Background: Curcumin can overcome CsA resistance. However, the molecular mechanism is unknown.
Results: Curcumin blocks T cell stimulation-induced Ca2+ mobilization and thereby prevents NFAT activation, a mechanism different from CsA.
Conclusion: Curcumin is an immunosuppressive phytochemical that blocks Ca2+ signaling.
Significance: The study demonstrates for the first time that curcumin is a potent inhibitor of NFAT activation via blocking Ca2+ signaling in T cells.
Keywords: Calcium Signaling, Cytokine, Drug Action, NF-κB (NF-κB), NFAT Transcription Factor, T Cell
Abstract
Curcumin is the active ingredient of the spice turmeric and has been shown to have a number of pharmacologic and therapeutic activities including antioxidant, anti-microbial, anti-inflammatory, and anti-carcinogenic properties. The anti-inflammatory effects of curcumin have primarily been attributed to its inhibitory effect on NF-κB activity due to redox regulation. In this study, we show that curcumin is an immunosuppressive phytochemical that blocks T cell-activation-induced Ca2+ mobilization with IC50 = ∼12.5 μm and thereby prevents NFAT activation and NFAT-regulated cytokine expression. This finding provides a new mechanism for curcumin-mediated anti-inflammatory and immunosuppressive function. We also show that curcumin can synergize with CsA to enhance immunosuppressive activity because of different inhibitory mechanisms. Furthermore, because Ca2+ is also the secondary messenger crucial for the TCR-induced NF-κB signaling pathway, our finding also provides another mechanism by which curcumin suppresses NF-κB activation.
Introduction
Curcumin (Diferuloylmethane) is a polyphenol product derived from the rhizomes of plant Curcuma longa widely used for flavoring additives and food preservatives in Asiatic countries. In old Hindu and in traditional Chinese medicine, Curcuma longa has been used for treatment of a variety of inflammatory conditions, skin wounds, hepatic, and biliary disorders (1). Research over the last 50 years demonstrates that curcumin possesses a variety of pharmacological effects against inflammation and infections from parasites, bacteria, and viruses (2, 3). Because of its potent anti-inflammatory properties, curcumin has actively been under clinical trials in various inflammatory conditions e.g. post-surgery, rheumatoid arthritis, osteoarthritis, dyspepsia, gastric ulcer, inflammatory bowel disease and pancreatitis (3, 4). Current studies indicate that curcumin may also have potential in prevention and treatment of tumors (6, 7).
Curcumin has been shown to suppress production of a number of inflammatory cytokines including TNF, IL-1, IL-8, IL-12, and chemokines (8–12). To a large part, the anti-inflammatory potential of curcumin stems from its antioxidant capacity (depending on the dose) leading to down-regulation of transcription factors, e.g. NF-κB, STAT-1, and STAT-3 (13–15). Along this line, pretreatment with curcumin was found to prolong skin graft survival in an animal model (16). A later study showed that curcumin could enhance the immunosuppressive activity of cyclosporine A (CsA)2 in rat cardiac allografts (17). Moreover, curcumin was found to be a potent inhibitor of CsA-resistant T cells in response to T cell activation (18). These studies imply that curcumin itself might have a direct immunosuppressive function.
Physiologically, T cell activation via T cell receptor (TCR) engagement initiates activation of SRC protein-tyrosine kinases (PTKs) LCK and FYN which in turn phosphorylate the TCR subunits at tyrosine residues. Phosphorylated TCR subunits function as docking sites for the recruitment of the PTK ZAP70 which subsequently phosphorylates several substrates, the linker for activation of T cells (LAT), SLP76 and phospholipase C-γ (PLC-γ). Activation of PLC-γ by phosphorylation leads to hydrolysis of phosphatidylinositol 4,5-bisphosphate to inositol 3,4,5-triphosphate (IP3) and diacylglycerol (DAG). DAG activates protein kinase Cθ (PKCθ)/NF-κB and the mitogen-activated protein kinases (MAPK) pathways, whereas, IP3 production triggers release of Ca2+ from intracellular stores in the endoplasmic reticulum (ER) (19, 20). Depletion of Ca2+ stores is then sensed by stromal interaction molecule 1 (STIM1), an ER-resident sensor, which triggers entry of Ca2+ through the Ca2+ release-activated calcium (CRAC) channels in the plasma membrane (20, 21). Increase in cytoplasmic Ca2+ levels triggers activation of the Ca2+-dependent serine/threonine phosphatase calcineurin, which in turn activates the nuclear factor of activated T cells (NFAT) by dephosphorylation and nuclear translocation. NFAT is a key regulator of T cell function and is essential for activating most T cell cytokine genes (20, 22).
The well-known NFAT inhibitors are CsA and FK506 that have been used in organ transplantation to prevent graft-versus-host disease (23). Both drugs target calcineurin (24). Because curcumin could overcome CsA-resistant T cells (18), we asked whether curcumin suppresses NFAT activation and if so by what mechanism curcumin overcomes CsA resistance. In this study, we show that curcumin exerts its immunosuppressive activity by suppressing NFAT activation via a mechanism different from CsA and FK506. We demonstrate that curcumin blocks T cell-stimulation-induced Ca2+ mobilization and thereby prevents calcium signaling in lymphocyte activation.
EXPERIMENTAL PROCEDURES
Cells and Cell Culture
The Jurkat T leukemia cells were cultured in RPMI 1640 medium (Sigma) supplemented with 10% FCS, 50 μg/ml gentamicin, 6 mm HEPES (Sigma), and 2 mm l-glutamine (Sigma) at 37 °C and 5% CO2. Human peripheral blood T cells were prepared as described previously (25). Briefly, peripheral blood mononuclear cells were prepared by Ficoll-Paque (Pharmacia) density centrifugation. Adherent cells were removed by adherence to plastic culture vessels for 1 h. T cells were isolated from the peripheral blood mononuclear cells by rosetting with 2 amino-ethylisothio-uronium-bromide-treated sheep red blood cells. For inductions, cells were stimulated by α-CD3 (OKT3 10 μg/ml) or PMA (5 ng/ml) (Sigma) plus ionomycin (1 μm) (Merck, Darmstadt, Germany) for the indicated time. Curcumin (> 99%) was purchased from Alexis Biochemicals (San Diego, CA).
