Background: P2X7 receptors are thought to be primarily involved in inflammatory signaling.
Results: Transient (1–4 min) high ATP induced delayed (hours) cell death in macrophages from WT and TLR2/4, Casp1, or Panx1 knock-out mice.
Conclusion: Transient P2X7 receptor activation triggers macrophage-selective apoptotic cell death independent of TLR signaling, Casp1, and Panx1.
Significance: P2X7 receptors function foremost as death triggers in macrophages.
Keywords: ATP, Caspase, Cell Death, Cell Signaling, Macrophages, p2x7, Pannexin, Toll-like Receptors (TLR)
Abstract
The function of P2X7 receptors (ATP-gated ion channels) in innate immune cells is unclear. In the setting of Toll-like receptor (TLR) stimulation, secondary activation of P2X7 ion channels has been linked to pro-caspase-1 cleavage and cell death. Here we show that cell death is a surprisingly early triggered event. We show using live-cell imaging that transient (1–4 min) stimulation of mouse macrophages with high extracellular ATP ([ATP]e) triggers delayed (hours) cell death, indexed as DEVDase (caspase-3 and caspase-7) activity. Continuous or transient high [ATP]e did not induce cell death in P2X7-deficient (P2X7−/−) macrophages or neutrophils (in which P2X7 could not be detected). Blocking sustained Ca2+ influx, a signature of P2X7 ligation, was highly protective, whereas no protection was conferred in macrophages lacking caspase-1 or TLR2 and TLR4. Furthermore, pannexin-1 (Panx1) deficiency had no effect on transient ATP-induced delayed cell death or ATP-induced Yo-Pro-1 uptake (an index of large pore pathway formation). Thus, “transient” P2X7 receptor activation and Ca2+ overload act as a death trigger for native mouse macrophages independent of Panx1 and pro-inflammatory caspase-1 and TLR signaling.
Introduction
P2X receptors are cation-selective channels, and seven genes encoding the subunits P2X1 to P2X7 have been identified (1, 2). The P2X7 receptor stands out from the others because it is resistant to desensitization (3), and prolonged activation induces membrane permeability to large molecules (<∼1 kDa) such as thee 375-Da fluorescent probe Yo-Pro-1 (2, 4, 5). Pannexin-1, a putative hemichannel, has been implicated as the P2X7 receptor-activated large pore pathway (6, 7). The P2X7 receptor is predominantly expressed in immune cells (8). It was previously denoted as the pro-cytolytic P2Z receptor, but cloning in 1996 revealed that it belonged to the P2X family (9). Since that time, P2X7 has emerged as a potentially important second stimulus for Toll-like receptor 4 (TLR4)-dependent IL-1β and IL-18 secretion. First, priming of macrophages with the TLR4 2 ligand lipopolysaccharide (LPS) induces intracellular accumulation of pro-IL-1β and pro-IL-18. Second, P2X7 receptor activation promotes assembly of the “inflammasome” and caspase-1-dependent cleavage and release of biologically active IL-1β and IL-18. This scenario is well established in both in vivo (10) and in vitro models (11–20).
Typically, pro-inflammatory IL-1β and IL-18 cytokine processing and release can be detected after about 20–30 min of P2X7 receptor stimulation, and cell death, commonly indexed as lactate dehydrogenase release, is moderately low under such conditions (10–12, 15). In contrast, prolonged (>30 min) P2X7 receptor stimulation is well known to be lethal (4, 11, 15). Hence, cell death is generally assumed to be a late event in relation to inflammatory cytokine processing. However, the extracellular [ATP] is tightly controlled by ectonucleotidases (21), and it is difficult to imagine a situation in which macrophage P2X7 receptors are stimulated by high [ATP]e for a long duration (15–20 min) after sensing bacteria via TLR4. Brief (5 min) stimulation of LPS-primed macrophages with millimolar ATP concentrations is a more likely signaling scenario. Indeed, brief stimulation of P2X7 receptors is sufficient to initiate the processing of IL-1β in macrophages (16, 18).
The aim of this study was to elucidate the role of P2X7 receptors in innate immune cells. We used resident macrophages from mice deficient in various genes (Casp1, P2rx7, Tlr2, Tlr4, P2ry2, and Panx1) and real-time single-cell imaging to elucidate, in particular, a link between transient P2X7 receptor activation and cell death.
EXPERIMENTAL PROCEDURES
Materials
Chemicals were obtained from Sigma-Aldrich unless stated otherwise. ATP was added from a 100 mm stock solution in Dulbecco's phosphate-buffered saline (PBS; pH 7.4). The fluorogenic substrate for caspase-3 (and caspase-7) DEVD-NucView488 was added from a 1 mm stock solution in dimethyl sulfoxide (DMSO) (BioTrend, Germany). z-VAD-fmk (20 mm stock in DMSO) was obtained from R&D Systems, and both tetramethylrhodamine, ethyl ester (TMRE) and fluo-3/AM were from Invitrogen. Calpeptin (Tocris Bioscience) was added from a 100 mm stock in DMSO. Yo-Pro-1 iodide (Mr 629) was added from a 1 mm stock in DMSO (Invitrogen). Yo-Pro-1 is a monomeric cyanine dye with a cationic side chain.
Knock-out Mice
P2X7−/−, P2Y2−/−, Casp1−/−, Panx1 (22), and TLR2/4 double knock-out (dKO) mice were backcrossed onto a C57BL/6 genetic background. Casp1−/− mice were kindly supplied by Arturo Zychlinsky (Max-Planck-Institut für Infektionsbiologie, Berlin, Germany) (23) and Leo Joosten (Nijmegen Institute for Infection, Inflammation and Immunity), and P2X7−/− mice were provided by GlaxoSmithKline (24).
Resident Peritoneal Macrophages
Resident macrophages were isolated and seeded into fibronectin-coated μ-Slide I chambers (Ibidi, Martinsried, Germany), as described previously (25, 26). In brief, mice were killed by overdose with isoflurane. A 24-gauge catheter (BD Insyte-W, BD Infusion Therapy Systems) was inserted into the peritoneum, and resident peritoneal cells were harvested by lavage with 8 ml of ice-cold Hanks' buffered salt solution (Invitrogen). After centrifugation at 360 × g for 5 min, cells were resuspended in RPMI 1640 medium (Biochrom AG, Berlin, Germany) containing 10% heat-inactivated fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. μ-Slide I chambers (Ibidi), which have a channel volume of 100 μl, were filled with the cell suspension and incubated at 37 °C in air with 5% CO2. After 2 h, nonadherent cells were removed by washing the channel with 2 ml of fresh medium. Experiments were performed after 1–2 days of incubation, and the medium was switched to bicarbonate-free RPMI 1640 containing 20 mm Hepes (Biochrom AG). Hanks' buffered salt solutions were used for single-cell Ca2+-imaging experiments. EGTA (0.5 mm) was added to Ca2+-free Hanks' solution. Alternatively, nominally Ca2+-free RPMI 1640 medium was made by adding 5 mm EGTA and subsequently titrating the pH back to 7.4. In selected experiments, macrophages were primed (pretreated) with LPS by incubation in medium containing 1 μg/ml LPS from Escherichia coli 0111:B4 (L3012, Sigma) for 4 h.
