the TMDs (in fact, in the central/outer parts of the TMs). Although the locations of the two putative gates that allow ABC proteins to function as active transporters are not known, if evolutionarily conserved then we would propose that they may be found within the membrane-spanning parts of these proteins.
Despite its functional uniqueness within the ABC family, our results suggest that CFTR still undergoes conformational changes in its TMDs that resemble an alternating access mechanism, and as with other ABC proteins that the switch between outward facing and inward facing conformations of the TMDs is still driven by ATP interactions with the NBDs. However, in contrast to current models (5, 8, 15, 16), our direct functional data correlate the open state with an inward facing conformation and the closed state with an outward facing conformation. This discrepancy with functional data should be taken into account in structural and mechanistic models of CFTR function.
Supplementary Material
This work was supported by the Canadian Institutes of Health Research.

This article contains supplemental Figs. S1 and S2.
- ABC
- ATP-binding cassette
- CFTR
- cystic fibrosis transmembrane conductance regulator
- MTS
- methanethiosulfonate
- MTSES
- [2-sulfonatoethyl]methanethiosulfonate
- NBD
- nucleotide binding domain
- TES
- N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid
- TM
- transmembrane α-helix
- TMD
- transmembrane domain.
REFERENCES
- 1. DeFelice L. J., Goswami T. (2007) Transporters as channels. Annu. Rev. Physiol. 69, 87–112 [DOI] [PubMed] [Google Scholar]
- 2. Kos V., Ford R. C. (2009) The ATP-binding cassette family: a structural perspective. Cell Mol. Life Sci. 66, 3111–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Locher K. P. (2009) Review: structure and mechanism of ATP-binding cassette transporters. Phil. Trans. R. Soc. B 364, 239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rees D. C., Johnson E., Lewinson O. (2009) ABC transporters: the power to change. Nat. Rev. Mol. Cell Biol. 10, 218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gadsby D. C. (2009) Ion channels versus ion pumps: the principal difference, in principle. Nat. Rev. Mol. Cell Biol. 10, 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gadsby D. C., Vergani P., Csanády L. (2006) The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature 440, 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hwang T. C., Sheppard D. N. (2009) Gating of the CFTR Cl− channel by ATP-driven nucleotide-binding domain dimerisation. J. Physiol. 587, 2151–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller C. (2010) CFTR: break a pump, make a channel. Proc. Natl. Acad. Sci. U.S.A. 107, 959–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mio K., Ogura T., Mio M., Shimizu H., Hwang T. C., Sato C., Sohma Y. (2008) Three-dimensional reconstruction of human cystic fibrosis transmembrane conductance regulator chloride channel revealed an ellipsoidal structure with orifices beneath the putative transmembrane domain. J. Biol. Chem. 283, 30300–30310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang L., Aleksandrov L. A., Riordan J. R., Ford R. C. (2011) Domain location within the cystic fibrosis transmembrane conductance regulator protein investigated by electron microscopy and gold labelling. Biochim. Biophys. Acta 1808, 399–404 [DOI] [PubMed] [Google Scholar]
- 11. Rosenberg M. F., O'Ryan L. P., Hughes G., Zhao Z., Aleksandrov L. A., Riordan J. R., Ford R. C. (2011) The cystic fibrosis transmembrane conductance regulator (CFTR): three-dimensional structure and localization of a channel gate. J. Biol. Chem. 286, 42647–42654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mornon J. P., Lehn P., Callebaut I. (2008) Atomic model of human cystic fibrosis transmembrane conductance regulator: membrane-spanning domains and coupling interfaces. Cell Mol. Life Sci. 65, 2594–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Serohijos A. W., Hegedus T., Aleksandrov A. A., He L., Cui L., Dokholyan N. V., Riordan J. R. (2008) Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc. Natl. Acad. Sci. U.S.A. 105, 3256–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alexander C., Ivetac A., Liu X., Norimatsu Y., Serrano J. R., Landstrom A., Sansom M., Dawson D. C. (2009) Cystic fibrosis transmembrane conductance regulator: using differential reactivity toward channel-permeant and channel-impermeant thiol-reactive probes to test a molecular model for the pore. Biochemistry 48, 10078–10088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mornon