Determination of IL-2 and IFN-γ Proteins
Freshly isolated peripheral blood T cells (1 × 106/ml) were stimulated by PMA (5 ng/ml) plus ionomycin (1 μm) in the absence or presence of curcumin for 24 h. Supernatants were tested for the presence of IL-2 and IFN-γ using an enzyme-linked immunosorbent assay (ELISA) specific for human IL-2 and IFN-γ proteins (Becton Dickinson Company, Heidelberg, Germany) according to the manufacturer's instruction.
Flow Cytometry Analysis of CD69 Surface Expression
Cells were washed twice with PBS and stained with fluorescent-tagged antibody to CD69 (mAb, FITC; BD Biosciences, Heidelberg, Germany) on ice in the dark. After 30 min of staining, cells were washed twice with PBS and analyzed by FACScan (BD Biosciences).
Preparation of Nuclear Proteins, Total Cell Lysates, and Immunoblotting
Nuclear proteins were prepared as described previously (25). For cell lysates, T cells were lysed in ice-cold RIPA buffer (50 mm Tris-HCl, pH 8.0, 120 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 1 mm PMSF, 25 mm NaF, 0.1% SDS, 1 mm DTT, 0.2 mm Na3V04, and 20 μl/ml protease inhibitors) for 20 min at 4 °C. Equal amounts of proteins were run on 10% SDS-PAGE gels, transferred onto Hybond-ECL nitrocellulose membrane (GE Healthcare, Munich, Germany). The blot was blocked with 5% BSA in PBS/0.1% Tween 20 for 1 h at room temperature. The blot was washed 3 × 10 min with PBS/Tween 20 and developed with HRP-coupled antibodies in 5% milk powder in PBS/0.1% Tween 20 for 1 h at room temperature, washed again 3 × 10 min at room temperature followed by enhanced Chemiluminescence Reagent Plus (PerkinElmer Life Sciences, Rodgau). The following antibodies were used: the antibodies against NFκB p65 (A, sc-109), p50 (E-10, sc-8414) and YY1 (H-10, sc-7341) antibody from Santa Cruz Biotechnology, Inc. (Santa Cruz, Heidelberg, Germany); the anti-c-Jun monoclonal antibody from BD-Bioscience-Phamingen (Heidelberg, Germany); the anti-active p38 antibody from NEB (Frankfurt a. Main, Germany); the phosphor-SAPK/JNK (G9, 9255) monoclonal antibody from Cell signaling (Europe) and the anti-NF-ATc1 monoclonal antibody (7A6) from Alexis Biochemicals (Grünberg, Germany). For stripping, membranes were incubated for 30 min at 56 °C in a buffer containing 62.5 mm Tris-HCl, pH 6.7, 2% SDS, and 100 mm β-mercaptoethanol.
Confocal Laser Scan Microscopy (LSM)
Jurkat T cells stimulated with PMA/ionomycin for 30 min in the presence or absence of curcumin were fixed with Cytofix/Cytoperm (BD Pharmingen) and washed with Perm/Washing solution (BD Pharmingen) for three times at 4 °C. The cells were incubated with anti-NF-ATc1 for 30 min, washed three times with Perm/Washing solution as before and then stained with anti-IgG Cy3 antibodies for 30 min. The cells were washed with Perm/Washing solution for three times again and subjected to LSM. Slides were coated with poly-l-lysine to increase adhesion and prevent cell motility during microscopy. The nucleus was stained with DAPI. Confocal LSM was performed using a Zeiss LSM 510 UV microscope operating with an argon ion laser (488 nm) and a HeNe laser (543 nm) (Zeiss, Jena, and Oberkochen, Germany).
Plasmid Constructs and Transient Transfections
Luciferase reporter construct containing the human IL-2 (−300/+47), the human IFN-γ (−854/+7) promoter, and the multiple NF-AT, AP-1, and NF-κB luciferase constructs were generated previously (25). Jurkat T cells were transfected by electroporation as previously described (25). After overnight recovery, the cells were divided and treated with different doses of curcumin and then further cultured in the absence or presence of PMA (5 ng/ml) and ionomycin (1 μm) for 8 h. Luciferase activity was determined in 10 μl of cell extract using the luciferase assay substrate (Promega Corp., Mannheim, Germany) with a Duolumat LB9507 luminometer (Berthold, Bad Wildbad, Germany).
Determination of Intracellular Calcium
Cells were loaded with 1 μm Fluo-4 (Invitrogen, Darmstadt, Germany) at 37 °C in the dark for 20 min followed by treated with different doses of curcumin for 10 min and then, washed three times with PBS to remove the extracellular Ca2+ and suspended in PBS. The release of Ca2+ was measured by flow cytometry in a FACScan (Becton Dickinson, Sunnyvale, CA) in FL-1 after adding ionomycin (0.5 μm) or αCD3 (OKT3 10 μg/ml). For determination of influx of extra-cellular Ca2+, the intracellular Ca2+ stores were first depleted in the absence of extracellular Ca2+ (in PBS) by either ionomycin or αCD3 for 7 min. The cells were then treated with or without curcumin for 30 s and 1 mm Ca2+ was added back.
Calcineurin Phosphatase Assay
Calcineurin phosphatase activity was measured using a calcineurin assay kit (Merck). Each reaction (total 50 μl) contained 50 mm Tris, pH 7.5, 100 mm NaCl, 6 mm MgCl2, 0.5 mm CaCl2, 0.5 mm DTT, 0.025% Nonidet P-40, 0.25 μm calmodulin, and 40 units of calcineurin. The reaction was initiated at 30 °C by adding the phosphopeptide substrate to a final concentration of 0.15 mm. In the case of calcineurin inhibition, 5 or 10 μg/ml curcumin or 400 ng/ml CsA plus 200 ng/ml cyclophilin A (BIOMOL Research Laboratories, Inc) was included in the reaction mixture.