Bone Marrow-derived Neutrophils
Bone marrow cells were flushed from the hind leg femurs of mice using Hanks' buffered salt solution (Invitrogen) containing 10% FCS and Hepes (pH 7.4). After filtration via a cell strainer with 70-μm pores (BD Falcon, BD Biosciences), the cell suspension was centrifuged at 1200 rpm for 10 min. During this time, a density gradient was prepared in a round bottom 14-ml tube (BD Falcon, BD Biosciences) by layering 4-ml Histopaque-1119 underneath a 4-ml Histopaque-1077 via a long syringe needle. The bone marrow cell pellet was resuspended in 1 ml of solution, layered onto the Histopaque density gradient, and centrifuged at 1800 rpm (without using brakes) for 30 min at room temperature. The granulocyte layer, sandwiched between the Histopaque-1077 and -1119, was removed using a pipette, washed once, and resuspended in 10 ml of conditioned medium containing RPMI 1640 medium (Biochrom AG), 20% heat-inactivated fetal calf serum, 10% culture supernatant from WEHI-3B cells (mouse myelomonocytic leukemia cell line; ATCC TIB-68), 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were cultured overnight at 37 °C (5% CO2). The following morning, cells were centrifuged at 1200 rpm for 8 min and resuspended in 10 ml of Hepes-Ringer solution. Subsequently, cells were seeded into μ-Slide I chambers (Ibidi) freshly coated with fibronectin. After allowing 5–10 min for adherence, the medium was replaced with bicarbonate-free RPMI 1640 containing 20 mm Hepes (Biochrom AG), but no fetal calf serum.
Bone Marrow-derived Macrophages
For selected experiments, bone marrow-derived macrophages were used to produce glass bottom WillCo (WillCo Wells) dishes (40-mm glass diameter and 0.17-mm thickness) with highly confluent macrophages. The femurs of mice were cleared of tissue and completely fractured in the middle of the shaft (diaphysis) using a surgical scalpel blade (number 21). Bone marrow cells were flushed out of each bone fragment using ∼5 ml of Dulbecco's modified Eagle's medium (DMEM), injected via a 90° bent 23-gauge needle. The cell suspension, collected in a 50-ml Falcon tube, was centrifuged for 8 min at 1100 rpm and 4 °C. The supernatant was discarded, and the pellet was resuspended in 1 ml of lysis buffer for 5 min (before recentrifugation) to induce hemolysis. The lysis buffer contained: 155 mm NH4Cl, 10 mm KHCO3, and 0.1 mm EDTA (pH 7.4). The suspension was then centrifuged for 10 min at 300 × g and room temperature. The supernatant was aspirated, and the cells were washed once using 10 ml of Dulbecco's PBS and centrifuged (300 × g for 8 min at room temperature). Next, the pellet was resuspended in 30 ml of incubation medium, which consisted of DMEM, 2% glutamine, 1% kanamycin, 1% nonessential amino acids, 10% heat-inactivated FCS, and 20 ng/ml recombinant mouse colony-stimulating factor 1 (macrophage), which is also called macrophage colony-stimulating factor (R&D Systems). The resuspended cells were incubated (37 °C, 5% CO2) in 30-ml Teflon bags for 6 days. After this period, the cells were resuspended after placement on ice for 15 min and transferred to glass-bottomed WillCo dishes. After 2 h (to allow cell adhesion), the dishes were washed with RPMI 1640 medium (Biochrom) containing 10% heat-inactivated FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Before use, the cells were incubated overnight.
Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analyses
Living macrophages were labeled with Alexa Fluor 488-conjugated anti-mouse F4/80 antibodies (clone CI:A3-1; catalogue number MCA497A488, AbD Serotec, Oxford, UK), and F4/80+ cells (∼30% of total) were isolated using a BD FACSAria II cell sorter (BD Biosciences). Bone marrow-derived granulocytes were colabeled with Alexa Fluor 488-conjugated anti-Gr-1 and phycoerythrin-conjugated anti-CD11b antibodies, and Gr-1high/CD11bhigh cells (neutrophils) were purified by cell sorting. The anti-mouse Gr-1 (Ly-6G) antibody (clone RB6-8C5) was obtained from eBioscience (catalogue number 53-5931-82). RNA was isolated using the RNeasy mini kit and protocol from Qiagen (Hilden, Germany). cDNA was synthesized using SuperScript III reverse transcriptase. In the case of pannexins, the thermocycling protocol for the PCR was as follows: 94 °C for 4 min, then 29 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 45 s. The following primers were used (product sizes shown in parentheses): Panx1 (249 bp), forward, CATTGACCCCATGCTACTCC; reverse, TCAGCCACAGAAGTCACAGG; Panx2 (269 bp), forward, GAGAAAAAGCATACCCGCCAC; reverse, GGGTGAGCAGACATGGAATGA; and Panx3 (268 bp), forward, CCTCACAAGGCTCTTCCCTA; reverse, ACCGCTCTACCAAGGGAAAT (27). PCR for P2X7 was performed using the following protocol: 94 °C for 5 min, then 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s. The primer pair for P2X7 (195 bp) was: forward, CCCTGCACAGTGAACGAGTA; reverse, AGACAGGTCGGAGAAGTCCA.
Time-lapse Video Microscopy
Macrophages were placed on the stage of an inverted Axio Observer microscope (Carl Zeiss MicroImaging, Göttingen, Germany) maintained at 37 °C by a temperature-controlled XL incubator (Zeiss). Differential interference contrast (DIC) and fluorescence images were obtained via a 63×/1.40 oil immersion objective lens and charge-coupled device camera (AxioCam MRm, Zeiss) controlled by AxioVision software (Zeiss). Typically, time-lapse images were captured every 15 s or 1–2 min (6–12 h of recording).
Live-cell Imaging of Caspase-3/7 Activity
To detect active caspase-3 and caspase-7 (caspase-3/7), cells were incubated with the nonfluorescent caspase-3/7 substrate DEVD-NucView488 (Biotium). Following enzymatic cleavage, the released NucView488 moiety becomes green fluorescent upon binding to DNA (28). In selected experiments, macrophages were coloaded with TMRE by incubating the cells with 200 nm TMRE (diluted from a 20 mm stock solution in DMSO) for 5 min at 37 °C.
Western Blot
Macrophages were lysed in buffer containing 100 mm NaCl, 2 mm MgCl2, 1 mm dithiothreitol, 1% Nonidet P-40, 10% glycerol, 5 mm NaF, 1 mm Na3VO4 (sodium orthovanadate), the protease inhibitors leupeptin, aprotinin, and Pefabloc (each at 10 μg/ml), and 50 mm Tris-HCl (pH 7.4). Proteins were separated by 6–15% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Roche Applied Science, Mannheim, Germany). Membranes were blocked for 1 h at room temperature in TBS containing 5% nonfat dry milk and 0.05% Tween 20 followed by overnight incubation with anti-cleaved caspase-3 (17-kDa fragment) antibodies (catalogue number 9661), diluted 1:1000 (obtained from Cell Signaling Technology via New England Biolabs). For detection, horseradish peroxidase-conjugated secondary antibodies (Dianova, Hamburg, Germany) were used in combination with SuperSignal West Pico chemiluminescence substrate (Perbio, Bonn, Germany). Blots using anti-β-actin antibody (Sigma-Aldrich) were performed to control sample loading.
Nuclear Staining and Cytochrome c Labeling
Macrophages were fixed with 4% paraformaldehyde in Dulbecco's PBS for 15 min at 37 °C followed by permeabilization with 0.1% Triton X-100 in PBS, containing 5% normal goat serum, for 10 min. Cells were incubated with Alexa Fluor 555-conjugated anti-cytochrome c antibodies (BD Pharmingen) for 30 min. Subsequently, the nuclei were stained by incubating cells with 280 nm 4′,6-diamidino-2-phenylindole (DAPI), a nucleic acid stain, for 90 s. Note that NucView488, similar to DAPI, is a nucleic acid stain, which allows assessment of nuclear morphology (28).