J. P., Lehn P., Callebaut I. (2009) Molecular models of the open and closed states of the whole human CFTR protein. Cell Mol. Life Sci. 66, 3469–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Csanády L., Vergani P., Gadsby D. C. (2010) Strict coupling between CFTR's catalytic cycle and gating of its Cl− ion pore revealed by distributions of open channel burst durations. Proc. Natl. Acad. Sci. U.S.A. 107, 1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Hiani Y., Linsdell P. (2010) Changes in accessibility of cytoplasmic substances to the pore associated with activation of the cystic fibrosis transmembrane conductance regulator chloride channel. J. Biol. Chem. 285, 32126–32140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang W., El Hiani Y., Linsdell P. (2011) Alignment of transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore. J. Gen. Physiol. 138, 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mense M., Vergani P., White D. M., Altberg G., Nairn A. C., Gadsby D. C. (2006) In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer. EMBO J. 25, 4728–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li M. S., Demsey A. F., Qi J., Linsdell P. (2009) Cysteine-independent inhibition of the CFTR chloride channel by the cysteine-reactive reagent sodium (2-sulphonatoethyl) methanethiosulphonate. Br. J. Pharmacol. 157, 1065–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qian F., El Hiani Y., Linsdell P. (2011) Functional arrangement of the 12th transmembrane region in the CFTR chloride channel pore based on functional investigation of a cysteine-less CFTR variant. Pflügers Arch. 462, 559–571 [DOI] [PubMed] [Google Scholar]
- 22. Wang W., Linsdell P. (2012) Conformational change opening the CFTR chloride channel pore coupled to ATP-dependent gating. Biochim. Biophys. Acta 1818, 851–860 [DOI] [PubMed] [Google Scholar]
- 23. Holstead R. G., Li M. S., Linsdell P. (2011) Functional differences in pore properties between wild-type and cysteine-less forms of the CFTR chloride channel. J. Membr. Biol. 243, 15–23 [DOI] [PubMed] [Google Scholar]
- 24. Zhou J. J., Li M. S., Qi. J., Linsdell P. (2010) Regulation of conductance by the number of fixed positive charges in the intracellular vestibule of the CFTR chloride channel pore. J. Gen. Physiol. 135, 229–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Z. R., Song B., McCarty N. A. (2005) State-dependent chemical reactivity of an engineered cysteine reveals conformational changes in the outer vestibule of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 280, 41997–42003 [DOI] [PubMed] [Google Scholar]
- 26. Beck E. J., Yang Y., Yaemsiri S., Raghuram V. (2008) Conformational changes in a pore-lining helix coupled to cystic fibrosis transmembrane conductance regulator channel gating. J. Biol. Chem. 283, 4957–4966 [DOI] [PubMed] [Google Scholar]
- 27. Bai Y., Li M., Hwang T. C. (2010) Dual roles of the sixth transmembrane segment of the CFTR chloride channel in gating and permeation. J. Gen. Physiol. 136, 293–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou J. J., Fatehi M., Linsdell P. (2008) Identification of positive charges situated at the outer mouth of the CFTR chloride channel pore. Pflügers Arch 457, 351–360 [DOI] [PubMed] [Google Scholar]
- 29. Hwang T. C., Sheppard D. N. (1999) Molecular pharmacology of the CFTR Cl− channel. Trends Pharmacol. Sci. 20, 448–453 [DOI] [PubMed] [Google Scholar]
- 30. Linsdell P. (2006) Mechanism of chloride permeation in the cystic fibrosis transmembrane conductance regulator chloride channel. Exp. Physiol. 91, 123–129 [DOI] [PubMed] [Google Scholar]
- 31. Bai Y., Li M., Hwang T. C. (2011) Structural basis for the channel function of a degraded ABC transporter, CFTR (ABCC7). J. Gen. Physiol. 138, 495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fatehi M., St Aubin C. N., Linsdell P. (2007) On the origin of asymmetric interactions between permeant anions and the cystic fibrosis transmembrane conductance regulator chloride channel pore. Biophys. J. 92, 1241–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Linsdell P., Zheng S. X., Hanrahan J. W. (1998) Non-pore-lining amino acid side chains influence anion selectivity of the human CFTR Cl− channel expressed in mammalian cell lines. J. Physiol. 512, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCarty N. A., Zhang Z. R. (2001) Identification of a region of strong discrimination in the pore of CFTR. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L852–867 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.