RESULTS
Curcumin Suppresses T Cell Activation
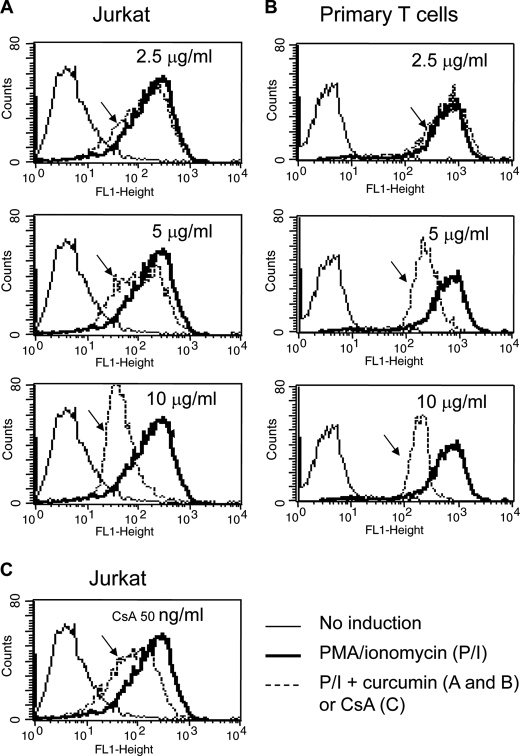
To investigate the role of curcumin on T cell activation, we used the Jurkat T cell line and peripheral blood T cells (PBT) freshly isolated from healthy donors as model systems. Jurkat or PBT were stimulated with ionomycin, a Ca2+ stimulus, in combination with PMA, which mimics antigen signals for T cell activation, in the absence or presence of different concentrations of curcumin. The effect of curcumin on the status of T cell activation was evaluated by the cell surface expression levels of CD69, an early activation marker of T cells (26). The experiment showed that PMA/ionomycin stimulation resulted in increase in CD69 expression in Jurkat and PBT cells. However, in the presence of curcumin (2.5–10 μg/ml equivalent to 7–28 μm), CD69 expression levels were reduced in a dose-dependent manner (Fig. 1, A and B). 5 μg/ml of curcumin (equivalent to 14 μm) reduced CD69 expression to a level comparable to that treated with 50 ng/ml of CsA (Fig. 1C). Suppression of CD69 expression correlated with reduced expression levels of the T cell cytokine IL-2 and IFN-γ (Fig. 2A). High doses of curcumin induces apoptotic cell death in PBT (> 30 μm) and Jurkat T cells (50 μm) (27, 28). At the doses used (2.5–10 μg/ml equivalent to 7–28 μm), no apoptotic cell death was seen (data not shown). These data confirm that curcumin suppresses T cell activation.
FIGURE 1.
Curcumin suppresses CD69 expression in activated T cells. A and B, curcumin suppresses CD69 expression in T cells. Jurkat (A) and freshly isolated human peripheral blood T cells (B) were stimulated with PMA (5 ng/ml) and ionomycin (1 μm) in the absence or the presence of 2.5 to 10 μg/ml curcumin as indicated. After 24 h stimulation, the cells were analyzed for CD69 surface expression levels by FACS. C, CsA suppresses CD69 expression. Jurkat T cells were stimulated with PMA and ionomycin in the absence or presence of CsA (50 ng/ml) as in A and B. The reduced CD69 expression is indicated by arrows. Results are representative of two independent experiments.
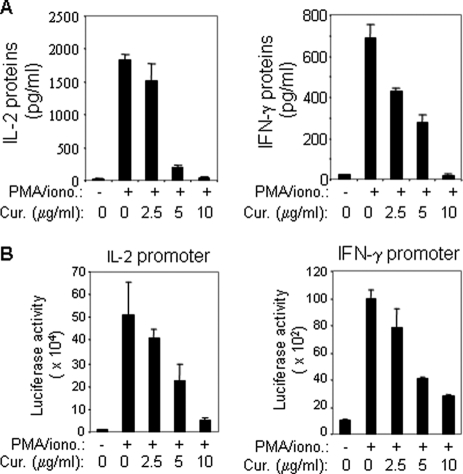
FIGURE 2.
Curcumin suppresses cytokine expression in T cells by down-regulation of promoter activity. A, curcumin inhibits IL-2 and IFN-γ protein expression in primary peripheral T cells. Freshly isolated human peripheral blood T cells were stimulated with PMA (5 ng/ml) and ionomycin (0.5 μm) in the absence or presence of different concentrations of curcumin as indicated. After 24 h stimulation, the supernatants were analyzed for IL-2 and IFN-γ protein production by ELISA. Results are representative of two independent experiments. B, curcumin down-regulates promoter activities of IL-2 and IFN-γ. Luciferase constructs containing the human IL-2 and IFN-γ promoter were transfected into Jurkat T cells. After overnight recovery, transfected cells were split and pre-incubated with different concentrations of curcumin as indicated for 1 h and then stimulated with PMA and ionomycin or left unstimulated for 8 h. The promoter activity was given as luciferase activity. Results are representative of two independent experiments each in triplicate transfections.
Curcumin Suppresses NFAT-mediated Transcriptional Activity
We next investigated the mechanisms by which curcumin down-regulates IL-2 and IFN-γ protein expression. Luciferase reporter plasmids containing promoters of the human IL-2 and IFN-γ were subjected to transient transfection studies into Jurkat T cells. Transfected cells were then stimulated with PMA/ionomycin in the presence or absence of different concentrations of curcumin. Consistent with the protein expression data, the promoter activities of IL-2 and IFN-γ were down-regulated by curcumin in a dose-dependent manner (Fig. 2B). Therefore, curcumin suppresses cytokine expression at the transcriptional levels.
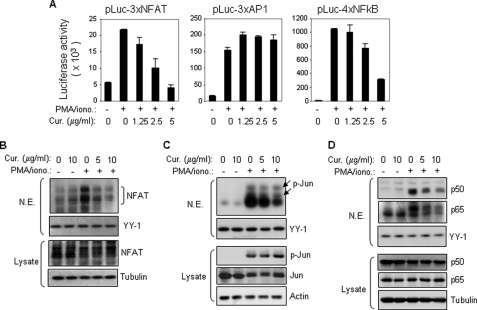
Most of inflammatory cytokine genes, including IL-2 and IFN-γ, are activated by the inducible transcription factors NFAT and NF-κB (29, 30). Curcumin has been shown to inhibit NF-κB activity in various cell types including T cells and, therefore, inhibition of NF-κB has been suggested to be the main mechanism of curcumin-mediated suppression of cytokine expression (31). Because NFAT is a key regulator of T cell function and has been shown to be essential for activating most T cell cytokine genes (32, 33), we examined the effect of curcumin on NFAT activity. A luciferase reporter construct containing multiple copies of the NFAT-binding elements derived from the IL-2 promoter was transiently transfected into Jurkat T cells, and the transfected cells were stimulated with PMA/ionomycin in the absence or presence of curcumin. The experiment showed that the transcriptional activity mediated by the NFAT elements was down-regulated by curcumin in a dose-dependent manner (Fig. 3A, left panel). The IL-2 NFAT element is composed of a combined NFAT and AP-1 (Fos/Jun) binding sequence (33). However, the same concentrations of curcumin did not suppress the transcriptional activity mediated by the consensus binding sequences for AP-1 (Fig. 3A, middle panel). Consistent with other studies, NF-κB-mediated transcriptional activity was down-regulated by curcumin (Fig. 3A, right panel). This study indicates that besides NF-κB, curcumin may inhibit NFAT transcriptional activity.