Single-cell Cytosolic [Ca2+] Measurements
A glass coverslip seeded with macrophages was sealed onto the bottom of a Perspex bath (volume, 100 μl) using silicone lubricant (Fine Science Tools). Macrophages were imaged via a 40×/1.40 oil objective lens and superfused at 1 ml/min. To monitor intracellular [Ca2+], cells were incubated for 15 min with 10 μm fluo-3/AM. To reduce the rate of fluo-3 loss, solutions contained 0.8 mm probenecid, and experiments were performed at room temperature (20–23 °C). In each experiment, a single macrophage (selected with a bilateral iris) was excited at 488 nm, whereas fluorescence was detected at 530 ± 15 nm using a microscope-based spectrofluorometer system (Photon Technology International, Seefeld, Germany). Fluorescence signals were normalized with respect to the resting fluorescence intensity (F0) and expressed as F/F0. Solutions were rapidly switched by means of miniature three-way valves (The Lee Co., Westbrook, CT).
Statistical Analysis
Normality and homoscedasticity were tested using the Kolmogorov-Smirnov and Levene tests, respectively. A one-way analysis of variance was performed using an α value of 0.05. The post hoc Scheffé test was used to compare groups. In the case of two independent groups, an unpaired t test was used to test for statistical significance. Statistical analyses were performed using SPSS software, and data are presented as means ± S.E.
RESULTS
Continuous ATP Application
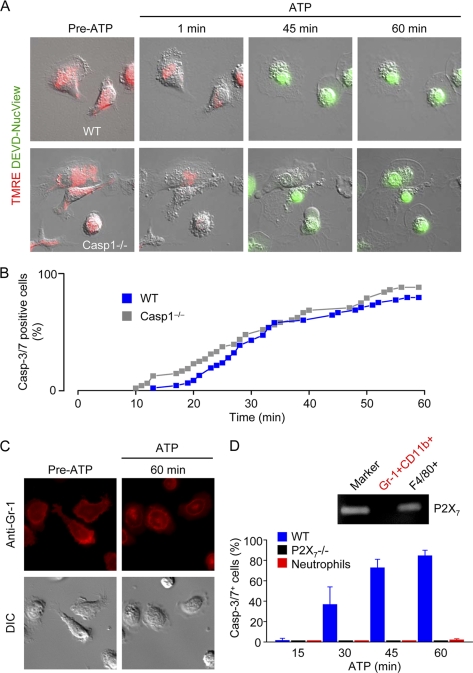
We initially tested whether prolonged P2X7 receptor stimulation activated caspase-3/7 and induced cell death in resident peritoneal macrophages using live-cell time-lapse imaging. After preloading with TMRE to monitor mitochondrial membrane potential, macrophages were continuously incubated in medium containing the cell-permeable and nonfluorescent probe DEVD-NucView488 to detect caspase-3/7 activity. Application of high [ATP]e (3 mm), required to activate P2X7 receptors, induced microblebbing of wild-type (WT) and Casp1−/− macrophages (Fig. 1A) within 60 s and dissipated the mitochondrial membrane potential in all cells within 2–3 min. Subsequently, between 10 and 60 min, one cell after the other became caspase-3/7-positive (Fig. 1B), indicated by the appearance of green fluorescence; the nonfluorescent probe DEVD-NucView488 is cleaved by caspase-3/7 (DEVDases), and the NucView488 moiety becomes fluorescent upon binding to RNA and DNA (28).
FIGURE 1.
Continuous ATP application. A, simultaneous measurement of mitochondrial membrane potential (mitoΔΨ; red fluorescence) and caspase-3/7 activity (green fluorescence) in WT and caspase-1 deficient (Casp1−/−) macrophages challenged with 3 mm ATP (P2X7 receptor ligand). Images are 70 × 70 μm. B, cumulative plot of WT and Casp1−/− macrophage cell death, indexed as caspase-3 and caspase-7 (Casp-3/7) activity, during continuous stimulation with 3 mm ATP in medium containing the cell-permeable and nonfluorescent caspase-3/7 substrate DEVD-NucView488. Following caspase activation, the cleavage product NucView488 binds to DNA and becomes highly fluorescent. C, prolonged ATP application has no effect on the viability of neutrophils (Gr-1+ cells). D, summary of data obtained using macrophages isolated from WT, Casp1−/−, or P2X7−/− mice (n = 5 independent experiments (56–73 cells) per group) or neutrophils (n = 4 independent experiments; 61 cells).
In contrast to macrophages, prolonged application of ATP (3 mm) did not induce microblebbing, activation of caspase-3/7, or cell death in bone marrow-derived neutrophils (Gr-1+ cells) (Fig. 1C). This could be explained by a lack of P2X7 receptors in this cell type. Indeed, we could not detect P2X7 mRNA in Gr-1high/CD11bhigh cells (neutrophils) purified by cell sorting. Macrophages isolated from P2X7-deficient mice were similarly insensitive to prolonged ATP application (Fig. 1D). Hence, prolonged stimulation with high [ATP]e induces the activation of caspase-3/7 and cell death in WT and Casp1−/− macrophages, but has no effect on the viability of P2X7−/− macrophages and neutrophils (summarized in Fig. 1D). Thus, in principle, a modest time window is available for P2X7-mediated pro-IL-1β processing before caspase-3/7 activity and cell destruction manifest. However, it is unlikely that macrophages are confronted with high [ATP]e for prolonged periods. Other unidentified factors may increase the sensitivity of P2X7 ion channels to ATP. Alternatively, transient increases of ATP above 500 μm may be physiologically more relevant.
Transient ATP Application
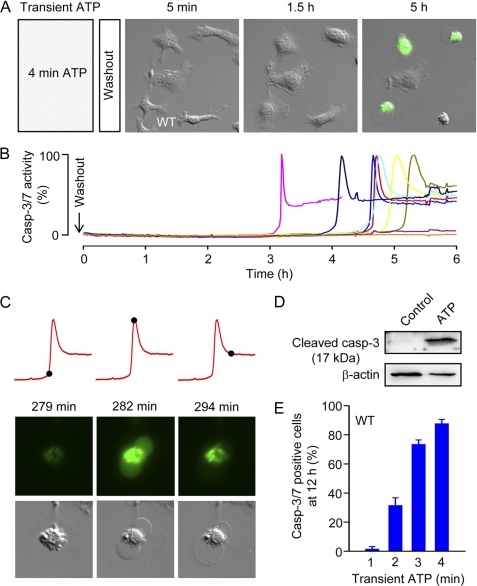
Next, we tested whether transient (1–4 min) P2X7 receptor stimulation is sufficient to induce cell death in macrophages. Macrophages were stimulated with 3 mm ATP for 1, 2, 3, or 4 min. Following washout of ATP, macrophages were incubated in medium containing DEVD-NucView488, and fluorescent and DIC images were taken every 1 min for 6 h. After application of ATP for 4 min and washout, the macrophages had “fuzzy” edges (probably due to cell swelling) and a paucity of lamellipodia (Fig. 2A and supplemental Video 1). However, within 1–2 h, the cells resumed a normal morphology characterized by broad lamellipodial membrane extensions and peripheral ruffling (Fig. 2A and supplemental Video 1). This pseudo-recovery period was interrupted by abrupt cell contraction and dynamic membrane blebbing, characteristic of apoptosis, followed by concurrent caspase-3/7 activity and massive membrane blebbing, indicating secondary necrosis (Fig. 2, A–C, and supplemental Video 1; for comparison, see the supplemental Video accompanying the review by Taylor et al. (29)). The kinetics of caspase-3/7 activation in individual cells is shown in Fig. 2B.
FIGURE 2.
Transient ATP application. A, delayed caspase-3/7 activation following a 4-min challenge with 3 mm ATP. B, kinetics of caspase-3/7 (Casp-3/7) activation in individual macrophages, including the five shown above. Images are 100 × 100 μm. C, typical morphological changes accompanying transient ATP-induced delayed caspase-3/7 (DEVDase) activation. The red traces show cell fluorescence as a function of time (as in B above), and the black spots indicate the time points 279, 282, and 294 min, respectively, after washout of ATP (applied for 4 min). The morphology of the macrophage corresponding to these time points is shown below. D, Western blot analysis of macrophage lysates obtained 3 h after sham treatment (Control) or 4 min ATP (3 mm) challenge, using anti-cleaved (activated) caspase-3 antibody. E, relation between duration of ATP challenge and cell death, indexed as caspase-3/7-positive cells at 12 h (n = 11–18 independent experiments (48–113 cells) per group).