FIGURE 3.
Curcumin inhibits NFAT transcriptional activity by blocking its nuclear expression. A, curcumin inhibits NFAT-mediated transcription. Jurkat T cells were transfected with luciferase constructs containing multiple copies of the NFAT binding element derived from the IL-2 promoter or the consensus DNA binding element of either AP1 or NF-κB. After overnight recovery, the cells were split and pre-incubated with the indicated amounts of curcumin for 0.5 h and then stimulated with PMA (5 ng/ml) and ionomycin (0.5 μm) or left unstimulated. Luciferase activity was determined after 8 h stimulation. Data are representative of three independent experiments performed in triplicate. B, curcumin prevents stimulation-induced nuclear expression of NFAT determined by Western blot. Jurkat T cells were stimulated with PMA and ionomycin for 2 h in the absence or presence of different concentrations of curcumin as indicated. Nuclear proteins were isolated and the expression levels of NFAT were analyzed by Western blot with the specific mAb against NFATc1. YY1 was used for an equal loading control. Total cell lysates were used as an additional control for total amounts of NFAT expression in the experiment. C and D, as controls, Western blot was also carried out in parallel with antibodies against Jun and NF-κB (p50/p65), respectively. Total cell lysates were used as additional controls for total amounts of Jun and NF-κB expression in the experiment.
Curcumin Prevents T Cell Stimulation-induced NFAT Nuclear Translocation
To investigate the mechanism by which curcumin inhibits NFAT-mediated transcription, we examined the effect of curcumin on nuclear expression of NFAT. Jurkat T cells were stimulated with PMA/ionomycin for 2 h in the absence or presence of different concentrations of curcumin and nuclear proteins were isolated for Western blot analysis with specific antibodies against NFATc1. The experiment showed that NFATc1 was initially absent in the nucleus and rapidly expressed in the nucleus after 2 h of PMA/ionomycin stimulation. However, in the presence of curcumin, the nuclear NFATc1 expression levels were strongly impaired (Fig. 3B). Reduced nuclear expression of Jun and NF-κB (p50/p65) was also observed in the presence curcumin (Fig. 3, C and D). However, the levels of phosphorylated (activated) Jun were not affected by curcumin treatment (Fig. 3C). This may explain why AP-1-mediated transcriptional activity was not impaired by curcumin (Fig. 3A, middle panel). These experiments demonstrate that curcumin inhibits NFAT transcriptional activity by preventing its nuclear translocation.
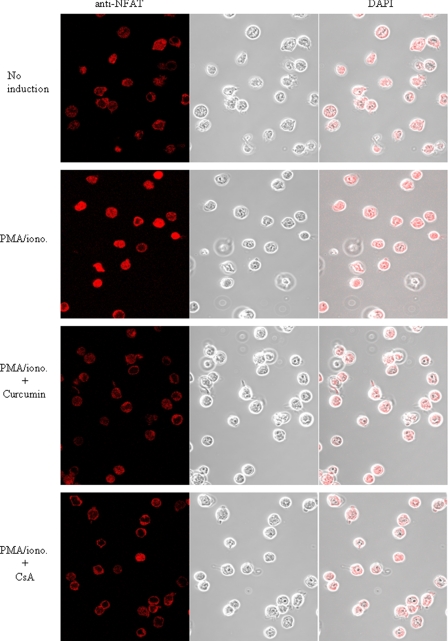
To confirm the suppressive effect of curcumin on nuclear translocation of NF-AT in vivo, we performed confocal LSM analysis. The LSM analysis showed that NFATc1 was translocated from the cytoplasm into the nucleus upon PMA/ionomycin stimulation of Jurkat T cells. However, in the presence of curcumin, the nuclear expression of NFATc1 was blocked (Fig. 4). These data demonstrate that curcumin can inhibit NFAT nuclear expression in stimulated T cells.
FIGURE 4.
Curcumin prevents stimulation-induced nuclear expression of NFAT determined by confocal LSM. Jurkat T cells were treated as described in Fig. 3B. The cellular and nuclear expression levels of NFATc1 were visualized by confocal LSM.
Curcumin Does Not Inhibit NFAT Nuclear Expression by Inhibition of Calcineurin or by Enhancing Activities of JNK and p38
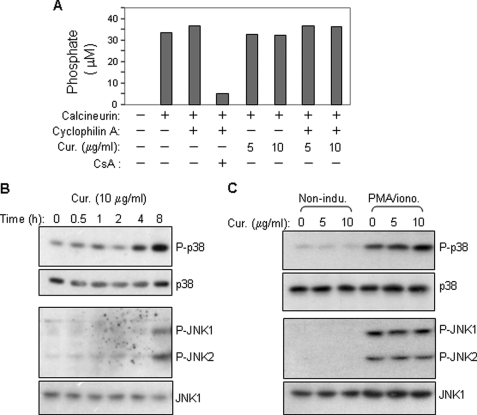
The NFAT family of proteins is activated by the calcium/calmodulin-dependent phosphatase calcineurin that dephosphorylates and promotes nuclear translocation of NFAT (33). Therefore, we asked whether curcumin inhibits NFAT activity by inhibition of calcineurin function. To address this question, an in vitro phosphatase assay was performed in the presence or absence of curcumin. The experiment showed that no reduction in calcineurin activity was seen in the presence of curcumin (Fig. 5A). In contrast, CsA strongly inhibited calcineurin phosphatase activity (Fig. 5A). Therefore, curcumin does not directly inhibit calcineurin activity.
FIGURE 5.
Curcumin does not directly inhibit calcineurin phosphatase activity and does also not enhance activities of the NFAT kinases p38 and JNK in activated T cells. A, curcumin has no direct inhibitory effect on calcineurin phosphatase. Calcineurin phosphatase activity was measured using an in vitro calcineurin assay kit in the absence or presence of curcumin. B, kinetic analysis of the effect of curcumin on p38 and JNK activity. Jurkat T cells were treated with curcumin (10 μg/ml) for different time periods. Total cell lysates were subjected to Western blot with antibodies against phosphorylated p38 (p-p38) and JNK (p-JNK1/2). C, curcumin does not enhance PMA/ionomycin-induced phosphorylation of p38 and JNK. Jurkat T cells were stimulated with PMA (5 ng/ml) plus ionomycin (1 μm) for 2 h in the absence or presence of different concentrations of curcumin. Total cell lysates were analyzed by Western blot. All data are representative of two independent experiments.
It has been shown that NFAT activation can be inhibited by the cellular MAPK JNK and p38 that phosphorylate and promote NFAT nuclear export (34, 35). Therefore, we asked whether curcumin inhibits NFAT activity by enhancing JNK and p38 activity. To address this question, Jurkat T cells were treated with curcumin for different time periods (Fig. 5B) or in combination with T cell stimulation with PMA/ionomycin (Fig. 5B). Kinetic analysis showed that curcumin alone induced phosphorylation of JNK and p38, but at a rather late time point (8 h) (Fig. 5B). As shown in Fig. 3B, inhibition of NFAT nuclear expression was seen at 2 h of curcumin treatment. In addition, curcumin did not significantly enhance the PMA/ionomycin-induced phosphorylation of JNK and p38 (Fig. 5C). These experiments ruled out the possibility that curcumin inhibits NFAT nuclear expression through enhancing JNK/p38-mediated nuclear export.
Curcumin Inhibits T Cell Activation-induced Ca2+ Mobilization
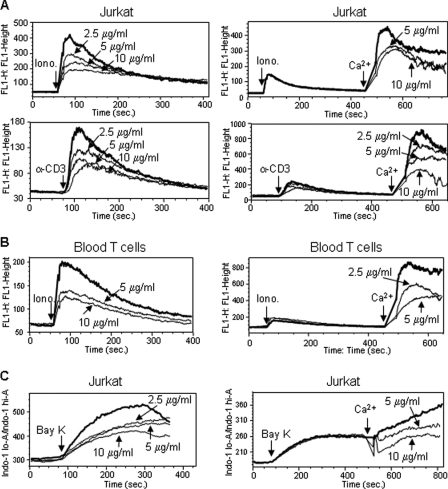
Because NFAT is a Ca2+-regulated transcription factor, we asked whether curcumin inhibits NFAT activity by interfering with T cell activation-induced Ca2+ signal transduction. To investigate this question, Jurkat T cells were loaded with the Ca2+ indicator Fluo-4 and then stimulated with ionomycin or α-CD3 antibody (which mimic TCR stimulation) in the absence or presence of curcumin. In the absence of curcumin, a rapid rise in cytosolic Ca2+ concentration was seen immediately after stimulation of the cells with ionomycin or with α-CD3 (Fig. 6A, left panel). In the presence of curcumin, the ionomycin- and α-CD3-induced increase in cytosolic Ca2+ concentrations was inhibited in a dose-dependent manner (Fig. 6A, left panel). These data suggest that curcumin blocks Ca2+ efflux from intracellular Ca2+ stores. To investigate whether curcumin could also block Ca2+ influx from outside the cell, Jurkat T cells were suspended in medium lacking Ca2+ and then stimulated with ionomycin or α-CD3 to deplete the intracellular Ca2+ store. After 7 min of Ca2+ depletion, cells were treated without or with different concentrations of curcumin for 30 s and, then, Ca2+ was added into the medium. A rapid uptake of Ca2+ into the cells was seen following Ca2+ supply (Fig. 6A, right panel). Curcumin was shown to also reduce Ca2+ influx from extracellular space (Fig. 6A, right panel). Similar results were obtained when the experiments were carried out with PBT freshly isolated from healthy donors (Fig. 6B).
FIGURE 6.
Curcumin blocks Ca2+ mobilization in activated T cells. A, effect of curcumin on Ca2+ mobilization in Jurkat T cells. Jurkat T cells were loaded with 1 μm fluorescent Ca2+-indicator Fluo-4 in PBS in the absence of extracellular Ca2+ followed by pretreated with different doses of curcumin for 10 min. Cells were washed with PBS. The release of Ca2+ from the intracellular stores was measured by flow cytometry after adding ionomycin (1 μm) or αCD3 (OKT3 10 μg/ml) (left panels). For determination of influx of extracellular Ca2+, the intracellular Ca2+ stores were first depleted in the absence of extracellular Ca2+ by ionomycin or by αCD3 for 7 min. The cells were then treated with or without curcumin for 30 s. The Ca2+ influx was measured after adding 1 mm Ca2+ (right panels). B, effect of curcumin on Ca2+ mobilization in peripheral blood T cells. Blood T cells were treated as in A. C, effect of curcumin on Ca2+ mobilization in Jurkat T cells stimulated with (±)-Bay K 8644. Jurkat T cells were loaded with 1 μm indol-1 and pretreated without or with curcumin as in A. The Ca2+ levels were monitored by flow cytometry after adding 100 μm (±)-Bay K 8644. All data are representative of at least two independent experiments.
The L-type voltage-gated calcium (L-type Cav) channel, known to mediate Ca2+ mobilization in excitable cells, has recently been shown to also play a critical role in Ca2+ mobilization in non-excitable cells including T lymphocytes (36, 37). Therefore, we asked whether curcumin interferes with l-type Cav-mediated Ca2+ mobilization. To address this question, Jurkat T cells were stimulated with the 1,4-dihydropyridine L-type channel agonist (±)-Bay K 8644 in the absence or presence of curcumin. The experiments showed that curcumin could block L-type Cav-mediated Ca2+ mobilization (Fig. 6C). These experiments demonstrate that curcumin exerts its immunosuppressive effect by blocking the T cell activation-induced Ca2+ signaling pathway.
Curcumin and CsA Cooperatively Down-regulate T Cell Activation
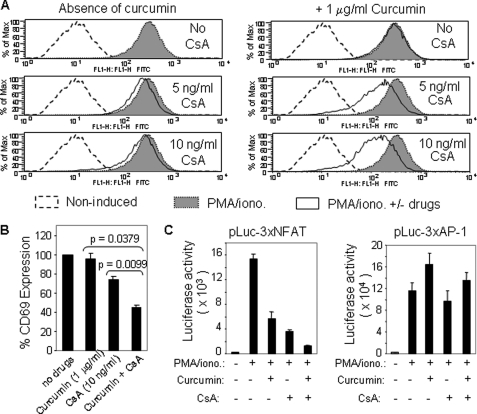
Because curcumin inhibits NFAT activation through a mechanism different from CsA, we predict that curcumin may enhance the immunosuppressive activity of CsA. To prove this prediction, we examined the CD69 expression levels of Jurkat T cells stimulated with PMA/ionomycin in the absence or presence of different concentrations of CsA with or without curcumin. As predicted, the experiments showed that even at a very low dose (1 μg/ml, equivalent to 2.5 μm) curcumin could significantly enhance the suppressive effect of CsA on CD69 expression (Fig. 7, A and B). To further investigate that this observation was due to enhanced inhibition of NFAT activity, the NFAT-luciferase reporter plasmid was transfected into Jurkat T cells, which were then treated with either CsA or curcumin alone or in combination. Indeed, the NFAT-mediated transcriptional activity could be further down-regulated by combination treatment with curcumin and CsA (Fig. 7C, left panel). As negative control, curcumin and CsA did not show a cooperative suppression of the AP-1-mediated transcriptional activity (Fig. 7C, right panel). These experiments demonstrate that curcumin can serve as an adjuvant of CsA.