The sequence of events described above (and shown in Fig. 2, A–C) was also observed in bone marrow-derived macrophage plated at high (>80%) confluency (supplemental Fig. 1). The macrophage purity was high, as indicated by anti-F4/80 labeling of living macrophages (supplemental Fig. 1A). At such high confluency, individual macrophages are in close contact with neighboring cells. However, following transient stimulation with high [ATP]e, confluency is decreased due to the retraction of lamellipodia (supplemental Fig. 1, B and C).
Activation of DEVDase (caspase-3/7) activity and massive blebbing consistently coincided, as shown in the example in Fig. 2C. Note that the cell becomes diffusely green due to the binding of NucView488 to RNA, and then NucView488 redistributes to DNA (28). Thus, in addition to reporting DEVDase activity, NucView488 provides a “view” of nuclear morphology. The nucleus of macrophages appeared condensed or fragmented, indicative of apoptosis, during delayed cell death (supplemental Video 1). As in the case of prolonged P2X7 receptor stimulation, delayed caspase-3/7 activation and massive blebbing following transient high [ATP]e were end-stage apoptotic events. To confirm that transient high [ATP]e leads to caspase-3 activation, we performed Western blot (Fig. 2D). Fully cleaved caspase-3 (17-kDa fragment) could be detected 4 h after transient high [ATP]e. Susceptibility to delayed caspase-3/7 activation and cell death increased steeply as a function of the duration of ATP application (Fig. 2E).
Loss of Cytochrome c and Nuclear Contraction
Cytochrome c was localized to the mitochondria in fixed and permeabilized macrophages labeled with Alexa Fluor 555-conjugated anti-cytochrome c antibodies (supplemental Fig. 2A). Apoptotic macrophages fixed 3 h after transient (4 min) ATP challenge were characterized by loss of mitochondrial cytochrome c and contracted nuclei (supplemental Fig. 2, B and C).
Absence of Delayed Cell Death in P2X7−/− Macrophages
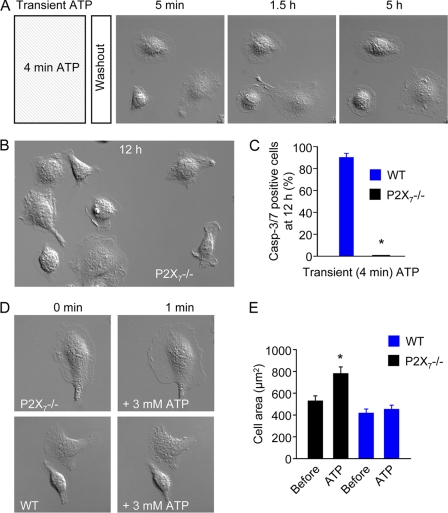
No apoptotic changes, such as microblebbing and caspase-3/7 activation, could be detected in P2X7−/− macrophages following 4 min of stimulation with millimolar ATP (Fig. 3, A–C, and supplemental Video 2). Instead of forming microblebs, P2X7−/− macrophages generated large lamellipodial membrane protrusions in response to high [ATP]e, such that cell area increased by ∼50% (Fig. 3, D and E). These observations confirm that transient millimolar ATP induces delayed cell death specifically via the activation of P2X7 receptors.
FIGURE 3.
Transient high [ATP]e induces cell spreading but not delayed cell death in P2X7−/− macrophages. A, lack of delayed caspase-3/7 (Casp-3/7) activation in P2X7−/− macrophages challenged with 3 mm ATP for 4 min. Images are 100 × 100 μm. B, P2X7−/− macrophages 12 h after transient challenge with 3 mm ATP. Image is 100 × 150 μm. C, summary data (n = 4 independent experiments (29 cells) in the WT group; n = 4 independent experiments (35 cells) in the P2X7−/− group). D, high ATP (3 mm) induces lamellipodial membrane protrusions in P2X7−/− but not WT macrophages. E, mean cell areas of WT (n = 30) and P2X7−/− (n = 22) macrophages before and 1 min after application of 3 mm ATP. *, p value < 0.05.
Transient ATP-induced Cell Death Is Independent of Caspase-1 and TLR Signaling
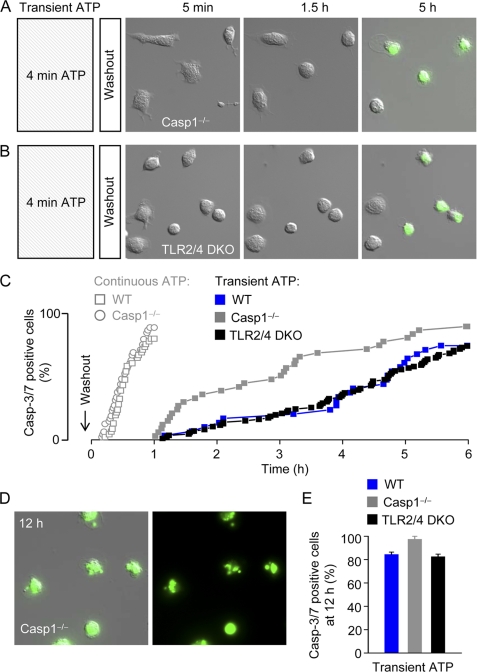
P2X7 receptor stimulation has been shown to activate caspase-1, and we speculated that this pro-inflammatory and pro-apoptotic enzyme could play a role in delayed ATP-induced cell death. However, Casp1−/− macrophages were clearly not protected from delayed P2X7-dependent cell death (Fig. 4A and supplemental Video 3). In addition, we found that macrophages isolated from TLR2 and TLR4 double knock-out (TLR2/4 dKO) mice were not protected from ATP-induced cell death (Fig. 4B). The cumulative death rates of individual WT, Casp1−/−, and TLR2/4 dKO macrophages (solid symbols) are plotted in Fig. 4C. It can be seen that one cell after the other becomes caspase-3/7-positive (cell death end point) starting at around 1 h after washout of ATP. For comparison, the data from Fig. 1B, showing the cumulative death rates during continuous ATP application, are superimposed (open symbols). Most cells die within 1 h of continuous high [ATP]e. As in the case of WT cells, the nucleus or Casp1−/− and TLR2/4 dKO macrophages became fragmented during delayed cell death (for example, Fig. 4D), and by 12 h after washout, less than ∼20% of WT, Casp1−/−, and TLR2/4 dKO cells survived (Fig. 4E). Thus, the data in Fig. 4 indicate that TLR signaling and cleavage of pro-caspase-1 to active caspase-1 are not required in the P2X7-triggered signaling cascade culminating in caspase-3/7 activation and cell death.
FIGURE 4.
Transient high [ATP]e induces cell death in Casp1−/− and TLR2/4 double knock-out macrophages. A, delayed caspase-3/7 activation and massive blebbing in Casp1−/− macrophages following a 4-min challenge with 3 mm ATP. Images are 100 × 100 μm. B, delayed caspase-3/7 activation and massive blebbing in TLR2/4 dKO macrophages following a 4-min challenge with 3 mm ATP. Images are 100 × 100 μm. C, cumulative plot of caspase-3/7 (Casp-3/7) activation in individual macrophages isolated from WT, Casp1−/−, and TLR2/4 dKO mice. For comparison, the cumulative plots (from Fig. 1B) of WT and Casp1−/− macrophages during continuous high ATP application are superimposed (open symbols). D, apoptotic Casp1−/− cells 12 h after transient high [ATP]e. The left image shows an overlay of DIC and green (NucView488) fluorescence images, whereas the right image shows the fluorescence image alone. The Images are 100 × 100 μm. E, summary data (n = 4–12 independent experiments (34–127 cells)).
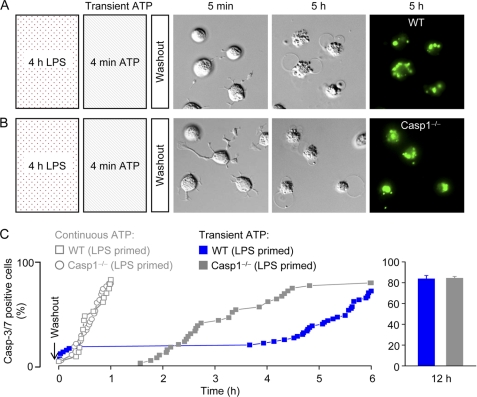
Effects of LPS Priming on Transient ATP-induced Cell Death
Priming with LPS (1 μg/ml for 2 h) has previously been reported to render WT macrophages susceptible to cell death (indexed as lactate dehydrogenase release) within 30 min of a brief ATP (5 mm for 5 min) challenge, whereas Casp1−/− cells are protected (15). We therefore tested whether LPS priming (1 μg/ml for 4 h) potentiated transient (4 min) ATP-induced caspase-3/7 activation and cell death. Similar to unprimed macrophages (Fig. 4), transient ATP-induced delayed cell death was observed in both WT and Casp1−/− macrophages primed with LPS (Fig. 5). However, ∼18% of LPS-primed WT macrophages became caspase-3/7-positive within 30 min (Fig. 5C) of ATP washout, as compared with 0% in unprimed WT cells (Fig. 4C). In the case of Casp1−/− macrophages, ATP-induced delayed caspase-3/7 activation and cell death were similar in unprimed (Fig. 4C) and LPS-primed (Fig. 5C) cells.
FIGURE 5.
Effects of LPS priming on cell death induced by continuous or transient ATP application. A, delayed caspase-3/7 activation and massive blebbing in LPS-primed (1 μg/ml for 4 h) WT macrophages following a 4-min challenge with 3 mm ATP. Images are 70 × 70 μm. B, delayed caspase-3/7 activation and massive blebbing in LPS-primed Casp1−/− macrophages following a 4-min challenge with 3 mm ATP. Images are 70 × 70 μm. C, cumulative plot of caspase-3/7 (Casp-3/7) activation in individual LPS-primed macrophages isolated from WT and Casp1−/− mice. For comparison, the cumulative plots of LPS-primed WT and Casp1−/− macrophages during continuous high ATP application are superimposed (open symbols). Data are from n = 2–3 independent experiments.
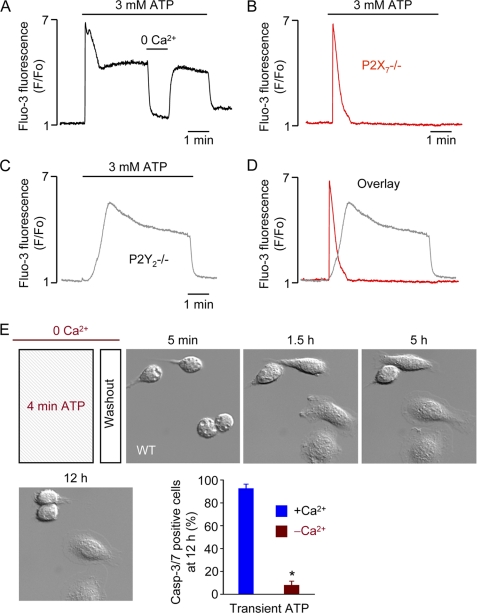
Massive Ca2+ Influx Is Critical Determinant of P2X7-mediated Delayed Cell Death
Superfusion of macrophages with ATP concentrations up to 300 μm has previously been reported to induce a large Ca2+ transient attributable to P2Y2 receptor activation and a small Ca2+ influx signal via P2X1 and P2X4 receptors (30). In single-cell Ca2+ recordings, we found that high [ATP]e (3 mm) induced a sustained increase in cytosolic [Ca2+] following the initial Ca2+ transient (Fig. 6A). The elevated Ca2+ plateau could be interrupted by superfusing the cell with Ca2+-free medium. We presumed that the sustained Ca2+ component was solely due to P2X7 receptor activation. Indeed, there was no Ca2+ plateau in P2X7−/− macrophages superfused with high [ATP]e (Fig. 6B). Instead, ATP induced a single Ca2+ spike in P2X7−/− macrophages, consistent with P2Y2 receptor activation. The lack of sustained Ca2+ influx in P2X7−/− macrophages is good evidence, together with the lack of microblebbing and cell death, that the functional P2X7 splice variant found in some tissues (31) is not (functionally) expressed in macrophages. Using P2Y2−/− macrophages, we could “isolate” the P2X7-mediated sustained Ca2+ signal (Fig. 6C). An overlay of the P2Y2- and P2X7-mediated Ca2+ signaling components is shown in Fig. 6D.
FIGURE 6.
P2X7 receptor-mediated Ca2+ signaling and cell death. A, cytosolic Ca2+ response, indexed as fluo-3 fluorescence, of a single WT macrophage superfused with 3 mm ATP. The cell was superfused for a short period with nominally Ca2+-free (0 Ca2+) medium as indicated. B, cytosolic Ca2+ response of a single P2X7−/− macrophage superfused with 3 mm ATP. C, superfusion of a P2Y2−/− macrophage with high ATP reveals the P2X7-mediated Ca2+ component. D, overlay of Ca2+ transients induced by high ATP in P2X7−/− and P2Y2−/− macrophages. E, macrophages are protected from delayed cell death when the trigger ATP is applied in nominally Ca2+-free (0 Ca2+) medium. Images are 100 × 100 μm. *, p value < 0.05.
Ca2+ has been recognized as a death trigger for a long time (32), and we speculated that the striking Ca2+ influx and Ca2+ overload mediated by P2X7 receptor activation may play a role in transient high [ATP]e-induced cell death. Consistent with this notion, application of millimolar ATP in Ca2+-free (0 Ca2+) medium protected macrophages from delayed cell death (Fig. 6E). That is, the transient (4 min) application of ATP was bracketed with Ca2+-free medium, and under these conditions, a single Ca2+ spike, similar to that seen in P2X7−/− cells (Fig. 6B), is evoked. Hence, the duration of P2X7 ligation and accompanying sustained Ca2+ influx are important determinants of death signaling.
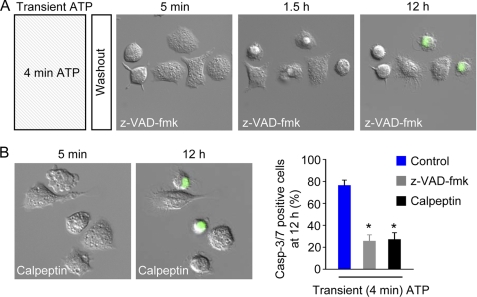
Pan-caspase and Calpain Inhibitors Attenuate Transient ATP-induced Delayed Cell Death
In the presence of the cell-permeable pan-caspase inhibitor z-VAD-fmk (40 μm), macrophages were partially protected from delayed cell death induced by 4 min of exposure to 3 mm ATP (Fig. 7A). Cells were similarly protected by calpeptin (100 μm), an inhibitor of the calpain family of Ca2+-activated proteases (Fig. 7B). These inhibitor experiments indicate that calpain and caspases contribute to transient ATP-induced cell death signaling cascade.
FIGURE 7.
Inhibitors of proteases (caspases and calpains) attenuate high ATP-induced delayed cell death. A, time-lapse images (100 × 100 μm) of WT macrophages in the continued presence of the pan-caspase inhibitor z-VAD-fmk (40 μm) following washout of 3 mm ATP (applied for 4 min). B, time-lapse images (100 × 100 μm) of WT macrophages in the continued presence of the calpain inhibitor calpeptin (100 μm) following washout of 3 mm ATP (applied for 4 min). Right panel, summary data (n = 4–8 independent experiments; 39–99 cells). *, p value < 0.05. Casp-3/7, caspase-3/7.