FIGURE 7.
Curcumin enhances the immunosuppressive activities of CsA. A, curcumin and CsA synergize to inhibit CD69 expression in activated T cells. Jurkat T cells were stimulated with PMA/ionomycin in the absence or the presence of either CsA alone or CsA plus 1 μg/ml curcumin. After 17 h stimulation, the cells were analyzed for CD69 surface expression levels by FACS. B, data from A are presented as bar charts. C, curcumin and CsA cooperatively inhibit NFAT transcriptional activity. The NFAT and AP1 luciferase reporter plasmids were transfected into Jurkat T cells. After overnight culture, the cells were split and pre-incubated with solvents, curcumin, CsA, or curcumin plus CsA for 30 min and then stimulated with PMA/ionomycin or left unstimulated. Luciferase activity was determined 8 h after stimulation. Data are representative of three independent experiments performed in triplicate transfections.
DISCUSSION
So far, the anti-inflammatory and immunosuppressive function of curcumin has been largely attributed to its anti-oxidative activity and to inhibition of NF-κB (7, 31). In this study, we identified a new action of curcumin, namely, its function as an NFAT inhibitor through blocking the Ca2+ signaling pathway. This finding provides an additional mechanism for curcumin-mediated anti-inflammatory and immunosuppressive functions.
NFAT is a central regulator of T cell function essential for activation of most T cell cytokine genes (32, 33). We show that curcumin-mediated inhibition of cytokine expression in activated T cells involves inhibition of NFAT activation via blocking T cell activation-induced increase in Ca2+ mobilization. The major pathway that induces intracellular Ca2+ levels in lymphocytes is through store-operated calcium entry (SOCE) and CRAC channels (20, 21). TCR ligation leads to activation of PLC-γ, which, in turn, hydrolyzes phosphatidylinositol 4,5-bisphosphate to IP3 and DAG. IP3 binds to and opens IP3 receptors in the membrane of the ER resulting in release of Ca2+ from intracellular Ca2+ stores. Depletion of Ca2+ stores is then sensed by STIM1 that triggers entry of Ca2+ through the CRAC channels in the plasma membrane (20, 21). We show that curcumin inhibits the increase in cytosolic Ca2+ levels induced by ionomycin, a Ca2+ ionophore which induces release of Ca2+ from ER, or by α-CD3 antibody, which mimics TCR stimulation. These data suggest that curcumin function as a channel blocker. Indeed, a study using porcine cerebellar microsomal membranes to investigate the effect of curcumin on IP3-induced Ca2+ release demonstrates that curcumin is a potent inhibitor of the IP3 receptor with IC50 = 10 μm (38). Consistent with that study, we show that 5 μg/ml of curcumin (equivalent to 14 μm) blocked 50% of Ca2+ release from ER triggered by ionomycin or by α-CD3 (Fig. 6, A and B).
We also show that curcumin could prevent Ca2+ influx from the extracellular matrix after depletion of ER Ca2+ stores in T cells (Fig. 6, A and B). The major channel involved in the Ca2+ entry pathway from the extracellular matrix is the CRAC channel (20, 39). Whether curcumin directly interferes with CRAC channels remains an open question. Besides CRAC channels, a number of studies have shown that T cells express high levels of the L-type Cav1 pore-forming subunit. Recent evidence indicates that the L-type Cav channels are involved in Ca2+ entry during an immune response (40). We show that curcumin could inhibit cytosolic increase in Ca2+ levels induced by the L-type Cav channel agonist (±)-Bay K 8644 (Fig. 6C). Therefore, blocking L-type Cav function may be a mechanism by which curcumin prevents extracellular Ca2+ entry into the cells.
Following Ca2+ entry into the cells, NFAT is activated by the Ca2+-dependent serine/threonine phosphatase calcineurin which in turn dephosphorylates and promotes NFAT translocation from cytoplasm to nucleus (32, 33). Therefore, we examined whether curcumin inhibits NFAT activation via inhibition of calcineurin. However, curcumin, in contrast to CsA, does not directly inhibit calcineurin phophatase activity examined by an in vitro assay (Fig. 5A). We also show that curcumin has no effects on the activities of the NFAT kinases JNK and p38 (Fig. 5C). Therefore, curcumin-mediated inhibition of NFAT activity is unlikely through direct prevention of NFAT dephosphorylation or through acceleration of NFAT phosphorylation by JNK and p38.
Because curcumin suppresses NFAT activation through a mechanism that differs from that of CsA, this explains why curcumin could inhibit CsA-resistant T cells (18). This finding also provides the explanation for the observation that curcumin could prolong allografts observed in animal models (16, 17). CsA and FK-506 (Tacrolimus) are calcineurin inhibitors used to prevent graft-versus-host disease in organ transplantations. However, these drugs are quite toxic because of their ability to inhibit calcineurin in cells outside the immune system (41, 42). Therefore, it has precluded their use in other clinical situations such as allergy, inflammation, and autoimmune disease (41, 42). In comparison, pharmacological safety of curcumin has been demonstrated by its consumption for centuries and by several clinical trials (43, 44). Consistent with the early study showing that curcumin could enhance the immunosuppressive activity of CsA in rat cardiac allografts (17), we show that curcumin can enhance CsA-mediated suppression of T cell activation (Fig. 7). These data suggest that curcumin may be a promising adjuvant for CsA.