Pannexin-1 Is Not P2X7-dependent Large Pore Pathway
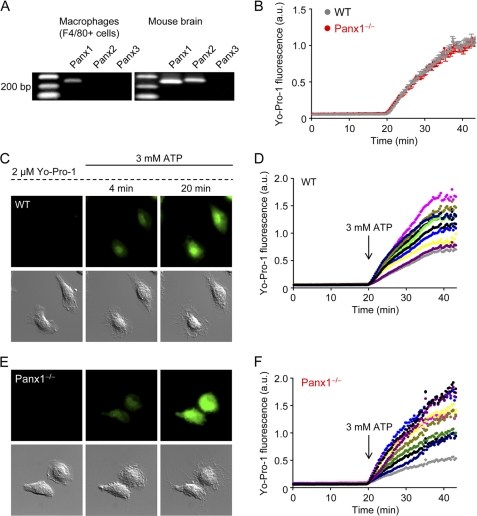
In addition to the ions Na+ (23 Da), K+ (39 Da), and Ca2+ (40 Da), activated P2X7 receptors become permeable to molecules up to a size of ∼900 Da. Pannexin-1 has been implicated as the molecular correlate of this P2X7-dependent large pore pathway and has been implicated in cell death (6, 33–36). In purified F4/80+ cells (macrophages), we could detect mRNA for Panx1, but not Panx2 or Panx3 (Fig. 8A). To investigate whether Panx1 is the large pore pathway accompanying P2X7 receptor ligation, we compared the rates of ATP-induced dye (Yo-Pro-1; Mr 375) uptake in single WT and Panx1−/− macrophages using time-lapse microscopy. Yo-Pro-1 is a green fluorescent DNA and RNA stain that is normally cell-impermeable. The mean rates of ATP-induced Yo-Pro-1 uptake by WT and Panx1−/− macrophages are shown in Fig. 8B. These results indicate that Panx1 is not the large pore pathway in native macrophages.
FIGURE 8.
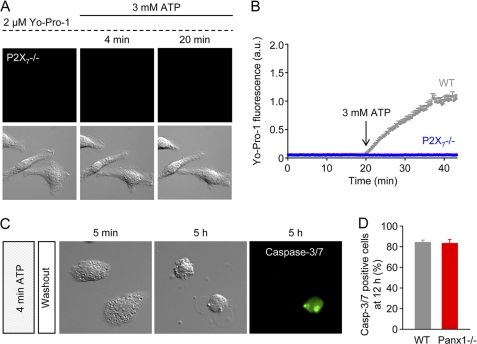
ATP-induced dye (Yo-Pro-1) uptake in WT and Panx1−/− macrophages. A, purified mouse peritoneal macrophages (F4/80+ cells) express Panx1, but not Panx2 or Panx3. B, summary of high ATP (3 mm)-induced Yo-Pro-1 uptake data obtained using macrophages isolated from WT (n = 30 cells; 3 independent experiments) or Panx1−/− mice (n = 30 cells; 3 independent experiments). a.u., arbitrary units. C, time-lapse Yo-Pro-1 fluorescence (green) and DIC images of WT macrophages challenged with 3 mm ATP. Yo-Pro-1 (2 μm), which becomes fluorescent upon binding to DNA and RNA, was present throughout the experiment. Images are 70 × 70 μm. D, kinetics of Yo-Pro-1 uptake in individual WT macrophages. After a 20-min baseline period, a high ATP (3 mm) was added. E, time-lapse Yo-Pro-1 fluorescence and DIC images of Panx1−/− macrophages challenged with 3 mm ATP. Images are 70 × 70 μm. F, kinetics of Yo-Pro-1 uptake in individual Panx1−/− macrophages.
The kinetics of Yo-Pro-1 uptake in individual cells is shown in Fig. 8 (C–F). During the initial 20-min incubation period with 2 μm Yo-Pro-1, there was no constitutive uptake of Yo-Pro-1 in WT macrophages (Fig. 8, C and D). However, application of 3 mm ATP induced Yo-Pro-1 uptake, consistent with the opening of a large pore (Fig. 8C and supplemental Video 4). Yo-Pro-1 uptake was not impaired in Panx1−/− macrophages. The time course of Yo-Pro-1 uptake by Panx1−/− macrophages is shown in Fig. 8, E and F (see also supplemental Video 5).
We tested whether stimulation of P2X7 receptors with a lower concentration of ligand, 500 μm instead of 3 mm ATP, was sufficient to induce Yo-Pro-1 uptake (supplemental Fig. 3). There was no obvious change in cell morphology after 20 min (supplemental Fig. 3A), or even 60 min, of continuous stimulation with 500 μm ATP. However, a weak increase in Yo-Pro-1 uptake could be detected (supplemental Fig. 3, A and B). The magnitude of Yo-Pro-1 fluorescence induced by 500 μm ATP was much less than that evoked by 3 mm (compare supplemental Fig. 3B with Fig. 8D). A similar weak increase in Yo-Pro-1 uptake was measured in Panx1−/− macrophages challenged with 500 μm ATP (supplemental Fig. 3, C and D). Thus, Panx1 is not responsible for the weak dye uptake response to 500 μm ATP. As in the case for WT macrophages, continuous stimulation of Panx1-deficient cells with 500 μm ATP also did not induce cell death.
Lack of ATP-induced Yo-Pro-1 Uptake in P2X7−/− Macrophages
We speculated that the stimulation of P2Y2 receptors may cause weak (low capacity) Yo-Pro-1 uptake, which is otherwise masked by the dominant effect of P2X7. However, this was not the case because no Yo-Pro-1 uptake could be detected when P2X7−/− macrophages were challenged with ATP (Fig. 9, A and B, and supplemental Video 6). These findings indicate that P2X7 receptors mediate the weak Yo-Pro-1 uptake induced by 500 μm ATP, as well as the robust dye uptake evoked by 3 mm ATP.
FIGURE 9.
Lack of Yo-Pro-1 uptake in P2X7−/− macrophages and Panx1 deficiency do not protect cells from transient high ATP-induced cell death. A, lack of Yo-Pro-1 uptake in P2X7−/− macrophages challenged with 3 mm ATP. Images are 70 × 70 μm. B, baseline Yo-Pro-1 fluorescence intensity, measured in individual cells ( n = 10), is unaffected by the application of 3 mm ATP (representative of 3 independent experiments). The mean response of WT cells (from Fig. 7) has been superimposed. a.u., absorbance units. C, delayed caspase-3/7 activation and cell death in Panx1−/− macrophages following a 4-min challenge with 3 mm ATP. Images are 70 × 70 μm. D, summary data of cell death in WT (2 independent experiments; n = 24 cells) and Panx1−/− (2 independent experiments; n = 25 cells) macrophages, assessed 12 h after a 4-min challenge with 3 mm ATP. Casp-3/7, caspase-3/7.
Panx1 Deficiency Does Not Protect Macrophages from Transient ATP-induced Cell Death
Panx1 activation has been implicated in cell death in response to P2X7 receptor ligands (33, 35). Therefore, we investigated whether Panx1−/− macrophages were protected from cell death (Fig. 9, C and D). Delayed transient ATP-induced cell death was clearly not reduced in Panx1−/− macrophages (Fig. 9, C and D). Thus, the pro-death signaling triggered by P2X7 receptor activation in native macrophages is not dependent on Panx1.