So far, the mechanism of curcumin-mediated suppression of NF-κB activation has been largely attributed to its anti-oxidant activity. The Ca2+ signaling pathway is also known to be crucial for TCR-induced NF-κB activation. It has been shown that the Ca2+-dependent phosphatase calcineurin cooperates with PKC to activate NF-κB in vivo (45). Both PKC and calcineurin were necessary, as inhibition of either one reverses the activation of the IKK complex and IKKα phosphorylation in vivo (45). The essential step in TCR-induced NF-κB activation is the formation of the CBM complex, which is composed of Carma 1, Bcl10 and Malt1. A most recent study shows that, in contrast to NFAT which is directly dephosphorylated by calcineurin, calcineurin regulates TCR-induced NF-κB activity by controlling the formation of the CBM complex (5). The study demonstrates that increased Ca2+ levels induced by ionomycin augmented the PMA-induced formation of the CBM complex and activation of NF-κB, whereas removal of Ca2+ by the Ca2+ chelator EGTA attenuated both processes (5). Therefore, inhibition of Ca2+ mobilization may contribute another mechanism by which curcumin suppresses NF-κB activation.
Taken together, by using Jurkat and primary human T lymphocytes, we demonstrate for the first time that curcumin is a potent inhibitor of NFAT activation and prevents NFAT activation by blocking Ca2+ signaling in T cells. Because Ca2+ is also the secondary messenger crucial for TCR-induced NF-κB signaling, our study also provides another mechanism by which curcumin suppresses NF-κB activation.
Footnotes
- CsA
- cyclosporine A
- TCR
- T cell receptor
- PTK
- protein-tyrosine kinases
- CRAC
- Ca2+ release-activated calcium
- LSM
- laser scan microscopy
- PMA
- phorbol myristate acetate
- NFAT
- nuclear factor of activated T cells
- IP
- inositol phosphate.
REFERENCES
- 1. Ammon H. P., Wahl M. A. (1991) Pharmacology of Curcuma longa. Planta Med. 57, 1–7 [DOI] [PubMed] [Google Scholar]
- 2. Araújo C. C., Leon L. L. (2001) Biological activities of Curcuma longa L. Mem. Inst. Oswaldo. Cruz. 96, 723–728 [DOI] [PubMed] [Google Scholar]
- 3. Jurenka J. S. (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev. 14, 141–153 [PubMed] [Google Scholar]
- 4. Goel A., Kunnumakkara A. B., Aggarwal B. B. (2008) Curcumin as “Curecumin”: from kitchen to clinic. Biochem. Pharmacol. 75, 787–809 [DOI] [PubMed] [Google Scholar]
- 5. Palkowitsch L., Marienfeld U., Brunner C., Eitelhuber A., Krappmann D., Marienfeld R. B. (2011) The Ca2+-dependent phosphatase calcineurin controls the formation of the Carma1-Bcl10-Malt1 complex during T cell receptor-induced NF-κB activation. J. Biol. Chem. 286, 7522–7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aggarwal B. B., Kumar A., Bharti A. C. (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 23, 363–398 [PubMed] [Google Scholar]
- 7. Strimpakos A. S., Sharma R. A. (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox. Signal. 10, 511–545 [DOI] [PubMed] [Google Scholar]
- 8. Xu Y. X., Pindolia K. R., Janakiraman N., Noth C. J., Chapman R. A., Gautam S. C. (1997) Curcumin, a compound with anti-inflammatory and anti-oxidant properties, down-regulates chemokine expression in bone marrow stromal cells. Exp. Hematol. 25, 413–422 [PubMed] [Google Scholar]
- 9. Abe Y., Hashimoto S., Horie T. (1999) Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 39, 41–47 [DOI] [PubMed] [Google Scholar]
- 10. Kang B. Y., Song Y. J., Kim K. M., Choe Y. K., Hwang S. Y., Kim T. S. (1999) Curcumin inhibits Th1 cytokine profile in CD4+ T cells by suppressing interleukin-12 production in macrophages. Br. J. Pharmacol. 128, 380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jang M. K., Sohn D. H., Ryu J. H. (2001) A curcuminoid and sesquiterpenes as inhibitors of macrophage TNF-α release from Curcuma zedoaria. Planta Med. 67, 550–552 [DOI] [PubMed] [Google Scholar]
- 12. Natarajan C., Bright J. J. (2002) Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J. Immunol. 168, 6506–6513 [DOI] [PubMed] [Google Scholar]
- 13. Singh S., Aggarwal B. B. (1995) Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane). J. Biol. Chem. 270, 24995–25000 [DOI] [PubMed] [Google Scholar]
- 14. Jobin C., Bradham C. A., Russo M. P., Juma B., Narula A. S., Brenner D. A., Sartor R. B. (1999) Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J. Immunol. 163, 3474–3483 [PubMed] [Google Scholar]
- 15. Plummer S. M., Holloway K. A., Manson M. M., Munks R. J., Kaptein A., Farrow S., Howells L. (1999) Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-κB activation via the NIK/IKK signaling complex. Oncogene. 18, 6013–6020 [DOI] [PubMed] [Google Scholar]
- 16. Jones E. A., Shoskes D. A. (2000) The effect of mycophenolate mofetil and polyphenolic bioflavonoids on renal ischemia reperfusion injury and repair. J. Urol. 163, 999–1004 [PubMed] [Google Scholar]
- 17. Chueh S. C., Lai M. K., Liu I. S., Teng F. C., Chen J. (2003) Curcumin enhances the immunosuppressive activity of cyclosporine in rat cardiac allografts and in mixed lymphocyte reactions. Transplant Proc. 35, 1603–1605 [DOI] [PubMed] [Google Scholar]
- 18. Ranjan D., Chen C., Johnston T. D., Jeon H., Nagabhushan M. (2004) Curcumin inhibits mitogen-stimulated lymphocyte proliferation, NFκB activation, and IL-2 signaling. J. Surg. Res. 121, 171–177 [DOI] [PubMed] [Google Scholar]
- 19. Baniyash M. (2004) TCR zeta-chain down-regulation: curtailing an excessive inflammatory immune response. Nat. Rev. Immunol. 4, 675–687 [DOI] [PubMed] [Google Scholar]
- 20. Feske S. (2007) Calcium signaling in lymphocyte activation and disease. Nat. Rev. Immunol. 7, 690–702 [DOI] [PubMed] [Google Scholar]