DISCUSSION
In the innate immune system, P2X7 receptors have emerged as potentially important modulators of inflammation on the basis that moderately prolonged (typically 15–20 min) stimulation of these ion channels induces caspase-1-dependent processing of pro-inflammatory cytokines (11–20). Here we identify a new paradigm in which transient (1–4 min) stimulation of P2X7 receptors triggers delayed (hours) macrophage death independent of caspase-1, TLR signaling, and pannexin-1. It was already known that prolonged (>30 min) stimulation of P2X7 receptors was lethal to cells, presumably due to massive perturbations of Na+, K+, and Ca2+ homeostasis and large pore formation (6, 18). The surprising finding that transient (1–4 min) P2X7 receptor activation triggers delayed effector caspase (caspase-3/7) activity and cell death suggests that ATP-gated P2X7 ion channels are essentially macrophage death receptors.
High [ATP]e (acting at P2X7 receptors) is one of many “danger signals” known to activate the inflammasome, a complex formed by the assembly of subsets of NLR (nucleotide-binding domain and leucine-rich repeat containing) proteins, such as NLRP3 (also called NALP3) (37), and the adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), encoded by the gene Pycard (38, 39). Inflammasomes bind to pro-caspase-1 and induce its proteolysis-dependent activation. Thus, the inflammasome, which activates pro-inflammatory caspase-1, can be considered as the inflammatory equivalent of the “apoptosome,” a protein complex that activates pro-apoptotic caspase-9 (40).
High [ATP]e (acting at P2X7 receptors) can also induce macrophage-specific cell death, and our data indicate that activation of P2X7 receptors for 1–4 min is sufficient to trigger apoptotic cell death. What was already known about the effects of transient P2X7 receptor stimulation on cell fate? In 2002, Le Feuvre et al. (15) reported that “a brief pulse of ATP (5 mm for 5 min) had no effect on basal lactate dehydrogenase release (measured in supernatant samples taken 0.5 and 2 h after stimulation) from control WT (mouse peritoneal) macrophages, and the cells completely excluded trypan blue (data not shown).” In light of our findings, revealed by live-cell imaging, that transient high [ATP]e leads to caspase-3/7 activation and cell death delayed by hours (Figs. 2 and 4), we presume that Le Feuvre et al. (15) would have detected loss of cell integrity (secondary necrosis) if they had followed the fate of the cells for a longer period. The authors, however, found that a brief pulse (5 min) of ATP variably induced massive lactate dehydrogenase release (∼40 and ∼100% of total release measured in two separate experiments, respectively) within 30 min from WT, but not caspase-1-deficient, macrophages that had been primed with LPS. In contrast, Pelegrin et al. (20) reported that application of 5 mm ATP for 20 min caused negligible lactate dehydrogenase release (2–4% of total) in mouse peritoneal macrophages primed with LPS for 4 h. Thus, the extent to which LPS priming sensitizes macrophages to ATP-induced cell death is unclear. In our study, we found that transient ATP similarly induced delayed cell death in WT, Casp1−/−, TLR2/4 dKO, and Panx1−/− macrophages, as well as in LPS-primed (4 h) WT and Casp1−/− cells. Cell death, indexed as caspase-3/7 activity, was negligible in the first 60 min after transient ATP application, except in LPS-primed WT macrophages, ∼20% of which became caspase-3/7-positive within 30 min. Thus, priming with LPS, and possibly other pathogen-associated molecular patterns or even macrophage infection, may shorten the delay between transient ATP stimulation and cell death in a caspase-1-dependent fashion.
Live-cell imaging revealed that the fate of macrophages after transient P2X7 receptor stimulation follows a distinct sequence of events. After washout of ATP, the cells appear to recover from the initial microblebbing and loss of lamellipodial membrane protrusive activity, but in the following hours, suddenly one cell after the other tightly contracts and further dynamic microblebbing manifests, followed by the formation of large, expansive blebs. The nuclei become contracted or fragmented, features typical for apoptosis (29). A consistent feature of delayed death in individual cells is that massive bleb formation coincides with the emergence of caspase-3/7 activity. We assume that the effector caspases caspase-3 and caspase-7 contribute to the massive blebbing. These effector caspases target multiple proteins of the cytoskeleton, including key components of actin filaments, intermediate filaments, and microtubules (29). The pan-caspase inhibitor z-VAD-fmk did not completely abrogate transient ATP-induced delayed cell death, indicating that the mode of death signaling is only partially caspase-dependent.
What is the function of P2X7 receptors expressed on macrophages? The major differences between P2X7 receptors and other members of the P2X family are (i) activation by high [ATP]e (0.5–3 mm rather than 0.1–100 μm range), (ii) lack of desensitization (P2X receptors typically desensitize within seconds), and (iii) the permeability transition to larger molecules upon stimulation. The requirement for high [ATP]e suggests that P2X7 ion channels are activated under extreme conditions, such as massive tissue injury. Our data indicate that the Ca2+ overload induced by several minutes of P2X7 receptor stimulation serves as a death trigger. Identifying pathophysiological scenarios in which [ATP]e and cytosolic [Ca2+] are increased, even for several minutes, remains a challenge in the fields of purinergic signaling and immunology. Unidentified endogenous or pathogen-associated factors may come into play to increase the affinity of P2X7 receptors for ATP. The contribution of P2X7 receptor-induced large pore formation to transient high [ATP]e-induced cell death remains unclear. Genetic deletion of pannexin-1, putative P2X7-mediated large pore pathway, did not affect the kinetics of ATP-induced Yo-Pro-1 uptake, and transient ATP-induced delayed cell death was unaffected. We speculate that instead of recruiting another transport pathway, the P2X7 channel protein probably changes configuration to allow the passage of larger molecules, although to date single-channel current recordings have not been able to reveal such dramatic changes in size selectivity (41).
In summary, there is considerable evidence linking P2X7 receptor function with caspase-1 and TLR4 signaling pathways, and prolonged P2X7 ion channel stimulation is known to induce cytolytic cell death. We now show, surprisingly, that death is an early triggered event, such that transient (1–4 min) high [ATP]e leads to delayed (hours) cell death. Moreover, our results clearly show that death signaling depends on the duration of trigger ATP and Ca2+ overload, but it is independent of pro-inflammatory caspase-1 activation and TLR signaling. Finally, we also show that pannexin-1 is not involved in P2X7-dependent Yo-Pro-1 uptake or transient high [ATP]e-induced delayed cell death.
This work was supported in part by the Deutsche Forschungsgemeinschaft (DFG) Grant SCHW 407/9-3 (to A. S.) and the Innovative Medizinische Forschung (IMF) Grant HA110710 (to P. J. H.) from the Westfälische Wilhems-Universität Münster.

This article contains supplemental Videos 1–6 and Figs. 1–3.
- TLR
- Toll-like receptor
- dKO
- double knock-out
- DMSO
- dimethyl sulfoxide
- TMRE
- tetramethylrhodamine, ethyl ester
- DIC
- differential interference contrast
- z-VAD-fmk
- carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone.
REFERENCES
- 1. North R. A., Surprenant A. (2000) Pharmacology of cloned P2X receptors. Annu. Rev. Pharmacol. Toxicol. 40, 563–580 [DOI] [PubMed] [Google Scholar]
- 2. Khakh B. S., North R. A. (2006) P2X receptors as cell-surface ATP sensors in health and disease. Nature 442, 527–532 [DOI] [PubMed] [Google Scholar]
- 3. North R. A. (2002) Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 [DOI] [PubMed] [Google Scholar]
- 4. Steinberg T. H., Silverstein S. C. (1987) Extracellular ATP4− promotes cation fluxes in the J774 mouse macrophage cell line. J. Biol. Chem. 262, 3118–3122 [PubMed] [Google Scholar]
- 5. Chessell I. P., Simon J., Hibell A. D., Michel A. D., Barnard E. A., Humphrey P. P. (1998) Cloning and functional characterization of the mouse P2X7 receptor. FEBS Lett. 439, 26–30 [DOI] [PubMed] [Google Scholar]
- 6. Pelegrin P., Surprenant A. (2006) Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 25, 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iglesias R., Locovei S., Roque A., Alberto A. P., Dahl G., Spray D. C., Scemes E. (2008) P2X7 receptor-Pannexin-1 complex: pharmacology and signaling. Am. J. Physiol. Cell Physiol 295, C752–C760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collo G., Neidhart S., Kawashima E., Kosco-Vilbois M., North R. A., Buell G. (1997) Tissue distribution of the P2X7 receptor. Neuropharmacology 36, 1277–1283 [DOI] [PubMed] [Google Scholar]
- 9. Surprenant A., Rassendren F., Kawashima E., North R. A., Buell G. (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272, 735–738 [DOI] [PubMed] [Google Scholar]
- 10. Solle M., Labasi J., Perregaux D. G., Stam E., Petrushova N., Koller B. H., Griffiths R. J., Gabel C. A. (2001) Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 276, 125–132 [DOI] [PubMed] [Google Scholar]
- 11. Perregaux D., Gabel C. A. (1994) Interleukin-1β maturation and release in response to ATP and nigericin: evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269, 15195–15203 [PubMed] [Google Scholar]
- 12. Grahames C. B., Michel A. D., Chessell I. P., Humphrey P. P.(1999) Pharmacological characterization of ATP- and LPS-induced IL-1β release in human monocytes. Br. J. Pharmacol. 127, 1915–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanz J. M., Di Virgilio F. (2000) Kinetics and mechanism of ATP-dependent IL-1β release from microglial cells. J. Immunol. 164, 4893–4898 [DOI] [PubMed] [Google Scholar]
- 14. Mehta V. B., Hart J., Wewers M. D. (2001) ATP-stimulated release of interleukin (IL)-1β and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J. Biol. Chem. 276, 3820–3826 [DOI] [PubMed] [Google Scholar]
- 15. Le Feuvre R. A., Brough D., Iwakura Y., Takeda K., Rothwell N. J. (2002) Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J. Biol. Chem. 277, 3210–3218 [DOI] [PubMed] [Google Scholar]
- 16. Brough D., Le Feuvre R. A., Wheeler R. D., Solovyova N., Hilfiker S., Rothwell N. J., Verkhratsky A. (2003) Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1β and IL-1α from murine macrophages. J. Immunol. 170, 3029–3036 [DOI] [PubMed] [Google Scholar]
- 17. Gudipaty L., Munetz J., Verhoef P. A., Dubyak G. R. (2003) Essential role for Ca2+ in regulation of IL-1β secretion by P2X7 nucleotide receptor in monocytes, macrophages, and HEK-293 cells. Am. J. Physiol. Cell Physiol. 285, C286–CC299 [DOI] [PubMed] [Google Scholar]
- 18. Kahlenberg J. M., Dubyak G. R. (2004) Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. Cell Physiol. 286, C1100–C1108 [DOI] [PubMed] [Google Scholar]
- 19. Qu Y., Franchi L., Nunez G., Dubyak G. R. (2007) Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179, 1913–1925 [DOI] [PubMed] [Google Scholar]
- 20. Pelegrin P., Barroso-Gutierrez C., Surprenant A. (2008) P2X7 receptor differentially couples to distinct release pathways for IL-1β in mouse macrophage. J. Immunol. 180, 7147–7157 [DOI] [PubMed] [Google Scholar]
- 21. Yegutkin G. G. (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signaling cascade. Biochim Biophys Acta 1783, 673–694 [DOI] [PubMed] [Google Scholar]
- 22. Anselmi F., Hernandez V. H., Crispino G., Seydel A., Ortolano S., Roper S. D., Kessaris N., Richardson W., Rickheit G., Filippov M. A., Monyer H., Mammano F. (2008) ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. U.S.A. 105, 18770–18775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J., et al. (1995) Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 [DOI] [PubMed] [Google Scholar]
- 24. Chessell I. P., Hatcher J. P., Bountra C., Michel A. D., Hughes J. P., Green P., Egerton J., Murfin M., Richardson J., Peck W. L., Grahames C. B., Casula M. A., Yiangou Y., Birch R., Anand P., Buell G. N. (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114, 386–396 [DOI] [PubMed] [Google Scholar]
- 25. Hanley P. J., Xu Y., Kronlage M., Grobe K., Schön P., Song J., Sorokin L., Schwab A., Bähler M. (2010) Motorized RhoGAP myosin IXb (Myo9b) controls cell shape and motility. Proc. Natl. Acad. Sci. U.S.A. 107, 12145–12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kronlage M., Song J., Sorokin L., Isfort K., Schwerdtle T., Leipziger J., Robaye B., Conley P. B., Kim H. C., Sargin S., Schön P., Schwab A., Hanley P. J. (2010) Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci. Signal 3: ra55. [DOI] [PubMed] [Google Scholar]
- 27. Huang Y. J., Maruyama Y., Dvoryanchikov G., Pereira E., Chaudhari N., Roper S. D. (2007) The role of pannexin-1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc. Natl. Acad. Sci. U.S.A. 104, 6436–6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cen H., Mao F., Aronchik I., Fuentes R. J., Firestone G. L. (2008) DEVD-NucView488: a novel class of enzyme substrates for real-time detection of caspase-3 activity in live cells. FASEB J. 22, 2243–2252 [DOI] [PubMed] [Google Scholar]
- 29. Taylor R. C., Cullen S. P., Martin S. J. (2008) Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 9, 231–241 [DOI] [PubMed] [Google Scholar]
- 30. del Rey A., Renigunta V., Dalpke A. H., Leipziger J., Matos J. E., Robaye B., Zuzarte M., Kavelaars A., Hanley P. J. (2006) Knock-out mice reveal the contributions of P2Y and P2X receptors to nucleotide-induced Ca2+ signaling in macrophages. J. Biol. Chem. 281, 35147–35155 [DOI] [PubMed] [Google Scholar]
- 31. Nicke A., Kuan Y. H., Masin M., Rettinger J., Marquez-Klaka B., Bender O., Górecki D. C., Murrell-Lagnado R. D., Soto F. (2009) A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. J. Biol. Chem. 284, 25813–25822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Orrenius S., Zhivotovsky B., Nicotera P. (2003) Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 4, 552–565 [DOI] [PubMed] [Google Scholar]
- 33. Locovei S., Scemes E., Qiu F., Spray D. C., Dahl G. (2007) Pannexin-1 is part of the pore-forming unit of the P2X7 receptor death complex. FEBS Lett. 581, 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pelegrin P., Surprenant A. (2007) Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1β release through a dye uptake-independent pathway. J. Biol. Chem. 282, 2386–2394 [DOI] [PubMed] [Google Scholar]
- 35. MacVicar B. A., Thompson R. J. (2010) Nonjunction functions of pannexin-1 channels. Trends Neurosci. 33, 93–102 [DOI] [PubMed] [Google Scholar]
- 36. Pelegrin P., Surprenant A. (2009) Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1β release through pyrophosphate. EMBO J. 28, 2114–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bauernfeind F., Bartok E., Rieger A., Franchi L., Núñez G., Hornung V. (2011) Cutting edge: reactive oxygen species inhibitors block priming, but not activation of the NLRP3 inflammasome. J. Immunol. 187, 613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davis B. K., Wen H., Ting J. P. (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pétrilli V., Dostert C., Muruve D. A., Tschopp J. (2007) The inflammasome: a danger-sensing complex triggering innate immunity. Curr. Opin Immunol. 19, 615–622 [DOI] [PubMed] [Google Scholar]
- 40. Riedl S. J., Salvesen G. S. (2007) The apoptosome: signaling platform of cell death. Nat. Rev. Mol. Cell Biol. 8, 405–413 [DOI] [PubMed] [Google Scholar]
- 41. Riedel T., Schmalzing G., Markwardt F. (2007) Influence of extracellular monovalent cations on pore and gating properties of P2X7 receptor-operated single-channel currents. Biophys. J. 93, 846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]