- 21. Hogan P. G., Lewis R. S., Rao A. (2010) Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 28, 491–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Müller M. R., Rao A. (2010) NFAT, immunity and cancer: a transcription factor comes of age. Nat. Rev. Immunol. 10, 645–656 [DOI] [PubMed] [Google Scholar]
- 23. Sigal N. H., Dumont F. J. (1992) Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu. Rev. Immunol. 10, 519–560 [DOI] [PubMed] [Google Scholar]
- 24. Ke H., Huai Q. (2003) Structures of calcineurin and its complexes with immunophilins-immunosuppressants. Biochem. Biophys. Res. Commun. 311, 1095–1102 [DOI] [PubMed] [Google Scholar]
- 25. Li-Weber M., Treiber M. K., Giaisi M., Palfi K., Stephan N., Parg S., Krammer P. H. (2005) Ultraviolet irradiation suppresses T cell activation via blocking TCR-mediated ERK and NF-κB signaling pathways. J. Immunol. 175, 2132–2143 [DOI] [PubMed] [Google Scholar]
- 26. Cho N. H., Feng P., Lee S. H., Lee B. S., Liang X., Chang H., Jung J. U. (2004) Inhibition of T cell receptor signal transduction by tyrosine kinase-interacting protein of Herpesvirus saimiri. J. Exp. Med. 200, 681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piwocka K., Zablocki K., Wieckowski M. R., Skierski J., Feiga I., Szopa J., Drela N., Wojtczak L., Sikora E. (1999) A novel apoptosis-like pathway, independent of mitochondria and caspases, induced by curcumin in human lymphoblastoid T (Jurkat) cells. Exp. Cell Res. 249, 299–307 [DOI] [PubMed] [Google Scholar]
- 28. Cipriani B., Borsellino G., Knowles H., Tramonti D., Cavaliere F., Bernardi G., Battistini L., Brosnan C. F. (2001) Curcumin inhibits activation of Vγ9Vδ2 T cells by phosphoantigens and induces apoptosis involving apoptosis-inducing factor and large scale DNA fragmentation. J. Immunol. 167, 3454–3462 [DOI] [PubMed] [Google Scholar]
- 29. Serfling E., Avots A., Neumann M. (1995) The architecture of the interleukin-2 promoter: a reflection of T lymphocyte activation. Biochim. Biophys. Acta. 1263, 181–200 [DOI] [PubMed] [Google Scholar]
- 30. Sica A., Dorman L., Viggiano V., Cippitelli M., Ghosh P., Rice N., Young H. A. (1997) Interaction of NF-κB and NFAT with the interferon-γ promoter. J. Biol. Chem. 272, 30412–30420 [DOI] [PubMed] [Google Scholar]
- 31. Gao X., Kuo J., Jiang H., Deeb D., Liu Y., Divine G., Chapman R. A., Dulchavsky S. A., Gautam S. C. (2004) Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem. Pharmacol. 68, 51–61 [DOI] [PubMed] [Google Scholar]
- 32. Im S. H., Rao A. (2004) Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol. Cells. 18, 1–9 [PubMed] [Google Scholar]
- 33. Macian F. (2005) NFAT proteins: key regulators of T cell development and function. Nat. Rev. Immunol. 5, 472–484 [DOI] [PubMed] [Google Scholar]
- 34. Gómez del Arco P., Martínez-Martínez S., Maldonado J. L., Ortega-Pérez I., Redondo J. M. (2000) A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J. Biol. Chem. 275, 13872–13878 [DOI] [PubMed] [Google Scholar]
- 35. Porter C. M., Havens M. A., Clipstone N. A. (2000) Identification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J. Biol. Chem. 275, 3543–3551 [DOI] [PubMed] [Google Scholar]
- 36. Kotturi M. F., Carlow D. A., Lee J. C., Ziltener H. J., Jefferies W. A. (2003) Identification and functional characterization of voltage-dependent calcium channels in T lymphocytes. J. Biol. Chem. 278, 46949–46960 [DOI] [PubMed] [Google Scholar]
- 37. Badou A., Jha M. K., Matza D., Mehal W. Z., Freichel M., Flockerzi V., Flavell R. A. (2006) Critical role for the beta regulatory subunits of Cav channels in T lymphocyte function. Proc. Natl. Acad. Sci. U.S.A. 103, 15529–15534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dyer J. L., Khan S. Z., Bilmen J. G., Hawtin S. R., Wheatley M., Javed M. U., Michelangeli F. (2002) Curcumin: a new cell-permeant inhibitor of the inositol 1,4,5-trisphosphate receptor. Cell Calcium. 31, 45–52 [DOI] [PubMed] [Google Scholar]
- 39. Parekh A. B. (2010) Store-operated CRAC channels: function in health and disease. Nat. Rev. Drug Discov. 9, 399–410 [DOI] [PubMed] [Google Scholar]
- 40. Matza D., Flavell R. A. (2009) Roles of Ca(v) channels and AHNAK1 in T cells: the beauty and the beast. Immunol. Rev. 231, 257–264 [DOI] [PubMed] [Google Scholar]
- 41. Sigal N. H., Dumont F., Durette P., Siekierka J. J., Peterson L., Rich D. H., Dunlap B. E., Staruch M. J., Melino M. R., Koprak S. L. (1991) Is cyclophilin involved in the immunosuppressive and nephrotoxic mechanism of action of cyclosporin A? J. Exp. Med. 173, 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dumont F. J., Staruch M. J., Koprak S. L., Siekierka J. J., Lin C. S., Harrison R., Sewell T., Kindt V. M., Beattie T. R., Wyvratt M. (1992) The immunosuppressive and toxic effects of FK-506 are mechanistically related: pharmacology of a novel antagonist of FK-506 and rapamycin. J. Exp. Med. 176, 751–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheng A. L., Hsu C. H., Lin J. K., Hsu M. M., Ho Y. F., Shen T. S., Ko J. Y., Lin J. T., Lin B. R., Ming-Shiang W., Yu H. S., Jee S. H., Chen G. S., Chen T. M., Chen C. A., Lai M. K., Pu Y. S., Pan M. H., Wang Y. J., Tsai C. C., Hsieh C. Y. (2001) Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 21, 2895–2900 [PubMed] [Google Scholar]
- 44. Sharma R. A., McLelland H. R., Hill K. A., Ireson C. R., Euden S. A., Manson M. M., Pirmohamed M., Marnett L. J., Gescher A. J., Steward W. P. (2001) Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 7, 1894–1900 [PubMed] [Google Scholar]
- 45. Trushin S. A., Pennington K. N., Algeciras-Schimnich A., Paya C. V. (1999) Protein kinase C and calcineurin synergize to activate IκB kinase and NF-κB in T lymphocytes. J. Biol. Chem. 274, 22923–22931 [DOI] [PubMed] [Google Scholar]